Abstract
Dimethyl disulfide (DMDS) was identified as a major volatile constituent of Brassica napus roots heavily infested by Delia radicum, the cabbage root fly. Attractiveness of this widespread compound was tested in the field in a naturally complex odorous environment. By using an original setup especially designed for ground dwelling beetles, different concentrations of the pure molecule as well as attractiveness of the natural blend emitted by the rotten part of infested roots were tested simultaneously. The use of general linear model (GLM) statistics permitted us to finely discriminate the responses among the different treatments. The main predators of D. radicum (i.e., two staphylinids Aleochara bilineata and Aleochara bipustulata and carabid beetles of the genus Bembidion) were significantly attracted by DMDS, but responded in different ways to the natural blend and to the different concentrations tested. The dose–response curves were similar for the two staphylinids. However, whereas A. bilineata was more attracted by the natural volatile blend than by its preferred DMDS concentration, A. bipustulata was attracted as much by the natural blend as by its preferred DMDS concentration. Carabid beetles exhibited a different response. They were not attracted by the natural blend, but responded to a wider range of DMDS concentrations that included low concentrations that did not attract the staphylinid beetles. These results are discussed according to the potential resources searched by each taxon studied and their specificity for the resources. The possible use of DMDS for enhancing biological control of D. radicum is mentioned.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs) emitted by plant–herbivore interactions are of importance for host or prey location by parasitoids and predators of phytophagous insects (e.g., Vinson et al. 1987; Dicke et al. 1990a, b; Turlings et al. 1995; Karban and Baldwin 1997). More than 1,000 VOCs are involved in such interactions (D’Alessandro and Turlings 2006), and in a single plant–herbivore complex 30–50 VOCs are frequently detected by chromatographic analysis. Some entomophagous arthropods are able to discriminate between minor qualitative or quantitative differences among this vast number of compounds, and use these differences as reliable cues to find hosts or prey. For example, sprouts infested by Plutella xylostella emit 10 additional VOCs compared to uninfested sprouts that emit 49 compounds. These qualitative differences are perceived by the parasitoid wasp Diadegma semiclausum that prefer the odor of infested plants (Bukovinszky et al. 2005). Among the myriad of VOCs identified, some are common and emitted in many plant arthropod interactions, whereas others are highly specific to a particular interaction. It is generally admitted that in tritrophic interactions that involve a carnivorous species specialized toward a particular phytophagous insect, the VOCs used by foragers are highly specific (Vet and Dicke 1992; Steidle and Van Loon 2003). Nevertheless, there is growing evidence that some specialist insects also use general cues such as β-farnesene (Mumm and Hilker 2005), methyl salicylate (de Boer and Dicke 2004), or green leaf volatiles (Whitman and Eller 1992). To understand how individuals use the cues of their environment in a way that can lead to an optimized foraging strategy (Bernays 1996), it is crucial to unravel how predators and parasitoids are able to use more or less specific compounds as reliable foraging cues. However, most studies that examine the effects of VOCs on the behavior of parasitoids or predators have been carried out in laboratory conditions (e.g., Vinson et al. 1987; Dicke et al. 1990a; Turlings et al. 1995; Sabelis et al. 2001; de Boer and Dicke 2004). Field studies are scarce (but see De Moraes et al. 1998 or Kessler and Baldwin 2001), and direct evidence for the potential of synthetic VOCs as field attractants for beneficial insects has only recently been obtained (James 2003, 2005).
Delia radicum, the cabbage root fly, is an economically important pest of cabbage crops (Finch 1989). Females lay their eggs in small clusters in the immediate vicinity of stems of Brassicaceae plants. Larval development takes place inside the roots, where larvae dig galleries while feeding, leading to root decay when infestation is heavy. A large part of egg and larval mortality in D. radicum is caused by predation by several species of carabidae and staphylinidae (Wishart et al. 1956; Hugues 1959; Coaker and Williams 1963; Luff 1987; Wright et al. 1960; Mowat and Martin 1981).
In this study, we investigated VOCs used in the field by the main natural enemies of D. radicum that are naturally found in cole crops (Finch 1989): small carabid beetles belonging to the genus Bembidion and the rove beetles Aleochara bilineata and A. bipustulata (Coleoptera : Staphilinidae). In both cases, adults are predators of D. radicum eggs and larvae, but the staphilinid larvae also develop as parasitoids of D. radicum pupae (Fuldner 1960). The two Aleochara species have been suggested previously as potential biological control agents against Delia antiqua, a pest of Alliaceae plants (Tomlin et al. 1985; Finch 1989). Whereas carabid beetles are generalist predators, A. bilineata and A. bipustulata, because they are also parasitoids of D. radicum, are more specialized. However, despite their close morphology and biology, the two staphilinids differ in host ranges. Whereas A. bilineata is a specialist of phytophagous and saprophagous Anthomyiidae dipterans, A. bipustulata has a wider host range, parasitizing not only Anthomyiidae species but also coprophagous and necrophagous non-Anthomyiidae dipterans. This difference in specificity should translate into a different use of volatile cues.
Only a few studies have examined the role of VOCs in host-finding behavior of D. radicum predators. In laboratory conditions, by using a Y-tube olfactometer, Royer and Boivin (1999) showed that volatiles coming from host-infested plants, as well as odors from larval frass and hydrosoluble volatiles coming from the larval tegument, were attractive to A. bilineata adults. However, the volatiles involved were not precisely identified. Another study highlighted the role of onion in mixed-crop strategies to improve the presence of D. radicum natural enemies, A. bilineata, A. bipustulata, and carabid beetles (Uvah and Coaker 1984). Nevertheless, it was unclear whether the observed effects were caused by onion volatiles or by confounding effects like the presence of additional resources. Finally, two field studies have revealed the ability to increase A. bipustulata catches in brassica plots that contain mustard seed meal (Ahlström-Olsson and Jonasson 1992; Riley et al. 2007). Which compounds, among the 14 identified in the natural odor source, could be responsible for this attraction was not specified.
To unravel the foraging cues used by A. bilineata, A. bipustulata and carabid beetles, to locate D. radicum, we first analyzed in the laboratory the VOCs emitted by the rotten part of Brassica napus roots that were heavily infested by D. radicum larvae. Then, by using an original bioassay designed for ground-dwelling beetles, we analyzed in the field the attractive effect on D. radicum natural enemies of various concentrations of the main VOC found compared to the odor emitted by naturally infested roots.
Methods and Materials
Natural Odor Source
Infested roots used for the chemical analysis of volatiles and for the bioassays were obtained from our D. radicum rearing facility at the University of Rennes (France). Infestations were carried out in a controlled room (21 ± 1°C, 60% RH, 16L:8D photoperiod). To obtain heavily infested material, swede roots (Brassica napus var. napobrassica) were placed on a bed of oven-dried sand in a glass plate and then put in 1 × 1 × 1 m Plexiglas™ cages that contained a few hundred D. radicum adults. After 3 d, the plates containing the roots harboring many eggs were removed and placed into an insect-proof empty cage. D. radicum larvae were left to develop in the roots for 3 wk before the material was used in the experiments. The high level of infestation chosen corresponds to what can be found in the field after the first emergence peak of the flies.
Identification of Major Compounds Emitted by Heavily Infested Roots
Three grams of the rotten part of heavily infested roots were put into a 30-ml glass vial closed with aluminum foil. Five repetitions of samples taken from different roots were carried out. Volatile sampling was obtained by inserting a solid-phase micro-extraction (SPME) fiber (1 cm long, 100 μm polydimethylsiloxane coating, SUPELCO 57300-U) through the aluminum sheet into the free space of the vial, where it was maintained for 15 min. Qualitative analysis was carried out with a Perkin–Elmer GC AutoSystem gas chromatograph (GC) coupled to a Perkin–Elmer Turbomass quadrupole mass spectrometer (MS). The GC column was a SGE BP1 capillary column (length 25 m, inner diameter 0.32 mm, film thickness 0.5 μm). The SPME fiber was inserted into the GC injector for thermal desorption in splitless mode for 2 min, and the split flow was adjusted to 30 ml/min. The injector was maintained at 250°C. The GC oven remained at 50°C for 2 min, then raised to 300°C at 5°C/min; helium was used as a carrier gas at a constant flow rate of 1 ml/min; the transfer line to the MS was thermostatted at 280°C, and the ion source at 180°C. MS spectra were recorded from 30 to 500 amu per 0.6 sec in the electron ionization mode at an electron energy of 70 eV and a filament emission of 200 μA. Identification of molecules was carried out by using the internal NIST library, our personal library, and was confirmed by using pure standards when possible.
Quantitative Measurement of Dimethyl Disulfide (DMDS)
All volatiles were collected by using a static headspace method. Headspace consisted of a 1-l airtight closed glass jar. A hole in the middle top of the jar permitted us to insert a SPME fiber for volatile sampling and the pure molecule dilutions for calibration. To allow odor concentration into the headspace, this hole was filled up with patafix™. Odors were sampled during 10 min, with the SPME fiber (same reference as above) at room temperature (24 ± 1°C).
Quantitative measurements were carried out with a Perkin–Elmer GC-FID AutoSystem gas chromatograph (GC) equipped with a flame ionization detector (FID). The GC column was a Perkin–Elmer PE-5ms (5% diphenyl dimethylpolysiloxane) capillary column, (length 20 m, inner diameter 0.18 mm, film thickness 0.18 μm). SPME injection and oven program were the same as described for the GC-MS used in the qualitative analysis.
For calibration, a constant quantity of 1 μl of solution that contained DMDS diluted in paraffin oil was inserted with a Pipetman™ through the hole of the jar, and deposited on a small piece of filter paper maintained inside the jar with Patafix™. As soon as the droplet was deposited, the hole was closed for 5 min, permitting the volatile to evaporate before SPME sampling. The net quantities of DMDS used for calibration were: 1, 0.5, 0.1, 0.05, 0.03, and 0.01 μl. For each of these volumes, the measurement was repeated three times with new prepared solutions. Calibration measurements were conducted over the whole period of chemical analysis. The results showed a strong correlation between the FID peak area and the volume of DMDS used (peak area = 8 × 10E-07 μl DMDS; R 2 = 0.9726).
Quantity of DMDS Emitted by Heavily Infested Roots
Infested roots were weighted and placed in the jar 1 hr before volatile sampling to allow odor concentration to build up. After this period, the quantity of DMDS emitted was measured as described above. Quantities emitted were expressed in nanoliters of DMDS produced per gram of fresh product per hour (nl g−1 hr−1). Measures were repeated on five different roots.
Quantity of DMDS Emitted by Field Traps
Two microliters of DMDS diluted in 18 μl of paraffin oil were deposited on a 1-cm2 piece of filter paper inside the trap (see trap description below). Traps were opened in the same way as in the field bioassay and kept 1, 5, or 24 hr on a work surface under an extractor at room temperature (24 ± 1°C). After this period, traps were placed into the 1-l jar and left to emit for 1 hr before SPME sampling. Measures were repeated on three different traps.
Field Bioassays
Bioassays were conducted on a 20 × 40 m broccoli plot in the experimental field station of La Rimbaudais in Saint Meloir des Ondes (48.65°N, 1.9°W), Brittany, France, in the heart of an important zone of cabbage production. Broccoli plants (Brassica oleracea Italica var. monopoly) were 8 wk old at the time of the experiment. Experiments were carried out on June 28 and 29 and July 4, 2006 under sunny weather (mean temperatures of 18.5, 18.3, and 22.5°C).
Setup
The setup consisted of a rectangular grid of 90 equally spaced mini-pitfall traps arranged over nine rows of culture with 10 traps per row (spacing between and among rows was 80 cm, Fig. 1). Accordingly, the position of each trap in the setup could be identified by its row number (1–9) and its column number (1–10). The location of the setup in the parcel was changed for each date the experiment was carried out.
Diagram showing the traps used to catch Aleochara bilineata, A. bipustulata, and carabid beetles of the genus Bembidion and their disposition in the field bioassays. A rectangular grid of 90 equally spaced traps was arranged over nine rows of broccoli plants with 10 traps per row (spacing between and among rows was 80 cm)
Traps were made of 35-ml plastic cups (33 mm high, 40 mm upper diameter, P100 Solocup™). They were filled with odor sources in the field station’s lab as follow: 1 g of the rotten part of an infested rutabaga root (R) or 20 μl of DMDS diluted in paraffin oil at the concentrations of 0% (C), 0.1% (D002), 1% (D02), 10% (D2), and 100% (D20), corresponding to a net quantity of 0, 0.02, 0.2, 2, and 20 μl of DMDS. As soon as an odor source was added, the cup was hermetically sealed with a piece of parafilm™. The 15 traps per treatment were randomly spatially distributed on the grid by using the internal function “sample” of R. Traps were placed in the field between 12:00 a.m. and 1:00 p.m. so that the upper part of the cup would be flush with the soil surface. After 1 hr, traps were carefully opened by piercing the piece of Parafilm™ in the middle with a precision forceps. Holes made this way were about 2 mm in diameter and permitted both odor diffusion and insect trapping. To limit variations caused by diel activity cycles, trapping was allowed for a 24-hr period without any perturbation in the field plot.
In parallel to the setup described above, a transect of 10 pitfalls (10 cm diameter) filled up with 50% alcohol and water, placed perpendicular to rows inside the parcel, was used to monitor sex ratios of A. bilineata and A. bipustulata in the field. These pitfalls will be referred to as “control pitfalls” in the “Results” section.
Recovery of Trapped Insects
Traps were brought to the lab for insect recovery. For each trap, we noted the treatment and position of the trap (row, column) as well as the number, species, and sex (determined with the presence of the male parameres, i.e., male genital apparatus) of A. bilineata and A. bipustulata, the number of other staphylinids, carabids, other coleopterans, dipterans, arachnids, and extra information such as dead individuals. All collected insects were grouped by date and treatment and conserved in alcohol for further investigation.
Statistical Analysis
Statistical analyses were done with R software (R Development Core Team 2006). The numbers of trapped insects were analyzed with general linear model (GLM) statistic using the numbers of individuals caught in each trap. The distribution of the data was consistent with a Poisson distribution, and the linking function “log” was chosen for these GLMs. Factor effects were analyzed with the function “anova.glm” of R, using a chi-squared test, on a complete model that contained the following effects: treatment (i.e., the five concentrations of DMDS: C, D002, D02, D2, and D20, and R), date, treatment × date interaction, row, column. Numbers of individuals belonging to the same taxon trapped in different odors were compared with an analysis of contrast, using the function “esticon” of the package “doBy” (Højsgaard 2004).
The goodness-of-fit of each model was assessed by a graphical observation of the quantile randomized residuals (Dunn and Smyth 1996), plotted against the normal distribution quantiles (normal probability plot) by using the function “qqnorm” (Becker et al. 1988). Quantile randomized residuals were calculated using the function “qresiduals” from the R package “statmod” (Smyth 2005). The use of randomized quantile residuals instead of residuals per se was more appropriate because of: (1) the use of a Poisson family for the GLM fit, and (2) the fact that there were only a few possible values for the response (typically 0, 1, 2, 3) (Dunn and Smyth 1996). For all the GLMs used, graphical representations revealed a good fit between the randomized quantile residuals and the normal probability plot. Furthering the analysis of the residuals, we made for each response at each date a spatial representation of the simplest model residuals over the set up. This representation permitted us to locate any spatial structuration of the residuals that would have revealed a non-independence of the number of insects caught in traps located close together.
Finally, to avoid all possible bias caused by interference inside a taxon (like aggregation behavior), all GLM analyses were carried out again in the presence/absence of analyzed taxa in each trap by using a binomial link function. The results (not shown) are similar to those obtained when using insect counts, thus supporting the conclusions presented herein.
Results
Identification and Quantification of Volatiles
The gas chromatograpy–mass spectrometry (GC-MS) analysis of volatiles trapped with SPME revealed a relatively simple profile essentially composed of sulfides (Fig. 2). The main volatile compound identified was dimethyl disulfide (DMDS). Other compounds such as dimethyl trisulfide, dimethyl tetrasulfide, 2-4-5-trithiahexane, and 2-4-dithiapentane were found in smaller amounts, sometimes as traces, or not at all, depending on the sample.
Among these volatiles, we chose to work with DMDS for several reasons: (1) In all cases, DMDS appeared to be one of the major compounds; (2) As an end product of tissue degradation and because of the high content of sulfur-containing compounds in this genus (notably all glucosinolates), DMDS is likely to be emitted by all attacked Brassicacae roots, regardless of the species infested. Thus, it could be a reliable marker of the presence of D. radicum.; (3) DMDS is known as an end product of Alliaceae tissue degradation (Auger et al. 1989b) and could be emitted by these plants also when attacked by D. antiqua, a host and prey of both staphilinids studied in this paper.
The quantitative measure of DMDS in infested roots revealed a highly variable concentration. The quantity of DMDS emitted per gram of fresh product in 1 hr, measured on six samples ranged from 0.5 to 38.2 nl, with a mean of 11.2 nl g−1 hr−1.
Quantity of DMDS Emitted by the Traps
The quantity of DMDS emitted by the traps we used decreased with time. With an initial volume of 2 μl pure DMDS inside the trap, the quantity emitted during the first 6 hr after opening was in the same range as what was emitted by 1 g of infested roots: 38.5 ± 13.2 nl/hr (±SE) between the first hour and the second hour, down to 8.0 ± 0.86 nl/hr (±SE) between the fifth hour and the sixth hour. After 24 hr, only traces were recovered.
Insects Caught in the Traps
The 270 traps caught a total of 473 arthropods distributed as follows: 123 A. bipustulata, 83 A. bilineata, 68 other staphylinids, 155 carabid beetles, 26 other coleopterans (essentially Halticinae), 11 dipterans (all belonging to the genus Delia), one ant, six Arachnids, plus hundreds of springtails (Hexapoda: Collembola) that were not taken into account. Of these arthropods, 32.8% were caught in the traps that contained infested roots, whereas only 6.6% were caught in control traps. The remaining 60.6% of individuals trapped were divided among the traps that contained one of the four DMDS concentrations. For each treatment, a certain number of traps did not catch any of the species we were interested in (N = 33, 26, 15, 11, 24, and 12 for T, D002, D02, D2, D20, and P, respectively).
A. bilineata
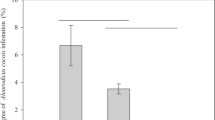
A total of 83 A. bilineata adults were caught (0.31 ± 0.06 per trap). The treatment effect was highly significant (Table 1, Fig. 3). Traps containing 0.02 or 0.2 μl of DMDS did not catch more A. bilineata than control traps. Traps containing 2 or 20 μl of DMDS caught a higher number of A. bilineata than control traps and were not significantly different from each other. Traps that contained infested root caught more A. bilineata than all the other treatments (Fig. 3).
The row and column effects were both significant, but without any obvious general pattern (none of the internal or the external rows or columns was responsible for this effect). This observation was consistent with the spatial representation of residuals that did not reveal any structuration.
Although odorous traps captured more females (N = 48) than males (N = 33), the sex ratio was not biased (binomial test, P = 0.120), and did not differ from that obtained from control pitfalls for the same week (58 females and 44 males, Chi-square test between attractive traps and control pitfalls: χ 2 = 0.030; 1 df, P = 0.861).
A. bipustulata
A total of 123 A. bipustulata adults were caught (0.46 ± 0.05 per trap). The treatment effect was highly significant (Table 1, Fig. 4). Traps that contained only 0.02 μl of DMDS did not catch more insects than control traps, whereas traps that contained 0.2, 2, and 20 μl of DMDS or traps that contained infested roots did. Traps with 2 μl of DMDS caught a higher number of insects than other DMDS concentrations and were not significantly different from traps that contained infested roots.
The influence of the date was significant for A. bipustulata (43 individuals caught between June 28 and 29, 52 between June 29 and 30, and 38 between July 4 and 5). As for A. bilineata, there was no effect of the interaction between the date and the treatment.
The row and column position of the traps had no effect (Table 1), in agreement with the spatial representation of residuals.
Sex ratios in the odorous traps were biased toward females (74 females and 49 males, binomial test, P = 0.030), but this was not caused by odors as a similar bias was observed in control pitfalls for the same week (control pitfall: 81 females and 56 males, Chi-square test between attractive traps and control pitfalls, χ 2 = 0.002; 1 df, P = 0.965).
Carabid beetles
A total of 155 carabid adults was caught (0.57 ± 0.06 per trap). All were identified a posteriori as Bembidion tetracolum (N = 91) or Bembidion lampros (N = 62) with the exception of one B. quadrimaculatum and one Microlestes minutulus. The treatment effect was highly significant (Table 1, Fig. 5). Surprisingly, the traps with the highest concentration of DMDS (20 μl) or naturally infested root odor had no significant effect on carabids, whereas traps with lower concentrations (2, 0.2 or even 0.02 μl of DMDS) caught more carabid beetles than control traps.
The influence of the date was highly significant, particularly because of the twice lower number of insects caught on July 4–5 (N = 27, compared to N = 67 and N = 61 captured on June 28–29 and 29–30, respectively). Nevertheless, there was no effect of the interaction between the date and the treatment.
The column effect was significant, although the row effect was not. As in the case of A. bilineata, however, no obvious pattern appeared. Furthermore, the observation of the spatial representation of residuals revealed no structuration.
Discussion
Field bioassays revealed the ecological importance of DMDS in the orientation behavior of the main predators of D. radicum eggs and larvae. This compound, frequent in sulfur-containing plants, was emitted in large amounts by brassica roots heavily infested by D. radicum. Our results show that DMDS attracts the staphylinids A. bilineata, A. bipustulata, and the carabids Bembidion tetracolum and B. lampros. Concentration of the pure molecule seems to play a role, influencing the response of the different predators. To our knowledge, this is the first field study that demonstrates that the concentration of one monomolecular odor influences the orientation behavior of different arthropod predators that belong to the same guild. These results shed new light on the use of general cues by foraging insects, and could also be helpful for enhancing biological control of D. radicum, which feeds on open field crops.
To date, the role of DMDS as a carnivorous insect attractant has been shown only in two tritrophic complexes. The first involves cabbage plants (Brassica oleracea spp. capitata), the phytophagous lepidoptera Plutella xylostella, its specialist parasitoid wasp Cotesia plutellae, and a generalist predator, the lacewing Chrysoperla carnea (Neuroptera) (Reddy et al. 2002). The second involves leek (Allium porum), the phytophagous specialist microlepidoptera Acrolepiopsis assectella, and its parasitoid wasp Diadromus pulchellus (Dugravot and Thibout 2006). In the first study, Reddy et al. (2002) found DMDS in the frass of P. xylostella that was attractive to C. plutellae and C. carnea in a Y-tube olfactometer setup. In the second, the parasitoid wasp D. pulchellus was attracted by a blend of odors coming from attacked leeks (that emit thiosulfinates) and odors of A. assectella larval frass (Dugravot et al. 2005; Dugravot and Thibout 2006), the latter material being known to emit a high concentration of DMDS (Auger et al. 1989a). Although DMDS appears to be present in interactions that involve sulfur-containing plants and their associate herbivorous species, little is known about its importance as a cue at the third trophic level (i.e., predators and parasitoids). This study is the first carried out in field conditions that demonstrates such a role for this molecule.
As an end product of sulfur-containing tissues, DMDS is likely emitted by all Brassicaceae infested by D. radicum, regardless of plant species. Interestingly, it is also an end product of Alliaceae (Auger et al. 1989b), which are frequently grown in the same geographical zones as cole crops and are attacked by D. antiqua, another important host (and prey) of both staphylinids studied here. Accordingly, the positive response of A. bilineata and A. bipustulata toward DMDS could allow them to locate two of their major preys and hosts (i.e., D. radicum and D. antiqua) regardless of the infested plant involved. Assessing attractiveness of DMDS in Allium crops would confirm this hypothesis.
The main difference we found between the two staphylinid species was their reaction toward the full blend of volatiles emitted by Brassica napus-infested roots. Whereas A. bilineata showed a preference for this more natural and complete odor, A. bipustulata was as much attracted by 2 μl of DMDS as by the natural odor source. One hypothesis is that the more specific the resource, the more specific the cues used to locate this resource should be (Vet and Dicke 1992; reformulated by Steidle and Van Loon 2003). Because A. bipustulata is more generalist than A. bilineata, notably in that its larvae can parasitize pupae of coprophagous and necrophagous flies, it could benefit from using a general cue like DMDS. Indeed, DMDS is not only emitted by decaying plant tissues that contain sulfur, but also by any decaying tissue that contains sulfur. Therefore, DMDS is most likely present in the microhabitat of the coprophagous and necrophagous hosts of A. bipustulata, thus making it a good general indicator of prey for this Aleochara species. It is notable that the “carrion” smell emitted by the inflorescence of some Araceae, which rely on necrophagous insects for pollination, consists mainly of dimethyloligosulfides (i.e., DMDS or dimethyl trisulfide) (Kite and Hetterschieid 1997). The presence of DMDS in decaying carrion could also explain why pure DMDS attracted carabid beetles (which, as generalists, are also necrophagous), while DMDS-containing odors of naturally infested roots did not.
The difference of attractivity of DMDS found between staphylinids and carabids could highlight the importance of the concentration of a cue in the environment for its use by foraging insects. Indeed, widespread volatile cues apparently lacking specificity could have informative properties that guide specialists to their specific resource based on the concentration of the volatile. In our experiments, the responsiveness of the different taxa toward the nonspecific cue DMDS went from a response to a wide range of concentrations for the generalist carabid beetles to a response to a narrower range of concentrations (close from what is emitted by the natural odor source) for the more specialist A. bilineata. Whereas carabid beetles responded to three of the four concentrations tested, A. bilineata responded to only two. The dose–response curve of the two staphylinid beetles shows the strongest response centered around what we found to be the natural quantity of DMDS emitted by an infested root, whereas the dose–response curve for carabid beetles seems wider and centered on the lowest concentration of the molecule. These findings are in accordance with the higher specialization of A. bilineata and A. bipustulata toward D. radicum, in comparison with the carabid beetles.
Associative learning (Vet and Dicke 1992; Dicke et al. 1990a; Takabayashi et al. 2006) or the presence/absence of a particular background odor (Bell 1990; Mumm and Hilker 2005; De Boer and Dicke 2006) could also play a role in attraction toward a nonspecific chemical cue like DMDS. Indeed, predators in a cabbage field could learn by experience that DMDS is often associated with sites where prey can be found. Also, DMDS could only be used when it is part of a full blend of decaying root odor. The extent to which learning or background odors may influence the response of the predators studied herein to different concentrations of DMDS remains to be investigated.
Because of the decreasing rate of volatile emission in our traps, and because of possible differences in the diel activity rhythms of the different predators, it remains possible that the different species caught in the same treatment were faced with different DMDS concentrations when trapped. To avoid this potential bias, future experiments should use a method that ensures the regular diffusion of odors, or work on a shorter time period. Another potential bias that could have influenced the distribution of the different species among the treatments is interference between these species. It is notable that carabid and staphylinid beetles were found in different traps most of the time. However, even if such avoidance occurred, the number of traps was high enough to allow the species to avoid each other without limiting their choice for their preferred treatment. In fact, for each treatment an important number of traps did not catch any insect.
In spite of the potential bias, the setup gave precise and reproducible results. GLM statistics permitted a clear discrimination between the different treatments, even with a low number of insects caught per trap. Furthermore, spatial analysis of residuals allowed checking the validity of assumptions, like the local character of attraction and the independence of data between two adjacent traps. Indeed, neither the effects of the row and columns where the traps were placed nor the spatial representation of residuals revealed any differentiation between the margins and the center of the setup (which could have been the case if individuals had been attracted from outside the setup). Likewise, there was no spatial structure of the residuals (i.e., spatial aggregation of smallest or highest residuals near a particular treatment) as would have been found if traps had influenced each other. Because of its high selectivity, the number of simultaneous comparisons allowed, and the power of the statistical analysis that can be conducted, this setup seems effective in testing local attraction for small ground-dwelling beetles (smaller than 3 mm) in realistic field conditions.
The use of our understanding of the chemical ecology of tritrophic interactions to enhance biological control practices is a motivating approach. To date, only the studies of James (2003, 2005) have shown the possibility of using synthetic VOCs for this purpose. By adding general cues such as methyl salicylate or cis-3-hexen-1-ol in plots, the number of flying beneficial arthropods caught in sticky traps increased. The results presented in this paper are the first that show attractivity of beneficial ground-dwelling beetles toward a synthetic odor in the field. Attractiveness of DMDS toward the main natural enemies of D. radicum could be useful for biological control. However, before DMDS can be used in the field, we need to define precisely the conditions under which it is attractive to predators and how it will influence their efficiency to control D. radicum populations. Ongoing lab and field experiments should give us such information.
References
Ahlström-Olsson, M., and Jonasson, T. 1992. Mustard meal mulch—a possible cultural method for attracting natural enemies of brassica root flies into brassica crops. IOBC/WPRS Bull. 15:171–175.
Auger, J., Lecomte, C., Paris, J., and Thibout, E. 1989a. Identification of leek-moth and diamondback-moth frass volatiles that stimulate parasitoid, Diadromus pulchellus. J. Chem. Ecol. 15:1391–1398.
Auger, J., Lecomte, C., and Thibout, E. 1989b. Leek odor analysis by gas-chromatography and identification of the most active-substance for the leek moth, Acrolepiopsis assectella. J. Chem. Ecol. 15:1847–1854.
Becker, R. A., Chambers, J. M., and Wilks, A. R. 1988. The New S Language. Wadsworth & Brooks/Cole.
Bell, W. J. 1990. Searching behavior patterns in insects. Annu. Rev. Entomol. 35:447–467.
Bernays, E. A. 1996. Selective attention and host-plant specialization. Entomol. Exp. Appl. 80:125–131.
Bukovinszky, T., Gols, R., Posthumus, M. A., Vet, L. E. M., and Van Lenteren, J. C. 2005. Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellen). J. Chem. Ecol. 31:461–480.
Coaker, T. H. and Williams, D. A. 1963. The importance of some carabidae and staphylinidae as predators of the cabbage root fly, Erioischia brassicae (Bouché). Entomol. Exp. Applicata 6:156–164.
D’Alessandro, M. and Turlings, T. C. J. 2006. Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst 131:24–32.
De Boer, J. G. and Dicke, M. 2004. The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J. Chem Ecol. 30:255–271.
De Boer, J. G. and Dicke, M. 2006. Olfactory learning by predatory arthropods. Anim. Biol. 56:143–155.
De Moraes, C. M., Lewis, W. J., Pare, P. W., Alborn, H. T., and Tumlinson, J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573.
Dicke, M., Sabelis, M. W., Takabayashi, J., Bruin, J., and Posthumus, M. A. 1990a. Plant strategies of manipulating predator–prey interactions through allelochemicals—prospects for application in pest control. J. Chem. Ecol. 16:3091–3118.
Dicke, M., Vanbeek, T. A., Posthumus, M. A., Bendom, N., Vanbokhoven, H., and Degroot, A. E. 1990b. Isolation and identification of volatile kairomone that affects acarine predator–prey interactions—involvement of host plant in its production. J. Chem. Ecol. 16:381–396.
Dugravot, S., and Thibout, E. 2006. Consequences for a specialist insect and its parasitoid of the response of Allium porrum to conspecific herbivore attack. Physiological Entomology 31:73–79.
Dugravot, S., Mondy, N., Mandon, N., and Thibout, E. 2005. Increased sulfur precursors and volatiles production by the leek Allium porrum in response to specialist insect attack. J. Chem. Ecol. 31:1299–1314.
Dunn, P. K. and Smyth, G. K. 1996. Randomized quantile residuals. J. Comput. Graph. Stat. 5:236–244.
Finch, S. 1989. Ecological considerations in the management of Delia pest species in vegetable crops. Annu. Rev. Entomol. 34:117–137.
Fuldner, D. 1960. Beitrage zur morphologie und biologie von Aleochara bilineata Gyll. und A. bipustulata L. (Coleoptera: Staphylinidae). Z. Morphol. Okol. Tiere 49:312–386.
Højsgaard, S. 2004. Some aspects of practical data analysis of “glm-type”data using R. <http://genetics.agrsci.dk/~sorenh/misc/DietOx.pdf>.
Hugues, R. D. 1959. The natural mortality of Erioischia brassicae (Bouché) (Diptera, Anthomyiidae) during the egg stage of the first generation. J. Anim. Ecol. 28:343–357.
James, D. G. 2003. Field evaluation of herbivore-induced plant volatiles as attractants for beneficial insects: Methyl salicylate and the green lacewing, Chrysopa nigricornis. J. Chem. Ecol. 29:1601–1609.
James, D. G. 2005. Further field evaluation of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. J. Chem. Ecol. 31:481–495.
Karban, R. and Baldwin, I. T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago. pp 319.
Kessler, A. and Baldwin, I. T. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144.
Kite, G.C. and Hetterschieid, W.L.A. 1997. Inflorescence odours of Amorphophallus and Pseudodracontium (Araceae). Phytochemistry 46:71–75.
Luff, M. L. 1987. Biology of polyphagous ground beetles in agriculture. Agric. Zool. Rev. 2:237–278.
Mowat, D. J. and Martin, S. J. 1981. The contribution of predatory beetles (Coleoptera: Carabidae and Staphylinidae) and seed-bed-applied insecticide to the control of cabbage root fly, Delia brassicae (Wied.), in transplanted cauliflowers. Hortic. Res. 21:127–136.
Mumm, R. and Hilker, M. 2005. The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem. Senses 30:337–343.
R Development Core Team 2006. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. <http://www.R-project.org>.
Reddy, G. V. P, Holopainen, J. K., and Guerrero, A. 2002. Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J. Chem. Ecol. 28:131–143.
Riley, K. J., Kuhlmann, U., Mason, P. G., Whistlecraft, J., Donald, L. J., and Holliday, N. J. 2007. Can mustard meal increase attacks by Aleochara spp. on Delia radicum in oilseed rape? Biocontrol Sci. Technol. 17:273–284.
Royer, L. and Boivin, G. 1999. Infochemicals mediating the foraging behaviour of Aleochara bilineata (Gyllenhal) adults: sources of attractants. Entomol Exp. Appl. 90:199–205.
Sabelis, M. W., Janssen, A., and Kant, M. R. 2001. Ecology—the enemy of my enemy is my ally. Science 291:2104–2105.
Smyth, G. 2005. Statmod: Statistical Modeling. R package version 1.2.2. http://www.statsci.org/r.
Steidle, J. L. M. and Van Loon, J. J. A. 2003. Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Entomol. Exp. Appl. 108:133–148.
Takabayashi, J., Sabelis, M. W., Janssen, A., Shiojiri, K., and Van Wijk, M. 2006. Can plants betray the presence of multiple herbivore species to predators and parasitoids? The role of learning in phytochemical information networks. Ecol. Res. 21:3–8.
Tomlin, A. D., Miller, J. J., Harris, C. R., and Tolman, J. H. 1985. Arthropod parasitoids and predators of the onion maggot (diptera, anthomyiidae) in southwestern ontario. J. Econ. Entomol. 78:975–981.
Turlings, T. C. J., Loughrin, J. H., Mccall, P. J., Rose, U. S. R., Lewis, W. J., and Tumlinson, J. H. 1995. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. U S A 92:4169–4174.
Uvah, I. I. I. And Coaker, T. H. 1984. Effect of mixed cropping on some insect pests of carrots and onions. Entomol. Exp. Appl. 36:159–167.
Vet, L. E. M. and Dicke, M. 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 37:141–172.
Vinson, S. B., Elzen, G. W., and Williams, H. J. 1987. The influence of volatile plant allelochemics on the third trophic level (parasitoids) and their herbivorous hosts. Insects–plants. Proceedings of the 6th Symposium on Insect–Plant Relationships (Pau, 1986):109–114.
Whitman, D. W. and Eller, F. J. 1992. Orientation of Microplitis croceipes (Hymenoptera, Braconidae) to green leaf volatiles—dose–response curves. J. Chem. Ecol. 18:1743–1753.
Wishart, G., Doane, J. F., and Maybee, G. E. 1956. Notes on beetles as predators of eggs of Hylemya brassicae (Bouché) (Diptera: Anthomyiidae). Can. Entomol. 88:634–639.
Wright, D. W., Hughes, R. D., and Worrall, J. 1960. The effect of certain predators on the numbers of cabbage root fly (Erioischia brassicae (Bouché)) and on the subsequent damage caused by the pest. Ann. Appl. Biol. 48:756–763.
Acknowledgments
The authors thank the staff of the experimental station of La Rimbaudais, Saint-Méloir-des-Ondes, France, for providing experimental fields; Romain Rouchet for help on the field experiment; Yannick Outreman and Manuel Plantegenest, Agrocampus, Rennes, France, for advice on statistical analysis; Roxina Soler, NIOO-KNAW, Heteren, The Netherlands, for useful comments on a previous version of the manuscript. This work was supported by a PhD grant to A. Ferry from the Region Bretagne and benefited from the financial help of the GDR d’écologie chimique CNRS no. G2827 for the chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferry, A., Dugravot, S., Delattre, T. et al. Identification of a Widespread Monomolecular Odor Differentially Attractive to Several Delia Radicum Ground-dwelling Predators in the Field. J Chem Ecol 33, 2064–2077 (2007). https://doi.org/10.1007/s10886-007-9373-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9373-3