Abstract
Ergoline alkaloids are constituents of Clavicipitaceous fungi living on Poaceae plants. Ergoline alkaloids as well as volatile oil are also present in Ipomoea asarifolia Roem. & Schult (Convolvulaceae). Treatment of this plant with two fungicides (Folicur, Pronto Plus) eliminates the ergoline alkaloids but not the volatile oil. Elimination of ergoline alkaloids occurs concomitantly with loss of fungal hyphae associated with secretory glands on the upper leaf surface of the Ipomoea plant. Our observations suggest that accumulation of ergoline alkaloids in the Convolvulaceae may depend on the presence of a plant-associated fungus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotaxonomists use natural products as characters to investigate evolutionary relationships between plant taxa. Their approach is based on the fact that related plant taxa usually produce similar or identical natural products (Hegnauer 1992). Not in every case, however, does a certain type of natural product occur in related taxa. For example, cyanogenic glycosides have been found not only in insects but also in plants(Nahrstedt 1996), β-lactam antibiotics are produced by bacteria and fungi (Lechevalier 1975), taxol occurs in higher plants (Wani et al. 1971; Walker and Croteau 2001), but is also present in microorganisms (Stierle et al. 1995; Strobel et al. 1996; Noh et al. 1999; Caruso et al. 2000; Wang et al. 2000), while ergoline alkaloids are typical of fungi belonging to the family Clavicipitaceae and two unrelated plant families, viz. Convolvulaceae and Poaceae (Clay 1991; Gröger and Floss 1998; Panaccione et al. 2001).
The sporadic occurrence of certain natural products brings into question the validity of chemotaxonomic observations. There are, however, three possible explanations for the occurrence of a certain type of natural product in unrelated taxa: (i) The biosynthetic pathways leading to certain types of natural product may have been repeatedly “invented” during evolution. (ii) During evolution, genes for the biosynthesis of natural products may have been transferred from one taxon to an unrelated taxon in a horizontal (lateral) gene transfer (Bushman 2002). (iii) Endophytic or epiphytic microorganisms (bacteria and/or fungi) that produce certain types of natural product may be associated with plants and sometimes even with more than one taxonomic group of plants.
While the first possibility seems to be unlikely for elaborate molecules (Mothes 1981) like taxol or ergoline alkaloids, the second and third possibilities seem to be feasible. Thus, a transfer of genes responsible for β-lactam antibiotics may have occurred from bacteria to fungi during evolution (Buades and Moya 1996). On the other hand, microorganisms producing secondary natural products may occur in or associated with plants. In fact, several such associations have been documented (Tan and Zou 2001). In such instances the synthesis of a natural product is not due to the plant but to the microorganism living in association with the plant. As an example, several different fungal species living on or with grass plants (Poaceae) produce alkaloids (Clay 1991; Gröger and Floss 1998; Panaccione et al. 2001).
Ergoline alkaloids, however, are also known to be present in various Convolvulaceae plants including Ipomoea asarifolia Roem. et Schult. Here we report the results of microscopic inspection of the I. asarifolia leaf surface, treatment of the plant with various fungicides and analysis of ergoline alkaloids and volatile oil occurring in these plants, and we propose the hypothesis that the presence of ergoline alkaloids as opposed to terpenoid compounds in I. asarifolia depends on the presence of a plant-associated fungus. This fungus may be closely associated with secretory glands on the upper leaf surface.
Materials and methods
Plant material
Ipomoea asarifolia plants were either grown from seeds or derived from stem cuttings which were rooted. The plants were kept in the greenhouse and employed 5 months after start of the culture. The seeds were collected by Dr. E. Eich, Berlin, in Ecuador in December 1991. The plants were formerly identified as Ipomoea piurensis O’Donnel (Jennet-Siems et al. 1994) but are now known to belong to the species Ipomoea asarifolia Roem. & Schult (Dr. E. Eich, Berlin, private communication).
Application of fungicides
Five groups, each consisting of six individual plants, remained either untreated (control) or were treated with one out of four different systemic fungicides exhibiting different modes of action. The fungicides were DuPont Benomyl (DuPont de Nemours, Bad Homburg, Germany), Folicur (Bayer CropScience, Leverkusen, Germany), Pronto Plus (Bayer CropScience, Leverkusen, Germany) and Switch (Syngenta Agro, Frankfurt a.M., Germany). The following concentrations were used: Benomyl 0.03%, Folicur 0.15%, Pronto Plus 0.15%, Switch 0.1%. The commercially supplied fungicides contained the following active principles (in parenthesis): DuPont Benomyl (benomyl 524 g kg−1), Folicur (tebuconazole 251 g l−1), Pronto Plus (tebuconazole 133 g l−1 and spiroxamine 250 g l−1), Switch (cyprodinol 375 g kg−1 and fludioxonil 250 g kg−1). Spray treatment on whole plants was carried out at 2-week intervals. The differently treated plants were kept separate in the greenhouse. They were harvested 18 weeks after start of the spray treatment.
Harvest of plants
Aerial plant material was collected and separated into leaves and stems. To ensure that leaves and stems were extracted in equal amounts, 30 g of each were then mixed, cut, frozen in liquid nitrogen and crushed with mortar and pestle. The plant material was freeze-dried and kept frozen at −80°C until further work-up.
Extraction of alkaloids
The plant material was homogenized in aqueous tartaric acid (300 ml, 1%) for 10 min using a blender. The extracted plant material was removed by centrifugation (15 min, 10,000 g, 5°C) and the pH of the supernatant adjusted to pH 8–9 using ammonia (25%). The basic solution was extracted 3 times with ethyl acetate (50 ml). The organic layers were collected, dried and evaporated. The alkaloid fraction was further purified using a solid-phase extraction system (SiOH column, 1.2 cm × 6.5 cm) following the supplier’s (Macherey-Nagel, Düren, Germany) recommendations.
Thin-layer chromatography of alkaloids
Alkaloids were separated on silica gel TLC plates using ethyl acetate (75 ml), ethanol (10 ml), CH2Cl2 (10 ml), ammonia (25%) (5 ml) as a solvent system. Alkaloids were stained with van Urk reagent.
Identification of alkaloids
Alkaloids were identified by HPLC–MS using an Agilent 1100 System (Agilent, Waldbronn, Germany) directly coupled to an Esquire 3000plus mass spectrometer (Bruker Daltonics, Bremen, Germany) in the positive electrospray ionisation (ESI pos.) mode with the following instrumental conditions:
-
Column: Luna C18(2): 3 μm, 2 mm × 100 mm (Phenomenex, Aschaffenburg, Germany); solvent A: 0.05% HCOOH, solvent B: acetonitrile; gradient: 0 min 10% B, 30 min 100% B, 35 min 100% B, 37 min 10% B, 42 min 10% B; flow: 0.3 ml min−1; temperature: 30°C.
-
MS parameters: scan range: 100–750; capillary voltage: 3.7 kV; capillary temperature: 325°C; carrier gas: N2.
Mass spectra were compared with those of authentic standards.
Quantitative estimation of alkaloids
A methanolic solution (100 μl) of alkaloids was diluted to 1 ml with water and 0.7 ml of this solution mixed with 0.7 ml of van-Urk reagent 1 [1 mg p-dimethylamino benzaldehyde in 1 ml of a mixture of conc. H2SO4 (0.5 ml) and H2O (0.5 ml)]. The solution was kept at room temperature for 60 min under dim light. The extinction was subsequently determined at 590 nm in a spectrophotometer and the extinction again read after addition of van-Urk reagent 2 (25 μl of a solution of 1 mg NaNO2 in 1 ml H2O). The difference between the two determinations is a measure of the amount of ergoline alkaloids present in a sample. A calibration curve with ergometrine (500 µg ml−1 methanol) was established and the amount of alkaloids in a given sample expressed as ergometrine.
Capillary electrophoresis of alkaloids
Capillary electrophoresis of alkaloid samples was carried out using a P/ACE station 5500 (Beckman) equipped with a diode array detector. Experimental details used were according to Frach and Blaschke (1998). The capillary had a length of 30 cm and an inner diameter of 74 μm. The voltage was set to 25 kV and samples run at 20°C. Injection of samples was carried out hydrodynamically within 2 s after the capillary had been rinsed with phosphoric acid (250 mM) and loading buffer.
Steam distillation of volatile oil
100 g leaf material, which had been cut into pieces, was suspended in 600 ml water in a round-bottomed flask. The flask was connected to a “Karlsruher Apparatur” as described in the European Pharmacopoea (Europäisches Arzneibuch 1997). The oil was trapped in 1 ml hexane during 3 h.
Identification of terpenoids
The volatile oil was investigated by GC–MS conducted with a Hewlett-Packard HP 5890 gas chromatograph (25 m fused silica capillary column with polydimethylsiloxane CPSil-5, 80°C starting temperature, 10°C min−1 temperature program to 230°C; He as carrier gas) coupled to a VG Analytical 70-250S mass spectrometer (ion source temperature 230°C). Identification of the volatile components was achieved by comparison of their mass spectra and retention indices with a spectral library established under identical experimental conditions (Hochmuth et al. 2002).
Light microscopy
For microscopic investigations a Leitz DMRB photomicroscope (Leica) equipped for Normarksi interference and epifluorescence was used. The secretory glands of the plant were examined after staining with Nile red (1 μl l−1, Sigma) with filter combination 490/15 as exciter, 500 chromatic beam splitter and BP 525 as 20-barrier filter, according to Broun and Somerville (2001). Fungal structures were visualised with aniline blue according to Ortega et al. (1998) with filter combination BP 340–380, 400 LP 425, and with Oregon Green-labelled wheat germ agglutinin (WGA; Molecular Probes, Leiden, The Netherlands), according to Werner et al. (2002), with filter combination 490/15, 500, BP 525/20.
Results
Phytochemical investigation of Ipomoea asarifolia led to the isolation and structure determination of chanoclavine-I (I), lysergic acid amide (i.e. ergine III; Fig. 1), lysergic acid α-hydroxyethylamide, ergobalansine and ergobalansinine (Jenett-Siems et al. 1994). With reference samples at hand and using an HPLC–ESI–MS system we confirmed the presence of these metabolites and in addition found lysergic acid, isolysergic acid amide (IV), ergometrine and elymoclavine (II). The presence of 8-hydroxylysergic acid amide in I. asarifolia is also likely but could not be proven due to lack of an authentic reference sample. The mass spectrum and its molecular ion (m/z 284), however, strongly suggested the presence of this compound.
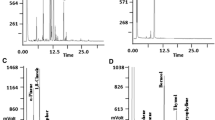
The alkaloid fraction of the plant was also analyzed by capillary electrophoresis, and is shown in Fig. 2. This technique was routinely used for analytical purposes. The electropherogram showed chanoclavine-I (I), elymoclavine (II), lysergic acid amide (III) and isolysergic acid amide (IV). Compounds II and III were not separated by this technique. This technique separated, however, epimers of ergoline alkaloids such as lysergic acid amide and isolysergic acid amide (Frach and Blaschke 1998).
Qualitative and quantitative investigation by capillary electrophoresis of alkaloids present in Ipomoea asarifolia plants after treatment with various fungicides at 2-week intervals for 18 weeks. I Chanoclavine-I, II elymoclavine, III lysergic acid amide, IV isolysergic acid amide. mAU milli absorption unit
To test whether these alkaloids are products of a plant-associated fungus or are produced by the plant itself, four groups of plants, each consisting of six individual plants, were treated with a regime of four different systemic fungicides. The alkaloid content was determined 18 weeks after start of the spray treatment.
The results are shown in Fig. 2 and Table 1. Control plants and those plants that were treated with Switch and Benomyl contained ergoline alkaloids whereas treatment with Folicur and Pronto Plus resulted in complete loss of alkaloids within 18 weeks (Table 1). The alkaloid pattern in plants treated with Switch and Benomyl, however, corresponded to that of the 18-week control (Fig. 2). The possibility that a peak (retention time 5.8 min) after treatment with Pronto Plus (Fig. 2) could be attributed to the presence of chanoclavine-I (I) or elymoclavine (II) was ruled out by co-chromatography. Thus, no trace of alkaloids was detectable in this case.
Microscopic inspection of the upper leaf surface showed secretory glands, usually composed of six to eight cells (Fig. 3), that stained with Nile red (Fig. 3b; Broun and Somerville 2001). Specific stains such as aniline blue for epi- and endophytic fungal hyphae (Fig. 3d; Ortega et al. 1998) and Oregon Green-labelled wheat germ agglutinin for chitin (Fig. 3c) revealed fungal hyphae mainly located around these secretory glands on the leaf surface. These hyphae were only seen in control plants or after treatment of plants with Switch and Benomyl but not when Pronto Plus or Folicur were employed. This association was tested as shown in Table 2. Among 500 inspected glands on different leaves of the plants, 93% (control) or 95.2% (Switch) or 88.6% (Benomyl) showed hyphae, but no hyphae were observed during inspection of 500 individual glands after treatment with Pronto Plus or Folicur.
Secretory glands on the upper leaf surface of I. asarifolia before (a) and after (b) staining with Nile red. c,d Fungal hyphae located around secretory glands on the upper leaf surface after staining with Oregon Green-labelled wheat germ agglutinin (c) or aniline blue (d). After treatment of plants with Pronto Plus or Folicur, hyphae, as opposed to secretory glands, are no longer detectable (not shown). Bar in a = 25 μm
In addition, the volatile oil contained in secretory glands was isolated by steam distillation and its components identified by combined GC–MS. The oil derived from the Folicur-treated plants contained two unknown compounds (Fig. 4) that did not appear in the control plants. The main components present in the volatile oil of the control as well as the Folicur-treated plants were represented by five sesquiterpenes identified as α-copaene (VI), (E)-β-caryophyllene (VII), α-humulene (VIII), germacrene D (IX) and δ-cadinene (X) (Fig. 4). In addition 53 minor terpenoid compounds were also found, 7 of which remained unidentified. Terpenoid compounds were present in both the Folicur-treated and in the control plants in almost identical amounts. This indicates that treatment with Folicur does not affect secondary metabolism in general but specifically accumulation of ergoline alkaloids.
Discussion
The co-occurrence of specific fungal hyphae as well as ergoline alkaloids and concomitant loss of both during treatment of Ipomoea asarifolia with two different fungicides (Pronto Plus, Folicur) indicates—but does not prove—that the accumulation of alkaloids in I. asarifolia depends on the presence of a plant-associated fungus. This situation is reminiscent of the plant/fungal association in Poaceae where fungi are known to be responsible for ergoline alkaloid production (Gröger and Floss 1998; Panaccione et al. 2001; Tudzynski et al. 2001).
The fungicides employed here are all systemic but inhibit different metabolic pathways in fungal metabolism. Benzimidazole fungicides (Benomyl) have a high affinity for tubulin proteins and disrupt mitosis by attacking the mitotic spindle (Davidse 1987). Switch contains two active constituents, the anilinopyrimidine cyprodinil and the phenylpyrrole fludioxonil. Cyprodinil inhibits the elongation of germ tubes at low concentrations. Several amino acids, particularly methionine, reverse the fungitoxicity of the anilinopyrimidines (Masner et al. 1994). Biochemical studies suggest that the biosynthesis of methionine is inhibited by affecting the cystathionine-β-lyase (Fritz et al. 1997). Folicur and Pronto Plus, on the other hand, are inhibitors of fungal sterol biosynthesis (Kuck and Thielert 1987; Tiemann et al.1997). Sterols are localised in cell membranes of the fungi conferring stability, controlling permeability and membrane function. This class of fungicides shows a broad spectrum of activity, being effective in the control of Ascomycetes, Basidiomycetes and Fungi imperfecti but not against Oomycetes. The fact that the hyphae and alkaloids are not eliminated by fungicides in every case (Switch, Benomyl) may be due to their selectivity depending on differences in their accumulation in the cell, different structures of the receptor target system, differences in the ability to detoxify a compound or different degrees of importance of a receptor or target system for survival of the fungus (Lyr 1995).
Our observations suggest that a fungus is involved in alkaloid biosynthesis, a view that has not been shared by all authors in the past. It has been claimed (Dobberstein and Staba 1969) that callus and cell suspension cultures raised from various members of the Convolvulaceae are producers of alkaloids. Callus and cell suspension cultures are presumed to be sterile and consequently it should be the plant cells that have the capacity to produce the alkaloids. This conclusion has been drawn from work with Ipomoea violaceae, Rivea corymbosa and Argyreia nervosa. However, in these studies, alkaloids have not been identified in a rigorous manner. The van-Urk reagent is non-specific and thin-layer chromatography is a technique with limited reliability in the identification of natural products. The use of this technique in attempts to identify ergoline alkaloids has also been criticized by Jenett-Siems et al. (1994). We have established callus and cell suspension cultures from I. violaceae, R. corymbosa and I. asarifolia and used thin-layer chromatography and capillary electrophoresis to check these plant cells for the presence of ergoline alkaloids. We occasionally observed van-Urk-positive compounds, but in no case did we observe any trace of ergoline alkaloids (data not shown). The limit of detection was 2 ng for chanoclavine-I (I), 25 ng for ergometrine and 50 ng for lysergic acid amide (III).
The presence of ergoline alkaloids in different organs of members of the Convolvulaceae has also been investigated. It has been found that particularly the plant embryos of R. corymbosa gave a positive van-Urk reaction. This was taken as evidence that embryos are axenic sites of ergoline synthesis. Simultaneously, seeds were investigated for the presence of fungi, which were found to reside in the seed coat but not in the embryo (Taber and Heacock 1962). “From this evidence it was considered reasonably certain that the alkaloids are a true metabolic product of the plant and not of an invading microbial parasite or contaminant” (Taber et al. 1963).
The possibility that alkaloids were synthesized in any other plant organ and translocated to the seeds and embryos, however, was not taken into account by these authors. Consequently, a different view of the occurrence of ergoline alkaloids in seeds was given by Mockaitis et al. (1973). Grafting experiments with I. violaceae and I. nil plants showed clearly that leaves are the principal sites for the biosynthesis of alkaloids, which are then translocated throughout the plant, with the major accumulation occurring in the seeds. Roots, seeds and stems of the plant are not sites of alkaloid synthesis (Mockaitis et al. 1973). These experiments and conclusions are in agreement with our observations. They show that the leaves and/or leaf-associated fungi are the sites of alkaloid synthesis.
Many related fungi are endophytes or epibionts that produce ergoline alkaloids (Clay and Frentz 1993; Porter 1994; Reddy et al. 1998; Lewis et al. 2002) Among these are Neotyphodium species, which infect grasses without causing any disease symptoms (Panaccione et al. 2001). It is conceivable that a fungus belonging to this genus is responsible for the alkaloid biosynthesis on and in cooperation with the I. asarifolia leaf. However, fungi of other genera also produce ergoline alkaloids (Gröger and Floss 1998), so it cannot be presumed that the fungus we observed is clavicipitaceous.
It is intriguing that the fungal hyphae were located on the leaf surface in close contact with secretory glands. A similar observation has been made for an undetermined epibiotic ascomycetous endophyte associated with Mentha piperita L. plants (Mucciarelli et al. 2002).
The terpenoid compounds occurring in Ipomoea asarifolia were identified in this study for the first time. It is an important observation that terpenoids were not removed by Folicur but were present in both Folicur-treated and control plants in similar amounts. This clearly shows that removal of ergoline alkaloids is a specific process and that Folicur does not interfere with the supply of hemiterpenoid biosynthetic building stones which are channelled into the biosynthesis of both ergoline alkaloids (Fig. 1) and terpenoid compounds.
The data presented here show that the erratic occurrence of ergoline alkaloids in the plant kingdom is probably no reason to question the validity of chemotaxonomic investigations because ergoline alkaloids are not a character typical of clavicipitaceous fungi, the Poaceae and Convolvulaceae, but in contrast they are very likely a character of a fungal taxon capable of colonizing certain higher plants in commensal or mutualistic symbiosis. Proof of this notion will have to await experiments in which Ipomoea plants devoid of alkaloids are inoculated with isolated endophytic and epibiotic fungi. This tool is now at hand.
References
Broun P, Somerville C (2001) Progress in plant metabolic engineering. Proc Natl Acad Sci USA 98:8925–8927
Buades C, Moya A (1996) Phylogenetic analysis of the isopenicillin-N-synthetase horizontal gene transfer. J Mol Evol 42:537–542
Bushman F (2002) Lateral DNA transfer. Mechanisms and consequences. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Caruso M, Colombo AL, Fedeli L, Pavesi A, Quadroni S, Saracchi M, Ventrella G (2000) Isolation of endophytic fungi and actinomycetes taxane producers. Ann Microbiol 50:3–13
Clay K (1991) Fungal endophytes, grasses and herbivores. In: Barbose P, Krischik VA, Jones CG (eds) Microbial mediation of plant–herbivore interactions. Wiley, New York, pp 199–226
Clay K, Frentz IC (1993) Balansia pilulaeformis, an epiphytic species. Mycologia 85:527–534
Davidse LC (1987) Biochemical aspects of benzimidazole fungicides-action and resistance. In: Lyr H (ed) Modern selective fungicides. Fischer, Jena, pp 245–257
Dobberstein RH, Staba EJ (1969) Ipomoea, Rivea and Argyreia tissue cultures: influence of various chemical factors on indole alkaloid production and growth. Lloydia 32:141–147
Europäisches Arzneibuch (1997) Amtliche deutsche Ausgabe, 3rd edn. Deutscher Apotheker Verlag, Stuttgart; Govi-Verlag-Pharmazeutischer Verlag, Eschborn, pp 129–130
Frach K, Blaschke G (1998) Separation of ergot alkaloids and their epimers and determination in sclerotia by capillary electrophoresis. J Chromatogr A 808:247–252
Fritz R, Lanen C, Colas V, Leroux P (1997) Inhibition of methionine biosynthesis in Botrytis cinera by the anilinopyromidine fungicide pyrimenthanil. Pestic Sci 49:40–46
Gröger D, Floss HG (1998) Biochemistry of ergot alkaloids—achievements and challenges. In: Cordell GA (ed) The alkaloids, vol 50. Academic Press, San Diego, pp 171–218
Hegnauer R (1992) Chemotaxonomie der Pflanzen. Birkhäuser, Basel
Hochmuth DH, Joulain D, König WA (2002) MassFinder Software and Data Bank, University of Hamburg. http://www.chemie.uni-hamburg.de/oc/koenig/massfinder.html
Jenett-Siems K, Kaloga M, Eich E (1994) Ergobalansine/ergobalansinine, a proline-free peptide-type alkaloid of the fungal genus Balansia, is a constituent of Ipomoea piurensis. J Nat Prod 57:1304–1306
Kuck KH, Thielert W (1987) On the systemic properties of HWG 1608, the active ingredient of the fungicides Folicur and Raxil. Pflanzenschutz Nachr Bayer 40:133–152
Lechevalier HA (1975) Production of the same antibiotics by members of different genera of microorganisms. Adv Appl Microbiol 19:25–45
Lewis EA, Bills GF, Heredia G, Reyes M, Arias RM, White JF Jr (2002) A new species of endophytic Balansia from Veracruz, Mexico. Mycologia 94:1066–1070
Lyr H (1995) Selectivity in modern fungicides and its basis. In: Lyr H (ed) Modern selective fungicides: properties, applications, mechanisms of action. Fischer, Stuttgart, pp 13–22
Masner P, Muster P, Schmid P (1994) Possible methionine biosynthesis inhibition by pyrimidinamine fungicides. Pestic Sci 42:163–166
Mockaitis JM, Kivilaan A, Schulze A (1973) Studies of the loci of indole alkaloid biosynthesis and alkaloid translocation in Ipomoea violacea plants. Biochem Physiol Pflanz 164:248–257
Mothes K (1981) The problem of chemical convergence in secondary metabolism. Sci Scientists 323–326
Mucciarelli M, Scannerini S, Bertea C M, Maffei M (2002) An ascomycetous endophyte isolated from Mentha piperita L.: biological features and molecular studies. Mycologia 94:28–39
Nahrstedt A (1996) Relationships between the defense systems of plants and insects. In: Romeo JT, Saunders JA, Barbosa P (eds) Recent advances in phytochemistry, vol 30. Plenum, New York, pp 217–230
Noh M-J, Yang J-G, Kim K-S, Yoon Y-M, Kang K-A, Han H-Y, Shim S-B, Park H-J (1999) Isolation of a novel microorganism, Pestalotia heterocornis. Biotechnol Bioeng 64:620–623
Ortega F, Steiner U, Dehne HW (1998) Induced resistance to apple scab: microscopic studies on the infection cycle of Venturia inaequalis (Cke.) Wint. J Phytopathol 146:399–405
Panaccione DG, Johnson RD, Wang J, Young CA, Damronykool P, Scott B, Schardl CL (2001) Elimination of ergovaline from a grass–Neotyphodium endophytic symbiosis by genetic modification of the endophyte. Proc Natl Acad Sci USA 98:12820–12825
Porter JK (1994) Chemical constituents of grass endophytes. In: Bacon CW, White JW Jr (eds) Biotechnology of endophytic fungi of grasses. CRC Press, Boca Raton, pp 103–123
Reddy PV, Bergen MS, Patel R, White JF Jr (1998) An examination of molecular phylogeny and morphology of the grass endophyte Balansia claviceps and similar species. Mycologia 90:108–117
Stierle A, Strobel G, Stierle D (1995) The search for a taxol-producing microorganism among the endophytic fungi of the pacific yew, Taxus brevifolia. J Nat Prod 58:1315–1324
Strobel G, Yang X, Sear J, Kramer R, Sidhu RS, Hess WM (1996) Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology 142:435–440
Taber WA, Heacock RA (1962) Location of ergot alkaloid and fungi in the seed of Rivea corymbosa (L.) Hall. f., “Ololiuqui”. Can J Microbiol 8:137–143
Taber WA, Vining LC, Heacock RA (1963) Clavine and lysergic acid alkaloids in varieties of morning glory. Phytochemistry 2:65–70
Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18:448–459
Tiemann R, Berg D, Krämer W, Pontzen R (1997) Biochemistry of the new fungicide KWG 4168 (spiroxamine). Pflanzenschutz Nachr Bayer 57:211–219
Tudzynski P, Correia T, Keller U (2001) Biotechnology and genetics of ergot alkaloids. Appl Microbiol Biotechnol 57:593–605
Walker K, Croteau R (2001) Taxol biosynthetic genes. Phytochemistry 58:1–7
Wang J, Li G, Lu H, Zheng Z, Huang Y, Su W (2000) Taxol from Tubercularia sp. strain TF5, an endophytic fungus of Taxus mairei. FEMS Microbiol Lett 193:249–253
Wani MV, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93:2325–2327
Werner S, Steiner U, Becher R, Kortekamp A, Zyprian E, Deising HB (2002) Chitin synthesis during in planta growth and asexual propagation of the cellulosic oomycete and obligate biotrophic grapevine pathogen Plasmopara viticola. FEMS Microbiol Lett 208:169–173
Acknowledgements
We thank Dr. D. Gröger (Halle, Germany) for reference samples, Dr. E. Eich (Berlin, Germany) for reference samples and seeds and Dr. Chris Schardl (Lexington, KY, USA) for helpful discussions. This work was supported by the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Wolfgang Steglich, München, on the occasion of his 70th birthday
Rights and permissions
About this article
Cite this article
Kucht, S., Groß, J., Hussein, Y. et al. Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 219, 619–625 (2004). https://doi.org/10.1007/s00425-004-1261-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-004-1261-2