Abstract
Stem cells are unspecialized cells having two key properties, the capacity of self-renewal and the ability of generating differentiated cells. Based on the different development phases of an organism, different stem cell types can be isolated. Among these, multipotent adult stem cells are committed cells able of differentiating into all mature cell lineages typical of the organ tissues in which they reside. The multipotent mesenchymal stem cells (MSCs), a type of adult stem cells, are a promising cell source for tissue engineering and cell-based therapies since their use in clinical applications does not imply neither ethical problems nor teratoma risk formation. Bone marrow has been the main source of human mesenchymal stem cells (hMSCs), even with the highly invasive isolation procedures and the low cell quantity obtained. Therefore, the identification of an alternative source of hMSCs has been an important issue, and in this context, the adipose and neonatal tissues, including placenta, are a promising choice for their anti-inflammatory, anti-fibrotic, and pro-regenerative characteristics. Placental and adipose-derived hMSCs could be a good option in severe muscle injury and particularly for sphincter incontinence regeneration. In this context, Lipogems®, rich in hMSCs from autologous lipoaspirated fat, is obtained in a closed disposable device system without using enzymes, additives, or other manipulations. Recently, the Lipogems® technique was used successfully to treat patients with fecal incontinence, according to the Helsinki Declaration and approved by the ethics committee. This safe and well-tolerated procedure did not have adverse events on the patients and improved their contractile activity of the anal sphincters and anocutaneous reflex.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
14.1 Stem Cell Biology
14.1.1 Stem Cell Classification
The German biologist Ernst Haeckel first introduced the term “stem cell” in the scientific literature in 1868 with the term “Stammzelle” (stem cell) to describe the unicellular ancestor progenitor of all organisms. In the nineteenth century, Theodor Boveri and Valentin Häcker instead used the same term to describe “cells committed to give rise to the germline” [1],and 4 years later, Edmund B. Wilson made the term “stem cell” universal by reviewing Hacker’s and Boveri’s work in his book entitled The Cell in Development and Inheritance [2].
Around 100 years later, Gail Martins of the University of California, Martin Evans and Matthew Kaufman of the University of Cambridge, independently isolated stem cells from mouse embryos and coined the term “embryonic stem cells” (ESCs). In 2007, Mario Capecchi, Martin Evans, and Oliver Smithies shared the Nobel Prize in Physiology and Medicine for the great achievement in the field of ESCs obtained in the mid-1980s. In 1995, Jamie Thompson of the University of Wisconsin cultured monkey ESCs for the first time and later, in 1999, human embryonic stem cells.
All tissues are composed by highly specialized cells derived from an initial pool of stem cells generated during early embryonic development, which provides a reserve for injured tissue repair and replaces the cells lost daily in the lifespan. Stem cells are unspecialized cells that have two key properties that distinguish them from other types of cells; they have the capacity of self-renewal and the ability of generating differentiated cells [3, 4]. These cells are capable of generating daughter cells for long periods identical to their mother cells (self-renewal). They are also able of differentiating, under specific physiological conditions, into many types of mature cell, which make up totally all our organs and tissues.
This area of interest includes different types of stem cells, which can be isolated during different phases of the development of an organism. In the initial stage of the embryo development, stem cells (ESCs) can be found in the blastocyst (50–100 cells), whereas in the adult stage, tissue stem cells can be found almost in all body tissue. These adult stem cells (ASCs) can also be found in the fetus and in babies. Finally, induced pluripotent stem cells (iPS cells) derived from specialized cells (e.g., skin cells) can be “in vitro” engineered, or “reprogrammed,” to become pluripotent cells like embryonic stem cells (Fig. 14.1).
Specifically, embryonic stem cells are derived from preimplanted embryos after the formation of the blastocyst [5]; this is made up of an outer layer of cells, an internal fluid-filled space, and an inner cell mass where the ESCs reside. They are defined “pluripotent” because of their ability to differentiate toward all the different types of body cells and tissues, except for extraembryonic organs such as the placenta, yolk sac, and umbilical cord. On the contrary, embryonic stem cells that immediately arise in the first few divisions of the fertilized egg, and defined “totipotent,” are able to totally generate a viable embryo including extraembryonic organs.
Instead, adult stem cells are committed cells able of differentiating into all mature cell lineages typical of the tissues or organs in which they reside and for this reason described as “multipotent.” For example, stem cells within the adult brain are able to differentiate in neurons and into other two types of cells, astrocytes and oligodendrocytes. Adult stem cells have been found in several organs, mostly those that continuously replenish themselves, such as the blood, skin, muscle, and liver, in large quantity but also in other, less regenerative organs such as the heart and brain.
Finally, induced pluripotent stem cells were “in vitro” produced in 2006 [6] by using viruses for the insertion inside somatic cells of four genes (Oct4, Sox2, c-myc, and Klf4) known to be important for the embryonic stem cell development. These pluripotent stem cells share many characteristics of embryonic stem cells, including the ability to differentiate toward all the cell types in the body. How these four “reprogramming” genes are able to induce pluripotency is not yet well known, and this question is the object of current studies [7]. In addition, recent research is concentrated on finding an alternative way to reprogram somatic cells using safer approaches in clinical sceneries [8, 9].
14.1.2 Stem Cells’ Potential Use: Advantages and Disadvantages
Several challenges must be addressed before stem cells can be used in regenerative medicine applications. The first important issue to be addressed is the identification, isolation, and growth of stem cells which are not easy procedures in the case of rare adult stem cells. The following reports and discusses the positive and negative aspects of the three main promising stem cells types, currently the object of worldwide research and investment.
Pluripotent embryonic stem cells that are easily isolated and have the advantage of an unlimited in vitro growth also have the capability of a great differentiating potential through strictly controlled processes. On the contrary, their clinical use has important limitations, due to their genetic instability, potential tumorigenic risk, and ethical considerations related to their origin [10]. For this last reason, in Europe there are rigorous laws that forbid destructive embryo research, while federal laws in the USA instead allow embryo use only in the case of it being discarded after in vitro fertilization [11].
Induced pluripotent stem cells, deriving from reprogrammed somatic cells with standard protocols, are able to differentiate into the three germ layer cell types [12], but still have a very low reprogramming efficiency. These cells could be a good option in autologous transplant applications, overcoming the tissue rejection; however, like the ES cells, they have an important genetic instability and a high tumorigenic [13] risk. Therefore, the standard and safe use in cell therapies of both stem cell types is still a target to reach which needs extensive research and effort.
Finally, multipotent adult stem cells can be used in autologous transplantation in which the patient’s own cells are expanded and differentiated in vitro. They are then implanted in the same person, avoiding the host’s immune rejection and protecting the patients from viral, bacterial, or other types of donor’s contamination. The disadvantage of ASCs is a very short life in culturing and expansion and a weaker differentiating potential in comparison with embryonic stem cells.
Although significant progress has been made in the stem cell research field and many preclinical studies have highlighted the great therapeutic potential of these cells, among the stem cells types, only the adult stem cells are currently used in some clinical applications. In particular, the bone marrow stem cells have been employed for more than 50 years, giving excellent results especially in the hematopoietic and immune system pathologies, which are addressed in the next section.
14.1.3 Current Clinical Applications Using Multipotent Adult Stem Cells
Presently, in some cases, this clinical protocol is replaced by autologous transplantation of stem cells; as a matter of fact, in the area of therapeutic implantation, it is very important to have a strong compatibility between the donor and the host tissue, in order to minimize the risk of rejection and at the same time deliver and engraft the stem cells to the target damaged tissue to improve the stem cell integration.
In successful clinical applications, the stem cells used were the blood (hematopoietic) stem cells from the bone marrow for the treatment of leukemia, lymphoma, and several inherited blood disorders. Umbilical cord blood, like bone marrow, is also collected as a source of blood stem cells and then used as an alternative to bone marrow transplantation, especially for the treatment of diseases in children. Other stem cell treatments which proved safe and effective involved bone, skin, and corneal diseases or injuries.
14.1.3.1 Bone Marrow Stem Cells in Transplants
Bone marrow stem cell therapy has been in routine use since the 1970s [14] and is able to treat a patient’s diseased blood. Although it presents a direct complication, due to the donor’s immune cells that sometimes can react to the patient’s tissues (graft-versus-host disease or GVHD) [15, 16], and an indirect complication, due to a risk of infection in chemotherapy pretreated patients [17], many thousands of people benefit from this kind of treatment every year.
14.1.3.2 Umbilical Cord Blood Stem Cells in Transplants
The umbilical cord blood stem cells (UCSCs) have the advantage of being less rejected by the immune system, compared to conventional bone marrow transplants. UCSCs, adequately cryopreserved in cell banks, are presently used for treating cancer blood disorders in children, such as leukemia, and genetic blood diseases like Fanconi anemia [18, 19].
14.1.3.3 Skin Stem Cells in Transplants
Skin stem cells have been used since the 1980s for the in vitro growth of new skin sheets for treating patients with severe burns [20]. However, the new skin has no hair follicles, sweat glands, or sebaceous (oil) glands, so this approach is used only for saving the lives of patients with third-degree burn over very large areas of their bodies [20].
14.1.3.4 Eye Stem Cells in Transplants
Clinical studies have shown that adult stem cells isolated from the limbus area of the eye can be used to repair damaged cornea. As matter of fact, the limbal stem cells can be taken from the patients, in vitro cultured and transferred back to their injured eye [20]. The treatment, safe and effective in early stage trials [20], is limited if both eyes have been seriously damaged for the impossibility to obtain the patients’ limbal stem cells. The safe and routine use of adult stem cells in clinical therapies needs a considerable research work and for this scope the public funds are required.
14.1.4 Multipotent Mesenchymal Stem Cells
Among the adult stem cells, multipotent mesenchymal stem cells (MSCs) are a promising cell source for tissue engineering and cell-based therapies due to their ability of self-renewal and of differentiating into specific cell lineages. Human Mesenchymal Stem Cells (hMSCs) have aroused great interest in the scientific community since their use in clinical applications does not imply neither ethical problems nor teratoma-risk formation. The number of clinical trials in which hMSCs have been tested has been increasing since 2004 [21], opening up their potential employment in the future treatment of numerous diseases, mainly tissue injuries and immune disorders. These non-hematopoietic adult stem cells, first isolated and studied by Friedenstein in 1971 [22], are able to differentiate into various mesoderm lineages, such as osteocytes, chondrocytes, and adipocytes, as well as ectodermic and endodermic cell lineages [23–27]. MSCs originate from the mesoderm but have a wide distribution in organs and can be isolated from many tissues such as the bone marrow, adipose tissue, muscle, liver, lung, and extraembryonic tissues [28–32].
These stem cells, involved in normal human tissue renewal, wound healing, and in physiological responses to injuries [33], have shown repairing effects for the treatment of damaged tissues and degenerative diseases [34–39]. In patients with cirrhosis disease due to hepatitis B, the autologous transplant of mesenchymal stem cells from the bone marrow (BM-MSCs) has showed encouraging results being able to improve the liver function [40, 41]. BM-MSCs have also provided positive responses in the treatment of muscular-skeletal diseases, periodontal tissue defects, diabetic critical limb ischemia, and burnt skin repair [42–44]. In addition, some preclinical studies have reported tissue regeneration through an anti-inflammatory effect of BM-MSCs in myocardial infarction treatment [45], cornea damage, and other tissue injuries, such as the brain, spinal cord [46], and lung [47–49].
For a long time, the main and traditional source of hMSCs for clinical application uses has been the bone marrow, but their employment is still limited not only because the procedures to isolate hMSCs are highly invasive and the cell quantity obtained is low but also because the proliferating and differentiating potential decreases as the donor’s age increases [50]. For this reason, the identification of an alternative source of hMSCs has been an important and necessary issue that still needs to be explored, and for this aim, a promising choice could be adipose tissue and neonatal tissues, including placenta.
14.1.5 hMSCs from Neonatal Tissue
Placental tissue is involved in important functions such as nutrition, respiration, and excretion and the maintenance of fetomaternal tolerance. It is made up of the chorionic plate, which is in close contact with the uterine decidua, and the fetal membranes (amnion and chorion), which spread from the borders of the chorionic plate and enclose the fetus in the amniotic cavity. The amniotic membrane (AM) encloses two types of stem cells, epithelial and mesenchymal, which have different embryological origins. The human amniotic epithelial cells (hAECs), derived from the embryonic ectoderm, form a continuous monolayer in contact with the amniotic fluid. The human amniotic mesenchymal stromal cells (hAMSCs), deriving from the embryonic mesoderm, are instead spread in the stromal layer underlying the amniotic epithelium. Stem cells deriving from AM have a great differentiating potential since these two layers originate at day 8–9 after fertilization, in a very early stage of the embryonic development. This has been extensively verified by several studies that report the capability of hAECs and hAMSCs to differentiate toward different cell lineages belonging to all three germ layers [51, 52]. The recovery of these stem cell types does not require any invasive procedures for the donor and does not rise any ethical issue; furthermore, the fact that the placenta is generally discarded after birth and is available in large supplies makes these stem cells an excellent candidate for their eventual use in cell therapy approaches [51]. The scientific interest for the use of these stem cells in regenerative medicine is also generated by their low immunogenicity characteristics; this is confirmed by the clinical applications that use the AM as biologic bandages in surgical procedures [53] for the treatment of corneal or conjunctival destructive loss [54].
The low immunogenic and the immunomodulatory properties of hAECs and hAMSCs can be explained by their low or limited levels of the HLA-ABC expression and the absence of the HLA-DR expression together with the co-stimulatory molecules [55–57]. All these immunological characteristics make them particularly suitable for the use in allogenic transplantations for the recovery of the damaged tissue through anti-inflammatory, anti-fibrotic, and pro-regenerative effects, minimizing the risk of rejection. This procedure is less invasive compared to autologous transplants and have all the advantages of allogenic transplantations in which the stem cells can be previously isolated and cryopreserved, making them readily available for possible clinical uses. This fact shortens the time of transplantation, offering the advantage of intervening timely on the damaged tissue before the fibrotic process irreversibly compromises the tissue regeneration.
14.1.5.1 Potential Use of hAMSC in Muscle Repair
The capability of muscle tissues to regenerate in response to injury stimuli represents an essential homeostatic process, in which the cell turnover plays an important role and in the case of small injuries due to contusions, the muscle is able to self-repair its damage through four correlated time-dependent phases: degeneration, inflammation, regeneration, and remodeling repair [58]. The injury of myofibers results in the rapid necrosis in which the influx of extracellular calcium induces the proteolysis of the myofibers [59]. The necrotic fibers activate an inflammatory response characterized by the recall of specific cell populations into the muscle [60]. The inflammatory response is then followed by a regenerative phase, characterized by satellite stem cell activation and by the presence of regenerating fibers [61]. In the final phase, the maturation of the regenerated myofibers, and the contraction and reorganization of the scar tissue occur, recovering the functional performance of the injured muscle [62]. On the contrary, in the case of severe muscle injuries, the muscle function results permanently damaged for the formation of dense scar tissue (fibrosis) [63, 64] that can diminish the ability of full recovery leading to muscle contracture and chronic pain [65]. Up to date, optimal treatment strategies for severe muscle injuries have not yet been identified, and for this scope, a new strategy needs to be developed. In this context, AM-derived mesenchymal stem cell could be a promising option for their anti-inflammatory, anti-fibrotic, and pro-regenerative intrinsic characteristics.
In our laboratory, the hAMSCs isolated from AM are being studied to investigate their possible use in severe muscle injury also with the goal of sphincter incontinence regeneration. These cells are isolated from the term amnion and dissected from the part connected to the umbilical cord to minimize the presence of maternal cells. Homogenous hAMSC populations are obtained by a two-step procedure: the amniotic membrane is treated with trypsin to remove hAECs and the remaining mesenchymal stem cells are then released by digestion with collagenase [66]. The quantity obtained from the term amnion is about one million hAMSCs [67], a great amount that is possible to cryopreserve. After isolation, the hAMSCs are characterized according to the minimal and univocal criteria indicated by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy [68]. The first cell requirement needed is the plastic-adherent ability when maintained in standard culture conditions; they must also express CD105, CD73, CD29, and CD90 and lack the expression of CD45 and CD31 surface molecules, and finally, they must show a differentiating potential toward osteoblast, adipocyte, and chondroblast lineages after specific in vitro chemical treatments. Based on this, we demonstrated by phase-contrast analysis on isolated hAMSCs the ability to adhere to plastic Petri dishes (Fig. 14.2a), and by trypan blue assay, they resulted able to exponentially grow from day 1 to day 4 (Fig. 14.2b). Actin fluorescence staining also revealed (Fig. 14.3) their typical fibroblast-like morphology (Fig. 14.3). The presence of MSC markers (CD90, CD44, CD73, CD54, CD105, and CD29) and a very low expression of hematopoietic markers such as CD31, CD34, and CD45 were also highlighted by their immunophenotypical characterization (Table 14.1). Moreover, a widespread expression of the mesenchymal ubiquitous Vimentin marker was revealed, by fluorescence microscopy analysis (Fig. 14.4) together with the capability to achieve osteogenic, adipogenic, and chondrogenic commitments when growing in appropriate and specific differentiating mediums as highlighted by specific assays (data not shown).
The mRNAs’ expression of early and late muscle differentiation markers has been also investigated in hAMSCs after the treatment of chemical and physical differentiating stimuli. The ongoing results confirmed their muscle commitment, suggesting their potential use in cell therapy leading us to suppose that these cells’ engraftment could be enhanced compared to other uncommitted transplanted stem cells. This hypothesis is presently under investigation in various muscles injury animal models in order to understand the most efficient differentiating level to be used to improve muscle repair.
14.1.6 hMSCs from Adipose Tissue
The adipose tissue was for a long time considered only for its energy storage function [69, 70], but in 1994 after the discovery of leptin, the first adipokine, it became clear that this tissue is also an endocrine organ playing an important role for several inflammatory diseases in physiopathology [71]. Adipose tissue is widely distributed in the adult human body and is found in the bone marrow; intra-articular, subcutaneous, and visceral depots; and ectopic sites such as intrahepatic and intramuscular tissues. The worldwide diffusion of obesity has contributed to increase the scientific interest toward this tissue. Even though the mature adipocytes are their main component, it is also composed by other cell types that contribute to its cellular heterogeneity. These different cell components are usually isolated from surgical specimens or lipoaspirates by collagenase enzyme digestions [72, 73] followed by centrifugation to separate the floating mature adipocytes from the remaining cells, forming a stromal vascular fraction (SVF) pellet [73]. It contains endothelial cells, macrophages, fibroblasts, B- and T-lymphocytes, myeloid cells, pericytes, smooth muscle cells, pre-adipocytes, and adipose-derived stem cells (ADSCs). After about 1 week of expansion in specific medium, it is possible to obtain from one milliliter of human lipoaspirate between 0.2 and 0.4 × 106 of ADSCs which are able to differentiate toward the adipocyte, chondrocyte, and osteoblast lineages [74, 75]. Since many patients routinely undergo liposuction annually, it is easy to isolate hundreds of million ADSCs from a single donor, making them particularly interesting for regenerative medicine applications. Recently, the International Federation for Adipose Therapeutics and Science (IFATS) have provided minimal criteria for the characterization of ASC based on functional and quantitative features [76], and many companies have developed closed system devices designed for ADSC isolation [77]. These automated devices have improved the methods to obtain reproducibility of results and their safety in clinical application uses.
The clinical translation of ADSCs still remains object of intensive research [78], but some very promising findings have been already reported. Finnish and collaborators at the Universities of Helsinki and Tampere, for example, used autologous human ADSCs to repair hard palate defect [79], reporting the encouraging results of a full recovery of the oral function and independent groups have shown similar results [80, 81].
14.2 Clinical Applications
14.2.1 Stem Cells and Sphincters Dysfunctions of the Pelvis
At present there are more than 350 clinical trials involving human MSCs for very different entities (www.clinicaltrials.gov). Most of these studies involve the use of mesenchymal stem cells from the bone marrow and adipose tissue, and no significant adverse effects were observed in all studies. Relatively few studies were performed to treat a degenerated sphincter muscle in humans with MSCs or MSC-like cells.
Based on the promising preclinical “in vitro” and “in vivo studies” [82–84], MSCs have been also investigated for their potential therapeutic applications in sphincter dysfunctions of the pelvic floor, both in the proctologic and in the urogynecological field [21, 85–88]. As a matter of fact, the use of MSCs for fecal and urinary incontinence treatments may be a major step forward in clinical efficacy with minimal risks, especially compared to surgical repair treatments [89–96].
The incontinence, both fecal and urinary, may result from the loss of the sphincter function due to muscle damage and peripheral nerve lesions, with various combinations of both. The rebuilding of the muscle fibers and nerve endings by a regenerative therapy employing mesenchymal stem cells is then an ideal treatment concept, especially because clinical use of these stem cells appears free of ethical concerns and risk of tumor formations [97].
Autologous and heterologous MSCs are used for “in vivo” studies on animals, while only autologous MSCs are employed in human trials. The most widely used technique for the production of adequate amounts of MSCs provides for their harvesting from several adult tissues, such as the bone marrow, muscle, and adipose tissue. Subsequently, MSCs are “in vitro” expanded, until desired cell numbers are achieved ready to transplantation to regenerative therapy [98]. However, the use of MSCs in clinical practice is still not widespread, and the clinical application in patients remains an important goal. Certainly, this is not due to the low consideration of physicians for this procedure but rather to the complex production method of expanding some types of stem cells and to their high costs [99].
The gynecological field of urinary incontinence was the first that received the attention of the scientific community, and only later researchers focused their interest on fecal incontinence. The first MSCs application in a rat urinary incontinence model was published in 2000 by Chancellor et al. [100], and only 8 years later Carr published the first study on patients affected by stress urinary incontinence [101].
14.2.2 Urinary Incontinence
14.2.2.1 Animal Models
Different animal models have been used to mimic the injuries that can produce urinary incontinence. The first model, introduced by Lin in 1998 [102], utilized vaginal distension in female rats to simulate the trauma of childbirth with damage to surrounding muscles and nerves. Subsequent other models have been developed to investigate the incontinence mechanism, including nerve injury (transection of pudendal or sciatic nerve), direct urethral injury (urethral transection or cryo-chemo injury), and pelvic ligament injury. However, female Sprague–Dawley rats are the most used in these experiments [103–105].
In preclinical studies reported, two main approaches have been used for MSC transplants: the systemic administration by intravenous injection and local injections by direct puncture [21, 106–108]. The advantage of the first method is characterized by the simplicity of the technique and the ability of MSCs to migrate “in vivo” to specific inflammatory tissue and concentrate at the site of the lesion, thanks to the capacity of MSCs to “homing” into the site of injury in several disease models. Cruz et al. in 2012 [109], and Dissaranan et al. in 2014 [110], showed, by intravenous injection of MSCs in a rat model, the homing of these cells in the urethra and a facilitated recovery of continence. However, additional studies have shown limits to this technique as reported by Rombouts and Ploemacher in 2003 [111] due to the fact that the increasing age and passage number in stem cell culture reduce the homing effect and the efficiency of MSCs engraftment. Furthermore, Fischer et al. in 2009 [112] reported that systemically infused MSCs often suffer from a first-pass effect where the larger cells become trapped in capillary beds of various tissues, decreasing their therapeutic bioavailability and functionality. Therefore, for increasing the number of mesenchymal stem cells and the efficiency of differentiation into the damaged sites, researchers have used local injections of MSCs, as reported by several pilot studies [43].
Functional analysis and histological examinations were performed to evaluate the therapy outcome, before and after treatment. Measurements of leak point pressure and bladder capacity were monitored to detect changes in urinary incontinence. To confirm the survival and differentiation of transplanted cells, histological sections of animal urethra were studied by immunohistochemistry–immunofluorescence analysis.
Although not all evaluation methods were uniform, in almost all studies performed on animal models of urinary incontinence, positive results are reported that showed both the improvement of the functional sphincter activity and the regeneration of new muscle and neuronal cells in the injured area.
Some of the most significant studies on animal model of urinary incontinence are summarized in Table 14.2.
14.2.2.2 Clinical Study
The clinical trial studies were performed using autologous muscle-derived mesenchymal stem cells (MDSCs) or adipose-derived mesenchymal stem cells (ADSCs) which can be obtained in large quantities from patients with an easy and low-invasive biopsy under local anesthesia. In these trials, the direct injection of MSCs was the most widely used procedure, performed by a local intrasphincteric injection by transurethral or periurethral approach using cystoscopy or ultrasonography guidance, both in females and in males. Clinical outcome was commonly based on 3-day leakage diary, 24 h pad test, quality-of-life score, and urodynamic test by urethral pressure measurement at rest and in squeezing.
In 2008, Carr et al. from the University of Toronto [101] reported the first clinical trial and published the results of 1-year follow-up on eight women in which urinary incontinence was treated with local injection of MDSCs. In this study, autologous muscle cells obtained from the thigh of patients, using a percutaneous needle technique, were expanded in culture and concentrated into a single-use dose containing 18–22 × 106 cells for injection in patients. This pilot study reported an improvement in urinary incontinence, especially between 3 and 8 months after the initial injection. Moreover, this study proved to be safe and with the absence of adverse events related to MDSCs transplant.
Later, Sebe et al. in 2011 [126], Gotoh et al. in 2013 [127], and other authors reported that MSCs are able to reduce urinary incontinence symptoms and improve quality of life of patients. Results of 11 clinical trials in a total of 456 women and 241 men, published in peer-reviewed journals [128], showed that MDSCs are safe for the treatment of urinary incontinence, suggesting their potential use in cell therapies. However, only a restricted number of studies have focused on the number of stem cells to be used, and in this context, a multicenter study of Carr, Chancellor, and colleagues, published from 2008 to 2014 [88, 100, 101, 129–133], reported that treatment outcomes depend on the number of transplanted cells. These authors reported that in all groups, there was a statistically significant reduction in stress leaks within 1–3 months of treatment that was maintained through the 12-month follow-up, suggesting that the efficacy of MSCs is related to cell dose. In particular patients who received higher doses (200 × 106 cells) appeared to have better efficacy outcomes than those who received lower doses (10 × 106) [133].
The most significant clinical studies on urinary incontinence are reported in Table 14.3.
14.2.3 Fecal Incontinence
14.2.3.1 Animal Models
Based on animal and clinical experiences of MSCs therapy for the treatment in urinary incontinence, in 2008 Lorenzi et al. and Kang et al. published the results of the first two in vivo studies with induced anal lesion.
In his study, Lorenzi treated an experimental rat model of anal sphincters injury followed by surgical repair with intrasphincteric injection of expanded rat bone marrow mesenchymal stem cells (BMSCs). The results indicate that, 30 days later, the injection of BMSCs led to an increase of muscle tissue in the injured area of the external and internal anal sphincter in which it is possible to observe abundant muscle cells of different sizes and irregularly disposed. Furthermore, functional studies highlighted an improved contractility of muscle fibers [135].
Kang injected 3 × 106 MDSCs into the anal sphincter in rats with cryoinjured anal sphincters, as a fecal incontinence model. One week after treatment, the anal sphincter was trimmed and functional tests and microscopic examination were evaluated. In the MDSCs injection group, contraction amplitude was higher than in the control group but not significantly. By immunohistochemical staining, regenerating muscle fibers were observed in variable orientation, both smooth and skeletal [136].
Two years later, White et al. published their study on 120 rats to estimate the effect of myogenic stem cells on contractile function of the external anal sphincter after transection with or without surgical repair. He noted that, in the sphincter repair group, injection of myogenic stem cells induced the enhancement of the contractile function [137]. Aghaee-Afshar et al. in 2009 [138] and Kajbafzadeh et al. in 2010 [139] focused their study on the use of stem cell transplantation in sphincter injury model without subsequent surgical repair. A stem cell injection was performed respectively 2 and 3 weeks after injury, and results were obtained at different intervals from the treatments, showing significant improvement in the electrical activity and in the mean resting pressure of the anal canal. Furthermore, histopathologic evaluation showed regenerated myotubes and a significant decrease in interstitial fibrosis.
In 2012, Pathi et al. [140] published an interesting randomized study on 204 female rats with external anal sphincter laceration and repair. Animals were treated with direct or intravenous injection of 4 × 106 heterologous BMSCs. The contractile function of sphincters and the parameters of wound healing were analyzed up to 21 days after injury, showing that the direct injection of MSCs into the injured anal sphincter leads to full functional recovery, while the intravenous injection did not fully rescue the compromised sphincter. Direct injection of BMSCs also increased, in the injured area, the expression of the RNA level of lysyl oxidase and TFG-Beta1, two genes involved in collagen, elastin, and matrix synthesis.
Some other significant studies were published in the last years. Salcedo and colleagues [141, 142], in two consecutive articles in 2013 and 2014 on animal model, with induced lesion of the anal sphincter, tested the efficacy of BMSCs via intramuscular or intravenous injected. In both studies, he reported a significant improvement in the anal pressure in BMSCs transplanted groups. However, in the first study, in which BMSCs were also injected into a group of rats with nerve injuries by pudendal crush, he did not report any functional improvement. Nevertheless, he also describes a marked decrease in fibrosis and scar tissue as effects of MSC transplantation.
The articles published in 2015 by a study group of Texas University examine the relationship between the muscular disruption and contractile force of sphincters after transection and repair of external anal sphincter. In intramuscular injection of the myogenic stem group, there was a substantial improvement in the generation of the contractile force, but there was no difference in the anal sphincter volume compared to the control. He suggests that stem cells might improve the contractile function through other cellular processes [143]. Montoya paper [144] reported an original study in which, after 2 weeks from a complete sphincter transection, rats were injected with a hydrogel matrix scaffold combined with myogenic stem cells. Neurophysiology tests and histologic examination, performed after 4 and 12 weeks, highlighted how, compared to the control groups, the addition of a biogel scaffold to the myogenic stem cells increases the contractile force and the histological evidence of sphincter restoration with steady improvement over time.
In Table 14.4 the studies on animal model of fecal incontinence reported in literature are summarized.
14.2.3.2 Clinical Study
Until today, only two pilot studies have been produced using MSCs to treat fecal incontinence in humans.
In 2013, Frudinger and colleagues published, for the first time, a study on ten women with damaged anal sphincter by obstetric trauma which were followed up for 1 year after treatment [145]. All patients, with a preventive endosonographic diagnosis of anterior defect of the external anal sphincter, were treated with autologous myoblast cells harvested from a pectoral muscle biopsy and cultured for 39 days. Muscle-derived stem cells were injected under ultrasonography guidance into external anal sphincter ends and also in the interposed scar tissue. Twelve to 14 individual injections of 0.5 ml for a total of 6–7 ml (the number of myoblasts is not specified) were administered, making sure not to treat directly the internal anal sphincter. No adverse events were observed, and the procedure was well tolerated. At 12 months, all patients stated that their symptoms had improved and the Rockwood Fecal Incontinence Quality of Life Scale [146] resulted significantly improved. The Wexner Incontinence Score [147] had a significant decrease from a mean value of 15.3–1.6. However, the initial significant increase in mean and maximal anal squeeze pressures seen at 1 and 6 months was transient and not sustained at 12 months. As matter of fact, between the 6 and 12 months assessment, also a significant decrease in mean and maximal anal resting pressure occurred. The author reported in an additional article published in 2015 the updates of the 5-year follow-up for the ten patients reporting significant improved symptoms of anal incontinence.
The second study on regenerative treatment in patients affected by fecal incontinence was produced by the group of Giori and coinvestigators. In this pilot study, performed according to the Declaration of Helsinki and approved by the ethics committee, they treated 15 patients (14 females and 1 male) with incontinence due to obstetric injury and anorectal-pelvic surgery. Preliminary results of this study were published in 2015 [148]. Actual experience of the authors in the use, for the first time, of Lipogems® technique to treat patients with fecal incontinence is described below.
14.2.4 Personal Experience with Technique Lipogems®
Lipogems® is a regenerative product by autologous lipoaspirated fat rich in mesenchymal stem cells, obtained in a completely closed system by a disposable device without using enzymes, additives, and other manipulations. Mild mechanical processes of microfracturing, washing, and filtration progressively reduce the size of the fragments of adipose tissue and remove oily substances and blood residues [149] (Fig. 14.5). Differently, from the mesenchymal cells expanded in laboratory, Lipogems micro-tissue is not a “pool” of individual stem cells, but it is composed of small adipose spherical clusters (400/900 μm) with micro-fragments of intact connective structure maintained viable by a stromal vascular fraction (SVF), particularly rich in pericytes and MSCs incorporated in their “natural niche tissue” [150, 151] and exposed on the surface of the vascular stroma. The micro-fragmented tissue enclosing stromal vascular fraction (SVF) can be ideally assimilated to a biologic matrix scaffold that facilitates the engraftment and the biological activity of MSCs, as evident in previous experience described by Montoya [144]. These properties contribute to make Lipogems® able to survive in a suffering tissue, facilitating the engraftment and the paracrine activity of the embedded cells when autologously inoculated in target tissues [152–154].
Lipogems device is a disposable closed system filled with physiologic solution. It reduces the size of lipoaspirate clusters after washing of oil, blood, and cellular debris. (A) sac with saline solution; (B) syringe with lipoaspirate; (C) washing chamber containing marbles for the emulsion of fluid and elimination of oil and blood; (D) mechanical filters, (E) syringe with clusters of microfractured adipose tissue rich in MSCs; and (F) sac with waste oil and blood
However, studies have shown that, when the Lipogems® product was cultured in vitro, it yielded a virtually pure population of hMSCs exhibiting the typical characteristics of surface markers isolated from other sources, including CD90, CD73, CD105, and CD44 [32, 155].
In clinical practice, Lipogems procedure is conducted in a single surgical session that includes three different steps: (1) harvesting of subcutaneous adipose tissue from abdomen or thigh of the patient, (2) processing of adipose tissue with Lipogems® device, and (3) reinjecting the product in the same patient under ultrasound guidance.
Unlike to Frudinger, who had injected MSC only in the area of the sphincter lesion [145], in our study, we injected Lipogems not only in the area of muscle defects but also in the intersphincteric space and around the external anal sphincter [148]. Moreover, because a lesion of the sphincter muscle that affects from outside, such as in childbirth, is always associated to a lesion of the peripheral nerve endings, we have found it useful to also stimulate a neural-regenerative effect injecting Lipogems® also along the course of peripheral pudendal nerve. An average of 340 cc of lipoaspirate was collected from subcutaneous fat of each patient. As a result of processing with the technique Lipogems®, an average of 87 cc of product was obtained, ready for injection in every patient. After treatment, the 15 patients of this series were followed up for 2 years. Wexner Incontinence Score, Rockwood Fecal Incontinence Quality of Life Scale, digital exploration, proctoscopy, endoanal ultrasound, and anorectal manometry were used before treatment and after 3, 6, 12, 18, and 24 months from the injection of ADSCs, to assess fecal incontinence. The procedure has proven to be safe and well tolerated. In all patients, there were no adverse events related to ADSCs injection. Only in one patient occurred a hematoma at the site of harvesting in the subcutaneous adipose tissue, which resolved spontaneously. Improvement both short and long term was observed in all patients.
Patients’ satisfaction for the treatment was very good, and the Fecal Incontinence Quality of Life Scale increased from a mean preoperative overall value of 53–102 after 3 months from treatment, essentially unchanged for the 2 years of follow up.
In the 15 patients, a significant improvement in the average values of the overall Wexner Incontinence Score was observed which decreased by a mean preoperative of 14.1 units to 3.4 units at 3 months and remained quite stable over the time of the study with a value of 4.1 at 24 months (Fig. 14.6). After treatment, the overall mean values of the anal pressure at rest and in squeeze improved in all the patients as reported in Fig. 14.7. In the graph, a remarkable increasing of the anal squeeze pressure during the entire second year of follow-up is evident. Ultrasound examinations showed a progressive reabsorption of the hyperechogenic Lipogems tissue from 3 to 12 months and increasing of muscle fibers with images of sphincter restoration at 12 and 24 months in several patients (Fig. 14.8). After treatment, physical examination and proctoscopy did not show any new pathological findings of the anorectal complex. At palpation the enhancement in the contractile activity of the anal sphincters and improvement of anocutaneous reflex, where it was lacking before treatment, was clearly observable.
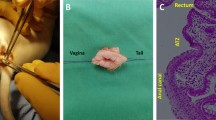
Endoanal ultrasonography images of a representative patient at preoperative, intraoperative stage and at 3, 6, 12, 24 months after treatment. (a) Preoperative stage. Lesion of the external and internal anal sphincter localized in the anterior area, evidenced by arrows (b) Intraoperative stage. Diffuse hyperechoic spots at the sites of inoculation of Lipogems. (c) 3rd month. Partial resorption of hyperechoic spots of Lipogems. (d) 6th month. Aspect of the early development of new muscle tissue at the site of the lesion. Are still evident few hyperechoic spots of Lipogems. (e, f) 12th and 24th month respectively. The image shows muscle restoration in the area of previous lesion of the anal sphincters evidenced by arrows. No more evidence of hyperechoic depots of Lipogems
From a detailed analysis of patients’ data, between the 3rd and the 12th month, we have observed a slight decline of the resting pressure associated with mild worsening of the Wexner index. This feature is, in our opinion, attributed to a temporary bulking effect of the “not stem cells” component of Lipogems, which is reabsorbed in a period between 3 and 12 months from treatment, as it usually occurs with the traditional techniques of biological lipofilling [156]. Thus, the clear increase in the contractile ability of the anal sphincter recorded by anorectal manometry after 12 months (Fig. 14.8e, f), when deposits of material inoculated disappeared and ultrasound images showed muscle restoration, is attributable to the effect of mesenchymal cells.
This is also indirectly confirmed by the results of Frudinger, who obtained a temporary increase in anal squeeze pressures not sustained at 12 months as reported in her first article. However, the extension of the follow-up described in the second publication has proven a slow and gradual improvement of incontinence, which remained unchanged through all the 5 years of observation [145, 157].
14.2.5 Final Considerations
On the basis of preclinical studies and clinical trials conducted both in the field of urinary incontinence and fecal incontinence, the use of autologous MSCs revealed to be safe, minimally traumatic, well tolerated, and effective in improving the symptoms of incontinence. In particular, new techniques like Lipogems®, which uses autologous stem cells derived from adipose tissue easy to harvest and prepare the product using a device directly in the operating room in the course of a single surgical time, make the use of mesenchymal stem cells of simple execution and that are inexpensive and widely applicable in clinical practice. Further investigations are necessary to clarify the efficacy and the biological mechanism of this very simple regenerative procedure, but a future association together with surgical repair of damaged anal sphincters is also possible.
References
Ramalho-Santos M, Willenbring H. On the origin of the term “stem cell”. Cell Stem Cell. 2007;1(1):35–8.
Wilson EB. The cell in development and inheritance. New York: The MacMillan Company; 1896.
Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17(1):387–403.
Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17(1):435–62.
Papaioannou V. Stem cells and differentiation. Differentiation. 2001;68(4–5):153–4.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Hussein SM, Nagy AA. Progress made in the reprogramming field: new factors, new strategies and a new outlook. Curr Opin Genet Dev. 2012;22(5):435–43.
Bayart E, Cohen-Haguenauer O. Technological overview of iPS induction from human adult somatic cells. Curr Gene Ther. 2013;13(2):73.
Rajasingh J. Reprogramming of somatic cells. Prog Mol Biol Transl Sci. 2012;111:51–82.
Robertson JA. Human embryonic stem cell research: ethical and legal issues. Nat Rev Genet. 2001;2(1):74–8.
Dhar D, Hsi-En Ho J. Stem cell research policies around the world. Yale J Biol Med. 2009;82(3):113–5.
Lee J-H, Lee JB, Shapovalova Z, Fiebig-Comyn A, Mitchell RR, Laronde S, Szabo E, Benoit YD, Bhatia M. Somatic transcriptome priming gates lineage-specific differentiation potential of human-induced pluripotent stem cell states. Nat Commun. 2014;5:5605.
Riggs JW, Barrilleaux BL, Varlakhanova N, Bush KM, Chan V, Knoepfler PS. Induced pluripotency and oncogenic transformation are related processes. Stem Cells Dev. 2012;22(1):37–50.
de la Morena MT, Gatti RA. A history of bone marrow transplantation. Hematol Oncol Clin North Am. 2011;25(1):1–15.
Martin PJ, Hansen JA, Storb R, Donnall Thomas E. Human marrow transplantation: an immunological perspective. Adv Immunol. 1987;40:379–438.
Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. J Allergy Clin Immunol. 2010;125(2):S324–35.
Bow EJ. Infection risk and cancer chemotherapy: the impact of the chemotherapeutic regimen in patients with lymphoma and solid tissue malignancies. J Antimicrob Chemother. 1998;41(4):1–5.
Vanichsetakul P. Clinical use of cord blood for stem cell transplantation. J Med Assoc Thailand. 2005;88:S93.
Goldstein G, Toren A, Nagler A. Transplantation and other uses of human umbilical cord blood and stem cells. Curr Pharm Des. 2007;13(13):1363–73.
Chen M, Przyborowski M, Berthiaume F. Stem cells for skin tissue engineering and wound healing. Crit Rev Biomed Eng. 2009;37(4–5):399–421.
Wei X, Yang X, Z-p H, F-f Q, Shao L, Y-f S. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34(6):747–54.
Friedenstein A, Kuralesova AI. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation. 1971;12(2):99–108.
Bianchi G, Borgonovo G, Pistoia V, Raffaghello L. Immunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells. Histol Histopathol. 2011;26(7):941.
Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4.
Granero-Molto F, Weis JA, Longobardi L, Spagnoli A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther. 2008;8(3):255.
Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28(3):585–96.
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, S-i T, Ide C, Y-i N. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309(5732):314–7.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Scherjon SA, Kleijburg‐van der Keur C, de Groot‐Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–45.
Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109(3):1298–306.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–9.
Tsai MS, Hwang SM, Chen KD, Lee YS, Hsu LW, Chang YJ, Wang CN, Peng HH, Chang YL, Chao AS. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells. 2007;25(10):2511–23.
Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41.
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–61.
Kim J-M, Lee S-T, Chu K, Jung K-H, Song E-C, Kim S-J, Sinn D-I, Kim J-H, Park D-K, Kang K-M. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007;1183:43–50.
Parekkadan B, Van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2(9), e941.
Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci. 2009;106(38):16357–62.
Amado LC, Saliaris AP, Schuleri KH, John MS, Xie J-S, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102(32):11474–9.
Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I–II clinical trial. Eur J Gastroenterol Hepatol. 2009;21(10):1199–205.
Peng L, Xie D, Lin BL, Liu J, Zhu H, Xie C, Zheng Y, Gao Z. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short‐term and long‐term outcomes. Hepatology. 2011;54(3):820–8.
Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92(1):26–36.
Rasulov M, Vasil’chenkov A, Onishchenko N, Krasheninnikov M, Kravchenko V, Gorshenin T, Pidtsan R, Potapov I. First experience in the use of bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med. 2005;139(1):141–4.
Yamada Y, Ueda M, Hibi H, Baba S. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: a clinical case report. Int J Periodontics Restorative Dent. 2006;26(4):363–9.
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63.
Zeng X, Y-s Z, Ma Y-h, Lu L-y, B-l D, Zhang W, Li Y, Chan WY. Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord injury. Cell Transplant. 2011;20(11–12):1881–99.
Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow‐derived mesenchymal stromal cells inhibit Th2‐mediated allergic airways inflammation in mice. Stem Cells. 2011;29(7):1137–48.
Ortiz LA, DuTreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci. 2007;104(26):11002–7.
Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Prockop DJ. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF‐α stimulated gene/protein 6. Stem Cells. 2011;29(10):1572–9.
Alves H, Van Ginkel J, Groen N, Hulsman M, Mentink A, Reinders M, Van Blitterswijk C, De Boer J. A mesenchymal stromal cell gene signature for donor age. PLoS One. 2012;7(8), e42908.
Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–11.
Parolini O, Soncini M, Evangelista M, Schmidt D. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen Med. 2009;4(4):275.
Azuara-Blanco A, Pillai C, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83(4):399–402.
Solomon A, Espana EM, Tseng SC. Amniotic membrane transplantation for reconstruction of the conjunctival fornices. Ophthalmology. 2003;110(1):93–100.
Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42(7):1539–46.
Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78(10):1439–48.
Magatti M, De Munari S, Vertua E, Gibelli L, Wengler GS, Parolini O. Human amnion mesenchyme harbors cells with allogeneic T‐cell suppression and stimulation capabilities. Stem Cells. 2008;26(1):182–92.
Crisco JJ, Jokl P, Heinen GT, Connell MD, Panjabi MM. A muscle contusion injury model biomechanics, physiology, and histology. Am J Sports Med. 1994;22(5):702–10.
Carosio S, Berardinelli MG, Aucello M, Musarò A. Impact of ageing on muscle cell regeneration. Ageing Res Rev. 2011;10(1):35–42.
Tidball JG. Inflammatory processes in muscle injury and repair. Am J Phys Regul Integr Comp Phys. 2005;288(2):R345–53.
Karpati G, Molnar MJ. Muscle fibre regeneration in human skeletal muscle diseases. In: Schiaffino S and Partridge T editors. Skeletal muscle repair and regeneration. Dordrecht: Springer; 2008. p. 199–216.
Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics. 2003;14(3):261–71.
Kääriäinen M, Kääriäinen J, Järvinen TL, Sievänen H, Kalimo H, Järvinen M. Correlation between biomechanical and structural changes during the regeneration of skeletal muscle after laceration injury. J Orthop Res. 1998;16(2):197–206.
Nozaki M, Li Y, Zhu J, Ambrosio F, Uehara K, Fu FH, Huard J. Improved muscle healing after contusion injury by the inhibitory effect of suramin on myostatin, a negative regulator of muscle growth. Am J Sports Med. 2008;36(12):2354–62.
Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg. 2002;84(5):822–32.
Moore R, Silver R, Moore J. Physiological apoptotic agents have different effects upon human amnion epithelial and mesenchymal cells. Placenta. 2003;24(2):173–80.
Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol Reprod. 1996;55(6):1253–60.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53(2):227–46.
Trayhurn P, Wood I. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33(Pt 5):1078–81.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32.
Rodbell M. The metabolism of isolated fat cells. J Biol Chem. 1964;239:375–80.
Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–60.
Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G. Immunophenotype of human adipose‐derived cells: temporal changes in stromal‐associated and stem cell–associated markers. Stem Cells. 2006;24(2):376–85.
Yu G, Wu X, Dietrich MA, Polk P, Scott LK, Ptitsyn AA, Gimble JM. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy. 2010;12(4):538–46.
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–8.
Hicok KC, Hedrick MH. Automated isolation and processing of adipose-derived stem and regenerative cells. In: Gimble JM, Bunnell BA, editors. Adipose-derived stem cells. New York/Dordrecht/Heidelberg/London: Springer; 2011. p. 87–105.
Gimble JM, Bunnell BA, Casteilla L, Jung JS, Yoshimura K. Phases I–III clinical trials using adult stem cells. Stem Cells Int. 2011;2010:2.
Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38(3):201–9.
Thesleff T, Lehtimäki K, Niskakangas T, Mannerström B, Miettinen S, Suuronen R, Öhman J. Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. 2011;68(6):1535–40.
Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, Hedrick MH, Berthold L, Howaldt H-P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Cranio-Maxillofac Surg. 2004;32(6):370–3.
Caplan AI. Adult mesenchymal stem cells: when, where, and how. Stem Cells Int. 2015:628767.
Santa María L, Rojas CV, Minguell JJ. Signals from damaged but not undamaged skeletal muscle induce myogenic differentiation of rat bone-marrow-derived mesenchymal stem cells. Exp Cell Res. 2004;300(2):418–26.
Bossolasco P, Corti S, Strazzer S, Borsotti C, Del Bo R, Fortunato F, Salani S, Quirici N, Bertolini F, Gobbi A. Skeletal muscle differentiation potential of human adult bone marrow cells. Exp Cell Res. 2004;295(1):66–78.
Feki A, Faltin D, Lei T, Dubuisson J-B, Jacob S, Irion O. Sphincter incontinence: is regenerative medicine the best alternative to restore urinary or anal sphincter function? Int J Biochem Cell Biol. 2007;39(4):678–84.
Tran C, Damaser MS. The potential role of stem cells in the treatment of urinary incontinence. Ther Adv Urol. 2014;7(1):22–40.
Lane FL, Jacobs S. Stem cells in gynecology. Am J Obstet Gynecol. 2012;207(3):149–56.
Wang H-J, Chuang Y-C, Chancellor MB. Development of cellular therapy for the treatment of stress urinary incontinence. Int Urogynecol J. 2011;22(9):1075–83.
Vaizey CJ, Norton C, Thornton MJ, Nicholls RJ, Kamm MA. Long-term results of repeat anterior anal sphincter repair. Dis Colon Rectum. 2004;47(6):858–63.
Baeten CG. Safety and efficacy of dynamic graciloplasty for fecal incontinence. Dis Colon Rectum. 2000;43(6):743–51.
Lehur P, Glemain P, des Varannes SB, Buzelin J, Leborgne J. Outcome of patients with an implanted artificial anal sphincter for severe faecal incontinence A single institution report. Int J Colorectal Dis. 1998;13(2):88–92.
Norderval S, Öian P, Revhaug A, Vonen B. Anal incontinence after obstetric sphincter tears: outcome of anatomic primary repairs. Dis Colon Rectum. 2005;48(5):1055–61.
Zorcolo L, Covotta L, Bartolo DC. Outcome of anterior sphincter repair for obstetric injury: comparison of early and late results. Dis Colon Rectum. 2005;48(3):524–31.
Dmochowski RR, Blaivas JM, Gormley EA, Juma S, Karram MM, Lightner DJ, Luber KM, Rovner ES, Staskin DR, Winters JC. Update of AUA guideline on the surgical management of female stress urinary incontinence. J Urol. 2010;183(5):1906–14.
Kuhn A, Eggeman C, Burkhard F, Mueller MD. Correction of erosion after suburethral sling insertion for stress incontinence: results and related sexual function. Eur Urol. 2009;56(2):371–7.
Kotb AF, Campeau L, Corcos J. Urethral bulking agents: techniques and outcomes. Curr Urol Rep. 2009;10(5):396–400.
Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11), e54.
Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2015: 496218.
Godara P, Nordon RE, McFarland CD. Mesenchymal stem cells in tissue engineering. J Chem Technol Biotechnol. 2008;83(4):397–407.
Chancellor MB, Yokoyama T, Tirney S, Mattes CE, Ozawa H, Yoshimura N, de Groat WC, Huard J. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn. 2000;19(3):279–87.
Carr L, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, Erickson J, Huard J, Chancellor M. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J. 2008;19(6):881–3.
Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52(1):143–51.
Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, Brubaker L. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn. 2000;19(1):53–69.
Chermansky CJ, Cannon TW, Torimoto K, Fraser MO, Yoshimura N, de Groat WC, Chancellor MB. A model of intrinsic sphincteric deficiency in the rat: electrocauterization. Neurourol Urodyn. 2004;23(2):166–71.
Kefer JC, Liu G, Daneshgari F. Pubo-urethral ligament injury causes long-term stress urinary incontinence in female rats: an animal model of the integral theory. J Urol. 2009;181(1):397–400.
Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AB, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–6.
Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4‐induced liver fibrosis in mice. Hepatology. 2004;40(6):1304–11.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–11.
Cruz M, Dissaranan C, Cotleur A, Kiedrowski M, Penn M, Damaser M. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gynecol Int. 2012;2012:612946.
Dissaranan C, Cruz MA, Kiedrowski MJ, Balog BM, Gill BC, Penn MS, Goldman HB, Damaser MS. Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplant. 2014;23(11):1395–406.
Rombouts W, Ploemacher R. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17(1):160–70.
Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox Jr CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–92.
Chermansky CJ, Tarin T, Kwon D-D, Jankowski RJ, Cannon TW, de Groat WC, Huard J, Chancellor MB. Intraurethral muscle-derived cell injections increase leak point pressure in a rat model of intrinsic sphincter deficiency. Urology. 2004;63(4):780–5.
Fu Q, Song X-F, Liao G-L, Deng C-L, Cui L. Myoblasts differentiated from adipose-derived stem cells to treat stress urinary incontinence. Urology. 2010;75(3):718–23.
Kinebuchi Y, Aizawa N, Imamura T, Ishizuka O, Igawa Y, Nishizawa O. Autologous bone‐marrow‐derived mesenchymal stem cell transplantation into injured rat urethral sphincter. Int J Urol. 2010;17(4):359–68.
Lim J-J, Jang J-B, Kim J-Y, Moon S-H, Lee C-N, Lee K-J. Human umbilical cord blood mononuclear cell transplantation in rats with intrinsic sphincter deficiency. J Korean Med Sci. 2010;25(5):663–70.
Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, Lue TF, Lin C-S. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12(1):88–95.
Xu Y, Song Y, Lin Z. Transplantation of muscle-derived stem cells plus biodegradable fibrin glue restores the urethral sphincter in a pudendal nerve-transected rat model. Braz J Med Biol Res. 2010;43(11):1076–83.
Zou XH, Zhi YL, Chen X, Jin HM, Wang LL, Jiang YZ, Yin Z, Ouyang HW. Mesenchymal stem cell seeded knitted silk sling for the treatment of stress urinary incontinence. Biomaterials. 2010;31(18):4872–9.
Kim S-O, Na HS, Kwon D, Joo SY, Kim HS, Ahn Y. Bone-marrow-derived mesenchymal stem cell transplantation enhances closing pressure and leak point pressure in a female urinary incontinence rat model. Urol Int. 2011;86(1):110–6.
Corcos J, Loutochin O, Campeau L, Eliopoulos N, Bouchentouf M, Blok B, Galipeau J. Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodyn. 2011;30(3):447–55.
Imamura T, Ishizuka O, Kinebuchi Y, Kurizaki Y, Nakayama T, Ishikawa M, Nishizawa O. Implantation of autologous bone-marrow-derived cells reconstructs functional urethral sphincters in rabbits. Tissue Eng Part A. 2011;17(7–8):1069–81.
Wu G, Song Y, Zheng X, Jiang Z. Adipose-derived stromal cell transplantation for treatment of stress urinary incontinence. Tissue Cell. 2011;43(4):246–53.
Zhao W, Zhang C, Jin C, Zhang Z, Kong D, Xu W, Xiu Y. Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur Urol. 2011;59(1):155–63.
Chun SY, Kwon JB, Chae SY, Lee JK, Js B, Kim BS, Kim HT, Yoo ES, Lim JO, Yoo JJ. Combined injection of three different lineages of early‐differentiating human amniotic fluid‐derived cells restores urethral sphincter function in urinary incontinence. BJU Int. 2014;114(5):770–83.
Sèbe P, Doucet C, Cornu J-N, Ciofu C, Costa P, de Medina SGD, Pinset C, Haab F. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: a prospective study. Int Urogynecol J. 2011;22(2):183–9.
Gotoh M, Yamamoto T, Kato M, Majima T, Toriyama K, Kamei Y, Matsukawa Y, Hirakawa A, Funahashi Y. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose‐derived regenerative cells: 1‐year outcomes in 11 patients. Int J Urol. 2014;21(3):294–300.
Gräs S, Klarskov N, Lose G. Intraurethral injection of autologous minced skeletal muscle: a simple surgical treatment for stress urinary incontinence. J Urol. 2014;192(3):850–5.
Carr LK, Herschorn S, Birch C, Murphy M, Robert M, Jankowski RJ, Pruchnic R, Wagner D, Chancellor MB. Autologous muscle-derived cells as a therapy for stress urinary incontinence: a randomized, blinded, multi-dose study. J Urol. 2009;181(4):546.
Carr L, Herschorn S, Birch C, Murphy M, Robert M, Jankowski R, Pruchnic R, Wagner D, Chancellor M. Aautologous muscle-derived cells as therapy for stress urinary incontinence: a randomized, dose-ranging trial. J Urol. 2010;183(4):e587–8.
Peters K, Kaufman M, Dmochowski R, Carr L, Herschorn S, Fischer M, Sirls L, Nagaraju P, Biller D, Ward R. 1340 Autologous muscle derived cell therapy for the treatment of female stress urinary incontinence: a multi-center experience. J Urol. 2011;185(4):e535–6.
Carr LK, Robert M, Kultgen PL, Herschorn S, Birch C, Murphy M, Chancellor MB. Autologous muscle derived cell therapy for stress urinary incontinence: a prospective, dose ranging study. J Urol. 2013;189(2):595–601.
Jankowski R, Werner S, Snyder S, Chancellor M, Kultgen P, Pruchnic R. Cell therapy for treatment of stress urinary incontinence in women: potential dose effect of autologous muscle-derived cells for urinary sphincter repair (AMDC-USR). Cytotherapy. 2014;16(4):S91.
Stangel‐Wojcikiewicz K, Jarocha D, Piwowar M, Jach R, Uhl T, Basta A, Majka M. Autologous muscle‐derived cells for the treatment of female stress urinary incontinence: a 2‐year follow‐up of a polish investigation. Neurourol Urodyn. 2014;33(3):324–30.
Lorenzi B, Pessina F, Lorenzoni P, Urbani S, Vernillo R, Sgaragli G, Gerli R, Mazzanti B, Bosi A, Saccardi R. Treatment of experimental injury of anal sphincters with primary surgical repair and injection of bone marrow-derived mesenchymal stem cells. Dis Colon Rectum. 2008;51(4):411–20.
Kang S-B, Lee HN, Lee JY, Park J-S, Lee HS, Lee JY. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum. 2008;51(9):1367–73.
White AB, Keller PW, Acevedo JF, Word RA, Wai CY. Effect of myogenic stem cells on contractile properties of the repaired and unrepaired transected external anal sphincter in an animal model. Obstet Gynecol. 2010;115(4):815–23.
Aghaee-Afshar M, Rezazadehkermani M, Asadi A, Malekpour-Afshar R, Shahesmaeili A, Nematollahi-mahani SN. Potential of human umbilical cord matrix and rabbit bone marrow-derived mesenchymal stem cells in repair of surgically incised rabbit external anal sphincter. Dis Colon Rectum. 2009;52(10):1753–61.
Kajbafzadeh A-M, Elmi A, Talab SS, Esfahani SA, Tourchi A. Functional external anal sphincter reconstruction for treatment of anal incontinence using muscle progenitor cell auto grafting. Dis Colon Rectum. 2010;53(10):1415–21.
Pathi SD, Acevedo JF, Keller PW, Kishore AH, Miller RT, Wai CY, Word RA. Recovery of the injured external anal sphincter after injection of local or intravenous mesenchymal stem cells. Obstet Gynecol. 2012;119(1):134–44.
Salcedo L, Mayorga M, Damaser M, Balog B, Butler R, Penn M, Zutshi M. Mesenchymal stem cells can improve anal pressures after anal sphincter injury. Stem Cell Res. 2013;10(1):95–102.
Salcedo L, Penn M, Damaser M, Balog B, Zutshi M. Functional outcome after anal sphincter injury and treatment with mesenchymal stem cells. Stem Cells Transl Med. 2014;3(6):760–7.
Fitzwater JL, Grande KB, Sailors JL, Acevedo JF, Word RA, Wai CY. Effect of myogenic stem cells on the integrity and histomorphology of repaired transected external anal sphincter. Int Urogynecol J. 2015;26(2):251–6.
Montoya TI, Acevedo JF, Smith B, Keller PW, Sailors JL, Tang L, Word RA, Wai CY. Myogenic stem cell-laden hydrogel scaffold in wound healing of the disrupted external anal sphincter. Int Urogynecol J. 2015;26(6):893–904.
Frudinger A, Kölle D, Schwaiger W, Pfeifer J, Paede J, Halligan S. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut. 2010;59(01):55–61.
Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, Lowry AC. Fecal incontinence quality of life scale. Dis Colon Rectum. 2000;43(1):9–16.
Jorge JMN, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
Giori A, Tremolada C, Vailati R, Navone S, Marfia G, Caplan A. Recovery of function in anal incontinence after micro-fragmented fat graft (Lipogems®) injection: two years follow up of the first 5 cases. CellR4. 2016;3(2):e1544.
Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063–77.
Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, Cavallini C, Fontani V, Ventura C. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant. 2013;22(7):1227–35.
Carelli S, Messaggio F, Canazza A, Hebda DM, Caremoli F, Latorre E, Grimoldi MG, Colli M, Bulfamante G, Tremolada C. Characteristics and properties of mesenchymal stem cells derived from micro-fragmented adipose tissue. Cell Transplant. 2014;24(7):1233–52.
Tremolada C, Palmieri G, Ricordi C. Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transplant. 2010;19(10):1217–23.
Jiang X-X, Zhang Y, Liu B, Zhang S-X, Wu Y, Yu X-D, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–6.
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–6.
Maurer MH. Proteomic definitions of mesenchymal stem cells. Stem Cells Int. 2011:704256.
Vaizey C, Kamm M. Injectable bulking agents for treating faecal incontinence. Br J Surg. 2005;92(5):521–7.
Frudinger A, Pfeifer J, Paede J, Kolovetsiou‐Kreiner V, Marksteiner R, Halligan S. Autologous skeletal muscle‐derived cell injection for anal incontinence due to obstetric trauma: a five‐year follow‐up of an initial study of ten patients. Colorectal Dis. 2015;9794–801.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ledda, M., Lisi, A., Giori, A. (2016). Stem Cells. In: Mongardini, M., Giofrè, M. (eds) Management of Fecal Incontinence. Springer, Cham. https://doi.org/10.1007/978-3-319-32226-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-32226-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32224-7
Online ISBN: 978-3-319-32226-1
eBook Packages: MedicineMedicine (R0)