Abstract
Soil is one of the major habitats of bacteria and fungi. In this arena their interactions are part of a communication network that keeps microhabitats in balance. Prominent mediator molecules of these inter- and intraorganismic relationships are inorganic and organic microbial volatile compounds (mVOCs). In this review the state of the art regarding the wealth of mVOC emission is presented. To date, ca. 300 bacteria and fungi were described as VOC producers and approximately 800 mVOCs were compiled in DOVE-MO (database of volatiles emitted by microorganisms). Furthermore, this paper summarizes morphological and phenotypical alterations and reactions that occur in the organisms due to the presence of mVOCs. These effects might provide clues for elucidating the biological and ecological significance of mVOC emissions and will help to unravel the entirety of belowground‚ volatile-wired’ interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inter- and intra-organismal communication strategies are symbolized by the three monkeys: the deaf, the mute, and the blind. Interestingly, one major communication path was not featured: the sense of smell. This is surprising since the sense of smell is well-established in many animals and plants. Vertebrates and invertebrates are able to detect minute amounts of volatiles even over very long distances; plants use volatiles to communicate with their pollinators as well as with plants of the same species or other plants (Baldwin et al., 2006; Dobson, 2006; Heil and Walters, 2009) (Fig. 1). The infochemicals used for these inter- and intra-organismal interactions are low molecular mass compounds with high vapor pressures, low boiling points, and a lipophilic character. All of these features facilitate evaporation. Consequently, these compounds disperse easily in the atmosphere and thus play essential biological/ecological roles in aboveground habitats. It was only recently recognized that belowground organisms are also opulent volatile producers and emitters. Therefore, a new research area focuses on volatile-based interactions in the soil. Here, we first describe the habitat soil with its characteristic structural prerequisites in relation to volatile-based communications. Then, we present a summary of volatile emissions of microbes (bacteria and fungi). In the final section, we discuss volatile-based bacterial and fungal interactions.
The Habitat Soil
The tremendous diversity of the bacterial and fungal kingdoms is paralleled by the heterogeneity of habitats these organisms are able to occupy. They appear ubiquitously around the world, successfully colonizing ecological niches and microhabitats (Dighton, 2003; Hawksworth and Mueller, 2005; Gasch, 2007). One of the major habitats for fungi and bacteria is soil, where they occur as free living organisms on the soil surface, in the soil core, or in association with belowground parts of living plants or organic material derived from dead plants and animals (Forster, 1988). Soil itself is a complex blend of weathered minerals and organic material mixed with biota. Fungi and bacteria hereby play a substantial role in the decomposition and breakdown of organic as well as inorganic materials, respectively (Dighton, 2003). These biomineralization processes contribute substantially to soil production, generating a continuous flow of nutrients for plant primary production. Therefore, functional soils should be regarded as a balanced complement of abiotic (mineral and organic) and biotic components (Nakas and Klein, 1980; Dighton, 2003). As part of the microbiotic soil community, fungi and bacteria form dynamic and enduring communities that are integrated into even more complex microecosystems, or they arise as transient communities to secondarily colonize substrates as long as degradable nutrients are available.
Soil Properties Influence Microenvironments Belowground
Microbial colonization of soil is determined mainly by its physicochemical properties (Dequiedt et al., 2011). These properties are influenced by texture, carbon content, and microstructure, which in turn affect the formation of macroaggregates and subsequently soil parameters such as porosity or air and water content. Soil texture is determined by its inorganic components and describes the proportional distribution of mineral particle sizes: sand (0.05–2 mm), silt (2–50 μm), and clay (<2 μm) (Cehnu and Stotzky, 2002; Brown, 2003; Conklin, 2005; Schafer, 2006). Texture, mineral composition, and particle shape give rise to certain particle arrangements (microaggregates) that determine soil microstructures (Cehnu and Stotzky, 2002; Alekseeva, 2007). These microstructures and the presence of organic matter contribute to the assembly and stabilization of macroaggregates >0.25 mm in size (Forster, 1988; Ranjard and Richaume, 2001). As a result, a complex network of void spaces is formed in soil, i.e., soil pores that can account for up to 50 % of the total soil volume (Ranjard and Richaume, 2001; Conklin, 2005; Standing and Killham, 2007). Their ability to retain water varies with their size and shape, so they are filled with different amounts of water and air. Depending on the air and water content, the chemical composition of aggregates, and the circulation within the pore network, numerous heterogenic microenvironments for microbial life are created. These vary in nutrient supply, aeration, availability of water, ionic composition, minerals, pH, redox potential, and surface composition (Forster, 1988; Ranjard and Richaume, 2001; Nannipieri et al., 2003).
Microhabitats Belowground
Microorganisms congregate in soil pores that provide a suitable microenvironment. Bacteria rely on the presence of organic and inorganic solutes in the aqueous phase of pores and on particle surfaces. The heterogeneity of these various microhabitats is probably the reason for the huge bacterial diversity in soil. Although the number of bacterial cells per gram of soil can easily exceed 1010 and estimates of the numbers of different species range from 103 to 105, only a rather small proportion of soils is actually colonized by bacteria (Gans et al. 2005; Roesch et al. 2007; citations in Heuer and Smalla 2012). Bacteria may occur as free living organisms, but are usually attached to solid surfaces as scattered individual cells, microcolonies, or biofilms. Fungi inhabit the same locations but other pore sizes. Water saturated micropores (Ø < 10 μm) are reserved for bacterial communities, where they escape predation and the effects of fungal antibiotics. Because of their size, fungi settle in macropores (Ø > 10 μm) found between and within macroaggregates. In addition, fungal hyphae can extend through aerated water-unsaturated pores to reach new pores and exploit new nutrient resources (Forster, 1988; Cehnu and Stotzky, 2002). The latter is especially important since soil in its entirety represents a nutrient-depleted habitat for microorganisms. Consequently, microorganisms aggregate near any suitable nutrient source, which creates colonization hotspots. Therefore, bacteria and fungi have to compete for the same resources and undergo interspecies interactions. On the macroscale, plant litter like dead leaves, stems, roots, wood, and bark as well as animal remains and fecal material are important sources of biodegradable organic material, while on the microscale cell-wall remains, lipids, polysaccharides, proteins, DNA and RNA, and metabolites contribute to temporary microhabitats (Forster, 1988; Nannipieri et al., 2003). The most lively and enduring microhabitat is the living plant root, which releases a wide variety of soluble, insoluble, or volatile metabolites that attract an exceptionally dense and diverse population of microbiota, including bacteria and fungi (Koske and Gemma, 1992; Chen et al., 2004; Gregory, 2006; Brimecombe et al., 2007; Nannipieri et al., 2007; Hussain and Hasnain, 2011). Bacteria adhere to the root surface itself (rhizoplane) and colonize a narrow soil zone around the plant root (rhizosphere) (Lenc et al., 2011). They benefit from a constant flow of organic substrates, but in return promote plant growth by providing soluble inorganic nutrients and producing growth-promoting factors (Brimecombe et al., 2007; Nannipieri et al., 2007; Compant et al., 2010). A special role is attributed to antagonistic bacteria, which are able to suppress the growth of various plant pathogenic fungi (Bhattacharyya and Jha, 2011). Mycorrhizal fungi (see Jung et al., 2012, this issue) also benefit from nutrients supplied by the plant root. More than 95 % of short roots of most terrestrial plants are colonized by symbiotic fungi, and these mycorrhizal fungi are surrounded by complex microbial communities. So called mycorrhiza helper bacteria (MHB) support mycorrhiza formation (Frey-Klett et al., 2007; Bonfante and Anca, 2009; Rigamonte et al., 2010). In addition, plant roots not only host beneficial but also attract detrimental organisms such as phytopathogens, which may harm plants and microbiota as well. Therefore, mycorrhizal fungi, their associated bacteria as well as rhizobacteria have to deal with a very complex and competitive rhizomicrobial milieu (Anderson, 1992; Bianciotto et al., 1996; Miransari, 2011). Bacteria and fungi closely intermingle in the mycorrhizosphere and mutually influence survival and colonization success as well as pathogenesis and virulence (Wargo and Hogan, 2006; Minerdi et al., 2008).
Volatiles as Medium for Interactions Belowground
Factors that regulate the dynamics and balance of symbiosis, cooperation, competition, and also coexistence in microbial communities have been investigated intensively. Phenomena like quorum-sensing and quorum-quenching (see Hartmann and Schikora, 2012, this issue), the impact of rhizobacterial and fungal antibiotics, effector molecules, and excreted enzymes have been recognized as effective regulatory principles (Walker et al., 2003, 2004; Chernin et al., 2011). The possible role of volatiles in bacterial-fungal interactions has been neglected for many years despite earlier reports on effective microbial volatiles (Stotzky and Schenk, 1976; Koske and Gemma, 1992). Prerequisite for volatile effectiveness is their release, emanation and distribution, and their perception by a target organism. This is ensured by the physicochemical properties of volatiles (low molecular weight, high vapor pressure, low boiling point), which facilitate distribution even over long distances (Farmer, 2001; Baldwin et al., 2006; Heil and Ton, 2008). However, does this also occur in soils? Yes, it does. Volatile distribution belowground takes place by diffusion and advection (Minnich and Schumacher, 1993). Volatiles can move through the network of soil pores since they are active in both gas and liquid phases and capable of revolatization after passing through water-saturated pores (Koske and Gemma, 1992; Aochi and Farmer, 2005; Asensio et al., 2008). However, due to their high vapor pressure, volatiles move primarily by vapor diffusion (Minnich and Schumacher, 1993). These processes are all influenced by inherent chemical properties of the volatile itself and physicochemical properties of the surrounding soil, which affect adsorption, desorption, and degradation. Adsorption/desorption depends on the polarity of the compound, the soil texture and spatial architecture, and the presence of water. On the microscale, increasing humidity reduces the adsorption of nonpolar volatiles to mineral surfaces; on the macroscale, nonpolar volatiles are increasingly sorbed by organic matter in moist or wet soils (Minnich and Schumacher, 1993; Ruiz et al., 1998; Aochi and Farmer, 2005; Insam and Seewald, 2010). Volatile compounds also are amenable to biodegradation. Owen et al. (2007) found rapid degradation of geraniol in the rhizosphere of Populus tremula, an observation they attributed to the activity of soil microorganisms. However, compared to compounds solely soluble in water, volatiles are less likely to be quickly biodegraded (Koske and Gemma, 1992). Mineral surfaces may serve as catalysts for chemical reactions that contribute to abiotic degradation. Highly specific clay surfaces react with volatiles that carry polar functional groups. Furthermore, volatiles also may be exposed to free-radical oxidation (Minnich and Schumacher, 1993; Insam and Seewald, 2010). Measurements of volatile exchange rates have revealed low volatile emission from soil, supporting the assumption that soil acts as a volatile sink (Stotzky and Schenck, 1976; Asensio et al., 2007).
Microbial Volatile Emission
A large number of bacterial species presently are known, and it is estimated that this number could reach a million (106). While many microorganisms have been isolated from aboveground habitats (i.e., plants, human skin and intestines, animals, and refuse, sewage, and aquatic habitats), a rich source of bacteria is the terrestrial and belowground biotope. Metagenomic approaches have demonstrated that the microbial diversity is larger in soils than in marine sediments or aquatic habitats (Will et al., 2010; Daniel, 2011). The capacity of bacteria and fungi to decompose, mineralize, and accumulate organic matter is extraordinary and has a significant impact on the carbon, nitrogen, phosphate and sulfur biogeochemical cycles (Naeem, 1997). Some of the metabolized compounds are emitted as volatile products that are readily utilized by other organisms of the microbial food chain or released into the underground habitat (Table 1). Soil microorganisms produce large quantities of highly diverse volatiles (Stotzky and Schenck, 1976; Linton and Wright, 1993; Leff and Fierer, 2008; Insam and Seewald, 2010 and citations therein). Volatile metabolites also are produced by the root system of plants, but in this review these sources will not be considered. Instead, the focus lies on bacterial and fungal volatile emissions and uptakes (Kesselmeier and Staudt, 1999; Wenke et al., 2009). The volatile compounds can be of organic (volatile organic compounds, VOCs) or inorganic nature, both presumably important for this habitat and capable of influencing organismic communities (McNeal and Herbert, 2009). The functions of the volatiles are diverse, e.g., i) they play a role in the food chain of the microbial loop because they are assimilated and incorporated into organic matter (bioconversion), ii) they influence physiological processes (e.g., laccase activity, nitrification, nitrogen mineralization), iii) they function as electron acceptors or donors to support metabolic reactions, iv) they play a role in quorum sensing/quenching, v) they act as defense compounds, vi) they are used as communication signals, or vii) their functions remain so far elusive (Table 1).
Volatiles Emitted from Bacteria
Inorganic Volatiles
Some producers and users of inorganic volatiles are summarized in Table 1, which is a brief extract from Gottschalk (1986) and Fuchs (2007). Carbon dioxide is a major inorganic volatile produced by all heterotrophic living organisms, and indeed much of the CO2 in the atmosphere originates from the huge microbial populations on earth, in both soil and aquatic habitats. Atmospheric CO2 is assimilated primarily by plants and oxygenic and anoxygenic phototrophic bacteria (cyanobacteria, Rhodospirillaceae [purple nonsulfur bacteria], Chromatiaceae [purple sulfur bacteria], Chlorobiaceae [green sulfur bacteria], and Chloroflexaceae [green nonsulfur bacteria]). The characteristic Calvin reactions and enzymes also are present in soil bacteria, such as Rhodospirillum rubrum, Thiobacillus intermedius, Ralstonia eutrophus, Pseudomonas facilis, to name a few. Chemolithotrophic microorganisms use ATP and the reducing power of inorganic substrates for the reduction of CO2. CO2 also is used by methanogenic bacteria such as Methanobacterium ruminatium and Methanobacterium thermoautrophicum for CH4 production (Gottschalk, 1986).
Anthropogenically released carbon monoxide results from incomplete reduction of wood and polymers of dead organic material, while microbial CO production is unknown. Aerobically grown Hydrogenomonas carboxydovorans and Selberia carboxyhydrogena can live on CO by oxidizing it to CO2. Some bacteria (e.g., Rhodospeudomonas sphaeroides, Methylosinus, Methylocystis) use the serine-isocitrate lyase pathway to form oxaloacetate from phosphoenol pyruvate (PEP) and CO2 (PEP carboxylase). As a result of this pathway, acetyl-CoA and finally succinate are formed from CH2O and CO2. Chemolithotrophic and phototrophic bacteria have in common the formation of cell material via CO2 reduction by using the reducing power from inorganic compounds. Energy sources can be H2, sulfide, ammonia, or nitrite.
Hydrogen is formed under anaerobic conditions during the fermentation of carbohydrates to short-chain fatty acids by Clostridium spp., Enterobacteriaceae (e.g., Escherichia, Salmonella, Shigella) and others. A group of chemolithotrophic bacteria (hydrogen-oxidizing bacteria), anoxygenic phototrophic bacteria, as well as methanogenic archaea utilize H2 as an electron donor.
Well-known volatile-dependent soil bacteria are the free-living and symbiotic nitrogen-fixing organisms. The latter are, for example, Rhizobium spp. and Frankia spp., and exist in partnerships with plants. These bacteria form bacteroids, and consequently, root nodules develop. The product of the nitrogenase is ammonia, which is usually not released but is efficiently incorporated into organic compounds by glutamate dehydrogenase, glutamine synthetase, and glutamate synthase. Soil-living clostridia (Clostridium spp.) and other bacteria (e.g., Peptococcus anaerobicus) ferment amino acids and nucleotides and live from these recycled carbon skeletons as well as ammonia. Recently, it was shown that Serratia, Pseudomonas, Stenotrophomonas, and Xanthomonas, when grown on complex media (NB or LB), emitted gaseous ammonia (or amines), which was detected in the headspace with Nessler’s reagent (Kai et al., 2010; Weise et al., 2012, Weise and Piechulla unpublished). Gaseous ammonia released from bacteria can modify, e.g., the antibiotic resistance of E. coli to tetracycline (Bernier et al., 2011). Apparently, increased intracellular polyamine levels alter the membrane permeability to antibiotics as well as resistance to oxidative stress. Another recent publication showed that ammonia could be sensed by Bacillus licheniformis, which was considered to be a first indication of bacterial olfaction (Nijland and Burgess, 2010). Although the nitrogen supply is usually a limiting factor in soil, it cannot be excluded that NH3 emission may occur in nature under confined protein-rich growth conditions (e.g., decomposition of carcasses, lysis of large microbial populations or plant materials, or land spreading of whey in agriculture). The amounts as well as the ecological consequences have not been investigated.
Denitrifying bacteria release nitrogen during respiration and reduction of nitrate (in some cases N2O instead of N2 is released). The group of nitrogen-evolving bacteria is quite diverse, including Alcaligenes faecalis, Bacillus licheniformis, Paracoccus denitrificans, and Pseudomonas stutzeri.
Most soil microorganisms use sulfate as their principal sulfur source, and the intrinsic enzyme system reduces sulfate to sulfide (sulfate assimilation). However, in anaerobic regions in the soil, sulfate is used by Desulfovibrio, Desulfomonas, Desulfuromonas, and Desulfotomaculum as a terminal electron acceptor, and consequently hydrogen sulfide is formed and released (dissimilatory sulfate reduction). The toxic end product H2S is used by chemolithotrophic bacteria as electron acceptor, e.g., Thiobacilli, and H2S can also be incorporated into O-acetylserine, an intermediate of amino acid biosynthesis. Furthermore, it also has been shown that H2S production in soil is due to the presence of cysteine (Morra and Dick, 1991). Only recently it was demonstrated that H2S production acts as a defense mechanism that protects bacteria from antibiotics (Shatalin et al., 2011).
The release of HCN from bacteria varies in different species (Stotzky and Schenck, 1976). Pseudomonas spp. (e.g., CHA0), Chromobacterium and Rhizobium typically emit this toxic inorganic volatile, while defective mutants (e.g., CHA207) do not (Blumer and Haas, 2000; Pessi and Haas, 2000; Kai et al., 2010; Blom et al., 2011b). Hydrogen cyanide inhibits several metal-containing enzymes, most significantly the cytochrome c oxidase of the respiratory chain. Therefore, this volatile can be toxic for most aerobic organisms living in the same habitat as Pseudomonades. It was reported that both the RHI/R- as well as the AHL-based quorum sensing system regulate HCN biosynthesis (Winson et al., 1995; Pessi and Haas, 2000). Consequently, bacterial population densities can be controlled by HCN levels.
The distribution and appearance of inorganic gaseous compounds in the soil determine the localization of other soil organisms, e.g., the oxidizers (nitrification) of ammonium occur in the upper sediment layers, followed by nitrate and sulfide oxidizers. In the deeper anaerobic layers, methanogenic and acetogenic bacteria reside. Many of the gaseous compounds are quickly recycled (e.g., H2) because producers and utilizers appear in nearby soil zones. Compounds emitted in excess are released into the atmosphere, for example, CO2, N2, and in some regions H2S.
Organic Volatiles (VOCs) (<120 D)
The smallest organic volatile compound is methane, the most reduced compound. Its formation is the terminal step in the food chain of methanogenic archaea (Gottschalk, 1986). They utilize CO2, CH2O, HCOOH, or CH3OH and H2 to synthesize methane. This soil-based methane production is of global importance; for example, tundra and rice fields contribute 40 % of atmospheric methane. In the soil, CH4 is a good substrate for obligate and facultative methylotrophs, which are often anaerobic organisms that grow in deeper soil layers. Bacterial production of the C1 volatile methanol has been described in Enterobacteriaceae such as Escherichia coli, Shigella flexneri, and Salmonella enterica (Bunge et al., 2008) and in Xanthomonas campestris (Weise et al., 2012). Methanol can be metabolized by methylotrophic bacteria including Hyphomicrobium species, some Pseudomonas species (P. oxalaticus), and Protaminobacter (Gottschalk, 1986). After an initial conversion into formaldehyde, a condensation with ribulose-5-phosphate forms dihydroxyacetone phosphate in the so–called ribulose-monophosphate cycle in Methylococcus and Methylomonas species. Yeasts, Zymomonas mobilis, lactic acid bacteria, and clostridia form ethanol (Gottschalk, 1986). Ethanol together with acetate is a good substrate for Clostridium kluyveri. Butanol and acetone are emitted e.g., by Clostridium acetobutylicum when enzymes of this pathway are activated under low pH conditions (Lütke-Eversloh and Bahl, 2011). Butanol also is formed by various microorganisms, and is considered a volatile organic compound (VOC). In the presence of butyrate and e.g., during glucose depletion butanol is a preferred product of butyrate metabolism. Many clostridia reduce acetone to isopropanol. Acetoin and 2,3-butanediol typically are produced during incomplete oxidation by Bacillus spp. (Gottschalk, 1986). Formed from pyruvate via α-acetolactate, both compounds are released under glucose abundance and taken up when glucose is depleted. Acetoin and 2,3-butanediol then can serve as a source for ATP production needed during the sporulation process. Butanediol production also is carried out by Enterobacteriaceae e.g., Serratia, Enterobacter, and Erwinia. Small molecular weight acids such as formate, acetate, propionate and butyrate are typical mixed acid fermentation products synthesized by Enterobacteriaceae, Clostridia, Propionibacteria, and e.g., Megasphaera elsdenii (Gottschalk, 1986). Small organic acids are utilized by many heterotrophic soil microorganisms.
Volatile Organic Compounds (>120 D) Emitted from Bacteria and Fungi
It is well-known that bacteria emit small molecular weight organic volatiles (<120 D, see above), but the frequent release of other compounds (120 to ca. 300 D) by microorganisms has only recently attracted attention. A literature search allowed the compilation of around 800 VOCs emitted by bacteria and fungi. Most compounds are in the range from 130 to 210 D (Fig. 2). In the ‘database of volatiles emitted by microorganisms (DOVE-MO),’ all VOC emitting microorganisms were compiled, including those in soil (literature search till December 2010, Kalderas, 2011). Since the origin of the microbes often was not well-documented, or it was difficult to assign microorganisms to a single habitat, we compiled all VOC emitting microorganims in DOVE-MO (Database of volatiles emitted by microorganisms) and present them in alphabetical order (bacteria: Table 2, fungi: Table 3). In total, 671 different VOCs are emitted by 212 bacterial species, and 335 VOCs from 96 fungal species are known. It is expected that future investigations in this new and developing research area will rapidly add organisms and VOCs to this database.
The volatile spectra of the microbes can be simple (<10 VOCs) as well as very complex (>50 VOCs) (e.g., Kai et al., 2007, 2010). Approximately 50 bacterial and ca. 30 fungal species presently are known that emit complex volatile mixtures. The number of detectable volatiles in a species blend increases when various techniques are applied (e.g., dynamic headspace volatile capture in open and closed airflow systems, different trapping materials, solid phase microextraction (SPME), gas chromatography combined with mass spectrometry (GC/MS), proton transfer reaction/mass spectrometry (PTR-MS), selected ion flow tube/mass spectrometry (SIFT-MS), secondary electron spray ionization/mass spectroscopy (SESI-MS), as well as analytical chemistry) (summarized in Wenke et al., 2012). Furthermore, the effects of growth media and conditions on the emission spectra have to be considered (Fiddaman and Rossall, 1994; Kai et al., 2010; Blom et al., 2011a).
The compiled information of volatile-producing microorganisms and their emission profiles was used to search for characteristic VOCs emitted by certain bacterial or fungal genera. The dominant classes of compounds emitted by fungi are alcohols (e.g., isomers of butanol, pentanol, octanol), hydrocarbons, ketones, terpenes, alkanes, and alkenes (Chiron and Michelot, 2005, Table 3). Prominantly emitted VOCs from bacteria are alcohols, alkanes, alkenes, and ketones, followed by esters and pyrazines, lactones, and sulfides (Wenke et al., 2012, Table 2). Some examples are given. Streptomyces species are especially rich in sesquiterpenes (Citron et al. 2012) and preferentially emit methylated short-chain alcohols and acids, while Pseudomonas species release C9-C16 alkanes/alkenes (Table 2). The product profiles of Bacteroides spp. and Lactobacillus spp. are rich in various C4 to C16 methylated carboxylic acids, C4 to C14 carboxylic acids, and small methylated alcohols (Table 2). Short-chain and long-chain acids are well-known carbon sources for many microorganisms, but the role of low molecular mass ketones and alcohols in the metabolic food chain is less clear (Table 1). N-acyl-l-homoserine lactones (AHL) are preferentially used as infochemicals (Ryan and Dow, 2008; Dickschat, 2009). Methylamine and other amines serve as good electron donors and carbon sources for many methylotrophic bacteria and methanogenic bacteria. The emission of indole from enterobacteria is well-known, but its ecological relevance is still speculative; an effect in indirect signaling has been indicated (Ryan and Dow, 2008). The sulfur containing compounds dimethyldisulfide (DMDS) and dimethyltrisulfide (DMTS) are often emitted from bacteria and fungi (Tables 2 and 3). While the organic sulfur compounds dimethylsulfide (DMS) and dimethylpropionate (DMSP) play central roles in the global sulfur cycles. This is apparently not the case for DMDS and DMTS (Schäfer et al., 2010). A clear picture on the biological or ecological relevance of the latter compounds is still missing since contrasting results have been obtained. DMDS had inhibitory effects on Arabidopsis thaliana in dual culture assays (IC50: 2.5 μmol) (Kai et al., 2010), while in another study it was shown that it could protect plants against fungal pathogens due to the induction of systemic resistance (Huang et al., 2012).
Prominent in bacterial emission profiles are pyrazines and β-phenylethanol. However, their biological functions are presently elusive. Even less understood is the biological and ecological relevance of the emission of extraordinary structures such as the terpene geosmin and sodorifen (Gerber and Lechevalier, 1965; Dickschat et al., 2005a; von Reuss et al., 2010). It is, for example, not known whether these volatiles act as communication signals or are used as carbon sources. Important future tasks are, therefore, elucidation of the plethora of bacterial and fungal VOCs and determination of their chemical structures and biological and ecological roles.
Volatile Mediated Bacterial-Fungal Interactions
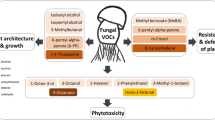
Bacterial and fungal volatiles may play multiple roles in microbial communities belowground. Although volatiles can serve as nutrient sources, under highly competitive but symbiotic conditions they are particularly important for antibiosis and signaling, and may serve as regulative principles in any ecosystem. Subsequently, interactions between bacteria and fungi can be beneficial or detrimental. In the latter situation, the term microbiostasis is used to describe the inability of bacteria and/or fungi to multiply in natural soils (Ho and Ko, 1982). Although nutrient depletion or suboptimal environmental conditions also may account for this effect, the involvement of microbial biogenic inhibitors, including volatiles, in microbiosis is widely accepted (Hora and Baker, 1972; Griffin et al., 1975; Stotzky and Schenck, 1976 and citations therein; Chuankun et al., 2004; Zou et al., 2007; Garbeva et al., 2011). The role of volatiles in signaling events within microbial communities has not yet been well-studied. Wheatley (2002) described volatiles as infochemicals that could mediate bacterial and fungal interactions. This was also proposed by Bending et al. (2006) for the mycorrhizal community. Fungi and plants produce volatile signal molecules that bacteria in the mycorrhizhospere may also synthesize, thereby affecting mycorrhiza formation. A similar situation has been described for the rhizobacterial community (Chernin et al., 2011). Volatiles of Pseudomonas fluorescens and Serratia plymuthica inhibited quorum-sensing in various other bacteria such as Agrobacterium, Chromobacterium, Pectobacterium, and Pseudomonas due to suppression of the transcription of N-acyl-homoserine lactone synthase genes.
Effects of Bacterial Volatiles on Fungi
Influence of Bacterial Volatiles on Germination and Mycelial Growth
The phenomenon of fungistasis was first described by Dobbs and Hinson (1953), which can be due to the negative influence of bacterial volatiles on germination and growth of soil-borne fungi. McCain (1966) showed that volatiles produced by Streptomyces griseus induced early sclerotia formation in Sclerotium cepivorum and Rhizoctonia solani, and reduced sporulation in Gloeosporium aridum. A strong inhibition of spore germination of Cladosporium cladosporioides was caused by but-3-en-2-one produced by Streptomyces griseoruber (Herrington et al., 1987). Zou et al. (2007) screened 1080 bacterial isolates for fungistatic activity. A total of 328 isolates belonging to the family of Rhizobiaceae, Xanthomonadaceae, Micrococcaceae, Alcaligenaceae, and to the order of Bacillales were identified as decreasing germination and mycelial growth of Paecilomyces lilacinus and Pochonia chlamydosporia. The spore germination of both fungi also was strongly inhibited by soil direct fungistasis and soil volatile fungistasis. Both effects correlated closely with impaired spore germination and disappeared after autoclaving. Several volatiles were identified, and trimethylamine, benzaldehyde, and N,N-dimethyloctylamine showed strong antifungal activity (Chuankun et al., 2004).
In order to identify bacterial isolates specifically antagonistic to plant pathogens, many in vitro experiments have been done. The experimental setup had to ensure that only volatile metabolites would influence fungal growth. Split Petri dishes (Fernando et al., 2005; Kai et al., 2007; Vespermann et al., 2007), separated agar patches (Alharbi et al., 2011), or the inversion of one bottom plate over a second one (Bruce et al., 2000) assured the exchange of volatiles solely in the headspace. Vespermann et al. (2007) and Kai et al. (2007 and 2008) conducted a comprehensive investigation using Bacillus subtilis, Pseudomonas fluorescens, Pseudomonas trivialis, Burkholderia cepacia, Staphylococcus epidermidis, Stenotrophomonas maltophilia, Stenotrophomonas rhizophila, Serratia odorifera, and Serratia plymuthica against pathogenic fungi, including Aspergillus niger, Fusarium culmorum, Fusarium solani, Microdochium bolleyi, Paecilomyces carneus, Penicillium waksmanii, Phoma betae, Phoma eupyrena, Rhizoctonia solani, Sclerotinia sclerotiorum, Trichoderma strictipile, and Verticillium dahliae. All rhizobacteria inhibited the mycelial growth of most fungi. The extent of inhibition depended on the individual bacteria-fungus combination. Noticeably, Fusarium solani turned out to be resistant against the bacterial volatiles. The spectra of bacterial volatiles produced included many unknown components; however, 2-phenylethanol, 1-undecene, dodecanal, dimethyl disulfide (DMDS), and dimethyl trisulfide (DMTS) could be identified (Kai et al., 2007). DMDS and 1-undecene indeed inhibited the growth of F. culmorum when applied as individual compounds in dual-culture tests (Kai et al., 2009). Several other reports also confirmed the antifungal action of volatiles produced by antagonistic rhizobacteria. Pseudomonas fluorescens and Pseudomonas pumila inhibited most effectively the growth of Gaeumannomyces graminis var tritici, the cause of take-all disease in wheat (Babaeipoor et al., 2011). Gluconacetobacter diazotrophicus decreased the growth of Fusarium oxysporum (Logeshwarn et al., 2011), Bacillus pumilus, Bacillus subtilis, and Bacillus cereus hindered growth of Botrytis mali (Jamalizadeh et al., 2010), and volatiles produced by Bacillus subtilis showed antifungal activity towards Rhizoctonia solani and Pythium ultimum (Fiddaman and Rossall, 1993) and Aspergillus alternate, Cladosporium oxysporum, Fusarium oxysporum, Paecilomyces lilacinus, Paecilomyces variotii, and Pythium afertile (Chaurasia et al., 2005). Bacillus spp. impaired the growth of Phytophthora sojae, which causes the soybean damping-off disease (Tehrani et al., 2002). Interestingly, the dual application of Bacillus pumilus and the mycorrhizal fungus Glomus mosseae improved the growth of mandarin plants, directly attributed in part to growth inhibition of fungal pathogens by rhizobacterial volatiles (Chakraborty et al., 2011). The volatiles 1-octen-3-ol, benzothiazol, and citronellol produced by Paenibacillus polymyxa strongly inhibited mycelial growth and impaired germination of eight fungal pathogens, including Botrytis cinerea (Zhao et al., 2011). Wan et al. (2008) investigated the effect of headspace volatiles of Streptomyces plantesis on phytopathogenic fungi. Two antifungal components were identified: 2-phenylethanol and a phellandrene derivative were responsible for the suppression of mycelial growth of Rhizoctonia solani, Sclerotinia sclerotiorum, and Botrytis cinerea. Ascospore germination was suppressed up to 90 % by volatiles released by Pseudomonas sp., which were isolated from canola and soybean plants (Fernando et al., 2005). Staphylococcus pasteuri showed a significant antifungal activity in vitro against Tuber borchii and inhibited ectomycorrhizal formation (Barbieri et al., 2005).
Many Pseudomonas species are known to produce HCN as an effective antifungal component (Voisard et al., 1989; Haas and Défago, 2005). Although HCN production could be correlated to fungistasis, its antifungal effect often could only be verified in vitro. Rhizobacterial isolates were screened for HCN production and diffusible antifungal metabolites, and tested against Verticilium dahliae and Rhizoctonia solani in dual-culture tests (Tehrani et al., 2001; Afsharmanesh et al., 2006), and subsequently used in greenhouse experiments. Interestingly, HCN producers showed the highest efficiency when applied to the soil, whereas non-producers were more efficient when applied to seeds. Antifungal properties also have been attributed to gaseous ammonia. Schippers et al. (1982) showed that ammonia release from soil as well as from an ammonium sulfate solution inhibited conidia germination of Botrytis cinerea and Penicillium nigricans. However, some fungi such as Fusarium culmorum and Verticillium nigrescens were not affected by ammonia. Furthermore, other volatiles released from the soil decreased conidia germination and tube growth of these two fungi. Similarly, Howell et al. (1988) identified ammonia to be the antifungal component in dual-culture tests using Enterobacter cloacae, Rhizoctonia solani, and Pythium ultimum.
Fungal growth promotion by bacterial volatiles has hardly ever been reported. Mackie and Wheatley (1999) and Wheatley (2002) selected four fungi as representative of a range of several habitats and challenged them in vitro with headspace volatiles of a variety of randomly selected soil bacteria. The response was unique for each fungal-bacterial combination, and revealed positive, negative, as well as neutral effects on radial growth of Trichoderma viride, Phanaerochaete magnoliae, Phytophthora cryptogea, and Gaeumannomyces graminis var tritici. Only P. cryptogea exhibited a significant increase in growth upon exposure to volatiles of certain bacterial isolates.
Impact of Bacterial Volatiles on Fungal Morphology
Several reports also have focused on morphological changes in fungi following bacterial volatile treatment. Fiddaman and Rosall (1993) observed abnormal hyphae with deformation and enhanced vacuolation in Rhizoctonia solani and Pythium ultimum exposed to volatiles produced by Bacillus subtilis. The same bacterial species caused hyphal and conidial deformations in Aspergillus alternaria, Cladosporium oxysporum, Fusarium osysporum, Paecilomyces lilacinus, Paecilomyces variotii, and Pythium afertile. Transverse and longitudinal septae completely disappeared in Aspergillus alternaria, and conidia became thick-walled and irregular in shape. Conidia formation was sometimes arrested, and in Cladosporium oxysporum, conidiophores became vegetative and stunted. Swelling of hyphae, vacuolization, and granulation lead finally to lysis of fungal mycelium in Fusarium oxysporum, Paecilomyces lilacinus, and Paecilomyces variotii (Chaurasia et al., 2005). Swollen terminal cells and bulging intercalary cells also were described for Tuber borchii upon exposure to volatiles emitted by Staphylococcus pasteuri and, finally, fungal mycelium showed swollen and contorted patterns when treated with 1-octen-3-ol (Barbieri et al., 2005). Benzothiazol caused a more frequent branching of the mycelium and increased conidia production, whereas citronellol only induced a slight hyphal contortion. All three compounds were components of the volatile mix produced by Paenibacillus polymyxa (Zhao et al., 2011).
Influence of Bacterial Volatiles on Mycorrhizal Fungi
Mycorrhiza is a complex symbiotic community including plant roots, mycorrhizal fungi, and associated bacteria (see Jung et al., 2012, this issue). Not only their physical contact but also the release of bioactive molecules, including volatiles, apparently play a regulatory role in a mycorrhizal network establishment (Bonfante and Anca, 2009). Associated bacteria comprise primarily the mycorrhiza helper bacteria (MHB) as well as rhizobacteria with beneficial or deleterious functions (Bonfante and Anca, 2009; Miransari, 2011). In 1991, Tylka et al. demonstrated that the MHB Streptomyces orientalis stimulated spore germination in Gigaspora margarita and Glomus mossae. Garbaye and Duponnois (1992) proposed that MHB directly stimulate the growth of Laccaria laccata by releasing volatile substances. Volatiles emitted by a bacterial isolate originally associated with Gigaspora margarita also promoted in vitro host fungus growth (Horii and Ishii, 2006), and volatile and diffusible compounds produced by MHB strains obtained from Glomus clarum spores stimulated or arrested spore germination, dependent on the bacterial species. Complete inhibition of spore germination, however, was only related to the volatiles (Xavier and Germida, 2003). Aspray et al. (2006) revealed that stimulation of mycorrhiza formation of Lactarius rufus required close proximity or contact. Volatiles of the MHB Paenibacillus sp. alone had significant negative effects on mycorrhiza formation. Furthermore, volatiles of the MHB Streptomyces spp., which actually promoted growth of the ectomycorrhizal fungus Amanita muscaria, did not affect mycelial extension rates (Schrey et al., 2005). The antagonist Bacillus subtilis JA inhibited significantly the spore germination and hyphal growth of a monoxenic strain of Glomus etunicatum in dual-culture experiments (Xiao et al., 2008), whereas volatiles produced by Klebsiella pneumonia promoted hyphae extension distantly located from the germinated spores of Glomus deserticola. Both organisms were indigenous to the roots of sea oats (Will and Sylvia, 1990).
Impact of Bacterial Volatiles on Fungal Enzyme Activities and Gene Expression
Mackie and Wheatley (1999) and Wheatley (2002) investigated the effect of bacterial volatiles on physiological properties of fungi by monitoring laccase and tyrosinase activity of Phanaerochaete magnoliae and Trichoderma viride upon exposure to volatiles of three selected soil bacteria isolates (A, B, C). Laccase activity completely ceased in P. magnolia in the presence of isolates A, B, C, whereas tyrosinase activity was inhibited only by the presence of isolate B. Isolate B was the only one to affect laccase activity in T. viride. The observed decrease in fungal growth correlated with decreased enzyme synthesis rather than inhibition of enzyme activity (Wheatley, 2002). Laccase activity in Rhizoctonia solani was induced after co-cultivation with Pseudomonas fluorescens. Due to the experimental setup, it was not possible to distinguish between effects of diffusible and volatile metabolites (Crowe and Olsson, 2001). Inhibition of enzyme activities may also be involved in the complete loss of pigmentation after treatment of Fusarium oxysporum with citronellol, a compound emitted by Paenibacillus polymyxa (Zhao et al., 2011). In contrast, Kai et al. (2009) observed a dark discoloration of the agar when fungi were exposed to rhizobacterial volatiles.
At present there are few reports that bacterial volatile components may affect gene expression. Minerdi et al. (2008, 2009) demonstrated an indirect volatile mediated effect of bacteria on fungal gene expression. The antagonistic wild type (WT) strain Fusarium oxysporum MSA35 lives in symbiosis with associated bacteria of the genera Serratia, Achromobacter, Bacillus, and Stenotrophomonas. Volatiles produced by the WT repressed the expression of two putative virulence genes of a pathogenic Fusarium oxysporum lactucae strain. When cured of the bacterial symbionts, the WT turned pathogenic and the sesquiterpene caryophyllene was no longer in the headspace of the cured WT. It also was not found in the headspace of the ectosymbionts, so this volatile seems to mediate a mechanism for the antagonistic properties of the Fusarium oxysporum WT. However, caryophyllene is only produced by the WT in the presence of the bacterial symbionts.
Possible Mechanisms of Actions of Volatiles
Presently, little is known about mechanisms of action and detoxification of bacterial volatiles in fungi. It is known that the cyanide ions from HCN are potent inhibitors of many metal-containing enzymes, in particular of copper-containing cytochrome c oxidases (Haas and Défago, 2005). However, it remains unclear how most volatiles develop their activity. One scenario relates to the production of melanin (Kai et al., 2009; Zhao et al., 2011). Melanins are known to reinforce the cell wall or accumulate on the cell surface where they develop antioxidative properties and scavenge free radicals. In fungi, melanins are synthesized via the polyketide synthase pathway (Jacobson, 2000), but phenol oxidizing enzymes such as laccases and tyrosinases may also be involved (Williamson, 1997). Intracellular laccases account for detoxification of chemicals (Champagne and Ramsay, 2010). In this regard, the increase of laccase activities reported by Crowe and Olsson (2001) might result from the presence of eligible volatile substrates, whereas the decrease in laccase and tyrosinase activity reported by Mackie and Wheatley (1999) might be a sign of impaired cell homeostasis. This again demonstrates that a deleterious bacterial volatile can be considered a toxin. Fungal cells respond to it as to any other biotic or abiotic stress factors. Whole-genome expression studies conducted in fungal model organisms including Saccharomyces cerevisiae, Candida albicans, and Schizosaccharomyces pombe have revealed that each species responded to environmental stress with an individual change in gene expression. Some species also expressed a common set of genes, referred to as environmental stress response (ESR) (Gasch, 2007). This can include the response to cell wall stress and/or oxidative and osmotic stress. Compounds like gaseous ammonia could be considered a stress factor, impairing cell homeostasis and triggering ESR. On the other hand, sub-inhibitory concentrations of ammonia might play a part in signaling. Ammonia released from bacterial strains has been shown to stimulate Bacillus licheniformis to form biofilms and pigmentation (Nijland and Burgess, 2010) and to increase the antibiotic resistance of various gram-positive and gram-negative bacteria (Bernier et al., 2011). Therefore, the ecological role of microbial volatiles may be intrinsically to serve as a signal molecule within and between species. They may also function as chemical ‘manipulators’ to alter central metabolic pathways, contribute to nutrient scavenging, and participate in developmental processes (Hibbing et al., 2010). Interestingly, ammonia also has been identified as a long-distance signal in Candida albicans, warning the colony of approaching starvation (Palková and Váhová, 2003). In this sense, the mode of actions of microbial volatiles should be assessed in more detail.
Effects of Fungal Volatiles on Bacteria
Bacteriostasis, similar to fungistasis, is the inability of bacteria to multiply in soil (Ho and Ko, 1982). Bacteriostasis is influenced by environmental factors such as nutrient supply and habitat conditions, but active volatile inhibitors also may be involved (Davis, 1976). It is known to date that bacteria produce volatiles that inhibit bacterial growth (Brown, 1973; Ko and Chow, 1977; Acea et al., 1988), and that volatiles produced by fungi also affect fungi (Stotzky and Schenck, 1976; Calvet et al., 1992; McAllister et al., 1996; Bruce et al., 2000; Martinez et al., 2004), but fungal volatiles acting on bacteria has not been reported (to the best of our knowledge).
Ecological Significance of Volatile Mediated Bacterial-Fungal Interactions
Suitable microenvironments in soils attract macro- and microbiota that colonize and form microhabitats, thereby creating dynamic microecosystems. Consequently, at least in densely and diversely populated habitats, bacteria and fungi are involved in a ‘networking’ community characterized by mutualism, commensalism, cooperation, antagonism, competition, and coexistence (Pal and McSpadden Gardener, 2006). Interactions between organisms can be specific or non-specific, but they are mostly multitrophic, thus keeping the microecosystem in balance. This is especially true for the mycorrhizosphere, where rhizobacteria, including plant growth promoting rhizobacteria, mingle with mycorrhizal fungi and their associated bacteria, free living bacteria and fungi, protozoa (amoeba) or metazoa (nematodes), including many phytopathogenic organisms. In this arena, interactions between bacteria and fungi could have a positive or a negative impact on third parties, which is useful if the weakened party is a pathogen and the strengthened party is a valuable member of the community. It is likely that volatile compounds are involved in these phenomena, since many bacterial volatiles affect phytopathogenic fungi directly or indirectly, i.e., as a result of bacterial-fungal interactions, pathogens are affected. In any case, the plant would benefit. An elucidation of this plant-fungus-bacterium network of interactions opens the way for biological control of plant diseases. An impressive example was given by Cao et al. (2011). They showed in vitro and in vivo that a GFP-tagged Bacillus subtilis strain, originally isolated from the rhizosphere of a non-infested cucumber plant, was able to successfully suppress the growth of Fusarium oxysporum f. sp. cucumerinum by colonizing the root and persisting on the rhizoplane, which is critical for an effective biocontrol in this case of cucumber wilt. Although not explicitly investigated, the authors proposed antibiosis caused by diffusible agents to be at least one mode of action. This, however, does not exclude volatile agents. Other experiments with a B. subtilis strain isolated from the rhizosphere of wheat and soybean showed that bacterial volatiles were involved in the biocontrol of Botrytis mali and Phytophthera sojae, respectively (Tehrani et al., 2002; Jamalizadeh et al., 2010). However, when using rhizobacteria as biocontrol agents, it is apparently important that the biocontrol strain is indigenous to the treated plant species in order to prevent damage of indigenous beneficial fungi (Will and Sylvia, 1990; Xiao et al., 2008).
Volatiles also might be involved in tritrophic interactions comprising bacteria, fungi, and nematodes. Paenibacillus polymyxa and P. lentimorbus exhibited strong antifungal activities, thereby interfering with the nematode-fungus interaction Meloidogyne incognita - Fusarium oxysporum, which significantly reduced nematode infestation of tomato plants (Son et al., 2009). In addition, soil bacteria, including one rhizobacterial strain, enhanced the nematophagous activity of the nematode-trapping fungus Arthrobotrys oligospora by increasing trap formation and predaceous activity (Duponnois et al., 1998). Volatile signaling cannot be excluded for either experiment.
In their entirety, the emission patterns of volatile metabolites of a belowground microecosystem reflect the dynamics of the community (McNeal and Herbert, 2009). Variations could be related to changes in the microenvironment such as pH, humidity, temperature, nutrient supply, and resulting changes in metabolic activities of micro- and macrobiota. In this respect, in vitro studies of volatile-mediated interactions between bacteria and fungi provide only limited access to the overall picture. Artificial test conditions might produce results that cannot be postulated uncritically for natural conditions. This especially applies to artificial growth media and nutrient supplies that influence metabolic activities as well as to “out of range” concentrations of the volatile mediators emitted (Nannipieri et al., 2003; Blom et al., 2011a). The crucial question is: are these concentrations found in the habitat? Since measurements of volatile concentrations in microhabitats are presently not available, in vitro testing is a useful tool to reveal substantial relationships between certain partners that might come into contact in a microecosystem. The consideration of environmental conditions and the verification of in vitro derived results in in situ/in natura experiments will give an overall picture regarding the role of volatiles in bacterial-fungal interactions and the implications of these interactions in community networks.
Conclusion and Perspectives
Volatiles are only a small proportion of the total number of metabolites produced by living organisms. However, because of their unique properties they are predestined to act as infochemicals in intra- and interspecies communications in the atmosphere as well as in the soil. This paper describes the wealth of microbial volatile emissions. The number of microbial volatiles (presently comprising around 800 compounds) and presumably of those with novel structures will increase significantly as this new research field expands. Just consider i) the large number of bacteria and fungi whose volatile profiles have yet not been obtained, ii) the various growth conditions that determine and alter the VOC profiles, and iii) the huge number of not yet identified or isolated microbes (106!!). This foreshadows the potential this research area has and where it may develop in the future. It seems very likely that only the “tip of the iceberg” of possible ‘volatile-wired’ interactions between underground bacteria and fungi (and elsewhere) has been seen. It will be a central task in the future to elucidate the plethora of bacterial and fungal VOCs and determine their biological and ecological roles in the soil. It also is quite likely that the naturally produced VOCs can be used as potent non-invasive indicators to study soil microbial ecosystems, including far-reaching spatiotemporal dynamics and environmental perturbations. Ultimately, these microbial volatiles – individually or in mixtures, chemically synthesized or biologically emitted - with their positive and/or negative effects on other organisms may develop into useful agricultural tools.
References
Acea, M. J., Moore, C. R., and Alexander, M. 1988. Survival and growth of bacteria introduced into soil. Soil Biol. Biochem. 20:509–515.
Afsharmanesh, H., Ahmadzadeh, M., and Sharifi-Tehrani, A. 2006. Biocontrol of Rhizoctonia solani, the causal agent of bean damping-off by fluorescent pseudomonads. Commun. Agric. Appl. Biol. Sci. 71:1021–1029.
Alekseeva, T. V. 2007. Soil microstructures and factors of its formation. Eurasian Soil Sci. 40:649–659.
Alharbi, S. A., Al-Harbi, N. A., Hajomer, S., Wainwright, M., and Aljohny, B. O. 2011. Study on the effect of bacterial and chemical volatiles on the growth of the fungus Aureobasidium pullulans. Afr. J. Microbiol. Res. 5:5245–5249.
Anderson, A. J. 1992. The influence of the plant root on mycorrhizal formation, pp. 37–64, in M. J. Allen (ed.), Mycorrhizal Functioning. Chapman & Hall, New York, NY.
Aochi, Y. O. and Farmer, W. J. 2005. Impact of soil microstructure on the molecular transport dynamics of 1,2-dichlorethane. Geoderma 127:137–153.
Asensio, D., Penuelas, J., Filella, I., and Llusia, J. 2007. On-line screening of soil VOCs exchange responses to moisture, temperature and root presence. Plant Soil 291:249–261.
Asensio, D., Owen, S. M., Llusia, J., and Penuelas, J. 2008. The distribution of volatile isoprenoids in the soil horizons around Pinus halepensis trees. Soil Biol. Biochem. 40:2937–2947.
Aspray, T. J., Eirian Jones, E., Whipps, J. M., and Bending, G. D. 2006. Importance of mycorrhization helper bacteria cell density and metabolite localization for the Pinus sylvestris–Lactarius rufus symbiosis. FEMS Microbiol. Ecol. 56:25–33.
Atmosukarto, I., Castillo, U., Hess, W. M., Sears, J., and Strobel, G. 2005. Isolation and characterization of Muscodor albus I-41.3 s, a volatile antibiotic producing fungus. Plant Sci. 169:854–861.
Babaeipoor, E., Mirzaei, S., Danesh, Y. R., Arjmandian, A., and Chaichi, M. 2011. Evaluation of some antagonistic bacteria in biological control of Gaeumannomyces graminis var tritici causal agent of wheat take-all disease in Iran. Afr. J. Microbiol. Res. 5:5165–5173.
Baldwin, I. T., Halitschke, R., Paschold, A., Von Dahl, C. C., and Preston, C. A. 2006. Volatile signaling in plant plant interactions: ‘Talking-Trees’ in the genomics era. Science 311:812–815.
Barbieri, E., Gioacchini, A. M., Zambonelli, A., Bertini, L., and Stocchi, V. 2005. Determination of microbial volatile organic compounds from Staphylococcus pasteuri against Tuber borchii using solid-phase microextraction and gas chromatography/ion trap mass spectrometry. Rapid Commun. Mass Sp. 19:3411–3415.
Beck, H. C., Hansen, A. M., and Lauritsen, F. R. 2002. Metabolite production and kinetics of branched-chain aldehyde oxidation in Staphylococcus xylosus. Enzyme Microb. Tech. 31:94–101.
Bending, G. D., Aspray, T. J., and Whipps, J. M. 2006. Significance of microbial interactions in the mycorrhizosphere. Adv. Appl. Microbiol. 60:97–132.
Bernier, S. P., Letoffe, S., Delepierre, M., and Ghigo, J. M. 2011. Biogenic ammonia modifies antibiotic resistance at a distance in physically separated bacteria. Mol. Microbiol. 81:705–716.
Bhattacharyya, P. N. and Jha, D. K. 2011. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microb. Biot.. doi:10.1007/s11274-011-0979-9.
Bianciotto, V., Minerdi, D., Perotto, S., and Bonfante, P. 1996. Cellular interactions between arbuscular mycorrhizal fungi and rhizosphere bacteria. Protoplasma 193:123–131.
Bjurman, J., Nordstr and, E., and Kristensson, J. 1997. Growth-phase-related production of potential volatile-organic tracer compounds by moulds on wood. Indoor Air 7:2–7.
Blom, D., Fabbri, C., Connor, E. C., Schiestl, F. P., Klauser, D. R., Bolle, R. T., Eberl, L., and Weisskopf, L. 2011a. Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ. Microbiol. 13:3047–3058.
Blom, D., Fabbri, C., Eberl, L., and Weisskopf, L. 2011b. Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly mediated due to hydrogen cyanide. Appl. Environ. Microbiol. 77:1000–1008.
Blumer, C. and Haas, D. 2000. Mechanism, regulation and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol. 173:170–177.
Bonfante, P. and Anca, I. A. 2009. Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu. Rev. Microbiol. 63:363–383.
Börjesson, T., Stöllman, U., and Schnürer, J. 1990. Volatile metabolites and other indicators of Penicillium aurantiogriseum growth on different substrates. Appl. Environ. Microbiol. 56:3705–3710.
Börjesson, T., Stöllman, U., and Schnürer, J. 1992. Volatile metabolites produced by six fungal species compared with other indicators of fungal growth on cereal grains. Appl. Environ. Microbiol. 58:2599–2605.
Brimecombe, M. J., De Leij, F. A. A. M., and Lynch, J. M. 2007. Rhizodeposition and microbial populations, pp. 73–110, in R. Pinton, Z. Varanini, and P. Nannipieri (eds.), The Rhizosphere: Biochemistry and Organic Substances at the Soil-plant Interface. Taylor & Francis, Boca Raton, Florida.
Brondz, I. and Olsen, I. 1991. Multivariate analyses of cellular fatty acids in Bacteroides, Prevotella, Porphyromonas, Wolinella, and Campylobacter spp. J. Clin. Microbiol. 29:183–189.
Brown, M. E. 1973. Soil bacteriostasis limitation in growth of soil and rhizosphere bacteria. Can. J. Microbiol. 19:195–199.
Brown, R. B. 2003. Soil texture, pp. 1-7, in Fact Sheet SL-29. Soil and Water Science Department, Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, University of Florida.
Bruce, A., Wheatley, R. E., Humphris, S. N., Hackett, C. A., and Florence, M. E. J. 2000. Production of volatile organic compounds by Trichoderma in media containing different amino acids and their effect on selected wood decay fungi. Holzforschung 54:481–486.
Bruce, A., Verrall, S., Hackett, C., and Wheatley, R. E. 2004. Identification of volatile organic compounds (VOCs) from bacteria and yeast causing growth inhibition of sapstain fungi. Holzforschung 58:193–198.
Bunge, M., Araghipour, N., Mikoviny, T., Dunkl, J., Schnitzhofer, R., Hansel, A., Schinner, F., Wisthaler, A., Margesin, R., and Mark, T. D. 2008. On-line monitoring of microbial volatile metabolites by proton transfer reaction-mass spectrometry. Appl. Environ. Microbiol. 74:2179–2186.
Calvet, C., Barea, J. M., and Pera, J. 1992. In vitro interactions between the vesicular-arbuscular mycorrhizal fungus Glomus mosseae and some saprophytic fungi isolated from organic substrates. Soil Biol. Biochem. 24:775–780.
Cao, Y., Zhang, Z., Ling, N., Yuan, Y., Zheng, X., Shen, B., and Shen, Q. 2011. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils 47:495–506.
Cehnu, C. and Stotzky, G. 2002. Interaction between microorganisms and soil particles: An overview, pp. 3–28, in P. M. Huang, J. M. Bollag, and N. Senesi (eds.), Interactions between soil particles and microorganisms. John Wiley & Sons, Hoboken, New York.
Chakraborty, U., Chakraborty, B. N., Allay, S., De, U., and Chakraborty, A. P. 2011. Dual application of Bacillus pumilus and Glomus mosseae for improvement of health status of mandarin plants. Acta Hortic. 892:215–230.
Champagne, P. P. and Ramsay, J. A. 2010. Dye decolorization and detoxification by laccase immobilized on porous glass beads. Bioresour. Technol. 101:2230–2235.
Chaurasia, B., Pandey, A., Palni, L. M. S., Trivedi, P., Kumar, B., and Colvin, N. 2005. Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol. Res. 160:75–81.
Chen, F., Ro, D. K., Petri, J., Gershenzon, J., Bohlmann, J., Pichersky, E., and Tholl, D. 2004. Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole. Plant Physiol. 135:1956–1966.
Chernin, L., Toklikishvili, N., Ovadis, M., Kim, S., Ben-ari, J., Khmel, I., and Vainstein, A. 2011. Quorum-sensing quenching by rhizobacterial volatiles. Environ. Microbiol. Rep. 3:698–704.
Chiron, N. and Michelot, D. 2005. Mushrooms odors, chemistry and role in the biotic interactions – a review. Cryptogr. Mycol. 26:299–365.
Chuankun, X., Minghe, M., Leming, Z., and Keqin, Z. 2004. Soil volatile fungistasis and volatile fungistatic compounds. Soil Biol. Biochem. 36:1997–2004.
Citron, C. A., Gleitzmann, J., Laurenzano, G., Pukall, R., and Dickschat, J. S. 2012. Terpenoids are widespread in actinomycetes: a correlation of secondary metabolism and genome data. Chem. Bio. Chem. 13:202–214.
Compant, S., Clément, C., and Sessitsch, A. 2010. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 42:669–678.
Conklin, A. R. 2005. Introduction to soil chemistry. John Wiley & Sons, Hoboken, New York.
Crowe, J. D. and Olsson, S. 2001. Induction of laccase activity in Rhizoctonia solani by antagonistic Pseudomonas fluorescens strains and a range of chemical treatments. Appl. Environ. Microbiol. 67:2088–2094.
Daniel, R. 2011. Soil-based metagenomics, pp. 83-92, in F. J. de Bruijn (ed.). Handbook of Molecular Microbial Mycology II: Metagenomics in Different Habitats. John Wiley & Sons, Inc.
Davis, R. D. 1976. Soil bacteriostasis: relation to bacterial nutrition and active soil inhibition. Soil Biol. Biochem. 8:429–433.
Dequiedt, S., Saby, N. P. A., Lelievre, M., Jolivet, C., Thioulouse, J., Toutain, B., Arrouays, D., Bispo, A., Lemanceau, P., and Ranjard, L. 2011. Biogeographical patterns of soil molecular microbial biomass as influenced by soil characteristics and management. Global Ecol. Biogeogr. 20:641–652.
Dickschat, J. S. 2009. Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 27:343–369.
Dickschat, J. S., Bode, H. B., Mahmud, T., Müller, R., and Schulz, S. 2005a. A novel type of geosmin biosynthesis in myxobacteria. J. Org. Chem. 70:5174–5182.
Dickschat, J. S., Bode, H. B., Wenzel, S. C., Müller, R., and Schulz, S. 2005b. Biosynthesis and identification of volatiles released by the myxobacterium Stigmatella aurantiaca. Chem. Biol. Chem. 6:2023–2033.
Dickschat, J. S., Helmke, E., and Schulz, S. 2005c. Volatile organic compounds from arctic bacteria of the cytophaga-flavobacterium-bacteroides-group: A retrobiosynthetic approach in chemotaxonomic investigations. Chem. Biodivers. 2:318–353.
Dickschat, J. S., Martens, T., Brinkhoff, T., Simon, M., and Schulz, S. 2005d. Volatiles released by a Streptomyces species isolated from the North Sea. Chem. Biodivers. 2:837–865.
Dickschat, J. S., Nawrath, T., Thiel, V., Kunze, B., Müller, R., and Schulz, S. 2007. Biosynthese des Duftstoffes 2-Methylisoborneol durch das Myxobakterium Nannocystis exedens. Angew. Chem. - Ger. Edit. 119:8436–8439.
Dickschat, J. S., Reichenbach, H., Wagner-Dobler, I., and Schulz, S. 2005e. Novel pyrazines from the myxobacterium Chondromyces crocatus and marine bacteria. Eur. J. Org. Chem. 19:4141–4153.
Dickschat, J. S., Wagner-Dobler, I., and Schulz, S. 2005f. The chafer pheromone buibuilactone and ant pyrazines are also produced by marine bacteria. J. Chem. Ecol. 31:925–947.
Dickschat, J. S., Wenzel, S. C., Bode, H. B., Müller, R., and Schulz, S. 2004. Biosynthesis of volatiles by the myxobacterium Myxococcus xanthus. Chem. Biol. Chem. 5:778–787.
Dighton, J. 2003. Fungi in ecosystem processes. Marcel Dekker, New York, NY.
Dobbs, C. G. and Hinson, W. H. 1953. A widespread fungistasis in soils. Nature 172:197–199.
Dobson, H. E. M. 2006. Relationship between floral fragrance composition and type of pollinator, pp. 147–198, in E. Pichersky and N. Dudareva (eds.), Biology of floral scents. Taylor & Francis Group, Boca Raton.
Duponnois, R., Ba, A. M., and Mateille, T. 1998. Effect of some rhizosphere bacteria for the biocontrol of nematodes of the genus Meloidogyne with Arthrobotrys oligospora. Fundam. Appl. Nematol. 21:157–163.
Ercolini, D., Russo, F., Nasi, A., Ferranti, P., and Villani, F. 2009. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl. Environ. Microbiol. 75:1990–2001.
Ezeonu, I. M., Price, D. L., Simmons, R. B., Crow, S. A., and Ahearn, D. G. 1994. Fungal production of volatiles during growth on fiberglass. Appl. Environ. Microbiol. 60:4172–4173.
Farag, M. A., Ryu, C. M., Summer, L. W., and Pare, P. W. 2006. GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67:2262–2268.
Farmer, E. E. 2001. Surface-to-air signals. Nature 411:854–856.
Fernando, W. G. D., Ramarathnam, R., Krishnamoorthy, A. S., and Savchuk, S. C. 2005. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 37:955–964.
Fiddaman, P. J. and Rossall, S. 1993. The production of antifungal volatiles by Bacillus subtilis. J. Appl. Bacteriol. 74:119–126.
Fiddaman, P. J. and Rossall, S. 1994. Effect of substrate on the production of antifungal volatiles from Bacillus subtilis. J. Appl. Bacteriol. 76:395–405.
Fischer, G., Schwalbe, R., Möller, M., and Ostrowski, R. 1999. Species-spezific production on microbial volatile organic compounds (MVOC) by airborne fungi from a compost facility. Chemosphere 39:795–810.
Forster, R. C. 1988. Microenvironments of soil microorganisms. Biol. Fertil. Soils 6:189–203.
Freeman, L. R., Silverman, G. J., Angelini, P., Merritt Jr., C., and Esselen, W. B. 1976. Volatiles produced by microorganisms isolated from refrigerated chicken at spoilage. Appl. Environ. Microbiol. 32:222–231.
Frey-Klett, P., Garbaye, J., and Tarkka, M. 2007. The mycorrhiza helper bacteria revisited. New Phytol. 176:22–36.
Fuchs, G. 2007. Allgemeine Hikrobiologie, 8th edn. http://www.kluweronline.com/issn/0098-0331.
Gans, J., Wolinsky, M., and Dunbar J. 2005. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science. 26;309(5739):1387–1390.
Garbaye, J. and Duponnois, R. 1992. Specificity and function of mycorrhization helper bacteria (MHB) associated with the Pseudotsuga menziesii – Laccaria laccata symbiosis. Symbiosis 14:335–344.
Garbeva, P., Hol, W. H. G., Termorshuizen, A. J., Kowalchuk, G. A., and De Boer, W. 2011. Fungistasis and general soil biostasis - A new synthesis. Soil Biol. Biochem. 43:469–477.
Gasch, A. P. 2007. Comparative genomics of the environmental stress response in ascomycete fungi. Yeast 24:961–976.
Gerber, N. N. and Lechevalier, H. A. 1965. Geosmin, an earthy-smelling substance isolated from actinomycetes. Appl. Microbiol. 13:935–938.
Gottschalk, G. 1986. Bacterial metabolism. Springer Verlag, Heidelberg.
Gregory, P. J. 2006. Roots, rhizosphere and soil: the route to a better understanding of soil science? Eur. J. Soil Sci. 57:2–12.
Griffin, G. J., Hora, T. S., and Baker, R. 1975. Soil fungistasis: elevation of the exogenous carbon and nitrogen requirements for spore germination by fungistatic volatiles in soil. Can. J. Microbiol. 21:1468–1475.
Gu, Y. Q., Mo, M. H., Zhou, J. P., Zou, C. S., and Zhang, K. Q. 2007. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 39:2567–2575.
Haas, D. and Défago, G. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3:307–319.
Hartmann, A., and Schikora, A. 2012. Quorum sensing of bacteria and trans-kingdom interactions of N-acyl homoserine lactones with eukaryotes. J. Chem. Ecol., this issue.
Hawksworth, D. L. and Mueller, G. M. 2005. Fungal communities: their diversity and distribution, pp. 27–37, in J. Dighton, J. F. White, and P. Oudemans (eds.), The fungal community. Taylor & Francis, Boca Raton, Florida.
Heil, M. and Ton, J. 2008. Long-distance signalling in plant defence. Trends Plant Sci. 13:264–272.
Heil, M. and Walters, D. R. 2009. Ecological consequences of plant defence signalling. Adv. Bot. Res. 51:667–716.
Herrington, P. R., Craig, J. T., and Sheridan, J. E. 1987. Methyl vinyl ketone: a volatile fungistatic inhibitor from Streptomyces griseoruber. Soil Biol. Biochem. 19:509–512.
Heuer, H. and Smalla, K. 2012. Plasmids foster diversification and adaptation of bacterial populations in soil. FEMS Microbiol. Rev. doi:10.1111/j.1574-6976.2012.00337.x
Hibbing, M. E., Fuqua, C., Parsek, M. R., and Peterson, S. B. 2010. Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8:15–25.
Hinton, A. and Hume, M. E. 1995. Antibacterial activity of the metabolic by-products of a Veillonella species and Bacteroides fragilis. Anaerobe 1:121–127.
Ho, W. C. and Ko, W. H. 1982. Characteristics of soil microbiostasis. Soil Biol. Biochem. 14:589–593.
Höckelmann, C. and Jüttner, F. 2004. Volatile organic compound (VOC) analysis and sources of limonene, cyclohexanone and straight chain aldehydes in axenic cultures of Calothrix and Plectonema. Water Sci. Technol. 49:47–54.
Höckelmann, C., Moens, T., and Friedrich, J. 2004. Odor compounds from cyanobacterial biofilms acting as attractants and repellents for free-living nematodes. Limnol. Oceanogr. 49:1809–1819.
Hora, T. S. and Baker, R. 1972. Soil fungistasis: microflora producing a volatile inhibitor. Trans. Br. Mycol. Soc. 59:491–500.
Horii, S. and Ishii, T. 2006. Identification and function of Gigaspora margarita growth-promoting microorganisms. Symbiosis 41:135–141.
Howell, C. R., Beier, R. C., and Stipanovic, R. D. 1988. Production of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Ecol. Epidemiol. 78:1075–1078.
Huang, C-J., Tsay, J-F., Chang, S-Y., Yang, H-P., Wu, W-S., and Chen, C-Y. 2012. Dimethyl disulfide is an induced systemic resistance-elicitor produced by Bacillus cereus C1L. Soc. Chem. Ind.; doi:10.1002/ps.3301
Hussain, A. and Hasnain, S. 2011. Interactions of bacterial cytokinins and IAA in the rhizosphere may alter phytostimulatory efficiency of rhizobacteria. World J. Microbiol. Biotechnol. 27:2645–2654.
Insam, H. and Seewald, M. S. A. 2010. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 46:199–213.
Jacobson, E. S. 2000. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 13:708–717.
Jamalizadeh, M., Etebarian, H. R., Aminian, H., and Alizadeh, A. 2010. Biological control of Botrytis mali on apple fruit by use of Bacillus bacteria, isolated from the rhizosphere of wheat. Arch. Phytopathol. Pl. 43:1836–1845.
Jelen, H. H. 2003. Use of solid phase microextraction (SPME) for profiling fungal volatile metabolites. Lett. Appl. Microbiol. 36:263–267.
Jelen, H. H., Mirocha, C. J., Wasowicz, E., and Kaminski, E. 1995. Production of volatile sesquiterpenes by Fusarium sambucinum strains with different abilities to synthesize trichothecenes. Appl. Environ. Microbiol. 61:3815–3820.
Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. 2012. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol., this issue.
Kai, M., Effmert, U., Berg, G., and Piechulla, B. 2007. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 187:351–360.
Kai, M., Vespermann, A., and Piechulla, B. 2008. The growth of fungi and Arabidopsis thaliana is influenced by bacterial volatiles. Plant Signal. Behav. 3:1–3.
Kai, M., Haustein, M., Molina, F., Petri, A., Scholz, B., and Piechulla, B. 2009. Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 81:1001–1013.
Kai, M., Crespo, E., Cristescu, S. M., Harren, F. J. M., and Piechulla, B. 2010. Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 88:965–976.
Kalderas, J. 2011. Erfassung, Analyse und Datenbank – Integration flüchtiger Metabolite von Pilzen und anderen Mikroorganismen. Diploma Thesis, University of Rostock
Kaminski, E., Stawicki, S., and Wasowicz, E. 1974. Volatile flavor compounds produced by molds of Aspergillus, Penicillum and fungi imperfecti. Appl. Microbiol. 27:1001–1004.
Kesselmeier, J. and Staudt, M. 1999. Biogenic volatile organic compounds VOC - an overview on emission, physiology and ecology. J. Atmos. Chem. 33:23–88.
Ko, W. H. and Chow, F. K. 1977. Characteristics of bacteriostasis in natural soils. J. Gen. Microbiol. 102:295–298.
Koske, R. E. and Gemma, J. N. 1992. Fungal reactions to plants prior to mycorrhizal formation, pp. 3–36, in M. J. Allen (ed.), Mycorrhizal functioning. Chapman & Hall, New York, NY.
Kurita-Ochiai, T., Fukushima, K., and Ochiai, K. 1995. Volatile fatty acids, metabolic by-products of periodontopathic bacteria, inhibit lymphocyte proliferation and cytokine production. J. Dent. Res. 74:1367–1373.
Labows, J. N., Mcginley, K. J., Webster, G. F., and Leyden, J. J. 1980. Headspace analysis of volatile metabolites of Pseudomonas aeruginosa and related species by gas chromatography-mass spectrometry. J. Clin. Microbiol. 2:521–526.
Lee, M. L., Smith, D. L., and Freeman, L. R. 1979. High resolution gas chromatographic profiles of volatile organic compounds produced by microorganisms at refrigerated temperatures. Appl. Environ. Microbiol. 37:85–90.
Leff, J. W. and Fierer, N. 2008. Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biol. Biochem. 40:1629–1636.
Lenc, L., Kwaśna, H., and Sadowski, C. 2011. Dynamics of the root/soil pathogens and antagonists in organic and integrated production of potato. Eur. J. Plant Pathol. 131:603–620.
Lin, H. C. and Phelan, P. L. 1992. Comparisons of volatiles from beetle-transmitted Ceratocystis fagacearum and four non-insect-dependent fungi. J. Chem. Ecol. 18:1623–1632.
Linton, C. W. and Wright, S. J. L. 1993. Volatile organic compounds: microbial aspects and some technical implications. J. Appl. Bacteriol. 75:1–12.
Logeshwarn, P., Thangaraju, M., and Rajasundari, K. 2011. Antagonistic potential of Gluconacetobacter diazotrophicus against Fusarium oxysporum in sweet potato (Ipomea batatus). Arch. Phytopathol. Pl. 44:216–223.
Lütke-Eversloh, T. and Bahl, H. 2011. Metabolic engineering of Clostridium acetobutylicum: recent advances to improve butanol production. Curr. Opin. Biotechn. 22:634–647.
Mackie, A. E. and Wheatley, R. E. 1999. Effects and incidence of volatile organic compound interactions between soil bacterial and fungal isolates. Soil Biol. Biochem. 31:375–385.
March, R. E., Richard, D. S., and Ryan, R. W. 2006. Volatile compounds from six species of truffle – head-space analysis and vapor analysis at high mass resolution. Int. J. Mass Spectrom. 249–250:60–67.
Martinez, A., Obertello, M., Pardo, A., Ocampo, J., and Godeas, A. 2004. Interactions between Trichoderma pseudokoningii strains and the arbuscular mycorrhizal fungi Glomus mosseae and Gigaspora rosea. Mycorrhiza 14:79–84.
Mattheis, J. P. and Roberts, R. G. 1992. Identification of geosmin as a volatile metabolite of Penicillium expansum. Appl. Environ. Microbiol. 58:3170–3172.
Matysika, S., Herbarth, O., and Mueller, A. 2008. Determination of volatile metabolites originating from mould growth on wall paper and synthetic media. J. Microbiol. Methods 75:182–187.
McAllister, C. B., Garcia-Garrido, J. M., Garcia-Romera, I., Godeas, A., and Ocampo, J. A. 1996. In vitro interactions between Alternaria alternata, Fusarium equiseti and Glomus mosseae. Symbiosis 20:163–174.
McCain, A. H. 1966. A volatile antibiotic by Streptomyces griseus. Phytopathology 56:150.
McNeal, K. S. and Herbert, B. E. 2009. Volatile organic metabolites as indicators of soil microbial activity and community composition shifts. Soil Sci. Soc. Am. J. 73:579–588.
Menetrez, M. Y. and Foarde, K. K. 2002. Microbial volatile organic compound emission rates and exposure model. Indoor Built Environ. 11:208–213.
Michalke, K., Wickenheiser, E. B., Mehring, M., Hirner, A. V., and Hensel, R. 2000. Production of volatile derivatives of metal (loid)s by microflora involved in anaerobic digestion of sewage sludge. Appl. Environ. Microbiol. 66:2791–2796.
Miller, A., Scanlan, R. A., Lee, J. S., and Libbey, L. M. 1973. Identification of the volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas fragi. Appl. Microbiol. 25:952–955.
Minerdi, D., Moretti, M., Gilardi, G., Barberio, C., Gullino, M. L., and Garibaldi, A. 2008. Bacterial ectosymbionts and virulence silencing in a Fusarium oxysporum strain. Environ. Microbiol. 10:1725–1741.
Minerdi, D., Bossi, S., Gullino, M. L., and Garibaldi, A. 2009. Volatile organic compounds: a potential direct long-distance mechanism for antagonistic action of Fusarium oxysporum strain MSA35. Environ. Microbiol. 11:844–854.
Minnich, M. and Schumacher, B. 1993. Behavior and determination of volatile organic compounds in soil: A literature review. US Environmental Protection Agency. EPA 600/R-93/140:1–104.
Miransari, M. 2011. Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl. Microbiol. Biotech. 89:917–930.
Morra, M. J. and Dick, W. A. 1991. Mechanisms of H2S production from cysteine and cystine by microorganisms isolated from soil by selective enrichment. Appl. Environ. Microbiol. 57:1413–1417.
Naeem, S. 1997. Species redundancy and ecosystem reliability. Conserv. Biol. 12:39–45.
Nakas, J. P. and Klein, D. A. 1980. Mineralization capacity of bacteria and fungi from the rhizosphere-rhizoplane of a semiarid grassland. Appl. Environ. Microbiol. 39:113–117.
Nannipieri, P., Ascher, J., Ceccherini, M. T., Landi, L., Pietramellara, G., and Renella, G. 2003. Microbial diversity and soil functions. Eur. J. Soil Sci. 54:655–670.
Nannipieri, P., Ascher, J., Ceccherini, M. T., Landi, L., Pietramellara, G., Renella, G., and Valori, F. 2007. Microbial diversity and microbial activity in the rhizosphere. Ciencia del suelo (ARGENTINA) 25:89–97.
Nawrath, T., Dickschat, J. S., Müller, R., Jiang, J., Cane, D. E., and Schulz, S. 2008. Identification of (8S, 9S, 10S)-8, 10-dimethyl-1-octalin, a key intermediate in the biosynthesis of geosmin in bacteria. J. Am. Chem. Soc. 130:430–431.
Nieminen, T., Neubauer, P., Sivela, S., Vatamo, S., Silfverberg, P., and Salkinoja-Salonen, M. 2008. Volatile compounds produced by fungi grown in strawberry jam. LWT- Food Sci. Technol. 41:2051–2056.
Nijland, R. and Burgess, J. G. 2010. Bacterial olfaction. Biotechnol. J. 5:1–4.
Owen, S. M., Clark, S., Pompe, M., and Semple, K. T. 2007. Biogenic volatile organic compounds as potential carbon sources for microbial communities in soil from the rhizosphere of Populus tremula. FEMS Microbiol. Lett. 268:34–39.
Pal, K. K. and McSpadden Gardener, B. 2006. Biological control of Plant Pathogens. The Plant Health Instructor doi:10.1094/PHI-A-2006-1117-02 http://www.apsnet.org/edcenter/advanced/topics/Pages/BiologicalControl.aspx.
Palková, Z. and Váhová, L. 2003. Ammonia signaling in yeast colony formation. Int. Rev. Cytol. 225:229–272.
Pelusio, F., Nilsson, T., Montanarella, L., Tilio, R., Larsen, B., Facchetti, S., and Madsen, J. 1995. Headspace solid-phase microextraction analysis of volatile organic sulfur compounds in black and white truffle aroma. J. Agric. Food Chem. 43:2138–2143.
Pessi, G. and Haas, D. 2000. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J. Bacteriol. 182:6940–6949.
Pittard, B. T., Freeman, L. R., Later, D. W., and Lee, M. L. 1982. Identification of volatile organic compounds produced by fluorescent pseudomonads on chicken breast muscle. Appl. Environ. Microbiol. 43:1504–1506.
Ranjard, L. and Richaume, A. 2001. Quantitative and qualitative microscale distribution of bacteria in soil. Res. Microbiol. 152:707–716.
Rigamonte, T. A., Pylro, V. S., and Duarte G. F. 2010. The role of mycorrhization helper bacteria in the establishment and action of ectomycorrhizae associations. Braz. J. Microbiol. 41:832–840.
Roesch, L. F., Fulthorpe, R. R., Riva, A., Casella, G., Hadwin, A. K., Kent, A. D., Daroub, S. H., Camargo, F. A., Farmerie, W. G., and Triplett, E.W. 2007. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 1:283–290.
Ruiz, R., Bilbao, R., and Murillo, M. B. 1998. Adsorption of different VOC onto soil minerals from gas phase; Influence of mineral, type of VOC, and air humidity. Environ. Sci. Technol. 32:1079–1864.
Ryan, R. P. and Dow, J. M. 2008. Diffusible signals and interspecies communication in bacteria. Microbiol. 154:1845–1858.
Ryu, C. M., Farag, M. A., Hu, C. H., Reddy, M. S., Wie, H. X., Pare, P. W., and Kloepper, J. W. 2003. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100:4927–4932.
Schafer, B. M. 2006. Particle shape, pp. 1249–1250, in W. Chesworth (ed.), Encyclopedia of soil science. Taylor & Francis, Boca Raton, Florida.
Schäfer, H., Myronova, N., and Boden, R. 2010. Microbial degradation of dimethyldisulphide and related C1-sulphur compounds: organisms and pathways controlling fluxes of sulphur in the biosphere. J. Exp. Bot. 61:315–334.
Schippers, B., Meijer, J. W., and Liem, J. I. 1982. Effect of ammonia and other soil volatiles on germination and growth of soil fungi. Trans. Br. Mycol. Soc. 79:253–259.
Schöller, E. G., Gürtler, H., Pedersen, R., Molin, S., and Wilkins, K. 2002. Volatile metabolites from actinomycetes. J. Agric. Food Chem. 50:2615–2621.
Schrey, S. D., Schellhammer, M., Ecke, M., Hampp, R., and Tarkka, M. T. 2005. Mycorrhiza helper bacterium Streptomyces AcH 505 induces differential gene expression in the ectomycorrhizal fungus Amanita muscaria. New Phytol. 168:205–216.
Schulz, S. and Dickschat, J. S. 2007. Bacterial volatiles: the smell of small organisms. Nat. Prod. Rep. 24:814–842.
Schulz, S., Fuhlendorff, J., and Reichenbach, H. 2004. Identification and synthesis of volatiles released by the myxobacterium Chondromyces crocatus. Tetrahedron 60:3863–3872.
Shatalin, K., Shatalina, E., Mironov, A., and Nudler, E. 2011. H2S: a universal defense against antibiotics in bacteria. Science. 334:986–990.
Sobik, P., Grunenberg, J., Boöroöczky, J., Laatsch, H., Wagner-Doöbler, I., and Schulz, S. 2007. Identification, synthesis, and conformation of tri- and tetrathiacycloalkanes from marine bacteria. J. Org. Chem. 72:3776–3782.
Son, S. H., Khan, Z., Kim, S. G., and Kim, Y. H. 2009. Plant growth-promoting rhizobacteria, Paenibacillus polymyxa and Paenibacillus lentimorbus suppress disease complex caused by root-knot nematode and Fusarium wilt fungus. J. Appl. Microbiol. 107:524–532.
Standing, D. and Killham, K. 2007. The soil environment, pp. 1–22, in J. D. van Elsas, J. K. Jansson, and J. T. Trevors (eds.), Modern soil microbiology. Taylor & Francis, Boca Raton, Florida.
Stoppacher, N., Kluger, B., Zeilinger, S., Krska, R., and Schuhmacher, R. 2010. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 81:187–193.
Stotzky, G. and Schenck, S. 1976. Volatile organic compounds and microorganisms. CRC Crit. Rev. Microbiol. 4:333–382.
Stritzke, K., Schulz, S., Laatsch, H., Helmke, E., and Beil, W. 2004. Novel caprolactones from a marine streptomycete. J. Nat. Prod. 67:395–401.
Sunesson, A. L., Vaes, W. H. J., Nilsson, C. A., Blomquist, G., andersson, B., and Carlson, R. 1995. Identification of volatile metabolites from five fungal species cultivated on two media. Appl. Environ. Microbiol. 61:2911–2918.
Sunesson, A. L., Nilsson, C. A., andersson, B., and Blomquist, G. 1996. Volatile metabolites produced by two fungal species cultivated on building materials. Ann. Occup. Hyg. 40:397–410.
Tehrani, A. S., Disfani, F. A., Hedjaroud, G. A., and Mohammadi, M. 2001. Antagonistic effects of several bacteria on Verticillium dahliae the causal agent of cotton wilt. Meded. Rijksuniv. Gent Fak. Landbouwkd. Toegep. Biol. Wet. 66:95–101.
Tehrani, A. S., Zebarjad, A., Hedjaroud, G. A., and Mohammadi, M. 2002. Biological control of soybean damping-off by antagonistic rhizobacteria. Meded. Rijksuniv. Gent Fak. Landbouwkd. Toegep. Biol. Wet. 67:377–380.
Tracey, R. P. and Britz, T. J. 1989. Freon 11 extraction of volatile metabolites formed by certain lactic acid bacteria. Appl. Environ. Microbiol. 55:1617–1623.
Tylka, G. L., Hussey, R. S., and Roncadori, R. W. 1991. Axenic germination of vesicular-arbuscular mycorrhizal fungi: effect of selected Streptomyces species. Phytopathology 81:754–759.
Van Lancker, F., Adams, A., Demulle, B., De Saeger, S., Moretti, A., Van Petheghem, C., and De Kimpe, N. 2008. Use of headspace SPME-GC-MS for the analysis of the volatiles produced by indoor molds grown on different substrates. J. Environ. Monit. 10:1127–1133.
Vespermann, A., Kai, M., and Piechulla, B. 2007. Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl. Environ. Microbiol. 73:5639–5641.
Voisard, C., Keel, C., Haas, D., and Défago, G. 1989. Cyanide production by Pseudomonas fluorescens helps to suppress black root of tobacco under gnotobiotic conditions. EMBO J. 8:351–358.
Von Reuss, S., Kai, M., Piechulla, B., and Francke, W. 2010. Octamethylbicyclo(3.2.1)octadienes from Serratia odorifera. Angew. Chem. Int. Ed. 49:2009–2010.
Walker, T. S., Bais, H. P., Grotewold, E., and Vivanco, J. M. 2003. Root exudation and rhizosphere biology. Plant Physiol. 132:44–51.
Walker, T. S., Bais, H. P., Deziel, E., Schweitzer, H. P., Rahme, L. G., Fall, R., and Vivanco, J. M. 2004. Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formations, and root exudation. Plant Physiol. 134:3210–3331.
Wan, M., Li, G., Zhang, J., Jiang, D., and Huang, H. C. 2008. Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biol. Control 46:552–559.
Wargo, M. J. and Hogan, D. A. 2006. Fungal-bacterial interactions: a mixed bag of mingling microbes. Curr. Opin. Microbiol. 9:359–364.
Weise, T., Kai, M., Gummesson, A., Troeger, A., Von Reuß, S., Piepenborn, S., Kosterka, F., Sklorz, M., Zimmermann, R., Francke, W., and Piechulla, B. 2012. Volatile organic compounds produced by the phytopathogenic bacterium Xanthomonas campestris pv. vesicatoria 85–10. Beilstein J. Org. Chem. 8:579–596.
Wei-Wei, L., Wie, M., Bing-Yu, Z., You-Chen, D., and Feng, L. 2008. Antagonistic activities of volatiles from four strains of Bacillus spp. and Paenibacillus spp. against soil-borne plant pathogens. Agr. Sci. China 7:1104–1114.
Wenke, K., Kai, M., and Piechulla, B. 2009. Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506.
Wenke, K., Weise, T., Warnke, R., Valverde, C., Wanke, D., Kai, M., and Piechulla, B. 2012. Bactertial volatiles mediating information between bacteria and plants, pp. 327–348, in G. Witzany (ed.), Biocommunication, signaling and communication in plants. Springer Verlag, Berlin, Heidelberg.
Wheatley, R. E. 2002. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 81:357–364.
Wheatley, R., Hackett, C., Bruce, A., and Kundzewiczd, A. 1997. Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. inhibitory to wood decay fungi. Int. Biodeterior Biodegradation 39:199–205.
Wiggins, R. J., Wilks, M., and Tabaqchali, S. 1985. Analysis by gas liquid chromatography of production of volatile fatty acids by anaerobic bacteria grown on solid medium. J. Clin. Pathol. 38:933–936.
Wilkins, K., Larsen, K., and Simkus, M. 2000. Volatile metabolites from mold growth on building materials and synthetic media. Chemosphere 41:437–446.
Will, M. E. and Sylvia, D. M. 1990. Interaction of rhizosphere bacteria, fertilizer, and vesicular-arbuscular mycorrhizal fungi with sea oats. Appl. Environ. Microbiol. 56:2073–2079.
Will, C., Nacke, H., Thürmer, A., and Daniel, R. 2010. Schlaglicht Biodiversität/Charakterisierung und Nutzung der bakteriellen Diversität in Bodenmetagenomen. Genomxpress 1.10:9–11.
Williamson, P. R. 1997. Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front. Biosci. 2:e99–e107.
Winson, M. K., Camara, M., Latifi, A., Foglino, M., Chhabra, S. R., Daykin, M., Bally, M., Chapon, V., Salmond, G. P., Bycroft, B. W., et al. 1995. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 92:9427–9431.
Wu, S., Krings, U., Zorn, H., and Berger, R. G. 2005. Volatile compounds from the fruiting bodies of beefsteak fungus Fistulina hepatica (Schaeffer: Fr.) Fr. Food Chem. 92:221–226.
Xavier, L. J. C. and Germida, J. J. 2003. Bacteria associated with Glomus clarum spores influence mycorrhizal activity. Soil Biol. Biochem. 35:471–478.
Xiao, X., Chen, H., Chen, H., Wang, J., Ren, C., and Wu, L. 2008. Impact of Bacillus subtilis JA, a biocontrol strain of fungal plant pathogens, on arbuscular mycorrhiza formation in Zea mays. World J. Microbiol. Biotechnol. 24:1133–1137.
Zhang, C. L., Wang, G. P., Mao, L. J., Komonzelazowska, M., Yuan, Z. L., Fu-Cheng Lin, F. C., Druzhinia, I. S., and Kubick, C. P. 2010. Muscodor fengyangensis sp. nov. from southeast China: morphology, physiology and production of volatile compounds. Fungal Biol. 114:797–808.
Zhao, L. J., Yang, X. N., Li, X. Y., Mu, W., and Liu, F. 2011. Antifungal, insecticidal and herbicidal properties of volatile components from Paenibacillus polymyxa Strain BMP-11. Agr. Sci. China 10:728–736.
Zhu, J., Bean, H. D., Kuo, Y. M., and Hill, J. E. 2010. Fast detection of volatile organic compounds from bacterial cultures by secondary electrospray ionization-mass spectrometry. Clin. Microbiol. 48:4426–4431.
Zou, C. S., Mo, M. H., Gu, Y. Q., Zhou, J. P., and Zhang, K. Q. 2007. Possible contribution of volatile-producing bacteria in soil fungistasis. Soil Biol. Biochem. 39:2371–2379.
Acknowledgments
The authors thank Prof. Hubert Bahl reading and correcting of Table 1 and the related chapter in the paper, Dr. Marco Kai for critical reading of the manuscript and for drawing Fig. 1, and Robert Penthin, who helped to develop the DOVE-MO database. We are grateful for the funding by the DFG (to BP153/26 and/28).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Effmert, U., Kalderás, J., Warnke, R. et al. Volatile Mediated Interactions Between Bacteria and Fungi in the Soil. J Chem Ecol 38, 665–703 (2012). https://doi.org/10.1007/s10886-012-0135-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0135-5