Abstract
As a contribution to the occurrence of ergot alkaloids in ergot from German rye and triticale, samples from the 2007 and 2008 harvests were analyzed. Twelve alkaloids—six pairs of main alkaloids and their corresponding epimers—were determined in extracts prepared under alkaline conditions by HPLC with fluorescence detection without preceding purification. The total alkaloid content was found to be 0.03–0.18% in ergot from rye (n = 19) and 0.06–0.22% in ergot from triticale (n = 4), respectively. Furthermore, single sclerotia (n = 40) were investigated in terms of alkaloid content and distributional pattern. The main alkaloids in ergot were ergocristine, ergotamine and ergocornine, although the alkaloid composition was highly variable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ergot intoxications (ergotism) caused by the sclerotia of the parasitic fungus Claviceps purpurea (Fries) Tulasne on nutrition relevant Poacea spp. have been well known since the Middle Ages. Even today, ergot alkaloid contents in food which give rise to pharmacological potential may occur occasionally. Above all, the alkaloid content in ergot sclerotia varies considerably in total amount and pattern (Hofmann 1964; Young 1981; Young and Chen 1982).
So far, the ergot contamination in cereals has still been regulated on a percentage basis in the European Union according to the Intervention Guideline (European Commission Regulation 2008), with 0.05% of sclerotia being the maximum permissible.
Earlier investigations of Canadian cereals observed that, within a single sclerotium, alkaloids were unevenly distributed while the alkaloid pattern remained nearly the same (Young 1981). The latter was also true for sclerotia of an individual ear, but the total amount of alkaloids varied. For sclerotia from the same field, both total alkaloid content and alkaloid pattern varied within a range of 0.011 to 0.452%.
Furthermore, an overall average alkaloid composition pattern for Canadian ergot (rye, wheat, triticale) was given as follows (Young and Chen 1982): ergocristine 31% – ergocristinine 13%; ergotamine 17% – ergotaminine 8%; α-ergo-cryptine 5% – α-ergocryptinine 3%; ergometrine 5% – ergometrinine 2%; ergosine 4% – ergosinine 2%; ergocornine 4% – ergocorninine 2% (rest: unidentified), with ergocristine being the major constituent followed by ergotamine.
In view of new EU regulations which will come into force soon, we investigated the composition and distribution of ergot alkaloids in German field samples from the 2007s and 2008s harvests.
Materials and methods
Chemicals
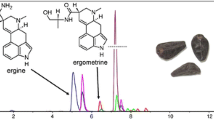
Alkaloids: ergometrine (as hydrogenmaleate) (EM), ergometrinine (EMI), ergosine (ES), ergosinine (ESI), ergotamine (as tartrate) (EA), ergotaminine (EAI), ergocristine (as mesylate) (ET), ergocristinine (ETI), ergocorninine (ECI) and α-ergocryptinine (EYI) originate from Novartis (previously Sandoz, Basel, Switzerland), α-ergocryptine (EY) and ergocornine (EC) from Sigma (St. Louis, Missouri, USA). All are compounds of a thin-film mixture furnished by the Federal Institute for Risk Assessment (BfR, Berlin, Germany) in a sealed ampoule intended to prepare multi-alkaloid standards.
All other chemicals and solvents used were of analytical or HPLC grade, purchased either from Merck (Darmstadt, Germany), Sigma-Aldrich (Steinheim, Germany) or Riedel-de Haën (Seelze, Germany) and were applied without further purification.
Silanisation of glassware in contact with standards and analyte was accomplished by SurfaSilTM Siliconizing Fluid (Pierce, Schwerte, Germany) according to the manufacturer’s instructions.
High purity water was produced by an arium 611 UV system (Sartorius, Göttingen, Germany).
Samples
Ergot samples (30–80 g) from each field were collected at random with regard to all sclerotia sizes and stored at −20°C. Two samples showed an unidentified grub infestation and its feeding canals.
Sample preparation
Based on the method of Müller et al. (2006, 2009, and additional private communication) for rye flour and products thereof, we made slight alterations for the analysis of pure ergot sclerotia. About 50 mg of a ground ergot sample (∼10 g, ≤0.5 mm, Ika MF10 mill; Staufen, Germany; and manually by a nutmeg grater for single sclerotia, respectively) were suspended in 50 ml of an extraction mixture consisting of ethyl acetate, methanol and aqueous ammonium hydroxide solution (25%) (75:5:4 v/v/v) (Müller et al. 2009). Due to possible phase separation, the extraction mixture was stirred while taking an aliquot from it. After 45 min of overhead rotation (approx. 42rpm/level 10 Rotierapparat RA 20; Gerhardt, Bonn, Germany), 1,000-, 100- and 20-µl aliquots of the supernatant solvent were transferred to a test tube and evaporated to dryness in a nitrogen stream (30°C). The residue was redissolved in 1,000 µl of the mobile phase [acetonitrile/aqueous ammonium carbamate solution (0.2 g/l), see below] in an ultrasonic bath, followed by vortexing, and 10 µl of the solution were used directly for HPLC analyis.
Standards
A stock solution containing ∼100 ng/ml per free alkaloid base was prepared by redissolving the alkaloids from a thin-film ampoule (see above) in the mobile phase. External calibration standards of the alkaloid mixture in a range of 1–50 ng/ml were obtained by dilution of the stock solution again in the mobile phase. Due to their light sensitivity, alkaloid standard solutions were prepared in amber glassware (graduated flasks, vials) and stored at −20°C.
HPLC-FLD
An Agilent 1100 system (Agilent Technologies, Waldbronn, Germany) was used, comprising membrane degasser, binary pump, autosampler, thermostated column compartment and fluorescence detector.
Elution: isocratic, mobile phase: acetonitrile/aqueous ammonium carbamate solution (0.2 g/l) 390:500 w/w (corresponding 500:500 v/v).
Flow: 0–15.0 min 0.8 ml/min; 15.1–31.0 min 1.5 ml/min; 31.1–35.0 min 0.8 ml/min [alternatively: A = as above, B = aqueous ammonium carbamate solution (0.2 g/l) 0–12.0 min 0.8 ml/min 90% A, 10% B; 12.1–16.0 min 0.8 ml/min 100% A; 16.1–35.0 min 1.5 ml/min 100% A; see text].
Column: Phenomenex (Aschaffenburg, Germany) Gemini Phenyl-Hexyl, 250 x 4.6 mm, 5 µm particle size
Column oven temperature: 30°C
Fluorescence detection: excitation λex 330 nm, emission λem 415 nm.
Results and discussion
Methods
Figure 1 shows the isocratic separation of a multi alkaloid standard mixture as well as a chromatogram of an ergot sample with a total run time of less than 35 minutes.
In some cases, we observed difficulties in quantifying ergosine due to interfering compounds. This became obvious when comparing the chromatograms of three different concentrations of each sample. The problem could be solved by applying a modified regime of eluent flow (alternative in brackets above).
The repeatability of the method was checked (n = 10; ISO 5725-2) and we found a relative standard deviation RSDr of 7.3% for the sum of the 12 alkaloids analysed and 7.4 (EY) – 12.4% (EA) for the single alkaloids, respectively. All others except EA, ETI (10.8%) and EMI (10.7%) were below 10%. Hence, these data meet with the demand for a RSDr ≤15% as a criterion for precision. However, the reproducibility has still to be examined.
Limits of detection (LOD) and limits of quantification (LOQ) were calculated according to the calibration method (DIN 32645) as follows [LOD (mg/kg)/LOQ (mg/kg)]: EM (0.95/3.61), EMI (1.77/6.66), ES (0.36/1.37), EA (1.13/4.73), EC (2.92/11.75), EY (3.43/13.61), ET (3.08/12.25), ESI (2.45/10.00), EAI (0.96/3.64), ECI (0.95/3.61), EYI (1.35/5.59), ETI (2.13/8.73).
Extraction efficiency was tested by repeated extraction of a sample. The analysis revealed that most of the determined alkaloids were either below their LOD or below their limit of identification. The overall residual alkaloid content was as low as 5% while some of the prevailing alkaloids in the sample examined remained within the ergot powder at a level of up to 10% (ET > ES > EA)
Application
We analyzed ergot samples grown on various rye and triticale cultivars from several German federal states (Tables 1, 2 and Fig. 2). Additionally, we scrutinized single sclerotia from a number of their selected specimens (n = 40).
The determined alkaloid content ranged from 280 to 1,826 µg/g or 0.03 to 0.18% (rye, n = 19) and from 572 to 2,214 µg/g or 0.06 to 0.22% (triticale, n = 4), respectively. While the alkaloid content and its pattern (i.e. the quantitative distribution of single alkaloid species) changed from sample to sample as had been expected, single sclerotia of one sample also showed a huge variability in either feature (Tables 3 and 4). Moreover, there was no correlation between alkaloid content and size of single sclerotia. The total determined alkaloid content of single sclerotia was found to be 2–4,178 µg/g or 0.0002–0.42% (rye, n = 33) and 5–3,759 µg/g or 0.0005–0.38% (triticale, n = 7), respectively.
Our results are in line with those cited above (Young and Chen 1982) except for the given overall values, since we found a lower average ergocristine content (ET 19%/ETI 9%), whereas the one of ergosine and ergocornine was higher (ES 10% – ESI 5%, EC 7% – ECI 4%), which might also be attributed to a narrower statistical basis of ours compared to the authors quoted.
It has been well known that alkaloid content and pattern in ergot is largely dependent on growing conditions, Claviceps spp., its strains and their geographical distribution, host plant, etc. There is little knowledge of how precisely these parameters influence the alkaloid production in field samples and nor do our results suggest a correlation between any of these. No attempt at interpreting results in terms of associating alkaloid content and pattern with the cultivar of the host plant has been made as this would require many more samples. However, Pažoutová et al. (2000) showed that Claviceps purpurea populations are not so host-specialized as had been previously assumed but rather habitat-specialized. Since our samples all originate from cultivated grain fields, i.e. open, sunny localities which can be dry at times, all of them would fall into their variable G1 isolate-group of C. purpurea producing variable amounts of ergotamin(in)e, ergocristin(in)e, ergocryptin(in)e and ergocornin(in)e.
With cereals containing the legal maximum of 0.05% ergot, it is clear that flour made therefrom could even contain more than 1,000 µg/kg total alkaloids—the latter would be the case assuming a medium content of 0.2% alkaloids in ergot sclerotia.
Since ergot contains variable amounts of different alkaloids (with diverging or additive receptor-mediated effects), any regulation based on the relative (percent) content of sclerotia in cereals can only be a convenient but gross measure. To avoid contamination of food with unacceptable levels of ergot alkaloids which might be precarious for sensitive individuals, maximum levels for this group of toxins should be alkaloid-specific. Exposure assessments to show the daily intake of a human being as well as toxicological evaluation of all prevailing individual ergot alkaloids and their effect in combination with each other are needed.
The results presented here demonstrate the need for further toxin-specific regulation. This also implies the need for an appropriate, collaboratively studied, official analytical method (and the commercial availability of all standards) according to the German Food and Feed Code [Lebensmittel-, Bedarfsgegenstände- und Futtermittelgesetzbuch (LFGB) § 64] which will come into effect in due course.
References
European Commission Regulation (EC) No 687/2008 (2008) Dated 18 July 2008 establishing procedures for the taking-over of cereals by intervention agencies or paying agencies and laying down methods of analysis for determining the quality of cereals (Codified version). Off J Eur Union L192:20–48
Hofmann A (1964) Die Mutterkornalkaloide. Ferdinand Enke, Stuttgart (reprint Nachtschatten Solothurn, 2000)
Müller C, Klaffke HS, Krauthause W, Wittkowski R (2006) Determination of ergot alkaloids in rye and rye flour. Mycotoxin Res 22:197–200. doi:10.1007/BF02946741
Müller C, Kemmlein S, Klaffke H, Krauthause W, Preiß-Weigert A, Wittkowski R (2009) A basic tool for risk assessment: a new method for the analysis of ergot alkaloids in rye and selected rye products. Mol Nutr Food Res 53:500–507. doi:10.1002/mnfr.200800091
Pažoutová S, Olšovská J, Linka M, Kolínská R, Flieger M (2000) Chemoraces and habitat specialization of Claviceps purpurea populations. Appl Environ Microbiol 66:5419–5425. doi:10.1128/AEM.66.12.5419-5425.2000
Young JC (1981) Variability in the content and composition of alkaloids found in Canadian ergot. I. Rye. J Environ Sci Health B 16:83–111. doi:10.1080/03601238109372242
Young JC, Chen Z (1982) Variability in the content and composition of alkaloids found in Canadian ergot. III. Triticale and barley. J Environ Sci Health B 17:93–107. doi:10.1080/03601238209372305
Acknowledgements
We wish to thank C. Müller (Federal Office of Consumer Protection and Food Safety, BVL) for advice and training in adopting the method developed at the Federal Institute for Risk Assessment (BfR) and supplying the alkaloid standards. In addition, we gratefully recognize the effort of the federal plant protection administration staff members to collect the analyzed ergot samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Appelt, M., Ellner, F.M. Investigations into the occurrence of alkaloids in ergot and single sclerotia from the 2007 and 2008 harvests. Mycotox Res 25, 95–101 (2009). https://doi.org/10.1007/s12550-009-0014-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-009-0014-2