Abstract
Ergot alkaloids are indole derivatives produced by a wide range of fungi, being considered medically important because of their significant effect on the central nervous system of mammals, due to their structural similarity to neurotransmitters. They are also considered mycotoxins due to the severe toxic effects of ergot-contaminated grains on human and animal health. This chapter summarizes different aspects of ergot alkaloids concerning their chemistry, biosynthesis, and bioactivity, discussing the pharmacological activity as well as some important aspects related to their toxicity, occurrence, and regulations. Finally, an overview of analytical methods for the determination of ergot alkaloids is included, whereby high-performance liquid chromatography coupled to fluorescence or mass spectrometer detection are the most widely used methods, although other techniques such as capillary electrophoresis or immunoassays have also been reported.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Ergot alkaloids
- Ergot alkaloid chemistry
- Fungi
- Biosynthesis
- Pharmaceutical properties
- Toxicology
- Mycotoxins
- LC–MS analysis
1 Introduction
Ergot alkaloids (EAs) are nitrogen-containing natural products belonging to indole alkaloids. They are secondary metabolites produced by a wide range of fungi of the families Clavicipitaceae (e.g., Claviceps ) and Trichocomaceae (including Aspergillus and Penicillium), which parasitize the seed heads of living plants at the time of flowering. Fungal infections are most common in rye and triticale that have open florets, but wheat and other small grains are also potential hosts together with grasses infected with endophytes. The fungal hyphae invade the ovule of the host grass and colonize the whole ovary. Around 3–4 weeks after infection, the wintering body of the fungus becomes visible and replaces the developing grain or seed. These alkaloid-containing wintering bodies, named sclerotia or ergot (derived from old French word “argot,” meaning cock’s spur since grains colonized with Claviceps often resemble the spurs on the legs of a rooster), are dark, crescent shaped, and protruding from the regular grains and represent the final stage of the disease [1]. The term ergot refers also to the common name for this disease of cereals and grains caused by these fungi. The sclerotia are harvested together with the cereals or grass and can contaminate cereal-based food and feed products with EAs, being especially important in seasons with heavy rainfall and wet soils [2]. EAs have also been identified in plants of the families Convolvulaceae, Poaceae, and Polygalaceae, in which recently investigations suggest that these compounds are produced by plant-associated fungi [3].
This family of indole derivatives with diverse structures is chemically very complex, showing different biological and pharmacological activities. They can be classified as micotoxins, which have been responsible for historic episodes of mass poisoning in the Middle Ages due to the consumption of grains, flour, or bread contaminated with EAs. Historic events associated with ergot poisoning include the first Crusade (1095), the cause of symptoms associated with witchcraft surrounding the Salem witch trials (1690s), and the interrupted Russian campaign (1720-22) under Peter the Great against the Ottoman Empire [4]. Ergot poisoning in humans and animals is known as ergotism. This disease, one of the oldest known, may cause strange hallucinations, the feeling of itchy and burning skin, gangrene, loss of hands and feet, and even death.
In modern times, the cause of the disease is well understood and improvements in agricultural practices and milling techniques (grading, sieving, and sorting) have removed the risk of severe epidemic outbreaks of ergotism . Mechanical means and other conventional techniques of industrial grain processing like dockage removing, separators, air screens, density separators, color sorting, and their combinations can significantly reduce EA levels in grain. Cleaning procedures become less reliable when the intact ergot sclerotia break into smaller fragments during transport or when dry climatic conditions produce fungal sclerotia which are similar in size to the grain [2]. Also, food processing, such as baking/pancake preparation with EA-contaminated flours, can produce a reduction of EA levels in the final product, as well as the effect of cooking and drying of some products like noodles or spaghetti. A small part of the EA loss is due to leaking into the cooking water, implying that EAs are to some extent heat sensitive, but depending on the EA content of the raw material, significant amounts of EAs may still remain in the final product [5].
In relation to animals, consumption of feedstuffs contaminated with EAs has a broad impact on many different physiological mechanisms that alters the homeostasis of livestock. These alterations on homeostasis cause an increased sensitivity in livestock to environment perturbations, which involve a reduced production and economic losses in livestock producers around the world [6]. Sclerotia can be removed from cereal grains by standard seed-cleaning techniques. Since the EAs are heat sensitive, they may be reduced during compound feed manufacture, where pellets generally leave the die at temperatures ranging from 60 °C to 95 °C [5]. Other strategies to reduce the risk of ergot infection in most cereal crops include changes in crop rotation, deeper plowing, application of fungicides, breeding for disease resistance, and crossing of natural rye with hybrid rye [1].
Following a request from the European Commission, in 2012, the Panel on Contaminants in the Food Chain (CONTAM Panel) was asked to deliver a scientific opinion on the risks to human and animal health related to the presence of EAs in food and feed [5]. Since the publication in 2005 of the European Food Safety Authority (EFSA) opinion on EAs in feed [7], no relevant information was identified that would alter the previous risk assessment. Estimates of exposure based on example diets and levels of EAs in cereal grains reported in Europe would suggest that under normal conditions the risk of toxicosis in livestock is low. Furthermore, the risk of ergotism in livestock as a result of consuming contaminated cereal grains, or compound feeds manufactured from them, is reduced where appropriate seed cleaning is carried out. In relation to humans, the CONTAM Panel performed estimates of both chronic and acute exposure for various age groups across European countries, concluding that while the available data do not indicate a concern for any population subgroup, the dietary exposure estimates are related to a limited number of food groups and a possible unknown contribution from other foods cannot be discounted. As recommendation, they suggest that efforts should continue to collect analytical data on occurrence of EAs in relevant food and feed commodities.
On the other hand, EAs present important applications in medicine, being included with both natural and semisynthetic origins, in different formulations. Their therapeutic potential was already recognized in the Middle Ages, using Claviceps sclerotia by midwives in support of childbirth or to induce abortion, according to medieval texts [1]. Their broad physiological effects are mainly based on their interactions with neurotransmitter receptors on the cells. Together with their traditional uses (prolactin inhibition, Parkinsonism, cerebrovascular insufficiency, venous insufficiency, thrombosis, migraine, uterine stimulation), new therapeutic applications have emerged (e.g., against schizophrenia, among others). EAs are also of social relevance because the semisynthetic alkaloid, lysergic acid diethylamide (LSD), is an illicit drug considered one of the most potent hallucinogen. Thus, over the years, EAs and derivatives have been synthesized by artificial parasitic cultivation on rye and saprophytic growth techniques [8, 9]. Today this uneconomic method has been replaced by submerged fermentation. Even after a century of research on EAs, the search still continues for new, more potent, and more selective EA derivatives.
Considering all of the abovementioned aspects, the developments in instrumental techniques in the last decades have led to separate and measure individual ergot compounds and their isomers, being of special interest in the monitoring and regulation of the contamination of cereal-based foods. There is a requirement therefore to measure EA in ergot sclerotia, infected cereals, forage grasses, processed foods, pharmaceutical preparations, illicit preparations, and body fluids and organs [10].
2 Chemistry
In 1920, Stoll isolated the first pure EA, ergotamine [11], and since then, more than 80 different EAs have been isolated, mainly from Claviceps spp. (over 70 EAs).
Natural EAs share a common tetracyclic ergoline ring system methylated on nitrogen N6 and substituted on C8 (Fig. 1). Most EAs have a double bond in position C8, C9 (Δ8,9-ergolenes) or in position C9, C10 (Δ9,10-ergolenes), with asymmetric centers at C5-C10 or C5-C8, respectively.
The configurations resulting from those centers of chirality are depicted in Fig. 2. The hydrogen at C5 has always β-configuration and only EA synthesized or prepared by isomerization of natural EAs can have α-configuration at C5. It reflects the derivation of these alkaloids from l-tryptophan (the amino acid precursor of the indole ring). The hydrogen at C10 (not existing in Δ9,10-ergolenes) can have α-configuration (trans-position relative to the hydrogen atom on C5) or β-configuration (cis-position relative to the hydrogen atom on C5). The stereochemistry C5-C10 has been represented by the use of roman number, I for trans-position and II for cis-position. However, this nomenclature has sometimes been misused: as example agroclavine-I that has C5-C10 cis-position [12]. Δ9,10-Ergolenes undergo epimerization, with respect to the center of symmetry at C8, resulting in rotating isomers: the left rotating (8R configuration) or β-Δ9,10-ergolenes and the right rotating (8S configuration) or α-Δ9,10-isoergolenes epimers [2, 13].
EAs are classified into four biogenetically related classes based on the substitutions at C8 and the structure of D-ring (Fig. 1) in the tetracyclic ergoline ring system [13–15]: clavine-type alkaloids, simple lysergic acid derivatives or ergoamides, ergopeptines, and ergopeptams.
2.1 Clavine-Type Alkaloids
Clavine-type alkaloids or clavines consist merely of the ergoline ring or its tricyclic precursors. They have been isolated from various fungal strains, especially in the family Trichocomaceae. Some of these metabolites are primary products in EA biosynthetic pathway and they can be precursors of other EAs [12, 16]. According to their structures, clavine-type alkaloids can be classified into six different groups: 6,7-secoergolenes, 6,7-secoergolines, Δ8,9-ergolenes, Δ9,10-ergolenes, ergolines, and alkaloids with modified ergoline structure [13]. Representative structures of clavine-type alkaloids are given in Fig. 3.
6,7-Secoergolenes and 6,7-secoergolines are tricyclic seco derivatives and show a structure in which the D-ring of ergoline system is not closed. 6,7-Secoergolenes have a double bond in position C8, C9, while 6,7-secoergolines have a saturated D-ring. Some important naturally occurring representatives of 6,7-secoergolenes are chanoclavine-I and its two isomers, chanoclavine-II and isochanoclavine-I. Dihydrochanoclavine-I and isodihydrochanoclavine-I are representatives of 6,7-secoergolines.
Clavine metabolites with a closed D-ring include Δ8,9-ergolenes (e.g., agroclavine and elymoclavine) that contain a double bond in position C8-C9, Δ9,10-ergolenes (e.g., lysergol and penniclavine) with a double bond in position C9-C10, and ergolines (e.g., festuclavine and fumigaclavine A) possessing a saturated D-ring.
Finally, alkaloids with modified ergoline structure have been also found in nature, but very few of them are produced by Claviceps spp. Paspaclavine, cycloclavine, and rugulovasine A are some of EAs included in this group.
2.2 Simple Lysergic Acid Derivatives or Ergoamides
Ergoamides are primary or secondary carbon acid amides of d-lysergic acid . In this group, paspalic acid and its derivatives (e.g., 10-hydroxy-trans-paspalamide and 10-hydroxy-cis-paspalamide) are also included, as d-lysergic acid is biosynthesized from the isomerization of paspalic acid.
The first simple lysergic acid derivative identified was ergometrine (also called ergonovine or ergobasine), and in its structure, d-lysergic acid is amidated with 2-aminopropanol. Ergometrine and its semisynthetic derivatives, methylergometrine (d-lysergic acid amidated with 2-aminobutanol) and methysergide (d-lysergic acid amidated with 2-aminobutanol and methylated on nitrogen N1), are the most important ergoamides.
Ergoamide derivatives of lysergic acid ( 8R-epimers ) are indicated by the suffix -ine (e.g., ergometrine); those that are derivatives of isolysergic acid (8S-epimers) are indicated by the suffix -inine (e.g., ergometrinine). Representative structures of ergoamides are given in Fig. 4.
2.3 Ergopeptines
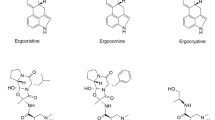
Ergopeptines or ergopeptides are d-lysergic acid peptides containing lysergic acid and three amino acids in their structure (Fig. 5). They are the most widely spread natural peptide-type EAs.
The cyclic part of the tripeptide results from the reaction of an α-hydroxyamino acid adjacent to lysergic acid with the carboxyl group of proline. Ergopeptines have l-proline at AA3 and the variability of ergopeptines is therefore given by the nature of AA1 and AA2. However, other configurations at AA3 have been described, such as ergobalansine and its corresponding epimer, ergobalansinine, where l-proline is substituted by l-alanine [17].
Ergopeptines can be classified into four subgroups based on the different amino acids AA1: ergotamines (AA1: alanine), ergoxines (AA1: α-aminobutyric acid), ergotoxines (AA1: valine), and ergoannines (AA1: isoleucine). Ergopeptines also undergo epimerization, and the isomers derived from d-lysergic acid and d-isolysergic acid are characterized by the suffix -ine and -inine, respectively. This EA class also includes derivatives with saturated D-ring (e.g., dihydroergotamine, dihydrocristine, or dihydroergosine) and they are subgrouped correspondingly.
2.4 Ergopeptams
The ergopeptams are tripeptidic non-cyclol EAs. Their structure is similar to that of ergopeptines except that l-proline at AA3 is exchanged by d-proline, and the tripeptide chain is a non-cyclol lactam (Fig. 6). It was suggested that ergopeptams are formed as a result of competitive epimerization at the last stage of the cyclopeptide biosynthesis [8].
The first ergopeptam isolated was ergocristam. This class of peptide-type alkaloids was originally found in small amounts in certain strains accompanying the ergopeptines. In addition, the probability of the existence of ergopeptams decreases with the decreasing volume of radical R1, which is explained by its high lability [8]. Later, ergopeptams have been found to predominate in some infected wild grasses from Norway [18].
Similar to ergopeptines, ergopeptams can be classified into four groups based on the AA1 type: ergotamams (AA1: alanine), ergoxams (AA1: α-aminobutyric acid), ergotoxams (AA1: valine), and ergoannams (AA1: isoleucine). Figure 6 shows the representative structures of ergopeptams. No isomers derived from d-isolysergic acid have been reported for ergopeptams, which is related to its high lability because these compounds readily decompose into simpler derivatives in the presence of bases [8]. However, the established nomenclature suggests that these hypothetical 8S-isomers are characterized by the suffix -inam and should be included into the corresponding groups.
2.5 Physicochemical Properties
The variability of EA compounds involves a wide range of physicochemical properties, although most EAs appear as colorless crystals that are readily soluble in various organic solvents, like acetonitrile, methanol, or organic/buffer mixtures [5, 19], and insoluble or only slightly soluble in water [9]. Moreover, EAs are neutral at higher pH values and positively charged at N6 in acidic solutions.
The most important characteristic of Δ9,10-ergolenes is their rapid epimerization with respect to the center of symmetry at C8, resulting in the right rotating (8S) and left rotating (8R) isomers. These EAs can epimerize from R to S forms and vice versa, especially in aqueous acidic or alkaline solutions [8], via enolization at C8 (Fig. 7), as C9-C10 double bond permits to form a large conjugated π-electron system [20, 21].
Epimerization scheme of ergot alkaloids (Adapted from Ref. [21])
The ratio of epimerization depends mainly on the nature of the amide substituent [22]; however, this epimerization is enhanced through exposure to strong light, prolonged storage, or contact with some solvents at high or low pH. The C8 epimers show different physicochemical properties, such as basicity and solubility [22]. Moreover, protonated pKa values, given by the N6 nitrogen, showed differences between both C8-epimers. They ranged between 5.5 (ergocristine) and 6.0 (ergometrine) for 8R-epimer, while pKa values of 8S-epimers ranged between 4.8 (ergocorninine) and 6.2 (ergometrinine) [11, 23].
Δ9,10-Ergolenes show natural fluorescence with excitation wavelengths of 254, 313, 325, or 366 nm and emission wavelength of 445 nm [24]. On the contrary, the rest of EAs without Δ9,10 double bond do not show native fluorescence, but they show ultraviolet absorption at 280 nm [13].
2.6 Stability
Most EAs melt with decomposition at high temperatures. Moreover, EAs are known to show high sensitivity to light, which leads not only to epimerization but also degradation [8, 22]. Δ9,10-Ergolenes add one molecule of water to C10 carbon of the lysergic acid moiety upon illumination, especially on irradiation with UV light, and acid catalysis. The reaction leads to a mixture of two diastereomers called lumi-derivatives: lumi-I-derivatives (e.g., lumi-ergotamine-I) or 10-α-hydroxy derivatives, with hydroxyl group being trans to the hydrogen atom in position 5 and lumi-II-derivatives (e.g., lumi-ergotamine-I) that have a cis-junction of rings C and D [22]. These lumi-derivatives are characterized by loss of fluorescence properties and lack of biological activity [25].
In addition, other degradation products called aci-derivatives are formed by acid-catalyzed isomerization of the 2′-carbon of the tricyclic peptide moiety of the ergopeptines [26, 27]. However, the aci-derivatives are formed at much slower rates than those for C8-epimers [27].
Stability of EAs is also affected by the epimerization process during storage, handling, and analysis. In order to minimize changes in the natural ratio of epimers and to improve the understanding of the factors affecting the epimerization, some studies have tried to explain the process using complex simulations [28]. However, the epimerization process is not yet well understood [10]. Some authors proposed different recommendations to avoid significant epimerization . EA standards are best stored below −20 °C in non-protic solvents or in the form of thin dry films, but they also have shown to be stable, in terms of epimerization and degradation, in chloroform at room temperature [21], over a period in excess of 12 months [29]. Solutions of EA standards in ethanol containing tartaric acid, ethylene glycol, 2-propanediol, and tartaric acid are also recommended [30].
The lability of EAs to decomposition must be taken into account during the technological treatments and the chemical and analytical handling of EAs in the development of stable medicinal forms, and in the storage of raw materials, semiproducts, parent substances, and ready-to-use medicinal preparations [8].
3 Biosynthesis
As it was shown in the previous sections, EAs are a large group of metabolites, showing very different structures. However, all of them share the first biosynthetic steps, consisting of tetracyclic ergoline ring system formation, except the simplest tricyclic compounds as 6,7-secoergolenes and 6,7-secoergolines. Biosynthesis of EAs has been investigated in detail for many years [1, 3, 31, 32], and although most of the biosynthetic pathways have been elucidated from the 1950s, some step remains largely unelucidated [33].
The variability of EAs and biosynthetic pathway depends largely on the EA producer fungi. As example, Clavicipitaceae typically produces either lysergic acid-derived EAs or dihydroergot alkaloids and several members of the Trichocomaceae produce alkaloids derived from festuclavine.
3.1 Ergot Alkaloid Producers
EAs are produced mainly by two orders of fungi , Eurotiales and Hypocreales, belonging to the phylum Ascomycota. Within the Hypocreales, EAs are associated exclusively with Clavicipitaceae family, although not all the members of the Clavicipitaceae produce EAs [31]. The fungal genera so far known to produce EAs in Clavicipitaceae family are Claviceps spp. [34, 35], Epichloë spp. (including their close relatives, the Neotyphodium spp.), Atkinsonella spp., Balansia spp., Periglandula spp. [36, 37], and Metarhizium spp. [36]. Within the Eurotiales order, the genera Aspergillus [38] and Penicillium [39] in Trichocomaceae family are also EA producers. Recently, it was demonstrated that Onygenales order belonging also to phylum Ascomycota, in particular Arthroderma genera (Trichophyton), is also related with EA production [40]. Moreover, EAs producing fungi occupy different ecological niches. Claviceps spp. are plant parasites and biotrophic symbionts, while Aspergillus fumigatus is an opportunistic pathogen of mammals [31, 41].
It was reported that three families of plants, i.e., Convolvulaceae [42, 43], Poaceae [44], and Polygalaceae [45], also produce EAs. For a long time, it was believed that horizontal gene transfer from fungi to higher plants had taken place during the evolutionary process. However, further investigations revealed that, at least in Poaceae and Convolvulaceae, the plant-associated fungi are likely responsible for EA production, since the treatment of these plants with fungicides led to elimination of associated fungus and simultaneous loss of alkaloids from the plant [3, 41, 46]. Fungi and plants form mutualistic symbiosis that consists of production of bioactive EAs by fungi to protect the host plant from insect, vertebrate herbivores and root nematodes, enhancements of drought tolerance and nutrient status, and improved growth, particularly of the root, while the fungi benefit from protected niche, nutrition, and dissemination from the plant [3, 41, 47]. The fungal symbionts are vertically transmitted through seed of the host plant, though the mechanism of how the fungi spread in the respective host plant remains unclear [41, 44, 48].
Claviceps purpurea is the most important of all the EA producers and it is otherwise known as the ergot fungus of rye and related grasses. In C. purpurea, EAs are found in the sclerotia, and their spectra vary strongly between different C. purpurea strains [49], although the main produced compounds are ergocristine, ergotamine, ergocornine, α- and β-ergocryptine, ergometrine, ergosine, ergocristinine, ergotaminine, ergocorninine, α- and β-ergocryptinine, ergometrinine, and ergosinine [5, 50–53]. Moreover, C. purpurea is morphologically a highly variable species with respect to sclerotial length and shape, color of the stomata, and conidial size and shape [54, 55].
Other important members of Claviceps spp. are C. africana, in which dihydroergosine is the principal EA found in its sclerotia [56]; C. fusiformis, related to agroclavine, elymoclavine, chanoclavine, penniclavine, and setoclavine production [57], but no d-lysergic acid derivatives [35, 58]; and C. gigantea [59], C. paspali, and C. hirtella, which are also mainly clavine producers [13].
On the other hand, A. fumigatus and Penicillium strains, including P. roqueforti, P. verrucosum, and P. commune, are fungi also associated to the production to clavines. Aspergillus has been related to the production of fumigaclavines A, B, and C, while Penicillium has been related to the production of fumigaclavines A and B, but not C [3].
3.2 Ergoline Ring Formation
The biosynthesis of the ergoline ring begins with the prenylation of l-tryptophan at position C4 with dimethylallyl diphosphate (DMAPP) as prenyl donor. This reaction is catalyzed by the prenyltransferase 4-dimethylallyltryptophan synthase (DMATS), also called FgaPT2 in A. fumigatus [33, 60–62], leading to the formation of 4-l-dimethylallyltryptophan (DMAT). The DMAT-forming reaction delivers the carbon skeleton of the ergoline ring system. After this first step, all enzymatic steps concern modifications and rearrangements leading to formation of rings C and D. The next step involves a N-methylation of the amino nitrogen of DMAT in the presence of S-adenosylmethionine (AdoMet) and is catalyzed by a 4-dimethylallyltryptophan N-methyltransferase EasF (FgaMT in A. fumigatus). The result is the formation of 4-dimethylallyl-l-abrine (4-DMA-l-abrine).
Chanoclavine-I was the next detected intermediate found in the biosynthesis of EAs [41, 63, 64]. Its accumulation is observed in many EA producers, and in some cases at relatively high concentrations [65]. Chanoclavine-I was obtained by decarboxylation and closure of ring C. This step would include at least three reactions, i.e., decarboxylation, cyclization, and hydroxylation [3], and the enzymes flavin adenine dinucleotide (FAD)-dependent oxidoreductase EasE (also called ccsA and FgaOx1 in A. fumigatus) and the catalase EasC (known as FgaCat in A. fumigatus) are needed [66, 67].
The next step is the oxidation of the hydroxyl group of chanoclavine-I to yield chanoclavine-I aldehyde. This reaction is catalyzed by the short-chain dehydrogenase/reductase (SDR) EasD (FgaDH in A. fumigatus). Chanoclavine-I aldehyde is the last shared intermediate and represents the branch point of the biosynthetic pathway of several fungi to produce agroclavine and festuclavine (and its 8S-stereoisomer, pyroclavine) [41, 65]. Agroclavine is usually the key intermediate in the formation of more complex EAs in Clavicipitaceae fungi, while festuclavine and pyroclavine are the substrates in Trichocomaceae fungi. The branch point is mainly controlled by the old yellow enzyme EasA (also called FgaOx3 in A. fumigatus and FgaOx3pc in P. commune) [68–70], and the divergence between fungi depends on the activities of different versions of this enzyme. The version of EasA (FgaOx3) found in A. fumigatus reduces the C8-C9 double bond in chanoclavine-I aldehyde to give the cyclized iminium intermediate in ring D formation [41, 71]. On the other hand, the versions of EasA found in most EA producers in the Clavicipitaceae do not permanently reduce the double bond; instead, these enzymes promote isomerization around the double bond. Finally, for the formation of agroclavine in the Clavicipitaceae or festuclavine (and its 8S-stereoisomer pyroclavine) in the Trichocomaceae, enzyme EasG (also called FgaFS in A. fumigatus and FgaFSpc in P. commune) is required to reduce the iminium ion [65, 72]. Moreover, versions of EasG found in A. fumigatus and P. commune differ in the proportion of the stereoisomers (festuclavine and pyroclavine). The formation of festuclavine was significantly higher in A. fumigatus, while P. commune produced higher concentration of pyroclavine [70].
Further investigation demonstrated that EasA was not necessary for the conversion of chanoclavine-I aldehyde to agroclavine in C. purpurea, at least in vitro experiments, and EasG was sufficient for the formation of agroclavine via a nonenzymatic adduct with reduced glutathione [73].
The mentioned steps of ergoline ring formation are shown in Fig. 8.
3.3 Fumigaclavine Formation
Festuclavine and its 8S-stereoisomer pyroclavine are the main substrates in the formation of fumigaclavines in Trichocomaceae fungi (Fig. 8). Both metabolites lead the formation of isomers 8R and 8S fumigaclavine B via a hydroxylation in A. fumigatus and P. commune, respectively. This reaction is probably catalyzed by the monooxygenase FgaP450-2 in A. fumigatus and its analogue FgaP450-2PC in P. commune [3]. The next step is the formation of fumigaclavine A catalyzed by the acetyltransferase FgaAT (FgaATPC) in the presence of acetyl-CoA [74]. Finally, fumigaclavine C is obtained by catalysis of the prenyltransferase FgaPT1 only in A. fumigatus [33]. Figure 9 shows the fumigaclavine biosynthetic pathway.
Fumiclavine biosynthetic pathway (Adapted from Ref. [33])
3.4 d-Lysergic Acid Formation
Although the formation of d-lysergic acid remains largely unelucidated, two important intermediates have been identified: elymoclavine and paspalic acid . It was proposed that the conversion of agroclavine to paspalic acid via elymoclavine involves two oxidation steps (2-electron and 4-electron oxidation) [31]. The C8-linked methyl group can be oxidized by the action of a cytochrome P450 monooxygenase CloA [75]. The different isoforms of CloA determine the level of oxidation, i.e., CloA of C. fusiformis catalyzes the 2-electron oxidation of agroclavine to elymoclavine, whereas CloA of C. purpurea and many other Clavicipitaceae catalyze a 6-electron oxidation of agroclavine to paspalic acid [35, 65]. Subsequently, paspalic acid is isomerized enzymatic or spontaneously to d-lysergic acid [76], which serves as the acyl component of ergoamides, ergopeptines, and ergopeptams. The biosynthetic pathway of d-lysergic acid is show in Fig. 10.
3.5 Formation of Ergoamides, Ergopeptines, and Ergopeptams
Most EA-producing Clavicipitaceae fungi produce more complex metabolites such ergoamides, ergopeptines, or ergopeptams. The diversity among them arises via an interesting combinatorial system involving two different pairs of peptide synthetases [65].
Ergopeptams are intermediate metabolites in the biosynthetic pathway of ergopeptines . Their formation is controlled by a nonribosomal peptide synthetase (NRPS) enzyme complex, which contains d-lysergyl peptide synthetases 1 and 2 (LPS1 and LPS2) [33, 41]. Firstly, d-lysergic acid is activated by LPS2 and subsequently LPS1 catalyzes its progressive elongation to the d-lysergyl mono-, di-, and tripeptide thioester intermediates, to get finally the d-lysergyl tripeptide lactam or ergopeptam [65, 77]. The next step is catalyzed by mono-oxygenase easH yielding an intermediate, which undergoes spontaneous cyclization that leads to the formation of ergopeptines [3].
Ergoamides , such as ergometrine, are also formed from d-lysergic acid. Ergometrine arises by interaction of LPS2 and LPS3 (a monomodular peptide synthetase that recognizes and activates l-Alanine) enzymes [78]. Figure 11 shows the biosynthetic pathway of ergoamides, ergopeptams, and ergopeptines.
Dihydroergot alkaloids , such as dihydroergosine, can also arise from festuclavine in C. africana and C. gigantea (Fig. 12). The CloA present in these fungi catalyzes the oxidation of festuclavine to dihydrolysergol in C. gigantea or to dihydrolysergic acid via dihydrolysergol in C. africana. Dihydrolysergic acid can follow the same biosynthetic pathway as that of other members of the Clavicipitaceae to produce dihydroergosine via LPS1, LPS2, and easH [4, 65, 79, 80].
Dihydroergot alkaloid biosynthetic pathway in C. africana and C. gigantean (Adapted from Ref. [65])
4 Bioactivity
EAs are particularly important for possessing a potent bioactivity. However, it is necessary to distinguish their valuable pharmacological properties and their toxic effects.
4.1 Pharmacological Activity
EAs have been reported to produce several effects including direct peripheral effects as uterotonic action or vasoconstriction, indirect peripheral effects as serotonin antagonism or adrenergic blockade, and central nervous effect as induction of hypothermia and emesis or control of the secretion of pituitary hormones [1, 81].
The effects of EAs are mainly responses mediated by neurotransmitters as noradrenaline, serotonin, or dopamine (5-hydroxytryptamine, 5-HT). The structure of these neurotransmitters fits well onto the d-lysergic acid ring structure (Fig. 13) [1]. Natural EAs possess the above-described effects to a greater or lesser degree, depending of the substituents attached to the carboxyl group at C8 of d-lysergic acid ring system that define agonistic or antagonistic mode (or a dual role as partial-agonist and antagonist) and the intensity of the interaction with receptors for these neurotransmitters [1, 61, 82, 83]. Peptide ergot alkaloids usually have high affinity for α-adrenergic receptors, while derivatives of d-lysergic acid amidated with small amino alcohols show high affinity for serotonin receptors [61]. Moreover, the biological activity of EAs depends largely on their configuration and epimers 8R and 8S differ in biological properties; 8R-isomers are biologically active, whereas the 8S-isomers are inactive [2].
Structural analogy between the tetracyclic ergoline system and dopamine, noradrenaline, and serotonin neurotransmitters (Adapted from Ref. [61])
The uterine contraction is the most known pharmacological effect . EAs as ergotamine and ergometrine were officially used for the first time in obstetrics to treat postpartum hemorrhage and to accelerate uterine involution in the puerperium [8, 81]. Later, it was demonstrated that although all natural EAs have qualitatively the same effects on uterus, ergometrine is most active and less toxic than ergotamine. So, ergometrine and its semisynthetic derivative methylergometrine replaced other EAs in obstetric applications [9]. Other important direct peripheral effect of EAs is their vasoconstrictor effect. The best-known drug of this type is ergotamine that has been widely used as migraine treatment due to its tonifying effect on the smooth muscle of the blood vessels [81]. However, methysergide, a semisynthetic EA and a serotonin antagonist, unlike ergotamine, has also been used in the treatment of migraine [84].
The indirect peripheral (humoral) effects are manifested in an adrenaline and noradrenaline antagonism, as well as serotonin antagonism. EAs are used in internal medicine as sympathetic agents due to their adrenolytic effect. EAs also present very diverse effects on the central nervous system, such as the reduction of the activity of the vasomotor center and the stimulation of sympathetic structures of the diencephalon, particularly the hypothalamus [81, 85].
The most important effects of natural ergopeptines are mainly their vasoconstrictive and sympatholytic–adrenolytic actions due to their high affinity for adrenergic receptors [1, 86]. However, slight modifications in the chemical structures produce changes in their biological activity. Dihydroergopeptines such as dihydroergotamine have an increased adrenolytic effect and reduced vasoconstrictive effect; thus, it is preferentially used for the treatment of migraine instead of ergotamine [1, 87–89]. The EAs are administered separately and in numerous complex compositions [8] as dihydroergotoxin, a mixture of three dihydroergopeptines (dihydroergocornine, dihydroergocristine, and dihydroergocryptine) that is used for the treatment of diseases associated with circulatory problems as high blood pressure and cerebral dysfunctions [1, 90, 91].
Clavine-type alkaloids have much less adrenolytic activity and show strong anti-serotoninergic action due to elevated affinity for serotonin (5-HT) receptors [61]. EAs, especially clavine-type alkaloids, possess activity inhibiting the growth of certain mammary tumors in animals and also in humans by blocking the release of prolactin from the anterior pituitary gland. In particular, clavine-type alkaloids with a C8 methyl group as festuclavine and agroclavine were shown to exhibit some inhibitory activity against cell proliferation in the L5178y mouse lymphoma system [92–94]. Recently, six EAs (agroclavine, ergosterol, ergocornine, ergotamine, dihydroergocristine, and 1-propylagroclavine tartrate) were investigated for their inhibitory activity toward a panel of cell lines of different tumor origins (ovarian carcinoma, brain tumor, prostate cancer, lung cancer, melanoma, colon cancer, renal carcinoma, breast cancer, or leukemia). 1-Propylagroclavine tartrate showed the strongest effect on tumor cells, especially against leukemia cell lines [95].
Serotonin agonist in the brain is also thought to be a key factor in hallucinogenic activities [31]. However, none of the naturally occurring EAs have typical hallucinogenic properties; such properties are confined to a number of semisynthetic derivatives of lysergic acid, as LSD [96], which has been used for psychedelic recreation [31].
Many EAs, including ergocryptine, produce a more or less pronounced dopaminergic effect . Bromination of ergocryptine in the 2-position (2-bromo-ergocryptine) strongly increases dopamine agonist activity. 2-Bromo-ergocryptine is semisynthetic derivative that is used for treatment of hyperprolactinaemia. Bromocriptine was also used for treatment of advanced breast cancer [97] and Parkinson’s disease due to its high affinity to dopaminergic receptors [98].
Limited information is available on the metabolism of EAs. They are rapidly cleared from the blood and the tissues with a high first pass effect in the liver [99]. In contrast, their physiological effect persists for a longer period of time [2].
Despite the beneficial pharmacological properties, natural and semisynthetic EAs also possess serious and unpredictable side effects and high instability, reducing their medical applications and being replaced by synthetic analogs. As example, ergocristine, ergocryptine, and ergocornine, despite having a similar activity spectrum to ergotamine, present some toxic effects, which prevents them from achieving the same clinical significance [81].
4.2 Toxicity
Intoxications induced by EAs have been known for many centuries. The most severe and frequent epidemic of ergotism took place during the Middle Ages in Europe, where the disease was called Holy Fire or St. Anthony’s Fire . It was caused by eating rye bread contaminated with C. purpurea, resulting in gangrene of limbs, disturbances in the function of the central nervous system, and ultimately death [100].
There are two symptomatic forms of ergotism: gangrenous and convulsive . The two distinct types of ergotism may be considered as acute and chronic varieties. The gangrenous form is caused by the extreme vasoconstrive properties of some EAs, which results in restriction of the blood flow to parts of the body (ischemia) [31, 101]. As a result, tingling effects are felt in fingers and toes followed in many cases by dry gangrene of the limbs and eventually loss of the limbs [102, 103]. In the convulsive form, tingling is followed by neurotoxic symptoms such as hallucinations, delirium, and epileptic-type seizures [104].
Outbreaks of ergotism tended to happen after cold, wet winters followed by warm spring weather, and arose mainly in areas where rye was commonly eaten. Also, some outbreaks were caused by other types of grain contaminated with ergot [101]. The gangrenous type was mostly seen in France and other European countries west of the Rhine and the convulsive one in Germany and Scandinavia [100, 101]. The symptoms of EAs poisoning vary, probably depending on the particular profiles of alkaloids present in the contaminated food. Clavines are thought to contribute substantially to convulsive ergotism, while the ergopeptines are known to produce similar symptoms and also to cause gangrenous ergotism [31, 101]. Moreover, it was proposed that a deficiency in vitamin A could be a causative factor inducing convulsive ergotism [105] and that EAs present at high concentrations in ergots could cause convulsive ergotism at a circulating concentration insufficient to produce peripheral ischemia [101].
In the late twentieth century, human poisoning from ergot was reported in France [106], India [107], and Ethiopia [108]; and the last recorded outbreak of gangrenous ergotism that occurred in the Arsi Zone (Ethiopia 2001) was attributed to the ingestion of barley containing ergotized wild oats [109]. While nowadays human poisoning from ergot has become of less concern, mainly owing to cleaning procedures at mills, EA contamination remains an important veterinary problem [2, 110]. There are numerous reports of poisoning of farm animals by ergot-contaminated feed [111] and by endophyte-infected grasses [112, 113]. Moreover, gangrenous ergotism has also been reported among free-living moose and roe deer in Norway [102, 114].
The toxic side effects of EAs have been studied in more detail, especially with respect to biological functions beyond the receptor interactions. In vivo studies regarding acute toxicity lead to different LD50 (lethal dose, 50 %) values depending on the used animal species, application form, and ergot alkaloid. Griffith et al. [115] reported a series of LD50 values (ranging between 0.9 and 275 mg/kg body weight) determined for several naturally occurring and semisynthetic EAs by subcutaneous and oral exposure in mouse, rat, and rabbit, demonstrating that rabbit is the most susceptible (LD50 values between 0.9 and 3.2 mg/kg). Sublethal acute exposure to EAs induces signs of neurotoxicity in mammals, including restlessness, miosis or mydriasis, muscular weakness, tremor, and rigidity. Moreover, tail gangrene was observed in rats after intraperitoneal injection of ergotoxin (ergocristine + ergocryptine + ergocornine). Recently, a case study of spontaneous tail necrosis in a rabbit colony has been reported, concluding that in order to avoid symptoms such as tail lesions and necrosis in younger rabbits, the mean EA content in such feeding must be controlled and kept as low as possible. However, controlled feeding trials under various conditions are necessary, to fully confirm that dietary EAs at concentration levels of around 500 μg/kg may act as a causative agent of mycotoxicosis in rabbits [116].
For in vitro experiments, only limited data are available for the single substances and their toxic effects on human cells [117]. Most data consist of receptor interaction analysis for single substances in dopamine overexpressing cells or tumor cells [118, 119]. Further experiments indicated a different toxic potential for peptide ergot alkaloids and lysergic acid amide alkaloids revealing that the cytotoxicity of EAs in human cell lines obviously depends on the type of alkaloids [120]. Mulac et al. described the apoptotic effect of some ergopeptines (especially ergocristine) [117]. Despite their lack of bioactivity, the 8S-epimers are considered to be mainly responsible for this effect, since they are preferentially accumulated in hepatic cell lines [121]. Therefore, it is important to consider both epimers when the EA contamination level has to be determined. Recently, it was demonstrated that ergometrine and its corresponding epimer ergometrinine exhibit cytotoxicity on animal smooth muscle cells, showing a positive correlation with alkaloid concentration [122].
EAs have a number of well-established effects on the reproductive process including prevention of pregnancy by interfering with implantation, embryotoxicity , developmental effects, and inhibition of lactation [115]. Several studies reported the effect of EAs reducing livestock reproductive performance with particular emphasis on the female gender [123, 124]. This is due to both direct and indirect effects of EA exposure through regional vasoconstriction and corresponding decreases in blood flow to reproductive tissues, decreases in dry matter intake, and/or increased body temperature [6]. Moreover, EAs inhibit milk production in humans, laboratory animals, and livestock animals [105, 125–127], effect linked by several authors to the decrease of prolactin (a protein hormone secreted mainly by the anterior pituitary gland) [5, 128]. However, later it was reported that decreased serum prolactin in lactating animals did not directly equate to decreased milk production [124, 129, 130]. 2-Bromo-ergocryptine, besides its indications in the treatment of Parkinson’s disease, prolactinoma, and hyperprolactinemia, has been described to inhibit lactation [131].
The high toxicity of EAs has led to frame these compounds as mycotoxins , and the interest in assessing the extent of the mycotoxins issue has increased in the last years. Despite improvements in agriculture practices and grain cleaning, generally it is only possible to remove up to 82 % of ergot by mechanical means with conventional grain cleaning equipment such as sieves and separators used during the harvesting process. So, different studies have demonstrated that EAs can still be present in cereal-based food and feed , sometimes in excessive amounts.
In this sense, De Saeger’s group at the University of Ghent carried out a large survey, in which 1,065 samples of cereals and cereal products intended for human consumption and animal feeding in Europe were analyzed. This study included rye, wheat, and multigrain-based food as well as rye, wheat, and triticale-based feed; and it was shown that 59 % of analyzed samples were contaminated with EAs at total levels ranging from 1 to 12,340 μg/kg [132]. Incidence of positive samples and the obtained alkaloid contents were in line with other published data. Storm et al. detected rye flour samples from Danish mills containing an average of 46 μg/kg of EAs with a maximum content of 234 μg/kg [133]; Crews et al. detected EAs in 25 of 28 samples, including all of 11 rye crispbreads that had up to 340 μg/kg [134]; and Müller et al. found EAs in 92 % of analyzed rye product samples with a maximum content of 739.7 μg/kg [19]. Reinhold et al. analyzed 500 food samples from Germany, and approximately 50 % were positive with a highest concentration of 1,063 μg/kg [135], whereas Masloff et al. reported twice higher maximum total EA content in surveys conducted in rye samples in Germany [136] and a maximum of 4,700 μg/kg was detected in Canadian wheat samples [137]. In most surveys, ergocryptine, ergocristine, and ergotamine including their C8-isomers were the most common EAs. Moreover, the main compound co-occurred with its corresponding 8S-epimers and in most cases the 8S-epimers had a higher maximum concentration compared with the main compounds [132].
Recently, Bryła et al. tested 65 samples, detecting EAs in 83 % of the tested rye grain, 94 % of rye flour, and 100 % of rye bran and flake samples. Measurable levels of alkaloids were found in the majority of the analyzed samples, particularly in rye flour, where a relatively high mass fraction of 1,215.5 μg/kg was found. Ergotamine, ergocornine, and ergosine were the most commonly found alkaloids, whereas ergometrinine and ergometrine were the least commonly found ones [138].
Regarding cereal-based infant foods, EAs also have been detected in 25 % of samples including oat, barley, soy and rice, and mixed-grain infant cereals from the Canadian retail marketplace. The incidence and overall mean level of EAs was highest in the barley-based samples (56 %, 18 μg/kg) [139].
Therefore, ergot infections of cereals are a severe problem of food security and consequently European Commission (EC), assisted by the EFSA , has established recommendations and directives that limit the maximum amount of ergot (i.e., sclerotia) that may be present in feed and food. So far, no regulatory limits for sclerotia have been set in the European Union (EU) for grain intended for human consumption. However, for intervention grain, a maximum level of 500 mg/kg has been set for ergot. For all feed containing unground cereals, the European Union Directive 2002/32/EC sets a maximum content of ergot of 1,000 mg/kg, whereas the maximum permissible level in the USA and Canada is 300 mg ergot per kg grain [2], and in Australia and New Zealand, a maximum level of 500 mg/kg of ergot sclerotia in cereal grains is applied [5].
There are currently no legislated limits for total EAs in food or feed; however, it is likely that limits for EAs will be included in future mycotoxin legislation [10]. Some countries have set guideline limits for EAs in cereals by deriving a limiting value for the maximum EA level from the maximum amount of sclerotia that may be present [140]. In this way, Germany and Switzerland have set limits for EAs in cereals for human consumption of 400–500 μg/kg and 100 μg/kg, respectively [140]. In Canada, the guideline limits for the total EA content in feed for poultry, swine, and chicks are 100, 600, and 9,000 μg/kg, respectively, whereas the guideline limits in animal feed in Uruguay is 450 μg/kg [5]. However, at the present, no country has established limits for individual EAs in food or feed.
According to EFSA, physical techniques to determine the contamination rate are often inaccurate as size, weight, and composition of the sclerotia may vary considerably [7]. In addition, sorting is impossible in processed feed materials, and there are significant variations of the total EA content within the sclerotia [141] and differences in the pattern of produced EAs. Hence, EFSA suggested replacing the physical methods by chemical analysis [7].
At present, the data on the toxicological properties of individual EAs are too limited to select individual marker toxins for monitoring the extent of contamination [7]. For that reason, EFSA has stated that more data on the variability of the EA patterns in European food and feed should be collected and that validated analytical methods for the quantification of EAs should be developed. In this way, the basic information needed for scientific risk assessment can be obtained and limits can be set for total and individual EAs via legislative regulations [104].
Recently, the European Commission has issued a recommendation to its member states to perform the monitoring on the presence of EAs in cereals and cereal products intended for animal feeding and in compound feed [142]. The European Commission has also requested the establishment of a relationship between the presence of EAs and the amount of sclerotia present, focusing the monitoring on the six main EAs, i.e., ergometrine, ergotamine, ergosine, ergocristine, ergocryptine, and ergocornine and their related epimers [142].
On the other hand, the interaction with neurotransmitter receptors could result in acute as well as longer-term effects, so EFSA has also established an acute reference dose (ARfD) of 1 μg/kg body weight and a tolerable daily intake (TDI) of 0.6 μg/kg body weight per day for EAs [5].
5 Determination of Ergot Alkaloids
Wide range of analytical methods has been proposed for determination of EAs from several matrices in pharmaceutical, forensic, and food areas. High-performance liquid chromatography (HPLC) and fluorescence detection (FLD) or tandem mass spectrometry (MS/MS) are the most widely used methods; however, other minor techniques including capillary electrophoresis (CE) or immunoassays are also available.
Regardless the analytical technique chosen for determination, a sample treatment is usually mandatory in order to remove interferences and pre-concentrate the analytes. Most of the reported analytical methods involve a liquid extraction followed by a clean-up by means of liquid–liquid extraction (LLE) or solid phase extraction (SPE) using different sorbents. Moreover, in order to compensate for the matrix effect, most methodologies include matrix-matched calibration.
Another aspect to be highlighted is the fact that during sample preparation and analysis, it is difficult to control the epimerization degree of EAs and both epimeric forms can interconvert. Attempts have been made to avoid this epimerization. Otherwise, it is necessary to determine both epimers and, alternatively, specify the EA content as a sum of both epimers for each EA [5].
The EFSA scientific opinion on EAs in food and feed [5] as well as a several reviews published during the last decade present a comprehensive overview of the different methodologies proposed for the determination of EAs, including sample preparation [2, 10, 24]. Thus, in the next sections, only most recent or relevant contributions in this field will be commented.
5.1 Capillary Electrophoresis
CE offers some advantages over liquid chromatography (LC), such as high efficiency, reduced analysis time, and low sample and reagent consumption, demonstrating its great potential for a wide range of compounds. However, depending on the analytes, sensitivity in CE needs to be improved, especially when UV detection is used.
CE analysis has been rarely applied for determination of EAs. Most of these works were developed in the 1990s, when CE was emerging as a promising analytical technique. Thus, Fanali et al. resolved for the first time a mixture of EA enantiomer derivatives (dl-terguride, dl-lisuride, dl-nicergoline, dl-isolysergic acid hydrazide and dl-1-methyl-10α-methoxy-dihydro-lysergol (dl-meluol), ergotamine, ergotaminine, ergometrine, and ergometrinine) using capillary zone electrophoresis (CZE), studying the effect of cyclodextrins as a chiral additive in the background electrolyte (BGE), on the migration time and the resolution [143]. CZE-UV was also used for simultaneous determination of ergotamine and caffeine in pharmaceutical dosage tablet formulations [144], ergovaline in the seeds of Festuca arundinacea (tall fescue) infected with fungus Acremonium coenophialum [145]. Also, Frach et al. proposed CE with laser-induced fluorescence (LIF) as an alternative to UV detection, improving limits of detection about 30-fold. Cyclodextrins , urea, and poly(vinyl alcohol) were included in the BGE, achieving the separation of ergometrinine, ergometrine, ergocorninine, ergocryptine, ergocornine, ergosine, ergocristinine, ergocristine, and ergotamine and their determination in sclerotia [146]. Cyclodextrins were also used as BGE modifiers in CZE for the determination of lisuride enantiomers, a chiral compound derived from EA and used for treatment of Parkinson’s disease. The method was used for enantiomeric purity checking of commercial lisuride pharmaceuticals, in order to determine the concentration of undesirable l-enantiomer [147].
Later on, CZE was also investigated for the separation of lysergic, isolysergic, and paspalic acid in pharmaceuticals. The method provided a detailed study describing the possibilities of CZE-UV as well as of mass spectrometry (MS) using quadrupole-time-of-flight (Q-TOF) as detection for the determination of these compounds. BGEs and detection conditions were carefully optimized regarding selectivity and analysis time as well as MS compatibility. The method was applied to the determination of these compounds in samples obtained from different stages of the manufacturing process such as fermentation broth, solution for precipitation, raffinate, or the raw product [148].
In these methods, sample treatment was based mainly on solid–liquid extraction using mixtures of different solvents or by simple dissolution in the case of pharmaceuticals. However, recently a new sample treatment based on cloud point extraction (CPE) prior to CE-UV for determination of ergotamine and ergometrine in cereal samples has been proposed. CPE is one of the nonpolluting phase separation techniques using surfactant at concentrations higher than its critical micellar concentration (CMC). Analytes are extracted from aqueous solutions into micelles. Afterward, the change on the experimental conditions that promotes the phase separation leads to a surfactant-rich phase with concentrated analytes on the one hand and aqueous solution saturated with surfactant monomers on the other. With CPE, a preconcentration factor of 22 of total EAs was achieved. This method was applied to the determination of EA in commercial flour samples, grain samples, and one cereal-based product for infant feeding [149].
5.2 Liquid Chromatography: Fluorescence Detection
The first attempts for chromatographic separation of EAs were carried out by normal phase HPLC and UV detection at different wavelengths [24]. Nowadays, reverse phase-based chromatography is the mode of choice used for the separation of EAs, mainly using C8 and C18-sorbent, because of limited separation provided by the normal phase procedures. Separation can be achieved with both isocratic and gradient mobile phases, and most methods use solvent systems of methanol–water or acetonitrile/water mixtures with added ammonium hydroxide, ammonium carbonate, ammonium carbamate, or triethylamine to provide alkaline pH conditions [5, 10].
Currently, FLD has replaced UV detection since most EAs possess native fluorescence, allowing increasing sensitivity and selectivity [8]. Δ9,10-Ergolenes can be effectively detected with an excitation and detection wavelength of 310 nm and 410 nm, respectively, while Δ8,9-EAs and EA with a saturated D-ring show maximal fluorescence with excitation at 272 nm and emission wavelength at 371 nm [31]. HPLC–FLD provides sufficient chromatographic resolution for the determination of major EAs, according to EFSA, and their corresponding epimers, with typical run times around 40–45 min [150, 151]. However, some compounds, such as α- and β-ergocryptine and similarly α- and β-ergocryptinine, have been reported as single compounds if they co-elute [10].
In the last decade, different applications of HPLC–FLD for the determination of EAs in food and feed have been reported, as well as clinical applications, usually after SPE.
Examples of food and feed analysis comprise a method for the determination of ergocornine, α-ergocryptine, ergocristine, ergometrine, and ergotamine and their C8-isomers in rye flour [133] and ergometrine, ergotamine, ergocristine, α-ergocryptine, and ergocornine analysis of cereals for animal feed [152], where extraction was carried out by liquid extraction under acidic conditions, followed by strong cation exchange (SCX) SPE [133, 152]. Also, 12 main EAs in rye and rye products were determined by HPLC–FLD, where extraction under basic conditions was followed by SPE using basic alumina cartridges [19, 150]. Finally, Köppen et al. reported a HPLC–FLD method to quantitate 12 priority EAs in rye flour and wheat germ oil. In this case, acidic and alkaline conditions were avoided during extraction, enabling minimized epimerization. Moreover, an improved SPE method using SCX material neutralized with sodium (Na + -SCX) was proposed, where EAs (in their protonated form) were eluted from the column by forming ion pairs with sodium hexanesulfonate, delaying epimerization for over 96 h [151]. Recently, a QuEChERS -based extraction has been proposed as sample treatment for the determination of ergovaline in tall fescue seed and straw followed by HPLC–FLD determination. This sample treatment (quick, easy, cheap, effective, rugged, and safe) is developed in two different steps: (i) an extraction/partitioning step and (ii) a clean-up based on dispersive SPE (d-SPE). In this work, 14 extraction solvents were tested and ammonium carbonate/acetonitrile (50/50, v/v) gave the highest and most consistent recovery (91–101 %), with no necessity of clean-up, eliminating the need for halogenated/chlorinated solvents. QuEChERS procedure was also compared with SPE using Ergosil, a chemically modified silica gel designed for the analysis of ergopeptine alkaloids, obtaining good agreement [153].
In addition to food analysis, Beaulieu et al. used HPLC–FLD to evaluate the diversity and distribution of EAs in seeds and seedlings and variation in alkaloid distribution among different morning glories. The compounds determined were ergobalansine, chanoclavine, lysergol, and ergometrine. In addition, cycloclavine, festuclavine, ergine, and lysergic acid α-hydroxyethylamide could be detected. Identification was confirmed by LC–MS. Before analysis, plant tissues were dried for 3 days at 40 °C and pulverized with 3-mm diam silica beads. The resulting fine powder was soaked in methanol for 3 days at 4 °C with daily vortexing to extract EAs [42]. Also, Nakamichi et al. measured methylergometrine (a postnatal uterotonic drug) in human breast milk using HPLC–FLD. Samples were diluted with McIlvaine buffer containing 5 % EDTA, and after centrifugation, the supernatant was loaded onto a mixed mode cation exchange (MCX) SPE cartridge. Recoveries from 93.5 % to 103.0 % were obtained [154].
5.3 Liquid Chromatography: Mass Spectrometry
Although HPLC–FLD is still a significant technique for the determination of EAs, LC–MS and, more recently, UHPLC–MS are becoming more and more relevant for the determination of mycotoxins, due to the MS capacity of an unambiguous compound identification, especially when high-resolution MS (HRMS) is used. Most of these methods use acidified acetonitrile/water mixtures as mobile phase in reverse mode, and equipment capable to perform MS/MS such as triple quadrupole or, less frequently, ion trap (IT) are commonly employed.
Due to the lack of available standards, most of the reported LC–MS/MS methods have been developed for the determination of the six major EAs (ergometrine, ergosine, ergotamine, ergocornine, ergocryptine, and ergocristine) and their corresponding -inine epimers (ergometrinine, ergotaminine, ergosinine, ergocristinine, ergocryptinine, and ergocorninine). De Saeger’s group determined these compounds in different food and feed samples by a method involving extraction under alkaline conditions and subsequent clean-up by a liquid–liquid partitioning procedure prior to LC–MS/MS analysis. The optimized sample clean-up and a careful selection of the sample solvent allowed minimizing the epimerization of the ergot alkaloids during analysis [132, 155]. This was also the methodology chosen for the in vitro binding efficacy study of a clay-based mycotoxin binder toward EAs [156]. Moreover, the same group reported the first molecularly imprinted polyme r (MIP) toward these compounds and its application in SPE for clean-up of barley samples before LC–MS/MS determination. Metergoline was used as template in the production of suspension polymerized beads used as selective sorbent, obtaining recoveries between 65 % and 79 % [157].
These six major EAs and four of their respective epimers were determined by UHPLC–MS/MS in rye and wheat. In this case, the analytes were extracted with acetonitrile–ammonium carbonate solution and the extract was clean-up with a commercial SPE column (Mycosep 150 Ergot) [104]. EAs were also determined in rye-based food products and ergot sclerotia isolated from rye grains by LC–IT–MS. In this case, neutral alumina-based SPE was selected for clean-up, avoiding the problems of matrix ions that may easily degrade the performance of the IT [138]. LC–IT–MS was also used for the determination of ergovaline in infected tall fescue, after liquid extraction [158].
Concerning ionization sources, electrospray ionization (ESI) has been preferred above other ionization techniques, such as atmospheric pressure photoionization (APPI) or atmospheric pressure chemical ionization (APCI), for determination of mycotoxins. In all these techniques, the sample is ionized at atmospheric pressure before entering the mass spectrometer, but in different ways: in ESI the ionization is achieved by application of a voltage to the spray tip; in APPI by reaction of aerosol droplets with photons produced by a UV lamp; and in APCI by gas-phase ion–molecule reactions. In a very recent paper, ESI and APPI have been compared as ionization sources in the LC–MS/MS determination of lysergic acid amide (LSA) and ergometrine in grass samples, after extraction with methanol [159]. The conclusion of this study was that the performance of APPI and ESI methods was comparable.
One of the main drawbacks of MS detection is matrix effect (signal suppression/enhancement) due to matrix component. In an interesting study, the effect of sample treatment, chromatographic separation, and ionization technique on the matrix effect in EA determination was studied [160]. Thus, LLE, d-SPE using primary secondary amine (PSA), and SPE with different sorbents, such as SCX, MycoSep Ergot multifunctional, and MIP, were compared. For all the procedures tested, no clear signal enhancement was noted, although, for the later eluting ergot alkaloids, MycoSep and SCX cartridges minimized signal suppression. ESI and APCI were also compared; signal suppression was observed in the ESI mode for almost all analytes (with ergometrine being the most susceptible) with no significant difference between ESI+ and ESI-. On the other hand, the use of APCI resulted in a very high signal enhancement for most of the EA. In the same study, LC and UHPLC were compared, concluding that UHPLC was more preferred for the later eluting compounds, as matrix effects were minimized. Other interesting conclusion of this study is that matrix effect varied significantly not only between grain types but also to a lesser extent within one grain type. This fact must be considered when selecting an appropriate blank sample for preparation of a matrix-matched calibration [160].
Multi-mycotoxin determination including EAs by LC–MS/MS has also been reported. For instance, 22 mycotoxins (including ergotamine and ergocornine) were determined in wheat, barley, oats, rye, and maize grain [161] and 26 mycotoxins (including aflatoxins, ochratoxins, fumonisins, trichothecenes, and EAs) in corn, rice, wheat, almond, peanut, and pistachio products using 13C-isotope-labeled internal standards for some of the mycotoxins and liquid extraction with acetonitrile/water [162]. In another work, 63 fungal and bacterial metabolites (including 11 EAs) were determined in commercial poultry feed from Nigeria. The samples were extracted in just one step with acidified acetonitrile, and no further clean-up was required [163]. QuEChERS were the method chosen for the simultaneous determination of 56 mycotoxins (including 12 EAs) in 343 samples of animal feed (non-fermented or fermented feeding stuffs, feeding stuff supplements, and complex compound feeds) by UHPLC–MS/MS, with the aim of estimating absolute mycotoxin concentrations and animal exposure to mycotoxins. In this case, a Q-Trap mass spectrometer was used, operating in both ESI+ and ESI- mode. Among other conclusions, a fairly high co-occurrence was noticed for deoxynivalenol and EAs [164]. The same MS (Q-Trap) and a simple “dilute and shoot” treatment were proposed for the LC–MS/MS determination of 295 fungal and bacterial metabolites (including several EAs). This method was validated in four different food matrices: apple puree for infants, hazelnuts, maize, and green pepper [165]. Other interesting studies involving liquid extraction and subsequent semiquantitative determination of mycotoxins (including EAs) in different samples also with LC–Q-Trap–MS/MS have been reported by Sulyok’s group; those studies comprise analysis of foods infected by molds (concluding that EAs occurred in all samples of dark bread/pastries at low ppb [166]), analysis of grain grown in exceptional climatic conditions [167], and grain dust from Norwegian grain elevators and compound feed mills [168].
HRMS, as Orbitrap or time-of-flight (TOF) MS analyzers, is becoming more and more popular for identification purposes in natural product analysis. In this sense, a QuEChERS-based extraction and UHPLC-Orbitrap MS with APCI have been proposed in the determination of four different groups of mycotoxins (including four EAs) in cereal-based products [169]. Moreover, HRMS has been used not only for the determination of major EAs but also for the identification of less studied or novel EA derivatives. In this regard, recently Arroyo-Manzanares et al. developed a method based on HRMS and IT-MS technology for the study of the fragmentation pattern of EAs and established a simple strategy for the identification of novel ergot alkaloid derivatives [170]. With this approach, besides the six most common ergot alkaloids and their corresponding epimers, 11 EA derivatives, for which commerical standards were not available, were identified. The same authors investigated the suitability of a Q-TOF-MS instrument based on the TripleTOF technology to provide simultaneously a quantitative analysis of common ergot alkaloids and the screening, detection, and identification of unexpected or novel EAs in rye samples [171]. On the other hand, Paulke et al. also used LC–HR–MS/MS for the identification of EAs in different “legal highs” derived from Argyreia nervosa, concluding that LSA/iso-LSA and ergometrine are the main ergot alkaloids present in these products, although a variety of additional EA could be identified, contributing to the pharmacological effects of these drugs [172, 173].
5.4 Immunological Methods
Immunological methods rely on the specificity of binding between antibodies and antigens. Radioimmunoassays (RIAs) and enzyme-linked immunosorbent assays (ELISAs) have been a rapid and inexpensive alternative for EAs determination. However, these methods are less specific and less accurate than HPLC–FLD or LC–MS methods [10].
ELISA has largely replaced RIA, since it has the advantage of not using radioisotopes, avoiding the associated disposal problems, with no sacrifice in sensitivity. Early assays used polyclonal antibodies , which recognized peptide EAs having a phenylalanine moiety, such as ergotamine, ergosine, and ergocristine [24, 174]. Nevertheless, these polyclonal antibodies were replaced by monoclonal antibodies [56, 175, 176] that provided the potential advantage of being more specific for the target hapten, since specific anti-target antibody producing hybridoma cell lines could be selected [13]. Monoclonal antibodies recognized a much wider range of EAs, any with an ergoline ring, since they hinder the antibody binding to the lysergic acid ring structure. However, many peptide EAs with large groups attached to the lysergic acid (ergocryptine, ergocristine, ergocornine, and ergotamine) are not amenable to ELISA [10, 24]. Molloy et al. compared monoclonal and polyclonal antibodies for determination of dihydroergosine in sorghum ergot and both assays were capable of detecting dihydroergosine concentrations above 0.01 mg/kg [56].
ELISA methods have frequently been used to determine the total concentration of EAs produced by endophytic fungi in fescue grass forage [175, 177, 178]. Sample preparation was based mainly on drying and grinding before diluting with phosphate-buffered saline with Tween 20. This was allowed to stand and the liquid portion of the sample was analyzed by ELISA [176]. Different commercial ELISA kits are available, providing LOD around 2 μg/kg [5]. These kits have been applied for the determination of total EAs in tall fescue [179] and in urine samples from lambs fed with tall fescue [180]. However, ELISA methods are not specific for individual EA and gave only an estimation of total EA content. Thus, when quantification of individual EA is required, analysis by HPLC–FLD or LC–MS is mandatory.
Immunological methods have been widely used to determine LSD in biological fluids, and commercial assays are also available [181]. Unfortunately, they are subject to cross-reactivity with structurally related and unrelated compounds potentially yielding false-positive results. So, the best practice following a positive LSD involves confirmation with MS [182].
5.5 Miscellaneous
Besides the liquid separation techniques and immunoassays previously commented, other techniques have been reported for EA determination.
Regarding separation techniques, gas chromatography (GC) was used for the analysis of low molecular weight clavine-type alkaloids and simple lysergic acid derivatives lacking hydrophilic functional groups [183–185], and it has mainly been applied in pharmaceutical and forensic areas. Nevertheless, this technique was not very useful for the determination of peptide EAs because of their high molar mass, low vapor tension, and heat instability, decomposing in a hot injector (225–300 °C) [13, 186]. Thus, most of the applications of GC–MS were published during the 1990s and concern the monitoring of LSD, proposing several derivatization strategies in order to improve volatility and stability and to reduce the peak tailing [187–190]. In food analysis, GC–MS was used for confirmation of identity of the alkaloids in grain foods [191], while Franzmann et al. investigated a method based on the determination of ricinoleic acid (as a characteristic components of ergot) by GC–FLD to estimate the distribution of EAs (determined by HPLC–FLD) in different milling fractions [53].
Thin layer chromatography (TLC) has also been proposed for the determination of naturally occurring EAs in extracts of ergots, grasses, grains, and feeds [24], although this technique has been clearly replaced by LC. TLC on silica gel was used to identify EAs (agroclavine-I and epoxyagroclavine I and their N–N dimers, such as dimer of epoxyagroclavine I and the mixed dimer of epoxyagroclavine I and agroclavine-I) in Penicillium fungi [37], and different metabolites produced by Penicillium fungi isolated from cheese-making and meat-processing plants included EAs (festuclavine and its isomers pyroclavin, costaclavin, and epicostaclavin) [192]. EAs were detected by UV absorbance after spraying the plates with Ehrlich’s reagent, while dimers were detected by luminescence in UV light at 366 nm and positive staining by Dragendorff’s reagent.
Another chromatographic method, such as supercritical fluid chromatography with UV detection at 280 nm or electron impact MS, has also been applied to the identification of a number of clavine alkaloids from Claviceps purpurea [193]. Also, Stahl et al. proposed the use of hybrid techniques such as capillary size exclusion chromatography performed under pressurized capillary electrochromatography (pCEC) on-line with MS for the separation of a crude extract of ergot fungus (secalis cornuti). This set up was compared with other one- and two-dimensional configurations of capillary HPLC [194].
Spectroscopic techniques have also been applied to the detection and quantification of EA. Thus, early methods for EA determination were based on colorimetric measures, where EAs reacted with p-dimethylaminobenzaldehyde under acid conditions, yielding an intensely colored blue solution, which could be measured at 580 nm. Other color reactions like addition of ferric chloride or sodium nitrite and combinations were also proposed [24]. Near-infrared (NIR) spectroscopy was also proposed to determine total EA content on tall fescue [195], and more recently, a method based on NIR hyperspectral imaging and multivariate image analysis has been reported for quantification of ergot bodies in cereals [196]. This method was intended for use in cereal conveyor belt systems at an industrial level.
6 Conclusion
Since the times of Holy Fire, EAs have been an interesting family of compounds, which have roused the interest of the scientific community. These compounds have, on the one hand, valuable pharmacological properties based on their interactions with neurotransmitter receptors on the cells. However, on the other hand, some natural and semisynthetic EAs also possess serious and unpredictable side effects. So, they are considered toxic compounds and have been framed as mycotoxins. Regarding their determination, a wide range of methods have been proposed in pharmaceutical, forensic, and food areas, being those based on liquid chromatography the most popular ones. LC–MS/MS deserves a special mention, as the recent advances in this technique have allowed not only an accurate quantification of major EAs in complex matrices but also the elucidation and identification of novel EA, not described before. EAs are still a challenge, and the elucidation of their biosynthesis pathway is still of great interest, especially because of their broad range of pharmaceutical uses.
Abbreviations
- 5-HT:
-
5-Hydroxytryptamine
- AA:
-
Amino acid
- AdoMet:
-
Adenosylmethionine
- APCI:
-
Atmospheric pressure chemical ionization
- APPI:
-
Atmospheric pressure photoionization
- ARfD:
-
Acute reference dose
- BGE:
-
Background electrolyte
- CE:
-
Capillary electrophoresis
- CMC:
-
Critical micellar concentration
- CONTAM Panel:
-
Panel on Contaminants in the Food Chain
- CPE:
-
Cloud point extraction
- CZE:
-
Capillary zone electrophoresis
- DMA:
-
Dimethylallyl
- DMAPP:
-
Dimethylallyl diphosphate
- DMAT:
-
Dimethylallyltryptophan
- DMATS:
-
Dimethylallyltryptophan synthase
- d-SPE:
-
Dispersive solid phase extraction
- EA:
-
Ergot alkaloid
- EC:
-
European Commission
- EFSA:
-
European Food Safety Authority
- ELISA:
-
Enzyme-linked immunosorbent assay
- ESI:
-
Electrospray ionization
- EU:
-
European Union
- FAD:
-
Flavin adenine dinucleotide
- FLD:
-
Fluorescence detection
- GC:
-
Gas chromatography
- HPLC:
-
High-performance liquid chromatography
- HRMS:
-
High-resolution mass spectrometry
- IT:
-
Ion trap
- LC:
-
Liquid chromatography
- LD50 :
-
Lethal dose 50 %
- LLE:
-
Liquid–liquid extraction
- LSA:
-
Lysergic acid amide
- LSD:
-
Lysergic acid diethylamide
- MIP:
-
Molecularly imprinted polymer
- MS/MS:
-
Tandem mass spectrometry
- MS:
-
Mass spectrometry
- MT:
-
Methyltransferase
- NIR:
-
Near infrared
- pCEC:
-
Pressurized capillary electrochromatography
- PSA:
-
Primary secondary amine
- Q-TOF:
-
Quadrupole time of flight
- QuEChERS:
-
Quick, easy, cheap, effective, rugged, and safe
- RIA:
-
Radioimmunoassay
- SCX:
-
Strong cation exchange
- SPE:
-
solid phase extraction
- TDI:
-
Tolerable daily intake
- TLC:
-
Thin layer chromatography
- TOF:
-
Time of flight
References
Tudzynski P, Neubauer L (2014) Ergot alkaloids. Chapter 14. In: Martín JF, García-Estrada C, Zeilinger S (eds) Biosynthesis and molecular genetics of fungal secondary metabolites. Springer, London
Krska R, Crews C (2008) Significance, chemistry and determination of ergot alkaloids: a review. Food Addit Contam 25:722–731
Wallwey C, Li S-M (2011) Ergot alkaloids: structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes. Nat Prod Rep 28:496–510
Young CA, Schardl CL, Panaccione DG, Florea S, Takach JE, Charlton ND, Moore N, Webb JS, Jaromczyk J (2015) Genetics, genomics and evolution of ergot alkaloid diversity. Toxins 7:1273–1302
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2012) Scientific opinion on ergot alkaloids in food and feed. EFSA J 10:2798 (158 pp.)
Klotz JL (2015) Activities and effects of ergot alkaloids on livestock physiology and production. Toxins 7:2801–2821
EFSA (2005) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ergot as undesirable substance in animal feed. EFSA J 225:1–27
Komarova EL, Tolkachev ON (2001) The chemistry of peptide ergot alkaloids. Part 1. Classification and chemistry of ergot peptides. Pharm Chem J 35:504–513
Kumar A, Bhansali RR (2007) Production of ergot alkaloids from Claviceps. In: Ramawat KG (ed) Biotechnology, second edition: secondary metabolites. CRC Press, Boca Raton
Crews C (2015) Analysis of ergot alkaloids. Toxins 7:2024–2050
Stoll A (1920) Zur kenntnis der mutterkornalkaloide. Verh Naturf Ges 101:190–191
Buchta M, Cvak L (1999) Ergot alkaloids and other metabolites of the genus Claviceps. In: Kren V, Cvak L (eds) Ergot: the genus Claviceps. Harwood Academic Publishers, London
Flieger M, Wurst M, Shelby R (1997) Ergot alkaloids – sources, structures and analytical methods. Folia Microbiol 42:3–30
Mukherjee J, Menge M (2000) Progress and prospects of ergot alkaloid research. Adv Biochem Eng Biotechnol 68:1–20
Bräse S, Gläser F, Kramer CS, Lindner S, Linsenmeier AM, Masters KS, Meister AC, Ruff BM, Zhong S (2013) Ergot alkaloids. Chapter 4. In: The chemistry of mycotoxins. Springer, Wien
Wallwey C, Li S-M (2012) Production, detection, and purification of clavine-type ergot alkaloids. In: Keller NP, Turner G (eds) Fungal secondary metabolism. Methods and protocols, vol 944, Methods in molecular biology. Humana Press, New York
Powell RG, Flattner RD, Yates SG (1990) Ergobalansine, a new ergot-type peptide alkaloid isolated from Cenchrus echinatus (sandbur grass) infected with Balansia obtecta, and produced in liquid cultures of B. obtecta and Balansia cyperi. J Nat Prod 53:1272–1279
Uhlig S, Petersen D (2008) Lactam ergot alkaloids (ergopeptams) as predominant alkaloids in sclerotia of Claviceps purpurea from Norwegian wild grasses. Toxicon 52:175–185
Müller C, Kemmlein S, Klaffke H, Krauthause W, Preiss-Weigert A, Wittkowski R (2009) A basic tool for risk assessment: a new method for the analysis of ergot alkaloids in rye and selected rye products. Mol Nutr Food Res 53:500–507
Lehner AF, Craig M, Fannin N, Bush L, Tobin T (2005) Electrospray [+] tandem quadrupole mass spectrometry in the elucidation of ergot alkaloids chromatographed by HPLC: screening of grass or forage samples for novel toxic compounds. J Mass Spectrom 40:1484–1502
Hafner M, Sulyok M, Schuhmacher R, Crews C, Krska R (2008) Stability and epimerisation behaviour of ergot alkaloids in various solvents. World Mycotoxin J 1:67–78
Rutschmann J, Stadler PA (1978) Chemical background. In: Berde B, Schild HO (eds) Ergot alkaloids and related compounds. Springer, Berlin
Maulding HV, Zoglio MA (1970) Physical chemistry of ergot alkaloids and derivatives. Ionization constants of several medicinally active bases. J Pharm Sci 59:700–701
Scott P (2007) Analysis of ergot alkaloids – a review. Mycotoxin Res 23:113–121
Saxton JE (1960) The indole alkaloids. In: Manske RHF (ed) The alkaloids: chemistry and physiology, vol 7. Academic, London
McPhail AT, Sim GA, Frey AJ, Ott H (1966) Fungal metabolites. Part V. X-ray determination of the structure and stereochemistry of new isomers of the ergot alkaloids of the peptide type. J Chem Soc B 377–395
Smith DJ, Shappell NW (2002) Technical note: epimerization of ergopeptine alkaloids in organic and aqueous solvents. J Anim Sci 80:1616–1622
Andrae K, Merkel S, Durmaz V, Fackeldey K, Köppen R, Weber M, Koch M (2014) Investigation of the ergopeptide epimerization process. Computation 2:102–111
Lauber U, Schnaufer R, Gredziak M, Kiesswetter Y (2005) Analysis of rye grains and rye meals for ergot alkaloids. Mycotoxin Res 21:258–262
Ware GM, Price G, Carter L, Eitenmiller RR (2000) Liquid chromatographic preparative method for isolating ergot alkaloids, using a particle-loaded membrane extracting disk. J AOAC Int 83:1395–1399
Schardl CL, Panaccione DG, Tudzynski P (2006) Ergot alkaloids-biology and molecular biology. Alkaloids Chem Biol 63:45–86
Panaccione DG (2010) Ergot alkaloids. In: Hofrichter M (ed) The mycota X. Springer, Berlin
Gerhards N, Neubauer L, Tudzynski P, Li S-M (2014) Biosynthetic pathways of ergot alkaloids. Toxins 6:3281–3295
Hulvova H, Galuszka P, Frebortova J, Frebort I (2013) Parasitic fungus Claviceps as a source for biotechnological production of ergot alkaloids. Biotechnol Adv 31:79–89
Lorenz N, Wilson EV, Machado C, Schardl CL, Tudzynski P (2007) Comparison of ergot alkaloid biosynthesis gene clusters in Claviceps species indicates loss of late pathway steps in evolution of C. fusiformis. Appl Environ Microbiol 73:7185–7191
Gao Q, Jin K, Ying SH, Zhang Y, Xiao G, Shang Y, Duan Z, Hu X, Xie XQ, Zhou G, Peng G, Luo Z, Huang W, Wang B, Fang W, Wang S, Zhong Y, Ma LJ, Leger RJS, Zhao GP, Pei Y, Feng MG, Xia Y, Wang C (2011) Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet 7:1–18
Kozlovsky AG, Zhelifonova VP, Antipova TV, Zelenkova NF (2011) Physiological and biochemical characteristics of the genus Penicillium fungi as producers of ergot alkaloids and quinocitrinins. Appl Biochem Microbiol 47:426–430
Ge HM, Yu ZG, Zhang J, Wu JH, Tan RX (2009) Bioactive alkaloids from endophytic Aspergillus fumigatus. J Nat Prod 72:753–755
Kozlovsky AG, Zhelifonova VP, Antipova TV (2013) Fungi of the genus Penicillium as producers of physiologically active compounds. Appl Biochem Microbiol 49:1–10
Wallwey C, Heddergott C, Xie X, Brakhage AA, Li SM (2012) Genome mining reveals the presence of a conserved gene cluster for the biosynthesis of ergot alkaloid precursors in the fungal family Arthrodermataceae. Microbiology 158:1634–1644
Jakubczyk D, Cheng JZ, O’Connor SE (2014) Biosynthesis of the ergot alkaloids. Nat Prod Rep 31:1328–1338
Beaulieu WT, Panaccione DG, Hazekamp CS, Mckee MC, Ryan KL, Clay K (2013) Differential allocation of seed-borne ergot alkaloids during early ontogeny of morning glories (Convolvulaceae). J Chem Ecol 39:919–930
Markert A, Steffan N, Ploss K, Hellwig S, Steiner U, Drewke C, Li SM, Boland W, Leistner E (2008) Biosynthesis and accumulation of ergoline alkaloids in a mutualistic association between Ipomoea asarifolia (Convolvulaceae) and a Clavicipitalean fungus. Plant Physiol 147:296–305
Schardl CL, Young CA, Pan J, Florea S, Takach JE, Panaccione DG, Farman ML, Webb JS, Jaromczyk J, Charlton ND, Nagabhyru P, Chen L, Shi C, Leuchtmann A (2013) Currencies of mutualisms: sources of alkaloid genes in vertically transmitted epichloae. Toxins 5:1064–1088
Scandola M, Games DE, Costa C, Allegri G, Bertazzo A, Curcuruto O, Traldi P (1994) Structural study of alkaloids from Securidaca longipedunculata roots II. Isolation and characterization by supercritical fluid chromatography/mass spectrometry. J Heterocycl Chem 31:219–224
Kucht S, Gross J, Hussein Y, Grothe T, Keller U, Basar S, Konig WA, Steiner U, Leistner E (2004) Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 219:619–625
Schardl CL (2001) Epichloë festucae and related mutualistic symbionts of grasses. Fungal Genet Biol 33:69–82
Panaccione DG, Beaulieu WT, Cook D (2014) Bioactive alkaloids in vertically transmitted fungal endophytes. Funct Ecol 28:299–314
Lorenz N, Haarmann T, Pazoutová S, Jung M, Tudzynski P (2009) The ergot alkaloid gene cluster: functional analyses and evolutionary aspects. Phytochemistry 70:1822–1932
Young JC, Chen ZJ (1982) Variability in the content and composition of alkaloid found in Canadian ergot. III. Triticale and barley. J Environ Sci Health B 17:93–107
Mainka S, Dänicke S, Böhme H, Ueberschär K-H, Liebert F (2007) On the alkaloid content of ergot (Claviceps purpurea). Landbauforschung Volkenrode 57:51–59
Appelt M, Ellner FM (2009) Investigations into the occurrence of alkaloids in ergot and single sclerotia from the 2007 and 2008 harvests. Mycotoxin Res 25:95–101
Franzmann C, Wachter J, Dittmer N, Humpf HU (2010) Ricinoleic acid as a marker for ergot impurities in rye and rye products. J Agric Food Chem 58:4223–4229
Pažoutová S (2002) The evolutionary strategy of Claviceps. In: White F, Bacon CW, Hywel-Jones NL (eds) Clavicipitalean fungi: evolutionary biology, chemistry, biocontrol and cultural impacts. Marcel Dekker, New York
Tudzynski P, Tenberge KB, Oeser B (1995) Claviceps purpurea. In: Kohmoto K, Singh US, Singh RP (eds) Pathogenesis and host specificity in plant diseases. Pergamon Press/Elsevier, Oxford
Molloy JB, Moore CJ, Bruyeres AG, Murray SA, Blaney BJ (2003) Determination of dihydroergosine in sorghum ergot using an immunoassay. J Agric Food Chem 51:3916–3919
Krishnamachari KA, Bhat RV (1976) Poisoning by ergoty bajra (pearl millet) in man. Indian J Med Res 64:1624–1628
Banks GT, Mantle PG, Szczyrbak CA (1974) Large-scale production of clavine alkaloids by Claviceps fusiformis. J Gen Microbiol 82:345–361
Agurell S, Ramstad E (1965) A new ergot alkaloid from Mexican maize ergot. Acta Pharm Suec 2:231–238
Lee SL, Floss HG, Heinstein P (1976) Purification and properties of dimethylallylpyrophosphate: tryptophan dimethylallyl transferase, the first enzyme of ergot alkaloid biosynthesis in Claviceps sp. SD 58. Arch Biochem Biophys 177:84–94
Tudzynski P, Correia T, Keller U (2001) Biotechnology and genetics of ergot alkaloids. Appl Microbiol Biotechnol 57:593–605
Metzger U, Schall C, Zocher G, Unsöld I, Stec E, Li SM, Heide L, Stehle T (2009) The structure of dimethylallyl tryptophan synthase reveals a common architecture of aromatic prenyltransferases in fungi and bacteria. Proc Natl Acad Sci U S A 106:14309–14314
Hassam SB, Floss HG (1981) Biosynthesis of ergot alkaloids. Incorporation of (17R)-[17-3H]- and (17S)-[17-3H] chanoclavine-I into elymoclavine by Claviceps. J Nat Prod 44:756–758
Abea M, Ohmomoa S, Ōhashia T, Tabuchia T (1969) Isolation of chanoclavine-(1) and two new interconvertible alkaloids, rugulovasine A and B, from the cultures of Penicillium concavo-rugulosum. Agric Biol Chem 33:469–471
Robinson SL, Panaccione DG (2015) Diversification of ergot alkaloids in natural and modified fungi. Toxins 7:201–218
Lorenz N, Olšovská J, Šulc M, Tudzynski P (2010) The alkaloid cluster gene ccsA of the ergot fungus Claviceps purpurea encodes the chanoclavine-I-synthase, an FAD-containing oxidoreductase mediating the transformation of N-methyl-dimethylallyltryptophan to chanoclavine-I. Appl Environ Microbiol 76:1822–1830
Goetz KE, Coyle CM, Cheng JZ, O’Connor SE, Panaccione DG (2011) Ergot cluster-encoded catalase is required for synthesis of chanoclavine-I in Aspergillus fumigatus. Curr Genet 57:201–211
Coyle CM, Cheng JZ, O’Connor SE, Panaccione DG (2010) An old yellow enzyme gene controls the branch point between Aspergillus fumigatus and Claviceps purpurea ergot alkaloid pathways. Appl Environ Microbiol 76:3898–3903
Cheng JZ, Coyle CM, Panaccione DG, O’Connor SE (2010) Controlling a structural branch point in ergot alkaloid biosynthesis. J Am Chem Soc 132:12835–12837
Matuschek M, Wallwey C, Wollinsky B, Xie X, Li SM (2012) In vitro conversion of chanoclavine-I aldehyde to the stereoisomers festuclavine and pyroclavine controlled by the second reduction step. RSC Adv 2:3662–3669
Xie X, Wallwey C, Matuschek M, Steinbach K, Li SM (2011) Formyl migration product of chanoclavine-I aldehyde in the presence of the old yellow enzyme FgaOx3 from Aspergillus fumigatus: a NMR structure elucidation. Magn Reson Chem 49:678–681
Wallwey C, Matuschek M, Xie X-L, Li S-M (2010) Ergot alkaloid biosynthesis in Aspergillus fumigatus: conversion of chanoclavine-I aldehyde to festuclavine by the festuclavine synthase FgaFS in the presence of the old yellow enzyme FgaOx3. Org Biomol Chem 8:3500–3508
Matuschek M, Wallwey C, Xie X, Li SM (2011) New insights into ergot alkaloid biosynthesis in Claviceps purpurea: an agroclavine synthase EasG catalyses, via a non-enzymatic adduct with reduced glutathione, the conversion of chanoclavine-I aldehyde to agroclavine. Org Biomol Chem 9:4328–4335
Liu X, Wang L, Steffan N, Yin WB, Li SM (2009) Ergot alkaloid biosynthesis in Aspergillus fumigatus: FgaAT catalyses the acetylation of fumigaclavine B. ChemBioChem 10:2325–2328
Maier W, Schumann B, Gröger D (1988) Microsomal oxygenases involved in ergoline alkaloid biosynthesis of various Claviceps strains. J Basic Microbiol 28:83–93
Haarmann T, Ortel I, Tudzynski P, Keller U (2006) Identification of the cytochrome P450 monooxygenase that bridges the clavine and ergoline alkaloid pathways. ChemBioChem 7:645–652
Walzel B, Riederer B, Keller U (1997) Mechanism of alkaloid cyclopeptide synthesis in the ergot fungus Claviceps purpurea. Chem Biol 4:223–230
Ortel I, Keller U (2009) Combinatorial assembly of simple and complex d-lysergic acid alkaloid peptide classes in the ergot fungus Claviceps purpurea. J Biol Chem 284:6650–6660
Barrow KD, Mantle PG, Quigley FR (1974) Biosynthesis of dihydroergot alkaloids. Tetrahedron Lett 16:1557–1560
Blaney BJ, Maryam R, Murray S-A, Ryley MJ (2003) Alkaloids of the sorghum ergot pathogen (Claviceps africana): assay methods for grain and feed and variation between sclerotia/sphacelia. Aust J Agric Res 54:167–175
Stoll A, Hofman A (1965) The ergot alkaloids. In: Manske RHF (ed) The alkaloids: chemistry and physiology, vol 8. Academic, London
Berde B, Stürmer E (1978) Introduction to the pharmacology of ergot alkaloids and related compounds. In: Berde B, Schild HO (eds) Ergot alkaloids and related compounds. Springer, Berlin
Stadler PA, Giger R (1984) Ergot alkaloids and their derivatives in medical chemistry and therapy. In: Krosgard-Larson P, Christensen CH, Kofod H (eds) Natural products and drug development. Munksgaard, Copenhagen
Hart C (1999) Forged in St. Anthony’s fire: drugs for migraine. Mod Drug Discovery 2:20–31
Gröger D (1972) Ergot. In: Kadis S, Ciegler A, Ajl SJ (eds) Microbial toxins, volume VIII: fungal toxins. Academic, London
Görnemann T, Jähnichen S, Schurad B, Latté KP, Horowski R, Tack J, Flieger M, Pertz HH (2008) Pharmacological properties of a wide array of ergolines at functional alpha1-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmakol 376:321–330
Villalon CM, de Vries P, Rabelo G, Centurion D, Sanchez-Lopez A, Saxena PR (1999) Canine external carotid vasoconstriction to methysergide, ergotamine and dihydroergotamine: role of 5-HT1B/1D receptors and α2-adrenoceptors. Br J Pharmacol 126:585–594
Willems EW, Trion M, de Vries P, Heiligers JOC, Villalon CM, Saxena PR (1999) Pharmacological evidence that α1- and α2-adrenoceptors mediate vasoconstriction of carotid arteriovenous anastomoses in anaesthetized pigs. Br J Pharmacol 127:1263–1271
Tfelt-Hansen PC, Koehler PJ (2008) History of the use of ergotamine and dihydroergotamine in migraine from 1906 and onward. Cephalalgia 28:877–886
de Groot AN, van Dongen PW, Vree TB, Hekster YA, van Roosmalen J (1998) Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gyneacology. Drugs 56:523–535
Wadworth AN, Crisp P (1992) Co-dergocrine mesylate: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in age-related cognitive decline. Drugs Aging 2:153–173
Ninomiya I, Klguchl T (1990) Ergot alkaloids. In: Brossi A (ed) The alkaloids, vol 38. Academic, San Diego
Eich E, Eichberg D, Miiller WEG (1984) Clavines. New antibiotics with cytostatic activity. Biochem Pharmacol 33:523–526
Eich E, Becker C, Eichberg D, Maidhof A, Miiller WEG (1986) Clavines as antitumor agents. Part 5. In vitro activity of semisynthetic festuclavines. Pharmazie 41:156–157
Mrusek M, Seo E-J, Greten HJ, Simon M, Efferth T (2015) Identification of cellular and molecular factors determining the response of cancer cells to six ergot alkaloids. Investig New Drugs 33:32–44
Fanchamps A (1978) Introduction to the pharmacology of ergot alkaloids and related compounds. In: Berde B, Schild HO (eds) Some compounds with hallucinogenic activity. Springer, Berlin
Barrett A, Morgan L, Raggatt PR, Hobbs JR (1976) Bromocriptine in the treatment of advanced breast cancer. Clin Oncol 2:373–377
Thobois S (2006) Proposed dose equivalence for rapid switch between dopamine receptor agonists in Parkinson’s disease: a review of the literature. Clin Ther 28:1–12
Moubarak AS, Piper EL, Johnson ZB, Flieger M (1996) HPLC method for detection of ergotamine, ergosine, and ergine after intravenous injection of a single dose. J Agric Food Chem 44:146–148
Van Dongen PWJ, de Groot ANJA (1995) History of ergot alkaloids from ergotism to ergometrine. Eur J Obstet Gynecol Reprod Biol 60:109–116
Eadie MJ (2003) Convulsive ergotism: epidemics of the serotonin syndrome? Lancet Neurol 2:429–434
Handeland K, Vikøren T (2005) Presumptive gangrenous ergotism in free-living moose and a roe deer. J Wildl Dis 41:636–642
Mohamed R, Gremaud E, Tabet JC, Guy PA (2006) Mass spectral characterization of ergot alkaloids by electrospray ionization, hydrogen/deuterium exchange, and multiple stage mass spectrometry: usefulness of precursor ion scan experiments. Rapid Commun Mass Spectrom 20:2787–2799
Kokkonen M, Jestoi M (2010) Determination of ergot alkaloids from grains with UPLC-MS/MS. J Sep Sci 33:2322–2327
Floss HG, Cassady JM, Robbers JE (1973) Influence of ergot alkaloids on pituitary prolactin and prolactin-dependent processes. J Pharm Sci 62:699–715
Fuller JG (1968) The day of St. Anthony’s fire. Signet, New York
Bhat RV, Roy DN, Tulpule PG (1976) The nature of alkaloids of ergoty pearl millet or bajra and its comparison with alkaloids of ergoty rye and ergoty wheat. Toxicol Appl Pharmacol 36:11–17
Demeke T, Kidane Y, Wuhib E (1979) Ergotism – a report on an epidemic, 1977–78. Ethiop Med J 17:107–113
Urga K, Debella A, Medihn YW, Agata N, Bayu A, Zewdie W (2002) Laboratory studies on the outbreak of gangrenous ergotism associated with consumption of contaminated barley in Arsi, Ethiopia. Ethiop J Heal Dev 16:317–323
Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16:497–516
Hogg RA (1991) Poisoning of cattle fed ergotised silage. Vet Rec 129:313–314
Porter JK (1995) Analysis of endophyte toxins: fescue and other grasses toxic to livestock. J Anim Sci 73:871–880
Miles CO, Lane GA, Di Menna ME, Garthwaite I, Piper EL, Ball OJ-P, Latch GCM, Allen JM, Hunt MB, Bush LP, Min FK, Fletcher I, Harris PS (1996) High levels of ergonovine and lysergic acid amide in toxic Achnatherum inebrians accompanying infection by an Acremonium-like endophytic fungus. J Agric Food Chem 44:1285–1290
Uhlig S, Vikoren T, Ivanova L, Handeland K (2007) Ergot alkaloids in Norwegian wild grasses: a mass spectrometric approach. Rapid Commun Mass Spectrom 21:1651–1660
Griffith RW, Grauwiler J, Hodel C, Leist KH, Matter B (1978) Toxicologic considerations. In: Berde B, Schild HO (eds) Ergot alkaloids and related compounds. Springer, Berlin
Korn AK, Gross M, Usleber E, Thom N, Köhler K, Erhardt G (2014) Dietary ergot alkaloids as a possible cause of tail necrosis in rabbits. Mycotoxin Res 30:241–250
Mulac D, Humpf H-U (2011) Cytotoxicity and accumulation of ergot alkaloids in human primary cells Dennis. Toxicology 282:112–121
Larson BT, Harmon DL, Piper EL, Griffis LM, Bush LP (1999) Alkaloid binding and activation of D2 dopamine receptors in cell culture. J Anim Sci 77:942–947
Larson BT, Samford MD, Camden JM, Piper EL, Kerley MS, Paterson JA, Turner JT (1995) Ergovaline binding and activation of D2 dopamine receptors in GH4ZR7 cells. J Anim Sci 73:1396–1400
Oda T, Kume T, Izumi Y, Takada-Takatori Y, Niidome T, Akaike A (2008) Bromocriptine, a dopamine D(2) receptor agonist with the structure of the amino acid ergot alkaloids, induces neurite outgrowth in PC12 cells. Eur J Pharmacol 598:27–31
Mulac D, Lepski S, Ebert F, Schwerdtle T, Humpf HU (2013) Cytotoxicity and fluorescence visualization of ergot alkaloids in human cell lines. J Agric Food Chem 61:462–471
Zhang X, Nan Z, Li C, Gao K (2014) Cytotoxic effect of ergot alkaloids in Achnatherum inebrians infected by the Neotyphodium gansuense endophyte. J Agric Food Chem 62:7419–7422
Strickland JR, Looper ML, Matthews JC, Rosenkrans JF, Flythe MD, Brown KR (2011) Board-invited review: St. Anthony’s fire in livestock: causes, mechanisms, and potential solutions. J Anim Sci 89:1603–1626
Porter JK, Thompson FN Jr (1992) Effects of fescue toxicosis on reproduction in livestock. J Anim Sci 70:1594–1603
Varga L, Lutterbeck PM, Pryor JS, Wenner R, Erb H (1972) Suppression of puerperal lactation with an ergot alkaloid: a double-blind study. Br Med J 2:743–744
Kopinski JS, Blaney BJ, Downing JA, McVeigh JF, Murray SA (2007) Feeding sorghum ergot (Claviceps africana) to sows before farrowing inhibits milk production. Aust Vet J 85:169–176
Kopinski JS, Blaney BJ, Murray SA, Downing JA (2008) Effect of feeding sorghum ergot (Claviceps africana) to sows during mid-lactation on plasma prolactin and litter performance. J Anim Physiol Anim Nutr 92:554–561
Zeilmaker GH, Carlsen RA (1962) Experimental studies on the effect of ergocornine methanesulphonate on the luteotrophic function of the rat pituitary gland. Acta Endocrinol 41:321–335
Schams D, Reinhardt V, Karg H (1972) Effects of 2-BR-alpha-ergocryptine on plasma prolactin level during parturition and onset of lactation in cows. Experientia 28:697–699
Walner BM, Booth NH, Robbins JD, Bacon CW, Porter JK, Kiser TE, Wilson R, Johnson B (1983) Effect of an endophytic fungus isolated from toxic pasture grass on serum prolactin concentrations in the lactating cow. Am J Vet Res 44:1317–1322
Bernard N, Jantzem H, Becker M, Pecriaux C, Bénard-Laribière A, Montastruc JL, Descotes J, Vial T, French Network of Regional Pharmacovigilance Centres (2015) Severe adverse effects of bromocriptine in lactation inhibition: a pharmacovigilance survey. BJOG 122:1244–1251
Malysheva S, Larionova D, Diana Di Mavungu J, De Saeger S (2014) Pattern and distribution of ergot alkaloids in cereals and cereal products from European countries. World Mycotoxin J 7:217–230
Storm ID, Have Rasmussen P, Strobel BW, Hansen HCB (2008) Ergot alkaloids in rye flour determined by solid-phase cation-exchange and high-pressure liquid chromatography with fluorescence detection. Food Addit Contam 25:338–346
Crews C, Anderson WAC, Rees G, Krska R (2009) Ergot alkaloids in some rye-based UK cereal products. Food Addit Contam Part B Surveill 2:79–85
Reinhold L, Reinhardt K (2011) Mycotoxins in foods in Lower Saxony (Germany): results of official control analyses performed in 2009. Mycotoxin Res 27:137–143
Masloff S (2006) Ergot alkaloids in foods. III. Ergot alkaloids in cereals and cereal products. J Verbr Lebensm 1:153–154
Fajardo JE, Dexter JE, Roscoe MM, Nowicki TW (1995) Retention of ergot alkaloids in wheat during processing. Cereal Chem 72:291–298
Bryła M, Szymczyk K, Jędrzejczak R, Roszko M (2015) Application of liquid chromatography/ion trap mass spectrometry technique to determine ergot alkaloids in grain products. Food Technol Biotechnol 53:18–28
Lombaert GA, Pellaers P, Roscoe V, Mankotia M, Neil R, Scott PM (2003) Mycotoxins in infant cereal foods from the Canadian retail market. Food Addit Contam 20:494–504
Bürk G, Hobel W, Richt A (2006) Ergot alkaloids in cereal products: results from the Bavarian health and food safety authority. Mol Nutr Food Res 50:437–442
Schoch C, Schlatter C (1985) Gesundheitsrisiken durch mutterkorn in getreide. Mitt Geb Lebensmittelunters Hyg 76:631–644
European Commission (2012) Commission Recommendation of 15 March 2012 on the monitoring of the presence of ergot alkaloids in feed and food. Off J Eur Union L77:20–21
Fanali S, Flieger M, Steinerova N, Nardi A (1992) Use of cyclodextrins for the enantioselective separation of ergot alkaloids by capillary zone electrophoresis. Electrophoresis 13:39–43
Aboul-Enein HY, Bakr SA (1997) Simultaneous determination of caffeine and ergotamine in pharmaceutical dosage formulation by capillary electrophoresis. J Liq Chromatogr Relat Technol 20:47–55
Ma Y, Meyer KG, Afzal D, Agena EA (1993) Isolation and quantification of ergovaline from Festuca arundinacea (tall fescue) infected with the fungus Acremonium coenophialum by high-performance capillary electrophoresis. J Chromatogr A 652:535–538
Frach K, Blaschke G (1998) Separation of ergot alkaloids and their epimers and determination in sclerotia by capillary electrophoresis. J Chromatogr A 808:247–252
Kvasnicka F, Bíba B, Cvak L (2005) Capillary zone electrophoresis separation of enantiomers of lisuride. J Chromatogr A 1066:255–258
Himmelsbach M, Ferdig M, Rohrer T (2014) Analysis of paspalic acid, lysergic acid, and iso-lysergic acid by capillary zone electrophoresis with UV- and quadrupole time-of-flight mass spectrometric detection. Electrophoresis 35:1329–1333
Felici E, Wang CC, Fernández LP, Gomez MR (2015) Simultaneous separation of ergot alkaloids by capillary electrophoresis after cloud point extraction from cereal samples. Electrophoresis 36:341–347
Müller C, Klaffke HS, Krauthause W, Wittkowski R (2006) Determination of ergot alkaloids in rye and rye flour. Mycotoxin Res 22:197–200
Köppen R, Rasenko T, Merkel S, Mönch B, Koch B (2013) Novel solid-phase extraction for epimer-specific quantitation of ergot alkaloids in rye flour and wheat germ oil. J Agric Food Chem 61:10699–10707
Ruhland M, Tischler J (2008) Determination of ergot alkaloids in feed by HPLC. Mycotoxin Res 24:73–79
Walker K, Duringer J, Craig AM (2015) Determination of the ergot alkaloid ergovaline in tall fescue seed and straw using a QuEChERS extraction method with high-performance liquid chromatography − fluorescence detection. J Agric Food Chem 63:4236–4242
Nakamichi T, Yawata A, Hojo H, Kagaya H, Kobayashi S, Chikuma T (2012) Monitoring of methylergometrine in human breast milk by solid-phase extraction and high-performance liquid chromatography with fluorimetric detection. Pharmazie 67:482–484
Diana Di Mavungu J, Malysheva SV, Sanders M, Larionova D, Robbens J, Dubruel P, Van Peteghem C, De Saeger S (2012) Development and validation of a new LC–MS/MS method for the simultaneous determination of six major ergot alkaloids and their corresponding epimers. Application to some food and feed commodities. Food Chem 135:292–303
Malysheva SV, Diana Di Mavungu J, Schoeters E, Larionova DA, Goryacheva IY, De Saeger S (2013) Rapid and sensitive LC-MS/MS determination of ergot alkaloids in buffered solutions: application to in vitro testing of a clay-based mycotoxin binder. World Mycotoxin J 6:105–115
Lenain P, Diana Di Mavungu J, Dubruel P, Robbens J, De Saeger S (2012) Development of suspension polymerized molecularly imprinted beads with metergoline as template and application in a solid-phase extraction procedure toward ergot alkaloids. Anal Chem 84:10411–10418
Najafabadi AS, Mofid MR, Mohammadi R, Moghim S (2010) Quantification of ergovaline using HPLC and mass spectrometry in Iranian Neotyphodium infected tall fescue. Res Pharm Sci 5:135–143
Jarmusch AK, Musso AM, Shymanovich T, Jarmusch SA, Weavil MJ, Lovin ME, Ehrmann BM, Saari S, Nichols DE, Faeth SH, Cech NB (2016) Comparison of electrospray ionization and atmospheric pressure photoionization liquid chromatography mass spectrometry methods for analysis of ergot alkaloids from endophyte-infected sleepygrass (Achnatherum robustum). J Pharm Biomed Anal 117:11–17
Malysheva SV, Diana Di Mavungu J, Goryacheva IY, De Saeger S (2013) A systematic assessment of the variability of matrix effects in LC-MS/MS analysis of ergot alkaloids in cereals and evaluation of method robustness. Anal Bioanal Chem 405:5595–5604
Martos PA, Thompson W, Diaz GJ (2010) Multiresidue mycotoxin analysis in wheat, barley, oats, rye and maize grain by high- performance liquid chromatography-tandem mass spectrometry. World Mycotoxin J 3:205–223
Liao C-D, Wong JW, Zhang K, Hayward DG, Lee NS, Trucksess MW (2013) Multi-mycotoxin analysis of finished grain and nut products using high-performance liquid chromatography − triple-quadrupole mass spectrometry. J Agric Food Chem 61:4771–4782
Ezekiel CN, Bandyopadhyay R, Sulyok M, Warth B, Krska R (2012) Fungal and bacterial metabolites in commercial poultry feed from Nigeria. Food Addit Contam 29:1288–1299
Zachariasova M, Dzuman Z, Veprikova Z, Hajkova K, Jiru M, Vaclavikova M, Zachariasova A, Pospichalova M, Florian M, Hajslova J (2014) Occurrence of multiple mycotoxins in European feeding stuffs, assessment of dietary intake by farm animals. Anim Feed Sci Technol 193:124–140
Malachová A, Sulyok M, Beltrán E, Berthiller F, Krska R (2014) Optimization and validation of a quantitative liquid chromatography–tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J Chromatogr A 1362:145–156
Sulyok M, Krska R, Schuhmacher R (2010) Application of an LC–MS/MS based multi-mycotoxin method for the semi-quantitative determination of mycotoxins occurring in different types of food infected by moulds. Food Chem 119:408–416
Uhlig S, Eriksen GS, Hofgaard IS, Krska R, Beltrán E, Sulyok M (2013) Faces of a changing climate: semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins 5:1682–1697
Straumfors A, Uhlig S, Eriksen GS, Heldal KK, Eduard W, Krska R, Sulyok M (2015) Mycotoxins and other fungal metabolites in grain dust from Norwegian grain elevators and compound feed mills. World Mycotoxin J 8:361–373
Malachova A, Dzuman Z, Veprikova Z, Vaclavikova M, Zachariasova M, Hajslova J (2011) Deoxynivalenol, deoxynivalenol-3-glucoside, and enniatins: the major mycotoxins found in cereal-based products on the Czech market. J Agric Food Chem 59:12990–12997
Arroyo-Manzanares N, Malysheva SV, Bussche JV, Vanhaecke L, Diana Di Mavungu J, De Saeger S (2014) Holistic approach based on high resolution and multiple stage mass spectrometry to investigate ergot alkaloids in cereals. Talanta 118:359–367
Arroyo-Manzanares N, Diana Di Mavungu J, Uka V, Gámiz-Gracia L, García-Campaña AM, De Saeger S (2015) An integrated targeted and untargeted approach for the analysis of ergot alkaloids in cereals using UHPLC – hybrid quadrupole time-of-flight mass spectrometry. World Mycotoxin J. doi:10.3920/WMJ2015.1900
Paulke A, Kremer C, Wunder C, Wurglics M, Schubert-Zsilavecz M, Toennes SW (2014) Identification of legal highs – ergot alkaloid patterns in two Argyreia nervosa products. Forensic Sci Int 242:62–71
Paulke A, Kremer C, Wunder C, Wurglics M, Schubert-Zsilavecz M, Toennes SW (2015) Studies on the alkaloid composition of the Hawaiian Baby Woodrose Argyreia nervosa, a common legal high. Forensic Sci Int 249:281–293
Shelby RA, Kelley VC (1990) An immunoassay for ergotamine and related alkaloids. J Agric Food Chem 38:1130–1134
Schnitzius JM, Hill NS, Thompson CS, Craig AM (2001) Semiquantitative determination of ergot alkaloids in seed, straw, and digesta samples using a competitive enzyme-linked immunosorbent assay. J Vet Diagn Investig 13:230–237
Shelby RA, Kelley VC (1991) Detection of ergot alkaloids in tall fescue by competitive immunoassay with a monoclonal antibody. Food Agric Immunol 3:169–177
Tunali B, Shelby RA, Morgan-Jones G, Kodan M (2000) Endophytic fungi and ergot alkaloids in native Turkish grasses. Phytoparasitica 28:375–377
Ayers AW, Hill NS, Rottinghaus GE, Stuedemann JA, Thompson FN, Purinton PT, Seman DH, Dawe DL, Parks AH, Ensley D (2009) Ruminal metabolism and transport of tall fescue ergot alkaloids. Crop Sci 49:2309–2316
Roberts CA, Kallenbach RL, Hill NS, Rottinghaus GE, Evans TJ (2009) Ergot alkaloid concentrations in tall fescue hay during production and storage. Crop Sci 49:1496–1502
Hopkins AA, Young CA, Panaccione DG, Simpson WR, Mittal S, Bouton JH (2010) Agronomic performance and lamb health among several tall fescue novel endophyte combinations in the South-Central USA. Crop Sci 50:1552–1561
Wiegand R, Klette KL, Stout PR, Gehlhausen JM (2002) Comparison of EMIT II, CEDIA, and DPC RIA assays for the detection of lysergic acid diethylamide in forensic urine samples. J Anal Toxicol 26:519–523
Saitman A, Park H-D, Fitzgerald RL (2014) False-positive interferences of common urine drug screen immunoassays. J Anal Toxicol 38:387–396
Agurell S, Ohlsson A (1971) Gas chromatography of ergot alkaloids. J Chromatogr 61:339–342
Barrow KD, Quigley FR (1975) Ergot alkaloids II. Determination of agroclavine by gas–liquid chromatography. J Chromatogr 105:393–395
Jegorov A (1999) Analytical chemistry of ergot alkaloids. In: Kren V, Cvak L (eds) Ergot: the genus Claviceps. Harwood Academic Publishers, London
Scott PM (1993) Gas chromatography of mycotoxins. In: Betina V (ed) Chromatography of mycotoxins – techniques and applications. Elsevier, Amsterdam
Bukowski N, Eaton AN (1993) The confirmation and quantitation of LSD in urine using gas-chromatography mass-spectrometry. Rapid Commun Mass Spectrom 7:106–108
Musshoff F, Daldrup T (1997) Gas chromatographic mass spectrometric determination of lysergic acid diethylamide (LSD) in serum samples. Forensic Sci Int 88:133–140
Nakahara Y, Kikura R, Takahashi K, Foltz RL, Mieczkowski T (1996) Detection of LSD and metabolite in rat hair and human hair. J Anal Toxicol 20:323–329
Nelson CC, Foltz RL (1992) Determination of lysergic-acid diethylamide (LSD), iso-LSD, and N-demethyl-LSD in body-fluids by gas-chromatography tandem mass-spectrometry. Anal Chem 64:1578–1585
Klug C, Baltes W, Kronert W, Weber R (1988) A method for the determination of ergot alkaloids in food. Z Lebensm Unters Forsch 186:108–113
Kozlovsky AG, Zhelifonova VP, Antipova TV, Baskunov BP, Ivanushkina NE, Ozerskaya SM (2014) Exo-metabolites of mycelial fungi isolated in production premises of cheese-making and meat-processing plants. Food Addit Contam Part A 3:300–306
Berry AJ, Games DE, Perkins JR (1986) Supercritical fluid chromatographic and supercritical fluid-mass spectrometric studies of some polar compounds. J Chromatogr 363:147–158
Stahl M, Jakob A, von Brocke A, Nicholson G, Bayer E (2002) Comparison of different setups for one- and two-dimensional capillary high-performance liquid chromatography and pressurized capillary electrochromatography coupled on-line with mass spectrometry. Electrophoresis 23:2949–2962
Roberts CA, Benedict HR, Hill NS, Kallenbach RL, Rottinghaus GE (2005) Determination of ergot alkaloid content in tall fescue by near- infrared spectroscopy. Crop Sci 45:778–783
Vermeulen P, Pierna JAF, van Egmond HP, Zegers J, Dardenne P, Baeten V (2013) Validation and transferability study of a method based on near-infrared hyperspectral imaging for the detection and quantification of ergot bodies in cereals. Anal Bioanal Chem 405:7765–7772
Acknowledgments
Natalia Arroyo-Manzanares received postdoctoral grant from the University of Granada.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this entry
Cite this entry
Arroyo-Manzanares, N., Gámiz-Gracia, L., García-Campaña, A.M., Diana Di Mavungu, J., De Saeger, S. (2017). Ergot Alkaloids: Chemistry, Biosynthesis, Bioactivity, and Methods of Analysis. In: Mérillon, JM., Ramawat, K. (eds) Fungal Metabolites. Reference Series in Phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-25001-4_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-25001-4_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25000-7
Online ISBN: 978-3-319-25001-4
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics