Abstract
Stress urinary incontinence (SUI) is highly prevalent and associated with a reduced quality of life. An intact rhabdosphincter at the mid-urethra is mandatory to maintain urinary continence. Adult stem cell injection therapy for the regenerative repair of an impaired sphincter is currently at the forefront of incontinence research. The implanted cells will fuse with muscle and release trophic factors promoting nerve and muscle integration. Hereby, we review the use of mesenchymal stem cell therapy for SUI and the experience with the development of muscle-derived stem cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are 17 million people in the USA and more than 200 million people worldwide who live with urinary incontinence [44, 51]. Incontinence occurs frequently from middle age onwards and is associated with a reduced quality of life [12]. Stress urinary incontinence (SUI) is the most common type of urinary incontinence. SUI happens when intra-abdominal forces suddenly increase, which makes bladder pressure exceed the urethral pressure. The risk factors for SUI include increased age, high parity, and obesity [22, 49, 61]. SUI could be further categorized into three conditions, including intrinsic sphincter deficiency (ISD), urethral hypermobility, or a combination of both [48]. ISD is characterized by failure of urethral closure. Urethral hypermobility occurs when the bladder neck muscle support is weak and the intra-abdominal pressure is unable to transmit to the proximal urethral. These mechanisms represent two extremes of the spectrum of SUI. However, the majority of these patients have both disorders in varying degrees [36].
Treatments for SUI include nonsurgical or surgical options. Pelvic floor muscle training usually serves as first-line therapy but promising results could only be achieved in carefully selected patients [25]. Pharmaceutical therapy has a limited role in SUI treatment. Duloxetine, a selective serotonin/norepinephrine reuptake inhibitor, is the only approved SUI medication in Europe, but it is not available in the USA [67]. Several surgical procedures are available for SUI treatment. Injection of bulking agents near the bladder neck can increase coaptation along the urethral and improve outlet resistance and urinary incontinence [24]. The use of carbon beads, silicon particles, polytetrafluoroethylene, autologous chondrocytes, and bovine collagen as bulking agents yields short-term success in treating SUI [4, 11, 24, 47, 60]. However, long-term efficacy is poor and complications such as chronic inflammatory reactions, particle migration, periurethral abscess, erosion of the urinary bladder or urethral, or obstruction with urinary retention are not uncommon [53, 54, 66]. Sling procedures or bladder neck suspensions are more efficacious but there are certain levels of morbidity [58, 64].
Recent advances in tissue engineering provide new therapeutic options in the field of SUI. Using the principles of cell transplantation, materials science, and biomedical engineering, we can develop biological substitutes to restore and maintain the normal function of damaged or lost tissue and organs [1]. The potential of stem cell (SC) therapy for the regenerative repair of the damaged rhabdosphincter is currently at the forefront of incontinence research [42, 71]. In the future, SUI treatment procedures may contain two clinic visits (Fig. 1). In the first visit, the patient will receive a small skeletal muscle biopsy using a small-caliber needle. The muscle sample will be sent to the approval facility or laboratory for stem cell preparation. Several weeks later, the patient will come back for a second visit to receive a stem cells injection into the his or her urethral sphincter under local anesthesia.
Diagram showing autologous stem cell injection therapy for stress urinary incontinence. Autologous stem cells are obtained with a biopsy of tissue, the cells are dissociated and expanded in culture, and the expanded cells are implanted into the same host. Used with permission from Wiley, Neurourol Urodyn 2007; 26(7):967
Neurophysiology and pathophysiology of continence control
The connective and musculature tissues surrounding the urethral contribute to passive and active resting tone, keeping the urethral from urine leakage. These two mechanisms, passive and active, accommodate by keeping the walls of the urethra apposed in response to increasing intra-abdominal pressure during coughing, weightlifting, sneezing, etc. In response to increasing intra-abdominal pressure, the vagina was lifted toward the pubic symphysis by pelvic muscles and it compressed the walls of the urethral against the pubis [16]. Bladder neck position in relation to other pelvic structures will also affect proper pressure transmission and continence.
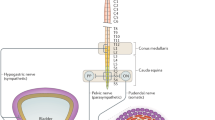
The external urethral sphincter (EUS), also known as rhabdosphincter, is composed of striated muscle fiber types I and II located in the mid-urethra [62]. In addition, smooth muscle fibers are also deposited in circular and longitudinal layers. The urethral muscles are controlled by three sets of peripheral nerves: somatic (pudendal nerves), sympathetic (hypogastric nerves), and parasympathetic (sacral pelvic nerves) (Fig. 2). The sympathetic pathways emerge from the thoracolumbar spinal cord, pass through the sympathetic chain ganglia, the inferior splanchnic nerves, the inferior mesenteric ganglia, and the hypogastric nerves to the pelvic plexus innervating urogenital organs. The parasympathetic preganglionic pathways originate in the sacral spinal cord and pass through the pelvic nerves to synapse on the distal ganglia in the target organs. The sacral somatic pathways are contained in the pudendal nerves, which provide innervation to the EUS. These three sets of nerves contain afferent axons arising from the lumbosacral dorsal root ganglia [15].
Diagram showing the sympathetic, parasympathetic, and somatic innervation of the urogenital tract. IMG inferior mesenteric ganglia, SCG sympathetic chain ganglia, DRG dorsal root ganglia, ISN inferior splanchnic nerves, EUS external urethral sphincter. Used with permission from Wiley, Neurourol Urodyn 2007; 26(7):967
EUS can be activated voluntarily or by spinal reflux mechanism elicited by bladder distension [8]. Onuf’s nucleus in the sacral spinal cord receives central nervous system impulses, which in turn release serotonin and norepinephrine in the synapses with the pudendal nerves to control EUS [8, 30]. The nerve-mediated active continence mechanisms during abdominal pressure elevation can be divided into two control areas. The first one is a central nervous system-controlled pathway through Onuf’s nucleus [33, 34], and the second one is the bladder-to-urethra spinal reflex in response to laughing, sneezing, or exercise [14, 32]. The reflex ark and the involvement of pelvic afferent pathways were investigated in a series of experiments by Kamo et al. [14, 32–34]. In the experiment with rat SUI model, an increase in the mid-urethral pressure is mediated by passive mechanisms as well as the active contractions of EUS and pelvic muscles when sneezing. In contrast, an increase in the proximal and distal urethral pressure during sneezing depends on the rising intra-abdominal and bladder pressure. The authors also demonstrated that the mid-urethral sneeze-induced continence reflex is impaired in the rat model of birth trauma created by vaginal distension.
Cell source for injection therapy
The ideal cells for tissue engineering and cell therapy should be easily procured from minimally invasive procedures, proliferate quickly in a well-controlled manner, provide sufficient quantities of cells, exhibit capabilities of differentiation to regenerate multiple tissues, and be able to be transplanted into an autologous host [2]. Multipotent SCs are the most common cell sources for cell therapy and tissue engineering. Two types of SCs are potentially useful for therapeutic purposes: embryonic SCs and adult SCs. Although both of these SCs own the remarkable properties of being able to differentiate into a large number of specific cell types and to self-renew [5], the usage of embryonic SCs are limited by problems of ethic consideration, potential for tumorigenicity, and government regulations [19, 46]. Mesenchymal stem cell (MSC) is a kind of adult SCs without significant ethical issues related to their usage. MSCs derived from embryonic mesoderm can be easily isolated from several adult tissues such as adipose tissue, bone marrow, muscle, amniotic fluid, placenta, umbilical cord, or liver [45]. MSCs are able to be cultured and expand in vitro easily, differentiate into cells derived from any germ layers, and release paracrine factors to affect the surrounding tissues [70]. Currently, bone marrow-derived stem cells (BMSCs), adipose-derived stem cells (ADSCs), and muscle-derived stem cells (MDSCs) are the stem cell sources applied in SUI therapy.
Bone marrow-derived stem cells
Bone marrow is the first source reported to contain MSCs. BMSCs have been widely studied and found to be capable of differentiating into adipogenic, osteogenic, myogenic, and chondrogenic cells [17, 20, 56]. Drost et al. used 5-azacytidine-exposed first vitro passage human BMSCs as cell source to culture and expanded for six passages. These myogenic differentiated cells expressed smooth and striated muscle antigens [18]. Corcos et al. injected autologous BMSCs into the injured urethral sphincter of Sprague–Dawley rats [13]. They also demonstrated that the rat BMSCs have the ability to differentiate their phenotype towards smooth and striated muscle with desmin expression and alpha-smooth muscle actin (SMA) up-regulation. The periurethral injection of BMSCs in a SUI animal model significantly improved Valsalva leak point pressure and restored the injured rhabdosphincter. Similar experiments and results were also demonstrated by Kim et al. [38]and Kinebuchi et al. [39]. However, for clinical use, bone marrow may be detrimental due to the highly invasive and painful procedures required for procurement, decline in differentiation potential and MSC number with increased age, and low yield of MSCs upon processing [37, 56]. In contrast, large numbers of MDSCs and ADSCs can be easily obtained using simple procedures [28, 77].
Muscle-derived stem cells
In the recent past, myoblast transfer therapy is hindered by the poor survival of muscle-derived cells upon injection. It was found that, while using traditional methods to obtain muscle precursor cells, less than 3% of cells are still present 1 h after being injected into a muscle [3]. New pre-plating techniques improved cell survival rates after transplant. Selection of a specific-muscle-derived cell population can be used as an approach to improve cell survival after myoblast-mediated ex vivo gene transfer approach and myoblast transplantation [57]. Such studies allow extensive investigations into the properties, differentiation capacity, interrelationship::, and functional heterogeneity of skeletal muscle progenitor cells [28, 63]. In comparison with the commonly recognized striated muscle precursor ‘myoblast’ cells, MDSCs display superiority in survival and engraftment of cells, restoration in impaired function, and resistance to oxidative apoptosis [52].
MDSC possess three qualities which make their usage more advantageous than that of other injectable bulking agents. First, since autologous cell transplantation will not induce an immunological reaction, the cells may persist longer than implanted foreign materials such as collagen [43, 73]. Furthermore, MDSCs also uniquely differ from fibroblasts or muscle cells since MDSCs will fuse to form post-mitotic multinucleated myotubes [21]. This limits the expansion of injected cells and reduces the risk of urinary tract obstruction that was observed with other sources such as fibroblasts [41]. Finally, MDSCS form myotubes and myofibers that could become innervated with the host muscle (Fig. 3). Instead of serving as a bulking agent only, MDSCs are physiologically capable of improving urethral sphincter function [9, 68, 72].
Hematoxylin–eosin staining revealed a atrophic proximal urethral sphincter in saline group (S) at 4 weeks compared with (b) normal, uninjured urethra. c, d MDCs (1 × 106) injected into denervated sphincter led to increased dorsolateral striated muscle masses with variable fiber orientation at the injection sites. e, f Fibroblasts (1 × 106) injected into denervated sphincter led to increased dorsolateral connective tissue masses at the injection sites. Images taken with ×10 (a–c and e) and ×20 (d, f) objectives. Used with permission from Elsevier, Urology 2006; 68(2):449
The feasibility and application of this concept was successfully conducted in rodent models of SUI [6, 10]. In these experiments, Chermansky et al. demonstrated the integration of MDSCs with damaged striated muscle in rat urethral. One week prior to MDSCs injection, intrinsic sphincter deficiency was created by cauterizing tissues lateral to the mid-urethral. At 4 weeks, incorporation of MDSCs was identified in the experimental group. LacZ staining confirmed that MDSCs had integrated with the striated muscle layer of the damaged urethral (Fig. 4). More importantly, the MDSC injection significantly improved the leak point pressure (LPP) without affecting bladder function. At 2, 4, and 6 weeks time points, the LPP was persistently elevated for the duration of the trial and comparable with that of uncauterized control rats (Fig. 5). This demonstrated the potential of the MDSC to take part in healing and restoring the function of the damaged sphincter [10].
Histologic findings of cauterized mid-urethra 4 weeks after Hanks’ balanced salt solution (HBSS) or MDC injection. a Hematoxylin–eosin stain of cauterized mid-urethra injected with HBSS. Reduced from ×400. Arrow points to disrupted striated muscle layer. b Hematoxylin–eosin stain of cauterized mid-urethra injected with MDCs. Reduced from ×400. Arrow points to intact striated muscle layer. c LacZ stain of cauterized mid-urethra injected with MDCs. Reduced from ×400. Arrows point to beta-galactosidase expressing MDCs, situated within the striated muscle layer of mid-urethra. Used with permission from Elsevier, Urology 2004; 63(4):782
Compared with cauterized rats injected with Hanks’ balanced salt solution (H) and matched respective to time, the increased leak point pressures (LPP) seen in each MDSC-injected groups (M) were significantly higher. Compared with control rats (C) and matched respective to time, LPPs seen in MDSC-injected groups at 4 and 6 weeks after MDSC injection were not statistically different. Used with permission from Elsevier, Urology 2004; 63(4):782
Kwon et al. compared the outcomes of injecting MDSCs or fibroblast or a mixture of both for the treatment of SUI in a previously established rat model [41]. Although the short-term LPP increment was similar in all treatment groups, urethral muscle contractility was significantly improved in the MDSC group only. Furthermore, a high-dose fibroblast injection will induce urinary retention but not with MDSC injection. These results suggested that a fibroblast injection can increase LPP by bulking effect but simultaneously make tissues less compliant.
Adipose-derived stem cell
Multipotent MSCs were identified within the stromal vascular fraction of human adipose tissue. These cells, also termed as ADSCs, are abundant in the human body and possess similar molecular signature markers and biological properties with BMSCs [55]. Clonal studies of ADSCs demonstrated that these cells exhibit multilineage differentiation into adipogenic, myogenic, chondrogenic, osteogenic, and neurogenic cells in the presence of specific induction factors [76, 77]. Rodriguez et al. cultured processed lipoaspirate cells in a smooth muscle differentiation medium and these smooth muscle-differentiated cells, but not their precursors, have the functional ability to contract and relax in the presence of pharmacological agents. These functional smooth muscle cells also express smooth muscle markers such as SMA, SM22, smoothelin, myosin heavy chain, and caldesmon [59]. Another study from the same research group revealed that ADSCs injected periurethrally exhibit in vivo survival and differentiation into smooth muscle cells. These ADSCs were tagged with fluorescent markers and were seen to be incorporated into host smooth muscle for up to 12 weeks after injection [27]. The feasibility of ADSC use was suggested through other study reports on improvement in LPP and retrograde urethral perfusion pressures (RUPP) in a rat model of SUI when animals were injected with ADSCs and biodegradable microbeads as carriers [74].
Zhao et al. designed a stem cell transplantation system, which contain ADSCs and release-controlled nerve growth factor (NGF), to enhance the therapeutic efficacy of ADSCs through periurethral injection in SUI rats [75]. NGF was encapsulated with poly(lactic-co-glycolic acid—PLGA) microspheres (PLGA/NGF) to control its release. Their results revealed that NGF could improve ADSC’s viability in vitro and in vivo for short-term observation. The ADSC injection with PLGA/NGF had induced a significant improvement in the amount of muscle and ganglia as well as in abdominal LPP and RUPP compared with other groups. This study suggested that the periurethral injection of autologous ADSCs with release-controlled NGF might be a potential treatment option for SUI.
Current human clinical experience
The first clinical series in the medical literature is with the use of muscle-derived cellular therapy from Canada. Carr et al. [7] from the University of Toronto performed the first North American clinical MDSC therapy trials. Eight patients received injection therapy of pure MDSCs obtained from muscle biopsies of the lateral thigh. The muscle tissue was further processed and expanded to the target dose. After the desired cell number has been achieved, quality tests were performed to assure that the cells met the criteria for cell viability, contaminants, and sterility. There were 18–22 × 106 MDSCs injected in an outpatient clinic at 3–5 weeks after muscle biopsy. Mean and median follow-up was 16.5 and 17 months, respectively. Three of these eight patients withdrew from the trial after 1 month of follow-up. The remaining five subjects got some improvement during follow-up, while one subject achieved total continence. The improvement in these subjects occurred between 3 and 8 months after the first injection. During follow-up, no serious adverse events were reported. Two of eight patients subsequently received midurethral tape placement. The previous MDSC injection did not negatively impact on the degree of tape placement difficulty and outcome of this procedure.
Strasser et al. initially presented autologous myoblasts and fibroblasts injection under transurethral ultrasound guidance into the rhabdosphincter, while fibroblast/collage suspension was injected into the submucosa to promote a sealing effect. The result was published in The Lancet in June 2007. However, an ethics issue was aroused due to an inspection which found that this study was not conducted according to Austrian law and to the standards of the International Conference on Harmonisation of Good Clinical Practice. The editors of The Lancet subsequently decided to retract the article because “the inspectors raise doubts as to whether a trial as described in The Lancet ever existed.” [40] Another SUI human trial study published in World Journal of Urology from the same group [65] was also retracted due to the same reason.
In Canada, Carr and associates conducted a randomized, blinded study of MDSC therapy in 29 female SUI patients [23]. In this trial, autologous MDSC transplantation was conducted through cystoscopic-assisted transurethral injection at different cell concentrations. A second injection was carried out 3 months after the first injection. In the patients who received two injections, half of them reported no leakage at 1 year of follow-up.
Surgery and cellular therapy discussion
The transvaginal tape (TVT) is the standard surgical option for SUI treatment at present. The objective cure rate of this procedure has been reported as from 85% to 89% at 3 or 5 years [29, 50]. However, Ward et al. [69] compared the objective and subjective outcomes after TVT insertion or abdominal colposuspension in the largest randomized, controlled study. Only 63% of the TVT group and 51% of the colposuspension group were cured at 2 years objectively. Moreover, the subjective cure rates were even lower at 43% and 37%, respectively. While suburethral sling procedures only provide mechanical support to the weakened pelvic floor muscles or fascias, MDSCs transplantation into the mid-urethra may not only serve as a bulking agent but also restore the contractile function of the EUS in the treatment of SUI. Further experiments on animal models may answer the questions if concurrent MDSCs injection and sling procedures could achieve better continence control than either single procedure. Furthermore, MDSC may be used in restoring continence in men receiving radical prostatectomy or who have other diseases which led to impaired sphincter function. Hoshi et al. [26] demonstrated promising results of treating post-prostatectomy incontinence using MDSC urethra injection in a rat model. Another trial involving autologous MDSC for the treatment of urinary incontinence in classic bladder extrophy children has shown promising results [31]. The efficacy of using MDSC in the treatment of fecal incontinence was investigated by Kang et al. [35]. A small number (3 × 106) of autologous MDSCs was injected into the cryoinjured anal sphincter in a rat model. Although there was no statistical difference in sphincter contractility in the treated or control groups, the MDSC-injected group demonstrated improved contractility in response to acetylcholine and KCL stimulations. Regeneration of smooth and striated myofibers at the sites of labeled MDSCs injections was also identified. Further experiments on a large number of MDSCs injections and a longer follow-up period may determine if MDSC transplantation could restore impaired anal sphincter function and be the treatment option for fecal incontinence.
Conclusion
According to the promising preclinical and clinical studies, the use of stem cells for SUI treatment may be a major step forward with clinical efficacy and minimal risks. However, several points have yet to be clarified. More preclinical studies should be conducted to improve cell survival and to maximize the function restoration in the target tissues. The costs and benefits of cellular therapy should be considered when comparing with the current standard of treatment, and the long-term efficacy and safety of new therapy should be further demonstrated. Furthermore, the current clinical trials came from a few research groups with a small case number. The proper cell number and injection locations and techniques should be further evaluated through randomized blinded studies. Our hope is that autologous cellular transplantation for sphincter regeneration and continence restoration will be the safe and preferred procedure for the treatment of SUI.
References
Atala A (2004) Tissue engineering and regenerative medicine: concepts for clinical application. Rejuvenation Res 7:15–31
Atala A (2009) Regenerative medicine and tissue engineering in urology. Urol Clin North Am 36:199–209, viii-ix
Beauchamp JR, Morgan JE, Pagel CN, Partridge TA (1999) Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol 144:1113–1122
Bent AE, Tutrone RT, McLennan MT, Lloyd LK, Kennelly MJ, Badlani G (2001) Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. Neurourol Urodyn 20:157–165
Brivanlou AH, Gage FH, Jaenisch R, Jessell T, Melton D, Rossant J (2003) Stem cells. Setting standards for human embryonic stem cells. Science 300:913–916
Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, Chancellor MB (2003) Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology 62:958–963
Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, Erickson J, Huard J, Chancellor MB (2008) 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 19:881–883
Chancellor MB, Perkin H, Yoshimura N (2005) Recent advances in the neurophysiology of stress urinary incontinence. Scand J Urol Nephrol 39:21–24
Chancellor MB, Yokoyama T, Tirney S, Mattes CE, Ozawa H, Yoshimura N, de Groat WC, Huard J (2000) Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn 19:279–287
Chermansky CJ, Tarin T, Kwon DD, Jankowski RJ, Cannon TW, de Groat WC, Huard J, Chancellor MB (2004) Intraurethral muscle-derived cell injections increase leak point pressure in a rat model of intrinsic sphincter deficiency. Urology 63:780–785
Chrouser KL, Fick F, Goel A, Itano NB, Sweat SD, Lightner DJ (2004) Carbon coated zirconium beads in beta-glucan gel and bovine glutaraldehyde cross-linked collagen injections for intrinsic sphincter deficiency: continence and satisfaction after extended follow-up. J Urol 171:1152–1155
Corcos J, Beaulieu S, Donovan J, Naughton M, Gotoh M (2002) Quality of life assessment in men and women with urinary incontinence. J Urol 168:896–905
Corcos J, Loutochin O, Campeau L, Eliopoulos N, Bouchentouf M, Blok B, Galipeau J (2010) Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodyn 30:447–455
de Groat WC (1998) Anatomy of the central neural pathways controlling the lower urinary tract. Eur Urol 34(Suppl 1):2–5
de Groat WC (2006) Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 147(Suppl 2):S25–S40
DeLancey JO (1994) Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 170:1713–20, discussion 1720-3
Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y (2005) Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 309:314–317
Drost AC, Weng S, Feil G, Schafer J, Baumann S, Kanz L, Sievert KD, Stenzl A, Mohle R (2009) In vitro myogenic differentiation of human bone marrow-derived mesenchymal stem cells as a potential treatment for urethral sphincter muscle repair. Ann NY Acad Sci 1176:135–143
Edwards RG (2007) A burgeoning science of embryological genetics demands a modern ethics. Reprod Biomed Online 15(Suppl 1):34–40
Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F (1998) Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279:1528–1530
Furuta A, Jankowski RJ, Pruchnic R, Yoshimura N, Chancellor MB (2007) The promise of stem cell therapy to restore urethral sphincter function. Curr Urol Rep 8:373–378
Hampel C, Wienhold D, Benken N, Eggersmann C, Thuroff JW (1997) Prevalence and natural history of female incontinence. Eur Urol 32(Suppl 2):3–12
Herschorn S, Carr LK, Birch C (2010) Autologous muscle-derived cells as therapy for stress urinary incontinence: a randomized blinded trial. Neurourol Urodyn 29:243–326
Herschorn S, Glazer AA (2000) Early experience with small volume periurethral polytetrafluoroethylene for female stress urinary incontinence. J Urol 163:1838–1842
Holroyd-Leduc JM, Straus SE (2004) Management of urinary incontinence in women: scientific review. JAMA, J Am Med Assoc 291:986–995
Hoshi A, Tamaki T, Tono K, Okada Y, Akatsuka A, Usui Y, Terachi T (2008) Reconstruction of radical prostatectomy-induced urethral damage using skeletal muscle-derived multipotent stem cells. Transplantation 85:1617–1624
Jack GS, Almeida FG, Zhang R, Alfonso ZC, Zuk PA, Rodriguez LV (2005) Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol 174:2041–2045
Jankowski RJ, Deasy BM, Huard J (2002) Muscle-derived stem cells. Gene Ther 9:642–647
Jeffry L, Deval B, Birsan A, Soriano D, Darai E (2001) Objective and subjective cure rates after tension-free vaginal tape for treatment of urinary incontinence. Urology 58:702–706
Kaiho Y, Kamo I, Chancellor MB, Arai Y, de Groat WC, Yoshimura N (2007) Role of noradrenergic pathways in sneeze-induced urethral continence reflex in rats. Am J Physiol Ren Physiol 292:F639–F646
Kajbafzadeh AM, Elmi A, Payabvash S, Salmasi AH, Saeedi P, Mohamadkhani A, Sadeghi Z, Nikfarjam L (2008) Transurethral autologous myoblast injection for treatment of urinary incontinence in children with classic bladder exstrophy. J Urol 180:1098–1105
Kamo I, Cannon TW, Conway DA, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N (2004) The role of bladder-to-urethral reflexes in urinary continence mechanisms in rats. Am J Physiol Ren Physiol 287:F434–F441
Kamo I, Kaiho Y, Canon TW, Chancellor MB, de Groat WC, Prantil RL, Vorp DA, Yoshimura N (2006) Functional analysis of active urethral closure mechanisms under sneeze induced stress condition in a rat model of birth trauma. J Urol 176:2711–2715
Kamo I, Torimoto K, Chancellor MB, de Groat WC, Yoshimura N (2003) Urethral closure mechanisms under sneeze-induced stress condition in rats: a new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol 285:R356–65
Kang SB, Lee HN, Lee JY, Park JS, Lee HS (2008) Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis Colon Rectum 51:1367–1373
Kayigil O, Iftekhar Ahmed S, Metin A (1999) The coexistence of intrinsic sphincter deficiency with type II stress incontinence. J Urol 162:1365–1366
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
Kim SO, Na HS, Kwon D, Joo SY, Kim HS, Ahn Y (2011) Bone-marrow-derived mesenchymal stem cell transplantation enhances closing pressure and leak point pressure in a female urinary incontinence rat model. Urol Int 86:110–116
Kinebuchi Y, Aizawa N, Imamura T, Ishizuka O, Igawa Y, Nishizawa O (2010) Autologous bone-marrow-derived mesenchymal stem cell transplantation into injured rat urethral sphincter. Int J Urol, official journal of the Japanese Urological Association 17:359–368
Kleinert S, Horton R (2008) Retraction—autologous myoblasts and fibroblasts versus collagen [corrected] for treatment of stress urinary incontinence in women: a [corrected] randomised controlled trial. Lancet 372:789–790
Kwon D, Kim Y, Pruchnic R, Jankowski R, Usiene I, de Miguel F, Huard J, Chancellor MB (2006) Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology 68:449–454
Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB (2003) The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 14:31–7, discussion 37
Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH, Lee SS (2004) Long term effects of muscle-derived stem cells on leak point pressure and closing pressure in rats with transected pudendal nerves. Mol Cells 18:309–313
Levy R, Muller N (2006) Urinary incontinence: economic burden and new choices in pharmaceutical treatment. Adv Ther 23:556–573
Lin Y, Yan Z, Liu L, Qiao J, Jing W, Wu L, Chen X, Li Z, Tang W, Zheng X, Tian W (2006) Proliferation and pluripotency potential of ectomesenchymal cells derived from first branchial arch. Cell Prolif 39:79–92
Lo B, Zettler P, Cedars MI, Gates E, Kriegstein AR, Oberman M, Reijo Pera R, Wagner RM, Wuerth MT, Wolf LE, Yamamoto KR (2005) A new era in the ethics of human embryonic stem cell research. Stem Cells 23:1454–1459
Maher CF, O’Reilly BA, Dwyer PL, Carey MP, Cornish A, Schluter P (2005) Pubovaginal sling versus transurethral Macroplastique for stress urinary incontinence and intrinsic sphincter deficiency: a prospective randomised controlled trial. BJOG 112:797–801
McGuire EJ, Lytton B, Pepe V, Kohorn EI (1976) Stress urinary incontinence. Obstet Gynecol 47:255–264
Milsom I, Ekelund P, Molander U, Arvidsson L, Areskoug B (1993) The influence of age, parity, oral contraception, hysterectomy and menopause on the prevalence of urinary incontinence in women. J Urol 149:1459–1462
Nilsson CG, Kuuva N, Falconer C, Rezapour M, Ulmsten U (2001) Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 12(Suppl 2):S5–S8
Norton P, Brubaker L (2006) Urinary incontinence in women. Lancet 367:57–67
Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, Tobita K, Keller BB, Cummins JH, Huard J (2005) Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther 12:1130–1141
Pannek J, Brands FH, Senge T (2001) Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol 166:1350–1353
Papa Petros PE (1994) Tissue reaction to implanted foreign materials for cure of stress incontinence. Am J Obstet Gynecol 171:1159
Peroni D, Scambi I, Pasini A, Lisi V, Bifari F, Krampera M, Rigotti G, Sbarbati A, Galie M (2008) Stem molecular signature of adipose-derived stromal cells. Exp Cell Res 314:603–615
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J (1998) Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol 142:1257–1267
Rezapour M, Ulmsten U (2001) Tension-free vaginal tape (TVT) in women with mixed urinary incontinence—a long-term follow-up. Int Urogynecol J Pelvic Floor Dysfunct 12(Suppl 2):S15–S18
Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ (2006) Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA 103:12167–12172
Sakamoto K, Sharma S, Wheeler JS (2007) Long-term subjective continence status and use of alternative treatments by women with stress urinary incontinence after collagen injection therapy. World J Urol 25:431–433
Sampselle CM, Miller JM, Mims BL, Delancey JO, Ashton-Miller JA, Antonakos CL (1998) Effect of pelvic muscle exercise on transient incontinence during pregnancy and after birth. Obstet Gynecol 91:406–412
Schroder HD, Reske-Nielsen E (1983) Fiber types in the striated urethral and anal sphincters. Acta Neuropathol 60:278–282
Seale P, Asakura A, Rudnicki MA (2001) The potential of muscle stem cells. Dev Cell 1:333–342
Sharifi-Aghdas F (2005) Surgical management of stress urinary incontinence. Urol J 2:175–182
Strasser H, Marksteiner R, Margreiter E, Mitterberger M, Pinggera GM, Frauscher F, Fussenegger M, Kofler K, Bartsch G (2007) Transurethral ultrasonography-guided injection of adult autologous stem cells versus transurethral endoscopic injection of collagen in treatment of urinary incontinence. World J Urol 25:385–392
Sweat SD, Lightner DJ (1999) Complications of sterile abscess formation and pulmonary embolism following periurethral bulking agents. J Urol 161:93–96
Sweeney DD, Chancellor MB (2005) Treatment of stress urinary incontinence with duloxetine hydrochloride. Rev urol 7:81–86
Tamaki T, Uchiyama Y, Okada Y, Ishikawa T, Sato M, Akatsuka A, Asahara T (2005) Functional recovery of damaged skeletal muscle through synchronized vasculogenesis, myogenesis, and neurogenesis by muscle-derived stem cells. Circulation 112:2857–2866
Ward KL, Hilton P (2004) A prospective multicenter randomized trial of tension-free vaginal tape and colposuspension for primary urodynamic stress incontinence: two-year follow-up. Am J Obstet Gynecol 190:324–331
Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25:2648–2659
Yiou R, Yoo JJ, Atala A (2003) Restoration of functional motor units in a rat model of sphincter injury by muscle precursor cell autografts. Transplantation 76:1053–1060
Yokoyama T, Pruchnic R, Lee JY, Chuang YC, Jumon H, Yoshimura N, de Groat WC, Huard J, Chancellor MB (2001) Autologous primary muscle-derived cells transfer into the lower urinary tract. Tissue Eng 7:395–404
Yokoyama T, Yoshimura N, Dhir R, Qu Z, Fraser MO, Kumon H, de Groat WC, Huard J, Chancellor MB (2001) Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol 165:271–276
Zeng X, Jack GS, Zhang R (2006) Treatment of SUI using adipose derived stem cells: restoration of urethral function. J Urol 175:291
Zhao W, Zhang C, Jin C, Zhang Z, Kong D, Xu W, Xiu Y (2010) Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur Urol 59:e1–e4
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228
Conflicts of interest
Chancellor MB is the consultant and patent holder of technology licensed to Cook MyoSite, no conflicts of interest for Wang HJ and Chuang YC.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript is an extended version of the Ulf Ulmsten lecture given by Dr. Michael Chancellor at the joint IUGA/ICS meeting in August 2010 in Toronto, Canada.
Rights and permissions
About this article
Cite this article
Wang, HJ., Chuang, YC. & Chancellor, M.B. Development of cellular therapy for the treatment of stress urinary incontinence. Int Urogynecol J 22, 1075–1083 (2011). https://doi.org/10.1007/s00192-011-1432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-011-1432-1