Abstract
Introduction and hypothesis
Cell therapy for stress urinary incontinence (SUI) management has been experienced with encouraging results.
Methods
We conducted an open prospective study on 12 women presenting severe SUI with fixed urethra, after previous failed surgical management. Patients underwent intrasphincteric injections of autologous progenitor muscular cells isolated from a biopsy of deltoid muscle. Primary endpoint focused on safety (measurement of Q max variation after 3 months). Secondary endpoints assessed side effects and efficacy.
Results
No variation was diagnosed on Q max measurements. Efficacy data show that three of 12 patients are dry at 12 months, seven other patients are improved on pad test but not on voiding diary, and two patients were slightly worsened by the procedure. Quality of life was improved in half of patients.
Conclusions
Cell therapy for severe multioperated cases of SUI is a mini-invasive, feasible, and safe procedure that can improve urinary condition in as a second line therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence (UI) is defined by the international continence society as a complaint of any involuntary leakage of urine [1]. Among millions of women suffering from UI in the world, it has been shown that a majority of them suffer from stress urinary incontinence (SUI) [2]. SUI is associated with social impact and a reduced long-term quality of life (QoL). The physiopathology of SUI is complex; SUI is caused by abnormalities of the urethral sphincter such as loss of anatomic support (hypermobility) or impaired sphincter functioning due to loss of elasticity and coaptation (intrinsic sphincter deficiency (ISD)) [3].
When ISD is predominant, i.e., not associated with urethral hypermobility, the external striated urethral sphincter (or rhabdosphincter) is the focus of the management of SUI. The goal of treatment for ISD is to correct incontinence without creating outlet obstruction. Previous work has emphasized the complexity of ISD management due to existence of controversial clinic and urodynamic definitions, incomplete understanding of physiopathology, and variability of etiologies [4]. Indeed, recognized causes of ISD as prior surgery (anti-incontinence procedures, hysterectomy), neurological lesions or injury, pelvic radiation or estrogen deficiency can be associated and lead to challenging situations. PFME are main conservative option for treating SUI, with moderate success [5]. Available pharmacological treatments (including duloxetin) remain unsatisfactory up to now [5, 6], and the existing surgical techniques (artificial urinary sphincter (AUS), suburethral slings, bulking agents) have proven efficacy in some cases [7] but can lead to severe adverse events, including sepsis, reoperation, and explantation [8, 9]. Therefore, there is still an unfulfilled medical need for more effective management of SUI related to ISD [10], especially in case of failure of currently available options, justifying the development of new treatments.
Autologous cell therapy, consisting in injections of progenitor muscular cells, has been used for more than 15 years in clinical trials mostly to treat other diseases such as muscular dystrophy or myocardium disorders [11, 12], with no major adverse events or toxicity. These treatments rely on the ability of adult progenitor muscular cells to self-renew and to differentiate to fuse with existing muscle fibers or to form new muscle fibers and consequently regenerate the tissue. Application of this therapy to a small muscle like the rhabdosphincter presents advantages over previous applications: the number of required cells is limited due to the small size of the rhabdosphincter, the use of autologous graft does not require costly and toxic adjuvant treatment, and the urethral sphincter is technically easy to reach [13]. This approach has been experienced in animals with promising results [14–18]. To our knowledge, this treatment has been experienced in women, and data of safety have been published [19].
We present here a study where the main objective was to evaluate safety of intrasphincteric injections of autologous progenitor muscular cells (cell-based product) in female subjects with severe and/or complex UI due to ISD, with history of failure of previous surgical management. Secondary objectives were to assess side effects and efficacy of the procedure in correcting SUI in these challenging cases.
Materials and methods
Patients
Between May 2006 and February 2008 an open prospective study on women presenting SUI due to ISD was conducted. This phase IIa study, open-labeled, non-controlled, has been done in a single center. The diagnosis of SUI due to ISD was made at enrolment with clinical examination and urodynamic data. The enrolment criteria are summarized in Table 1. The following data were preoperatively collected at the beginning of the study: age, body mass index (BMI), number of physiotherapy sessions done before enrolment, medical or surgical treatment already used for SUI, and past medical history. Preoperative evaluation also included urethrocystoscopy (to rule out strictures), electromyography, urethrocystography, biological examination, uroflowmetry, ultrasound (bladderscan), urine culture, completion of a validated questionnaire of quality of life (CONTILIFE) [20], pad test, bladder diary, cystometry, and urethral pressure profile (UPP) measurements.
Procedure
Patients underwent biopsy of deltoid muscle under local anesthesia with lidocain at standard dose. Through a 2-cm posterior incision of the skin above the left deltoid muscle (for right-handed patients), open muscle biopsies were taken from the deltoid muscle in order to obtain 1 to 2 g of muscle fibers. For each patient, a muscle biopsy was taken and was immediately transported to the facility (Cell Therapy Unit) certified to Good Manufactory Practice standards and authorized to produce myoblasts in order to prepare and condition this cell-based products. All the cell-based products were produced by Celogos company.
The cellular production process (3–4 weeks) includes several steps: cell extraction, two steps of cell amplification, cell freezing, and storage. The schematic diagram (Fig. 1) summarizes the production process, as well as the different quality controls performed at different steps of the cell-based product preparations. The final product was a progenitor muscle cell suspension to avoid any bulking effect after the periurethral injection. The vials of freezing cells (1 or 2 mL) were sent to the urology department and thawed immediately before administration to subjects.
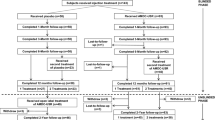
Subjects were hospitalized on an outpatient basis for muscular biopsy and for the cell-based product injection. This study was designed to have a single injection of three different concentrations of cells in order to evaluate a dose-related efficacy or dose-related adverse events. The patients were randomized in these three groups. The study was initiated by injecting the group with the lowest concentration of cells to carefully monitor potential adverse events. The G1, G2, and G3 groups, including four subjects each, were injected with 1 × 107, 2.5 × 107, and 5 × 107 cells, respectively. Cell-based product was administered via endourethral route under endoscopic control, under local anesthesia with xylocain, at 3 and 9 o’clock.
Study design
The protocol was reviewed and approved by an Independent Ethics Committee prior to the start of the study. Upon good safety results of the cell-based product injected in men (in a previous clinical trial), the committee allowed trial inclusion subjects.
Follow-up and assessment
Control visits were conducted at 1, 2, 3, 6, and 12 months post-operatively. At each visit, uroflowmetry, ultrasound (bladderscan), urine culture, CONTILIFE questionnaire, pad test, and bladder diary were evaluated. Furthermore, cystometry and UPP were performed at 3- and 12-month follow-up post-operatively.
The main criterion for evaluation of safety was reduced flow at the 3-month visit, which was diagnosed at uroflowmetry and defined as a Q max < 15 mL/s and significantly different from baseline. Other parameters to evaluate the safety were: urine culture, post-voiding residual (PVR) volume measured by supra-pubic ultrasound (increase of more than 100 mL compared to baseline), and bladder outlet obstruction measured by uroflowmetry and quantified according to ICS standards. In addition, an evaluation of all adverse events reported during the follow-up of this trial was done.
Secondary endpoints of the study deal with efficacy to assess the correction of SUI. This was first evaluated by a seven consecutive days of bladder diary with the following definitions: complete response if no leakage was noted, partial response if the leaking incidents decreased ≥50% compared to baseline, non-response otherwise. Other issues reflecting efficacy were 1 h-pad test (a responder was defined by a decrease in the pad weight >5 g compared to baseline), quality of life CONTILIFE questionnaire, and variation of maximal closure pressure (described as significant if it was >10 cm H2O compared to baseline).
Statistical analysis
Descriptive analyses were performed in intent-to-treat by dosage group (and across groups) for the primary endpoint (presence of dysuria). For all secondary endpoints, descriptive statistics were calculated by visit and dosage group (and across groups).
Results
Patients’ characteristics
Twelve patients were included and all patients completed the study. Mean age ± SD (range) was 58 ± 13.5 years (35–75 years), and mean BMI was 27.6 ± 4.7 (19.2–34.3). Patient characteristics are developed in Table 2. All patients were presenting pure SUI with a fixed urethra, no overactive detrusor or morphologic abnormalities. Eight of 12 patients were presenting severe incontinence (greater than or equal to five pads/day), two of 12 patients moderate incontinence (three to four pads/day), and two of 12 patients mild incontinence (one to two pads/day). All patients were presenting SUI and past medical history with numerous surgical interventions for incontinence (Table 3). With two exceptions, all patients did pelvic floor exercises for SUI, and the median (range) number of physiotherapy sessions before treatment was 20 (five to 60).
Safety
Muscle biopsies and urethral injections were completed without complications in all patients. No patient had a Q max < 15 mL/s at the 3-month visit, with no voiding difficulties noted. The differences between Q max at 3-month visit and Q max at baseline showed a dose-dependent pattern: ΔQ max value was +5, +1, and −4 for the G1, G2, and G3, respectively. Overall, no change in Q max was observed during the study; however, inter-individual variations were very large (Fig. 2). Pain, assessed by using visual pain scale (0–10), was moderate during the injection which spontaneously resolved. There was no immediate local complication due to injection whatever the dosage group. None of the subjects presented a significantly increased PVR volume, and no subject suffered from complete retention. Pressure-flow analysis showed absence of bladder outlet obstruction at 1and 3 months after injection. Occasional episodes of UTI were reported in one case at the 2- and 12-month follow-up visits and in two cases at both 3- and 6-month follow-up visits, on the basis of a positive urine culture. The three patients had a history of previous infections. All these infections were treated by antibiotics and completely resolved. No other adverse event related to the procedure was noted.
Efficacy
Results of the procedure are heterogeneous during follow-up (Table 2). Three patients, one in each group (number 3, 8, and 12) presented an important improvement of their condition at 12-month follow-up. Indeed, they were dry according to the pad test, and no leakage was declared on the 7 days of bladder diary by patient 3 and patient 12, and three episodes of leakage by patient 8. There was no correlation either with cell dosage or UI severity. It is to note that these results improved during the follow-up period, since these three patients were not dry at the three first visits. In the nine other patients, if pad test decreased for seven of them, the number of leaks remained comparable to baseline (Table 2). Quality of life according to the global score of the CONTILIFE questionnaire showed an increase not only in the three patients who responded to treatment but also in some patients who did not show an objective clinical response. Two patients were slightly worsened by the procedure. Concerning the profilometry, the maximal pressure closure increased in all dosage groups (three patients) at 3-month visit and one persisted at 12-month visit.
Discussion
The present study was undertaken to determine if a treatment using autologous muscle-derived cells should be safe and successful to treat SUI by ISD in women showing previous failure of previous surgical management. By its nature, and the limited number of subjects, this trial cannot yield ultimate evidence about risks/benefits of this innovative treatment method. Nevertheless, the intensive design, the inclusion of multi-operated and severe cases, selection of the assessment tools, and minimum of missing data allow to draw some preliminary conclusions concerning both safety and therapeutic potential of the product and its application procedure.
We first show here that autologous muscle cells could be easily obtained from patients in a clinical setting and conditioned for injection after tissue engineering and appropriate transport. There was no local complication due to biopsy or injection of the cell-based products whatever the dosage tested during all the follow-up periods. No patient was excluded from the study during follow-up. The main study endpoint was Q max; results after 1-year follow-up do not show any significant difference on Q max compared to baseline, no pressure-flow analysis abnormality and no increase in the PVR urine volume. It is noteworthy that the flow rate data show an inverse relationship of ΔQ max to the cell dose administered. It may indicate that the dosage of the cell-based product is not irrelevant. It may be interesting from the efficacy perspective. Side effects possibly related to the product were limited at one case of pain at injection point and three cases of infection of the urinary tract, treated by antibiotics. To our knowledge, other clinical studies in women, treated for SUI using a similar process showed close results, even if three of eight patients withdrew from the study after 1-month follow-up [19].
Data concerning efficacy must be analyzed regarding the severity of the cases included in our series. Indeed, patients recruited in the study have various age, medical history, and degree of incontinence, but most of them were greatly affected by their voiding condition and presented a failure of previous therapy. Eight of 12 patients managed in our study had severe SUI, and 11/12 had undergone several procedures, leading to failure of all the surgical available options, including AUS. It is to note that despite these conditions, three of 12 patients are socially dry at 12-month follow-up. The nine remaining patients had heterogeneous results, with merely no improvement of the urine leakage frequency.
Eight of 12 subjects improved in the global score of the CONTILIFE QoL questionnaire at the 12-month visit. On the other hand, profilometry did not show any systematic change during the study according to the pre-selected response criteria, and two patients had a worse condition than before at 12-month follow-up. As a whole, these results indicate that the evaluated treatment might have a therapeutic potential for achieving a partial control of incontinence symptoms, which need to be confirm. Furthermore, added to its safety, our technique is minimally invasive, on an outpatient basis.
Heterogeneity of the subjects partly explains the results on efficacy. Indeed, variable anatomic conditions make it difficult to inject the cell product exactly in the right place, especially under local anesthesia. The basic rhabdosphincter function is also patient-dependent, influencing the results. The outcome of the cells in the muscle tissue is also not certain concerning survival, engraftment, host tissue repair, and function [21]; however, we noted in our series that patients with the best results improved progressively during follow-up, with first complete responses observed after 3 months. This emphasizes that our results may be not due to a bulking effect. This also leads us to consider that cell product has a delayed biologic effect, and that pelvic floor muscle training should be beneficial after this procedure, by possibly helping the grafted cells to develop and take place in their environment in the rhabdosphincter. Concerning the cell product, no obvious evidence of a related-dose efficacy can be found for the studied items.
Other studies published in the literature show results close to ours. A safety study, using a similar technique of injection showed that this treatment do not generate severe side effects, and found improvement in one out of five patients who completed the study [19]. Unfortunately, the severity and history of the eight patients initially enrolled is not known.
According to our results, cell therapy seems clinically feasible and safe, and shows promising results in this initial experience of management of SUI by ISD in case of failure of other surgical management. Nevertheless, other studies are mandatory in order to evaluate the long-term efficacy of such procedures. These investigations should be performed on larger scales with long-term follow-up to give preciseness on the place of cellular therapy in the field of SUI management.
Abbreviations
- BMI:
-
Body mass index
- ISD:
-
Intrinsic sphincter deficiency
- PFME:
-
Pelvic floor muscle exercises
- PVR:
-
Post-voiding residual
- Q max :
-
Maximum urinary flow rate
- QoL:
-
Quality of life
- SUI:
-
Stress urinary incontinence
- UI:
-
Urinary incontinence
- UPP:
-
Urethral pressure profile
- UTI:
-
Urinary tract infection
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al (2003) The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61:37–49
Norton P, Brubaker L (2006) Urinary incontinence in women. Lancet 367:57–67
Pajoncini C, Costantini E, Guercini F, Bini V, Porena M (2003) Clinical and urodynamic features of intrinsic sphincter deficiency. Neurourol Urodyn 22:264–268
Haab F, Zimmern PE, Leach GE (1996) Female stress urinary incontinence due to intrinsic sphincteric deficiency: recognition and management. J Urol 156:3–17
Shamliyan TA, Kane RL, Wyman J, Wilt TJ (2008) Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med 148:459–473
Mariappan P, Alhasso A, Ballantyne Z, Grant A, N’Dow J (2007) Duloxetine, a serotonin and noradrenaline reuptake inhibitor (SNRI) for the treatment of stress urinary incontinence: a systematic review. Eur Urol 51:67–74
Wilson TS, Lemack GE, Zimmern PE (2003) Management of intrinsic sphincteric deficiency in women. J Urol 169:1662–1669
Kiilholma P, Chancellor MB, Makinen J, Hirsch IH, Klemi PJ (1993) Complications of Teflon injections for stress urinary incontinence. Neurourol Urodyn 12:131–137
Rogers RG (2008) Urinary stress incontinence in women. N Engl J Med 358:1029–1036
Pesce F (2004) Current management of stress urinary incontinence. BJU Int 94(S1):8–13
Torrente Y, Belicchi M, Marchesi C, Dantona G, Cogiamanian F, Pisati F et al (2007) Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant 16:563–577
Menasché P, Hagège AA, Scorsin M, Pouzet B, Desnos M, Duboc D et al (2001) Myoblast transplantation for heart failure. Lancet 357:279–280
Strasser H, Berjukow S, Marksteiner R, Margreiter E, Hering S, Bartsch G et al (2004) Stem cell therapy for urinary stress incontinence. Exp Gerontol 39:1259–1265
Praud C, Sebe P, Biérinx AS, Sebille A (2007) Improvement of urethral sphincter deficiency in female rats following autologous skeletal muscle myoblasts grafting. Cell Transplant 16:741–749
Peyromaure M, Sèbe P, Praud C, DeRocle G, Potin N, Pincet C et al (2004) Fate of implanted syngenic muscle precursor cells in striated urethral sphincter of female rats: perspectives for treatment of urinary incontinence. Urology 64:1037–1041
Yiou R, Yoo JJ, Atala A (2003) Restoration of functional motor units in a rat model of sphincter injury by muscle progenitor cell autografts. Transplantation 76:1053–1060
Yokoyama T, Yoshimura N, Dhir R, Qu Z, Fraser MO, Kumon H et al (2001) Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol 165:271–276
Chancellor MB, Yokoyama T, Tirney S, Mattes CE, Ozawa H, Yoshimura N et al (2000) Preliminary results of myoblasts injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractibility. Neurourol Urodyn 19:279–287
Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R et al (2008) 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 19:881–883
Amarenco G, Arnould B, Carita P, Haab F, Labat JJ, Richard F (2003) European psychometric validation of the CONTILIFE: a quality of life questionnaire for urinary incontinence. Eur Urol 43:391–404
Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B et al (2005) Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther 12:1130–1141
Conflicts of interest
Christelle Doucet is employed by Celogos. Christian Pinset is shareholder of Celogos and the inventor and the owner of the patent protecting the process of manufacturing cells. Sixtina Gil Diez de Medina received payment from HRA Pharma, a firm that holds shares in CELOGOS capital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Philippe Sèbe and Christelle Doucet have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sèbe, P., Doucet, C., Cornu, JN. et al. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: a prospective study. Int Urogynecol J 22, 183–189 (2011). https://doi.org/10.1007/s00192-010-1255-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-010-1255-5