Abstract
Metal-based nanoparticles (NPs) are one of the most manufactured nanomaterials and deserve singular attention given their continuous input to the environment, lack of degradation, and accumulation risk. In agricultural soils, the use of organic amendments and wastewater and the application of nanotechnology are important NP inputs. Metal-based NPs have beneficial applications as fertilizers and increase plant resistance to pathogens and environmental abiotic stressors. Ag-, Zn-, Cu-, Ti-, and Ce-based NPs are the most widely used to improve crop production. NPs can also have negative impacts, including phytotoxicity, lower nutrient content in plants, and soil microorganism toxicity. The potential NP interaction with other soil contaminants, including metals and organic compounds, is a major concern because it can modify the bioconcentration or affect the intrinsic toxicity of both substances with the consequent biological impact on plants. Exposure to NP-contaminant mixtures may induce unexpected toxic effects via several different mechanisms that affect the availability, uptake, and metabolic processes involved in the detoxification and degradation of compounds. However, the mechanisms underlying the effects of the NP-contaminant interaction on joint toxicity are poorly understood. This chapter covers some of the most relevant issues concerning the effects of metal-based NPs on plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Nanoparticles (NPs) are materials with the three dimensions below 100 nm and applications in a variety of sectors (e.g., biomedical, chemical, textile, food, agriculture). Their tiny size confers specific characteristics that can intensify their properties. In the natural environment, a small NP size can increase environmental negative impacts compared to the bulk form, but demonstrated beneficial applications as nano-remediators or nano-agrochemicals have been reported (Yang et al., 2019). NPs’ morphology is also crucial for showing toxicity in some cases. The coating of NPs’ surface and their encapsulation are common practices that can help their stability and change their reactivity and toxicity (Sturikova et al., 2018; Zeng et al., 2019). In the natural environment, NPs are subject to transformation processes like dissolution, aggregation, reduction/oxidation, sulfidation, and adsorption. Aging also drives NPs’ properties, including fate and toxicity (Fernández et al., 2021; Romero-Freire et al., 2017; Jośko et al., 2020; García-Gómez et al., 2020).
Overproduction, use, and abuse of NPs have rapidly led to them be released to several environmental compartments, which increases environmental threats to living organisms. The three major sources of terrestrial plant exposure to nanomaterials are air, water, and soil. In soil, the largest amounts accumulate (up to 1.5%, 7%, and 28% of total NPs’ production, respectively) (Liu et al., 2020). Atmospheric NPs can be easily deposited on various plant surfaces and infiltrate the plant system via stomatal apertures and across cuticles. The use of wastewater containing aged NPs is another source of NPs to plants. In agricultural soils, the use of amendments (manures, sludges, etc.) and the application of nanotechnology deliberately enable the input of NPs in agricultural environments. In recent decades, the agriculture has faced a wide range of challenges, such as climate change, salinity and drought, soil pollution, and the increasing food demand for a growing population. The use of nanomaterials in modern agriculture helps to gain maximum output from available resources and contributes to mitigate the aforementioned challengers (Rajput et al., 2021). Nonetheless, to promote sustainable progress, it is necessary to assess NPs’ toxicity to non-target organisms at the same time as NPs are being investigated and developed.
It is remarkable that NPs at nontoxic concentrations can still be hazardous because of their interaction with other contaminants present in the environment. Previous studies show that NPs can facilitate the intake of metals and organic compounds in plants and other organisms, which can lead to these chemicals’ increased toxicity (Deng et al., 2017; Naasz et al., 2018). In some cases, these indirect effects can be more significant than the direct impacts associated with NPs’ exposure. Thus acquiring knowledge of the potential effects that result from the interaction of NPs with co-existing organic and inorganic contaminants is critically important for evaluating and regulating the environmental impacts of NPs on plants.
The plant relation with NPs is very complex, and NPs’ absorption mechanisms in plants are still poorly understood. Plant systems provide a route for NP uptake, accumulation, and translocation that depends on the physiological properties, functionalization, and the form of exposure of NPs to plants (Agrawal et al., 2022). One of the most important limitations to impact plant uptake of NPs is particle size. Several studies establish 20–50 nm as the size limit for NPs to penetrate and move to plant tissues. In plants, NPs are firstly adsorbed on the root surface, and root exudates and transporter proteins can participate in uptake processes. Tiny NPs can diffuse through epidermal cell wall pores and enter the apoplastic and/or symplastic flow. The apoplast form takes place outside cell membranes through extracellular spaces, cell walls of adjacent cells, and xylem vessels. The symplastic form involves substances and water moving between the cytoplasm of adjacent cells. Larger NPs are first blocked, which results in osmotic pressure and capillary forces that finally help NPs to reach the endodermis by either crossing the cortex cells and diffusing through the apoplastic pathway or merging on symplastic route to penetrate the vascular system (Lv et al., 2019; Deng et al., 2014). The foliar NPs’ application implies crossing the cuticle layer, and uptake occurs via two routes: one for polar solutes by polar aqueous pores (hydrophilic pathway) and another for non-polar solutes via diffusion and permeation (lipophilic pathway) (Pérez-de-Luque, 2017; Ali et al., 2021).The cuticle serves as a primary barrier to prevent NPs larger than 5 nm from entering (Molina et al., 2021). This entrance does not prevent root damage because there is evidence for the transport of NPs from the aerial parts to roots (Chichiriccò & Poma, 2015).
Metal-based NPs are one of the most frequently manufactured nanomaterials due to their widespread uses, including environmental applications. Furthermore, given their non-biodegradable nature, significant amounts of these compounds are expected in soil.
This chapter focuses on the impacts that metal-based NPs have on plants. Indirect effects due to NPs are also discussed, such as changes in the plant-soil environment and the influence of co-occurrence with other soil contaminants like organics, metals/metalloids, and nanomaterials.
2.2 Nanoparticles and the Plant’s Environment
These new-age materials have the potential to alter biotic and abiotic systems, alterations that are governed mostly by the concentration and physiochemical properties of NPs. Of these, the most dominant are size, shape, and surface charge. Soil properties, mainly pH, organic matter content, cation exchange capacity, texture, moisture content, etc., have the capacity to modify the reactivity, fate, and, ultimately, the toxicity of NPs (Rawat et al., 2018; Gao et al., 2019; García-Gómez & Fernández, 2019). In soil, NPs may undergo several physical-, chemical-, and biological-mediated processes that lower their bioavailable concentration and, hence, their toxicity. In particular, aggregation, retention, adsorption or desorption, dissolution or precipitation, transformation, interaction with other molecules, or incorporation (ingestion-egestion) by organisms are common processes undergone by NPs in natural environments (Amde et al., 2017). Most of these processes depend on soil pH. Under acidic conditions, metallic NPs are transformed into ionic species at high rates, while alkaline environments help the aggregation of NPs. For example, ZnO NPs are differently reactive in acidic (pH 5.4) vs. alkaline (pH 8.3) soils, which results in positive germination and growth responses of nine plants in alkaline soil, but also in negative responses in acidic soil (García-Gómez et al., 2018c). CuO NPs are more toxic to barley at low pH, which is coincident with greater Cu dissolution from NPs (Qiu & Smolders, 2017). Hetero-aggregation induced by the pH of metal-based NPs with soil components enhances their electrostatic/steric stability, but hinders their diffusion and transport in soil (Dimkpa, 2018; Ju-Nam & Lead, 2016). Aggregation also involves a diminished particle surface made available for the release ions, which results in a lower dissolution rate that can attenuate their effects on biological systems.
Regardless of soil physiochemical properties and NP intrinsic characteristics, other factors influence the impact of NPs on plants. Root secretions contain organic molecules of high- and low-molecular weights (polysaccharides, fatty acids, amino acids, metal ions, etc.) that can modify the environment of the rhizosphere, the associated microbiome, and the fate of metal-based NPs (Ahmed et al., 2021). That is, NPs can be deposited on or adhered to the root surface, they can release free metal ions, and they can even be chemically modified as a result of the acids and oxidizing-reducing components of exudates (Gao et al., 2018; Zhang et al., 2017). Low-molecular-weight acid root exudates in rice largely determine the aggregation, sedimentation, and dissolution of CuO NPs (Peng et al., 2019). In cucumber, the binding of Cu NPs to synthetic root exudates significantly reduces both Cu uptake and accumulation (Huang et al., 2017). ZnO NPs applied to soybean plants transform into Zn2+ and Zn-citrate due to the lowering soil pH caused by the organic acids secreted by roots. Fe and Cu NPs precipitate as hydroxides (unavailable to plants) owing to exposure to root exudates (Dimkpa et al., 2015; Gao et al., 2018). In turn, the presence of metal-based NPs on the root surface can change the surface chemistry of roots, root secretions, and rhizosphere microbial composition and can, consequently, affect the uptake of nutrients in plants and soil properties. TiO2 and Fe3O4 NPs rise cysteine and methionine contents and induce alterations in phosphorous speciation in lettuce and wheat root exudates (Zahra et al., 2015; Rafique et al., 2018). Ag NPs apparently induce changes in the root exudates of wheat, cowpea, and mustard (Pallavi et al., 2016) and increased the abundance of diazotrophic bacteria in soil (Shah et al., 2014), while CuO NPs induced plant growth-promoting bacteria in the rhizosphere of red sage (Salvia miltiorrhiza) (Wei et al., 2021).
2.3 Positive Effects of Metal-Based NPs on Plants
At appropriate concentrations, metal-based NPs can promote plant growth. They can facilitate nutrient uptake and enhance the efficiency when acting as fertilizers through their slow release (Madzokere et al., 2021; Bindraban et al., 2015) and have the potential to increase plant tolerance to both pathogens and environmental abiotic stressors. Acting efficiently depends on plant species, type and dose of NPs, application method, and growing media (Ananthi et al., 2020). Of the nano-agrochemicals proposed to increase agricultural productivity, metal-/metalloid-based NPs are the commonest ones. Of these, mostly Zn and Cu oxide NPs, followed by Ti and Fe oxide NPs, are used in numerous commercial applications. Hence vast amounts of them will remain as residues (Ruttkay-Nedecky et al., 2017). In crop protection terms, ZnO NPs, Ag NPs, and Cu-based NPs are the most frequently studied ones for their antifungal and antibacterial toxicity (Worrall et al., 2018; Shang et al., 2019; Khan et al., 2019a).
Many studies have evaluated the efficiency of metal-based NPs as fertilizers (Beig et al., 2022; Adisa et al., 2019). Non-nutrient NPs, such as CeO2, TiO2, or SiO2, have positive impacts on plants. By way of example, TiO2 NPs enhance seed vigor and enzyme activities in maize (Shah et al., 2021) and increase P uptake in soybean (Hussain et al., 2021). SiO2 NPs positively affect maize seed germination by making larger amounts of nutrients available after altering the pH and conductivity of the growing medium (Suriyaprabha et al., 2012). Despite this, most fertilizer knowledge pays special attention to those that include micronutrients (Zn-, Fe- and Cu-based NPs). Biofortification by means of nanofertilizers with Zn is an effective method for removing zinc deficiency. ZnO NPs act as a micronutrient source, especially in calcareous soils where the available Zn concentration is generally very low, and slow steady zinc release is needed to adapt and match the plant growth stage (Almendros et al., 2022; Du et al., 2019). FeO NPs applied to lettuce at low concentrations increase the germination rate and root length (Delfani et al., 2014), and Fe2O3 improves the root growth of peanut plants (Rui et al., 2016). Cu NPs enhance shoot length in lettuce and also coriander germination (Verma & Khanam, 2020), and CuO NPs significantly improve wheat and maize yields (Seleiman et al., 2020). Recent reviews include tables that compile the fertilizer effect of several metal-based NPs by detailing NP concentration, crops, and impacts (Agrawal et al., 2022; Ahmed et al., 2021).
Additionally, new NPs have been proposed to overcome the impact of abiotic stress factors. Abiotic stress is a crucial global issue, and climate conditions and environmental contaminants are the primary causes of crop yield loss worldwide. The effects of metal-based NPs, along with other pollutants, are discussed in a specific section of this chapter. Regarding climate conditions, NPs play a beneficial role in overcoming both salinity and drought stress in plants by inducing the expression of several genes involved in stress response, such as those that enhance their antioxidant defense, trigger the signaling pathway of phytohormones, or alter root hydraulic conductance and water uptake (Zhao et al., 2020; Sarraf et al., 2022). It has been recently stated that several NPs also possess antioxidant “enzyme-like” activities: CeO2, Fe3O4, and Co3O4 NPs imitate catalase (CAT); CeO2, Fe3O4, Co3O4, MnO2, CuO, and Au NPs mimic peroxidase; CeO2 and Pt NPs mimic superoxide dismutase (SOD) activity (Sarraf et al., 2022; Liu et al., 2021). Other authors point out another possible way by which NPs reinforce plants’ self-protection against environmental conditions by demonstrating a noticeable rise in the level of some biochemicals like proline or tryptophan. These amino acids play an important role in osmotic adjustment, stomatal regulation, and reactive oxygen species (ROS) scavenging by protecting plants from dehydration (Helaly et al., 2014; Sun et al., 2020; Ramadan et al., 2022). Some examples of NPs that alleviate climatic stress effects on crops are as follows: ZnO NPs improve salt tolerance in tomato (Raghib et al., 2020) and okra (A. esculentus) (Alabdallah & Alzahrani, 2020); Ag NPs relieve saline stress in pearl millet (Khan et al., 2020); doped Fe2O3 NPs mitigate drought stress in B. napus by decreasing the amount of H2O2 and the peroxidation of membrane lipids (Palmqvist et al., 2017); a pretreatment of TiO2, followed by ZnO NPs, improves wheat tolerance to heat stress by enhancing glutathione peroxidase (GPX) and SOD activities, which allows H2O2 levels to lower and membrane stabilization to improve (Thakur et al., 2021). A modern review includes a very comprehensive study about the mechanisms involved in the relation between metal-based NPs and abiotic stress in plants (Sarraf et al., 2022).
An emerging research field is the application of metal-based NPs in agriculture to amplify the production of secondary metabolites in plants. Secondary metabolites are small organic molecules, such as alkaloids, terpenoids, coumarins, phenols, etc., which are derivatives of primary metabolism. They are not necessary for both growth and development, but perform special defensive physiological functions like resistance to diseases and insect pests, adaptation to environmental factors, or participation in biochemical processes related to the crop quality and flavor (Rana et al., 2021; Osbourn, 2000). NPs based on Mn, Cu, Zn, Al, Si, Ti, and Ag have been reported to increase the content of these metabolites. For example, 800 mg kg−1 of CuO NPs increases p-coumaric acid content in cucumber by 225-fold, while 100 mM of Ag NPs rises the anthocyanin level in A. thaliana by 18-fold (Predoi et al., 2020; Zhang et al., 2022). In addition to their protective function for plants, secondary metabolites promoted by NPs have the potential to be used as active ingredients for different purposes in agriculture, medicine, or food sectors (Rana et al., 2021; Predoi et al., 2020).
2.4 Negative Effects of Metal-Based NPs in Plants
The continuous deposition, low biodegradability, and long persistence of metal-based NPs in soils can adversely impact plants and soil organisms, and once these NPs come into contact with plants, they have the potential to alter plant physiology. The evaluation of NP phytotoxicity is a prior key condition for promoting nanotechnology applications and avoiding potential ecological hazards. The negative effects of metal-based NPs on plants are evidenced by the inhibition of the seed germination index (rate and time), alterations to root elongation, root tip morphology, shoot growth, delayed plant development and yield, and lower nutrient uptake, which cause a significant productivity and crop quality losses (Jan et al., 2022). With some exceptions, metal-based NPs are harmful at much higher concentrations than those expected to be found in the environment and those needed for correct plant development (Coman et al., 2019; García-Gómez et al., 2018c). Special attention should be paid to ZnO and CuO NPs because Zn and Cu are essential elements, and the differences between concentrations that act as fertilizers or toxics are small and depend mainly on both soil characteristics and plant species (Obrador et al., 2022; Baskar et al., 2018). Soil pH plays a fundamental role in the phytotoxicity of NPs of metallic origin. In this context, bean and tomato seeds have been grown in two agricultural soils of pH 8.3 and 5.4, in lysimeters, containing ZnO NPs’ concentrations ranging from 3 to 225 mg Zn kg−1 in a greenhouse experiment for 90 days. After 30 days in acid soil, bean plants died regardless of the Zn level, and tomatoes died at the highest dose. On the contrary in calcareous soil, all the tested concentrations allowed normal crop development (García-Gómez et al., 2017). Tables 2.1 and 2.2 provide some examples of phytotoxicity of non-essential and essential metal-based NPs, respectively. Given the large number of found results, only references published in recent years are cited.
Loss of nutritional value of the edible plant part is a negative issue linked with some metal-based NPs. Exposing tomato plants to several metallic NPs (TiO, Ag, Co, Fe3O4, CeO2, and Ni) leads to a reduction in nutrient elements like Mg, P, and S. Exposure of plants to CeO2 NPs results in the smaller amount of starch, antioxidants, glutelin, iron, lauric, and valeric acid in rice harvest grains and altered Mo micronutrient and sugar and phenolic contents, along with protein fractionation in fruit of cucumber plants (Ananthi et al., 2020).
The previously mentioned visible signs are macroscopic evidence of other biochemical, physiological, and molecular alterations of plant processes due to the stress caused by NPs being present at a high rate. As mentioned in the above section, low levels of metal NPs can increase plants’ protective antioxidant mechanisms to limit ROS generation and, hence, oxidative damage. In contrast at high exposure levels, the reaction of NPs with organelles of cells can lead to excessive ROS generation, and cells are unable to maintain normal physiological redox-regulated functions. Excess ROS damages cellular membrane integrity and induces protein denaturation, deficient enzymatic activity, loss of photosynthetic efficiency, and other genotoxic alterations like damaged DNA structure and chromosomal aberrations (Katarína et al., 2021; Yang et al., 2017; Budhani et al., 2019; Tripathi et al., 2017a).
The presence of metal-based NPs in soil may indirectly affect plant growth. The soil environment is a complex system in which each component (soil, soil biota, plants) is interconnected with one another. Some metal-based NPs can increase the abundance of beneficial microbes for soil health and plant development, but even at fertilizer doses, other NPs adversely affect soil microbiota. These are microbes, mainly bacteria and fungi, with key functions, such as plant growth promoters (rhizobacteria), producers of bioactive molecules, or those involved in cellulose/lignin degradation processes (Ameen et al., 2021).
NPs may also impair the soil microbiome involved in biogeochemical processes, mainly the degradation of organic compounds and the recycling of nutrients, including N, P, S, and C, which can ultimately affect plant development (García-Gómez et al., 2018a). Recent reviews include detailed data about the effects of several metal-based NPs on soil and beneficial plant-associated microorganisms (Ameen et al., 2021; Kalwani et al., 2022). For example, Ag NPs affect the symbiotic relation between fava bean (V. faba) with R. leguminosarum or G. aggregatum or a combination of both cultures. Moreover, Ag NPs significantly stunted nitrogenase activity, nodulation, mycorrhizal colonization, and glomalin content (Abd-Alla et al., 2016). Similarly, TiO2 NPs disrupt the Rhizobium–legume (garden pea) symbiosis system. TiO2 NPs induce morphological changes in pea roots, such as delayed nodulation development, which hence lead to the onset of nitrogen fixation and damage to the cell surface of Rhizobium leguminosarum (Fan et al., 2014).
NPs can indirectly impact the plant growth and development due to the combined action with other contaminants present in the exposure media. This issue is of major concern and is dealt with separately in the next section.
2.5 Nanoparticle Interactions with Co-existing Contaminants
The co-existence of NPs and other contaminants in the environment may result in unexpected toxic effects and changes in the accumulation of both NPs and convectional contaminants in plants. The majority of the works published in the literature deal with the influence of NPs on the toxicity/accumulation of these contaminants. The impact of other contaminants on NPs toxicity is examined to a lesser extent, although these studies are increasing in number. The third group of studies focuses on the joint toxicity of both pollutants (NPs and conventional contaminants) by taking into account the mutual interaction of chemicals in the biological effects of the mixture. Joint toxicity can be similar (additive), stronger (synergistic), or weaker (antagonistic) than that expected from the toxicity of individual components. The application of mathematical models, based on a two-factorial analysis of variance (ANOVA), an isobologram analysis, and toxic unit indices, allows the type of interaction to be determined (Cedergreen, 2014; Uwizeyimana et al., 2017). The application of these models to evaluate the joint toxicity of NP-chemical mixtures to plants is still scarce, although some exceptions exist (Ma et al., 2017).
The combined action of NPs and contaminants depends on several factors, such as the intrinsic properties of NPs and chemicals, crop species, experimental conditions (hydroponic or natural soil media), and exposure mode (direct to soil, foliar, seed treatment, etc.). Most studies have investigated the joint toxicity and bioaccumulation of metal-based NPs and co-contaminants on plants under hydroponic conditions, although the tests conducted with natural soil provide the most reliable data. These tests generally measure traditional endpoints, such as germination, growth, and development, as well as biomarkers of oxidative stress. Gene and protein expression measurements (Pagano et al., 2017), DNA alterations (Zhu et al., 2019), and metabolic profile changes (Lian et al., 2020) have been investigated to a lesser extent, even though they may help to reveal the mechanisms of interaction between contaminants. These assessments with chemical mixtures are generally made at much higher concentrations than realistic environmental concentrations to observe significant toxicity. They are also carried out with pristine nanomaterials despite NPs in the environment being subject to transformation processes (aging), which can affect the interactions of NPs with co-existing contaminants and, hence, their accumulation and toxicity (Jośko et al., 2021a; Servin et al., 2017b). However, studies with environmentally transformed NPs are very scarce.
2.6 Mechanisms Underlying the Influence of NP-Contaminant Interaction on the Joint Toxicity
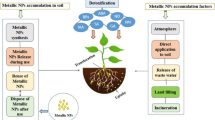
The mechanisms that underlie changes in toxicity due to co-exposure are complex, scarcely investigated, and poorly understood, especially those conducted in soil. They involve several processes that can individually or simultaneously occur. Combined exposure can alter both availability and degradation in exposure media, modify uptake and internalization in plant cells, and modulate the metabolic processes related to the mechanisms of action, detoxification, and excretion of components from mixtures (Naasz et al., 2018; Deng et al., 2017). Figure 2.1 summarizes the relevant mechanisms.
Many studies attribute co-exposure effects on the toxicity and accumulation of NPs and contaminants to changes in the availability of chemicals for organisms (Khan et al., 2019b; Zhang & Zhang, 2020; Adrees et al., 2020). NPs are characterized by high reactivity, a large specific surface area, and strong adsorption capacity. If contaminants are adsorbed to NPs or held in precipitating NP aggregates, the availability and bioaccumulation of these co-existing contaminants are likely to reduce (Bao et al., 2019; Ma et al., 2017). Co-contaminants can modify surface properties and/or transform the functional groups that coat NPs, which lead to changes in their electronegativity and promote the formation of homo- and heteroaggregates of NPs (Xiao et al., 2021). Both aggregation and adsorption processes can reduce bioavailability and slow down the dissolution of metal-based NPs and, therefore, the release of metal ions (Xiao et al., 2022) with consequent effects on plant toxicity. Indirectly co-present heavy metals can also induce excretion of root exudates, which affects NP aggregation (Sharifan et al., 2020). In soil, complex interactions (adsorption, competition) occur among NPs, co-contaminants, soil particles, and organic matter. The NPs and metals released from them can compete with other metals and contaminants for sorption sites, which might alter the availability of NPs and chemicals for plants under co-contamination conditions (Zhang et al., 2019; Naasz et al., 2018). NPs can also affect the formation of soil aggregates and can, thus, indirectly change heavy metal distribution in soils and their availability (Zhang & Zhang, 2020). In turn, NPs can be modified by edaphic and soil biotic factors.
The second mechanism of interaction focuses on the processes related to the uptake and translocation of metals (Skiba et al., 2020; Sharifan et al., 2020) and organic contaminants (Bao et al., 2019; De La Torre-Roche et al., 2013) in plants as a result of co-exposure. The uptake of xenobiotics by plants can be affected because the contaminants in the mixture can (i) compete for the same transporters and binding sites on the cell membrane; (ii) modify hydrophobicity or damage the cell membrane’s physical integrity; and (iii) alter the performance of membrane transport proteins, as well as the metabolic processes involved in the uptake and sequestration of substances in cellular compartments. In addition, the adhesion of NPs to the root surface can act as a physical barrier, which can hinder the uptake of other substances by plants. Organic compounds can change the electronegativity or affect the coating groups on the NP surface, which affects the nano-interaction with organism/cell surfaces.
Adsorption of metals and organic compounds on NP surfaces can display dual behavior with contradictory consequences. NPs can act as carriers of chemicals, which facilitate the entry of substances in cells (the Trojan horse-type phenomenon) (Naasz et al., 2018). Once inside organisms, the subsequent release of adsorbed contaminants can enhance the phytotoxic effects of these substances. Conversely, sorption of compounds to NPs can prevent chemicals from accumulating in plants if NPs reduce the availability of contaminants, as indicated above, or the NP-compound complex is negligibly internalized by plants. A third scenario can occur, in which NPs facilitate the uptake of compounds, but sorption is irreversible, and compounds remain attached to NPs inside organisms. In these cases, the toxicity of NP-contaminant combinations can be expected to diminish.
Finally, the impact of combined exposure can result from alterations to the metabolic processes involved in toxicity and detoxification mechanisms or those related to tolerance to contaminant stress (e.g., antioxidant enzymes involved in oxidative stress tolerance) (Jośko et al., 2021a; Kamali-Andani et al., 2022; Rizwan et al., 2019a, b). NPs can increase the toxicity of organic compounds by either facilitating transformation to compounds being more toxic than parents or hindering the interior degradation rate of these organic compounds and their excretion, which can imply a higher compound concentration in organisms (Deng et al., 2017).
2.7 Effects of Combined Exposure to NPs and Co-existing Contaminants on Their Accumulation and Toxicity to Plants
In plants, the interaction of NPs with pre-existing contaminants leads to changes in their biological effects (bioaccumulation and/or toxicity). Most studies have observed reduced chemical accumulation in plants in the presence of NPs (Hussain et al., 2019; Rizwan et al., 2019a, b). However in some exceptions, NPs promote the accumulation of metal ions (Xiao et al., 2022; Venkatachalam et al., 2017) and organic compounds (Bao et al., 2019). Changes observed in the bioaccumulation of contaminants do not always correlate with changes in toxicity in plants. Negative biological effects generally decrease with declining bioaccumulation (Ma et al., 2017; Ji et al., 2017; Hussain et al., 2019). In some cases, enhanced chemical accumulation in the presence of NPs does not lead to greater toxicity compared to individual treatments (Venkatachalam et al., 2017; Zhang et al., 2019). Some studies report changes in toxicity upon mixture exposure, but no changes in accumulation (Haisel et al., 2019).
2.7.1 The Interaction Between NPs and Metal/Metalloid
The phytotoxic effects of co-exposure to NP-contaminant mixtures on plants are tested mainly with metals as co-contaminants where the combination with Cd predominates (Table 2.3). Cd is one of the major pollutants in soils, and it is well-known that it affects the biochemical and physiological plant functions and can accumulate in edible tissues. Many authors have reported data collected from experiments performed in hydroponic media with conflicting results. For example, TiO2 NPs alleviate Cd toxicity (net photosynthetic rate and chlorophyll content) and decrease Cd uptake in roots and leaves of rice (Oryza sativa L.) (Ji et al., 2017). In turn, the presence of Cd significantly decreases Ti accumulation in rice roots. Similarly, Venkatachalam et al. (2017) report that phycomolecule-coated ZnO NPs (25 mg L−1) enhance seedling growth, reverse the oxidative stress symptoms induced by Cd and Pb, and induce desirable genomic alterations in Leucaena leucocephala. However, unlike the previous paper, NPs increase Cd and Pb accumulation in plant tissues. An opposite trend is indicated in a hydroponic study with Carex vulpina (Haisel et al., 2019), where ZnO NPs at low concentrations (10 or 50 μM of Zn) significantly aggravate the negative effect of Cd, which is reflected mostly in changes in the content of photosynthetic pigments. Exposure mode and contaminant levels are key factors in plant response to co-exposure to NPs and metals according to Lian et al. (2020), who studied the combined effect of TiO2 NPs and Cd on metal accumulation and toxicity to hydroponic maize (Zea mays L.). Root applications of TiO2 NPs and Cd synergistically inhibit plant growth and development, while the foliar spray of TiO2 NPs can partially protect plants from Cd stress. Similarly at low Cu concentrations (1 and 2 mg Cu L−1), TiO2 NPs enhance the toxicity and accumulation of Cu in soybean, whereas the effects caused by the co-presence of TiO2 NPs disappear at 5 and 20 mg Cu L−1 (Xiao et al., 2021). Cu adsorption in TiO2 NPs increases with a rising Cu concentration, with the subsequent reduction in the zeta-potential, aggregation, and sedimentation of TiO2 NPs. This fact can lead to a lower Cu and Ti concentration in hydroponic media, and, consequently, Cu toxicity can be alleviated.
The contaminant type also plays an important role in joint bioaccumulation. A hydroponic study with five forms of ZnO NPs (100 mg L−1) reports that NPs alter Cu, Mn, and Fe uptake and translocation in pea (Pisum sativum L.) plants, but effects are element-specific (Skiba et al., 2020). Similar behavior has been observed with two non-essential metals (Pb and Cd), where the influence of ZnO NPs on the accumulation of these metals in the edible tissue of three leafy green species is impacted by the co-contaminant nature (Sharifan et al., 2020). Additionally, metal-based NPs with oxidizing or reducing properties can regulate the oxidation states of some metals and, hence, their uptake and toxicity to plants (Cao et al., 2020). Combined exposure to CeO2 NPs or ZnO NPs and inorganic As species differently affects As(III)/As(V) accumulation and speciation in rice (Oryza sativa L.) (Wang et al., 2018c).
The joint toxicity and bioaccumulation of NPs and metals have been also studied in plants growing in soil. A fair number of studies conducted with Cd-contaminated soils indicate that ZnO NPs and Fe3O4 NPs applied by different routes (soil exposure, foliar spray, seed priming) mitigate Cd phytotoxicity to wheat (Triticum aestivum) (Hussain et al., 2018, 2019; Rizwan et al., 2019a, b). Decreased toxicity has been generally associated with reduced Cd accumulation in plants, which might be due to a drop in available Cd in soil. These outcomes are similar to those obtained in two experiments performed under water-limited conditions (Khan et al., 2019b; Adrees et al., 2020). Si NPs applied directly to soil or as foliar spray also promote yield and reduce Cd accumulation in wheat (Ali et al., 2019). Si NPs reduce Cd accumulation in plants by lowering Cd available concentrations in soil. With foliar applications, diminished Cd accumulation may be due to other causes, such as dilution effects because of increased growth or compartmentation into vacuoles, which restrict metal translocation to grain.
In contrast, co-exposure to ZnO NPs and Cd amplifies toxicity (root cell damage and increased oxidative stress) to Phytolacca americana L. (Xiao et al., 2022). In this study, Cd2+ promotes the release of Zn ions from ZnO NPs due to the interaction of Cd on NP surfaces, which can explain the increased toxicity of the mixture. In addition, ZnO NPs considerably increase Cd accumulation.
Interestingly, both synergistic and antagonistic effects of the ZnO NPs and Cd mixture appear in sweet sorghum (Sorghum bicolor) grown in soil depending on the contaminant concentration (Wang et al., 2018a). The mixture shows synergism at the two highest doses (250 and 500 mg Zn kg−1) of ZnO NPs. ZnO NPs are non-phytotoxic at the lowest dose (50 mg Zn kg−1) and show antagonistic interactions with Cd in plant growth. All the ZnO NPs’ doses significantly lower the Cd concentrations in sorghum shoots and roots, whereas the effect of Cd on Zn accumulation depends on the Zn rate. In addition to the application rate, the plant growth stage is an important factor for the biological effects that result from co-exposure. In a soil-rice system (Zhang et al., 2019), the main impact of NPs on Cd toxicity and bioaccumulation appears in the tillering stage, where ZnO NPs ameliorate toxic Cd effects (plant height). However, this effect diminishes over time and disappears in the fruiting stage.
2.7.2 The Interaction Between Different NPs
Only a few studies have investigated the impact of NP mixtures on plants, even though a variety of NPs may co-exist in the natural environment (Table 2.4). Two experiments in soilless culture media have assessed the effects of binary mixtures of metal-based NPs on plants with different results. In a germination assay with five different NPs (ZnO, CuO, TiO2, Cr2O3, and Fe2O3) and four plant species (cress, flax, wheat, and cucumber), Jośko et al. (2017) have found that co-exposure at 100 mg L−1 exerts significantly less toxicity (root growth inhibition) compared to single exposure and regardless of its components. In another study, binary combinations of five NPs have shown increased or decreased metal content and toxicity to zucchini (Cucurbita pepo L.) grown in vermiculite for 21 days depending on NP combinations (Pagano et al., 2017). Both experiments suggest that the differences in toxicity observed between simple and combined treatments, and between different binary NP mixtures, can be explained by distinct solubility and the ratio of the particulate/ionic forms that derive from NPs, as well as greater particle aggregation under combined stress conditions.
Two soil experiments have confirmed the influence of dose and exposure time on the toxicity magnitude of NP mixtures. Kamali-Andani et al. (2022) have observed that Se NPs modify the stress caused by CeO2 NPs on mung bean (Vigna radiata) plants grown under greenhouse conditions, but this effect depends on the foliar application rates of both NPs. The low concentrations of Se NPs (25 and 50 mg Se L−1) improve photosynthesis by increasing antioxidant activity and proline content, which lowers the levels of ROS and lipid peroxidation caused by CeO2 NPs. Other noteworthy studies indicate that the effects of co-exposure to CuO and ZnO NPs on toxicity and metal accumulation on soil-grown barley (Hordeum vulgare L.) vary with exposure time (7 and 30 days), although a general tendency is not easy to identify (Jośko et al., 2021a, b). Their findings reveal that co-exposure results in the downregulation of the genes related to the metal influx to cells. Interestingly, the binary mixtures of CuO and ZnO NPs have antagonistic effects on Zn and Cu availability in soil, whereas mixtures of their metal salts show synergism. Soil-extractable Zn and Cu concentrations weakly correlate with Cu and Zn contents in barley.
2.7.3 The Interaction Between NPs and Organic Compounds
Both decreases and increases in toxicity and contaminant accumulation in plants due to interactions between metallic NPs and organic compounds have been reported (Table 2.5). For example, in an interesting study, Ma et al. (2017) have investigated the joint effects of TiO2 NPs and tetracycline (TC) on rice (Oryza sativa L.) grown in hydroponic media for 10 days. Three mathematical models are applied to toxicity (plant growth, changes in oxidative stress enzymes, and macro-/micronutrient contents) data to establish the type of toxic interaction, i.e., synergistic, additive, or antagonistic, to result from co-exposure. The analyses indicate that TiO2 NPs and TC antagonistically interact, showing overall phytotoxicity alleviation compared to that expected of the toxicity of individual treatments. Decreased phytotoxicity is accompanied by low TC levels in plants. This is probably due to the sorption of the antibiotic into TiO2 NPs, which can decrease its availability for rice seedlings. However, Ti levels in rice shoots and roots rise in the combined treatment, which is attributed to the alteration of surface charges of TiO2 NPs caused by TC. In contrast, hydroponically exposed wheat (Triticum aestivum L.) to phenanthrene and ZnO (NPs and bulk) mixtures shows greater toxicity compared to individual treatments (Zhu et al., 2019). This effect is more evident in DNA damage in wheat root cells, especially for ZnO NPs. In another study, the plant response to the combined exposure to NPs and an organic contaminant strongly depends on the concentration of both xenobiotics (Zhang et al., 2020). At low concentrations (50 and 250 mg L−1), zero-valent iron (ZVI) NPs alleviate the toxicity (root length) of quinclorac herbicide (QNC) to Oryza sativa L. However, this effect disappears at the high ZVI NPs’ concentration (750 mg L−1), which is possibly due to the toxicity of ZVI NPs itself at this concentration. QNC content in both shoots and roots lowers compared to the tissues exposed to QNC alone, probably because ZVI NPs remove QNC from culture solution.
Experiments conducted simultaneously with other size particles and metal-based salt are particularly interesting because they allow the role of NP-specific properties in the interaction to be evaluated. Several works have evidenced that NPs’ co-exposure with metals or organic compounds can elicit different biological responses in plants to those caused by the other chemical forms. For example, De La Torre-Roche et al. (2013) have demonstrated that the effects caused by Ag NPs on the accumulation and translocation of dichlorodiphenyldichloroethylene (DDE) in soybean (Glycine max L.) and zucchini (Cucurbita pepo L.) grown in vermiculite differ from those caused by bulk or ionic Ag.
Similarly, the influence of oxytetracycline (OTC) on Fe accumulation in rice tissue (Oryza sativa L.) differs for plants exposed to ionic Fe or Fe2O3 NPs (Bao et al., 2019). OTC promotes Fe accumulation on root surfaces and shoots in Fe2O3 NPs treatments, which is the exact opposite result of Fe-EDTA treatments. The presence of ZnO (NPs and bulk) reduces phenanthrene accumulation in wheat (roots and leaves), but this effect is stronger for NPs than for bulk counterparts. This is probably due a stronger sorption capacity of NPs than bulk material (Zhu et al., 2019). Interestingly, ZnO (NPs and bulk) increases the detrimental effects of Cd on hydroponic Carex vulpina L. plants, whereas Zn salt protects plants against Cd-induced toxicity (Haisel et al., 2019). Although these results are not conclusive, they indicate some possible underlying mechanisms related to the NP properties inherent to their size that affect the interaction of NPs with conventional co-contaminants. This fact emphasizes the need to consider the combined action of NPs with other contaminants present in media to assess and regulate the environmental impacts of NP applications.
2.8 Conclusions
Metal-based NPs have many positive effects on plants which encourage their use to improve crop production and sustainable agriculture, although they also have detrimental effects. Among others, they may produce physicochemical soil alterations, modify the rhizosphere environment, and have toxic effects on plants and soil biota, particularly on beneficial microbial populations. Notwithstanding, the demonstrated fertilizing effects of metal-based NPs on crops, and the increased resistance ability of plants exposed to climatic stressor factors and pathogens, make nanotechnology a promising tool that is currently underused. Controversial results have been found in the published literature, which show positive or negative effects of NPs depending on many factors related not only to NPs’ properties and plant species, but also to culture media and exposure conditions. The potential effects of NPs on plants due to the interaction with other contaminants have been less studied. The results confirm the active interactions between NPs and co-existing contaminants, which can be synergistic or antagonistic depending on the intrinsic properties of NPs and co-contaminants, plant species, and, more importantly, the application rate. Other factors like exposure mode, plant growth stage, and exposure time also influence joint toxicity. From a risk perspective, the occurrence of synergistic interactions is the biggest concern.
One of the most evident difficulties that limits the use of NPs in agriculture is to compare the results between the studies performed under different experimental conditions that determine outcomes. Therefore, a more systematic approach with standardized protocols that defines the many involved parameters as much as possible is necessary. In addition, a gap has been detected in knowledge of the real joint effects of NP-chemical mixtures. Further studies are needed to acquire more knowledge about the mechanisms of NP interactions with co-existing contaminants, including a comparative study with bulk particles and their ionic counterparts. The possible applications and uses of nanotechnologies in agriculture require the joint effects of NPs and co-contaminants being taken into account to establish regulatory guidelines.
Future research into metal-based NPs will address the precise release of nutrients adapted to soil features and crop needs. NPs will regulate the uptake of beneficial and harmful chemicals by plants. Simultaneously, NPs will allow plants to enforce their defenses against external stress agents and to improve their potential in stimulating plants to produce natural active molecules. Ultimately in the near future, NPs will enable us to accomplish sustainable agriculture by reducing inputs and chemical residues in crops.
Abbreviations
- CAT:
-
Catalase
- DDE:
-
Dichlorodiphenyldichloroethylene
- GPX:
-
Glutathione peroxidase
- OTC:
-
Oxytetracycline
- QD:
-
Quantum dot
- QNC:
-
Quinclorac
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TC:
-
Tetracycline
- ZVI:
-
Zero-valent iron
References
Abd-Alla, M. H., Nafady, N. A., & Khalaf, D. M. (2016). Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: Implications for induction of autophagy process in root nodule. Agriculture, Ecosystems & Environment, 218, 163–177.
Adisa, I. O., Pullagurala, V. L. R., Peralta-Videa, J. R., Dimkpa, C. O., Elmer, W. H., Gardea-Torresdey, J. L., & White, J. C. (2019). Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environmental Science: Nano, 6, 2002–2030.
Adrees, M., Khan, Z. S., Ali, S., Hafeez, M., Khalid, S., ur Rehman, M. Z., Hussain, A., Hussain, K., Shahid Chatha, S. A., & Rizwan, M. (2020). Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere, 238, 124681.
Agrawal, S., Kumar, V., Kumar, S., & Shahi, S. K. (2022). Plant development and crop protection using phytonanotechnology: A new window for sustainable agriculture. Chemosphere, 299, 134465.
Ahmed, B., Rizvi, A., Ali, K., Lee, J., Zaidi, A., Khan, M. S., & Musarrat, J. (2021). Nanoparticles in the soil–plant system: A review. Environmental Chemistry Letters, 19, 1545–1609.
Akanbi-Gada, M. A., Ogunkunle, C. O., Vishwakarma, V., Viswanathan, K., & Fatoba, P. O. (2019). Phytotoxicity of nano-zinc oxide to tomato plant (Solanum lycopersicum L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environmental Technology & Innovation, 14, 100325.
Alabdallah, N. M., & Alzahrani, H. S. (2020). The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi Journal of Biological Sciences, 27, 3132–3137.
Ali, S., Rizwan, M., Hussain, A., Zia ur Rehman, M., Ali, B., Yousaf, B., Wijaya, L., Alyemeni, M. N., & Ahmad, P. (2019). Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiology and Biochemistry, 140, 1–8.
Ali, S., Mehmood, A., & Khan, N. (2021). Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. Journal of Nanomaterials, 2021, 6677616.
Almendros, P., González, D., Fernández, M. D., García-Gomez, C., & Obrador, A. (2022). Both Zn biofortification and nutrient distribution pattern in cherry tomato plants are influenced by the application of ZnO nanofertilizer. Heliyon, 8, e09130.
Amde, M., Liu, J.-f., Tan, Z.-Q., & Bekana, D. (2017). Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environmental Pollution, 230, 250–267.
Ameen, F., Alsamhary, K., Alabdullatif, J. A., & Alnadhari, S. (2021). A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicology and Environmental Safety, 213, 112027.
Ananthi, V., Mohanrasu, K., Boobalan, T., Anand, K., Sudhakar, M., Chuturgoon, A., Balasubramanian, V., Yuvakkumar, R., & Arun, A. (2020). An overview of nanotoxicological effects towards plants, animals, microorganisms and environment. In A. Krishnan & A. Chuturgoon (Eds.), Integrative nanomedicine for new therapies (pp. 113–146). Springer International Publishing.
Andersen, C. P., King, G., Plocher, M., Storm, M., Pokhrel, L. R., Johnson, M. G., & Rygiewicz, P. T. (2016). Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environmental Toxicology and Chemistry, 35, 2223–2229.
Apodaca, S. A., Tan, W., Dominguez, O. E., Hernandez-Viezcas, J. A., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2017). Physiological and biochemical effects of nanoparticulate copper, bulk copper, copper chloride, and kinetin in kidney bean (Phaseolus vulgaris) plants. Science of the Total Environment, 599–600, 2085–2094.
Bao, Y. Y., Ma, C. X., Hu, L., & Xing, B. S. (2019). Effect of individual and combined exposure of Fe2O3 nanoparticles and oxytetracycline on their bioaccumulation by rice (Oryza sativa L.). Journal of Soils and Sediments, 19, 2459–2471.
Baskar, V., Nayeem, S., Kuppuraj, S. P., Muthu, T., & Ramalingam, S. (2018). Assessment of the effects of metal oxide nanoparticles on the growth, physiology and metabolic responses in in vitro grown eggplant (Solanum melongena). 3 Biotech, 8, 362.
Beig, B., Niazi, M. B. K., Sher, F., Jahan, Z., Malik, U. S., Khan, M. D., Américo-Pinheiro, J. H. P., & Vo, D.-V. N. (2022). Nanotechnology-based controlled release of sustainable fertilizers. A review. Environmental Chemistry Letters, 20, 2709.
Bindraban, P. S., Dimkpa, C., Nagarajan, L., Roy, A., & Rabbinge, R. (2015). Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biology and Fertility of Soils, 51, 897–911.
Budhani, S., Egboluche, N. P., Arslan, Z., Yu, H., & Deng, H. (2019). Phytotoxic effect of silver nanoparticles on seed germination and growth of terrestrial plants. Journal of Environmental Science and Health, Part C, 37, 330–355.
Burachevskaya, M., Minkina, T., Mandzhieva, S., Bauer, T., Nevidomskaya, D., Shuvaeva, V., Sushkova, S., Kizilkaya, R., Gülser, C., & Rajput, V. (2021). Transformation of copper oxide and copper oxide nanoparticles in the soil and their accumulation by Hordeum sativum. Environmental Geochemistry and Health, 43, 1655–1672.
Cao, W. C., Gong, J. L., Zeng, G. M., Song, B., Zhang, P., Li, J., Fang, S. Y., Qin, L., Ye, J., & Cai, Z. (2020). Mutual effects of silver nanoparticles and antimony(iii)/(v) co-exposed to Glycine max (L.) Merr. in hydroponic systems: Uptake, translocation, physiochemical responses, and potential mechanisms. Environmental Science: Nano, 7, 2691–2707.
Cedergreen, N. (2014). Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS One, 9, e96580.
Chichiriccò, G., & Poma, A. (2015). Penetration and toxicity of nanomaterials in higher plants. Nanomaterials (Basel), 5, 851–873.
Coman, V., Oprea, I., Leopold, L. F., Vodnar, D. C., Coman C. (2019). Soybean Interaction with Engineered Nanomaterials: A Literature Review of Recent Data. Nanomaterials 9(9),1248. https://doi.org/10.3390/nano9091248
Corral-Diaz, B., Peralta-Videa, J. R., Alvarez-Parrilla, E., Rodrigo-García, J., Morales, M. I., Osuna-Avila, P., Niu, G., Hernandez-Viezcas, J. A., & Gardea-Torresdey, J. L. (2014). Cerium oxide nanoparticles alter the antioxidant capacity but do not impact tuber ionome in Raphanus sativus (L). Plant Physiology and Biochemistry, 84, 277–285.
De La Torre-Roche, R., Hawthorne, J., Musante, C., Xing, B., Newman, L. A., Ma, X., & White, J. C. (2013). Impact of Ag nanoparticle exposure on p,p′-DDE bioaccumulation by Cucurbita pepo (Zucchini) and Glycine max (Soybean). Environmental Science & Technology, 47, 718–725.
Delfani, M., Baradarn Firouzabadi, M., Farrokhi, N., & Makarian, H. (2014). Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Communications in Soil Science and Plant Analysis, 45, 530–540.
Deng, Y.-q., White, J. C., & Xing, B.-s. (2014). Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. Journal of Zhejiang University Science A, 15, 552–572.
Deng, R., Lin, D., Zhu, L., Majumdar, S., White, J. C., Gardea-Torresdey, J. L., & Xing, B. (2017). Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology, 11, 591–612.
Dimkpa, C. O. (2018). Soil properties influence the response of terrestrial plants to metallic nanoparticles exposure. Current Opinion in Environmental Science & Health, 6, 1–8.
Dimkpa, C. O., McLean, J. E., Martineau, N., Britt, D. W., Haverkamp, R., & Anderson, A. J. (2013). Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environmental Science & Technology, 47, 1082–1090.
Dimkpa, C. O., McLean, J. E., Britt, D. W., & Anderson, A. J. (2015). Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology, 24, 119–129.
Du, W., Gardea-Torresdey, J. L., Ji, R., Yin, Y., Zhu, J., Peralta-Videa, J. R., & Guo, H. (2015). Physiological and biochemical changes imposed by CeO2 nanoparticles on wheat: A life cycle field study. Environmental Science & Technology, 49, 11884–11893.
Du, W., Tan, W., Yin, Y., Ji, R., Peralta-Videa, J. R., Guo, H., & Gardea-Torresdey, J. L. (2018). Differential effects of copper nanoparticles/microparticles in agronomic and physiological parameters of oregano (Origanum vulgare). Science of the Total Environment, 618, 306–312.
Du, W., Yang, J., Peng, Q., Liang, X., & Mao, H. (2019). Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere, 227, 109–116.
Fan, R., Huang, Y. C., Grusak, M. A., Huang, C. P., & Sherrier, D. J. (2014). Effects of nano-TiO2 on the agronomically-relevant Rhizobium–legume symbiosis. Science of the Total Environment, 466–467, 503–512.
Fernández, M. D., Obrador, A., & García-Gómez, C. (2021). Zn concentration decline and apical endpoints recovery of earthworms (E. andrei) after removal from an acidic soil spiked with coated ZnO nanoparticles. Ecotoxicology and Environmental Safety, 211, 111916.
Frazier, T. P., Burklew, C. E., & Zhang, B. (2014). Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum). Functional & Integrative Genomics, 14, 75–83.
Gao, X., Avellan, A., Laughton, S., Vaidya, R., Rodrigues, S. M., Casman, E. A., & Lowry, G. V. (2018). CuO nanoparticle dissolution and toxicity to wheat (Triticum aestivum) in rhizosphere soil. Environmental Science & Technology, 52, 2888–2897.
Gao, X., Rodrigues, S. M., Spielman-Sun, E., Lopes, S., Rodrigues, S., Zhang, Y., Avellan, A., Duarte, R. M. B. O., Duarte, A., Casman, E. A., & Lowry, G. V. (2019). Effect of soil organic matter, soil pH, and moisture content on solubility and dissolution rate of CuO NPs in soil. Environmental Science & Technology, 53, 4959–4967.
García-Gómez, C., & Fernández, M. D. (2019). Impacts of metal oxide nanoparticles on seed germination, plant growth and development. In S. K. Verma & A. K. Das (Eds.), Comprehensive analytical chemistry (pp. 75–124). Elsevier.
García-Gómez, C., Babin, M., Obrador, A., Álvarez, J. M., & Fernández, M. D. (2015). Integrating ecotoxicity and chemical approaches to compare the effects of ZnO nanoparticles, ZnO bulk, and ZnCl2 on plants and microorganisms in a natural soil. Environmental Science and Pollution Research, 22, 16803–16813.
García-Gómez, C., Obrador, A., González, D., Babín, M., & Fernández, M. D. (2017). Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Science of the Total Environment, 589, 11–24.
García-Gómez, C., Fernández, M. D., García, S., Obrador, A. F., Letón, M., & Babín, M. (2018a). Soil pH effects on the toxicity of zinc oxide nanoparticles to soil microbial community. Environmental Science and Pollution Research, 25, 28140–28152.
García-Gómez, C., García, S., Obrador, A. F., González, D., Babín, M., & Fernández, M. D. (2018b). Effects of aged ZnO NPs and soil type on Zn availability, accumulation and toxicity to pea and beet in a greenhouse experiment. Ecotoxicology and Environmental Safety, 160, 222–230.
García-Gómez, C., Obrador, A., González, D., Babín, M., & Fernández, M. D. (2018c). Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Science of the Total Environment, 644, 770–780.
García-Gómez, C., García-Gutiérrez, S., Obrador, A., Almendros, P., González, D., & Fernández, M. D. (2020). Effect of ageing of bare and coated nanoparticles of zinc oxide applied to soil on the Zn behaviour and toxicity to fish cells due to transfer from soil to water bodies. Science of the Total Environment, 706, 135713.
Gonzalez-Moscoso, M., Juarez-Maldonado, A., Cadenas-Pliego, G., Meza-Figueroa, D., SenGupta, B., & Martinez-Villegas, N. (2022). Silicon nanoparticles decrease arsenic translocation and mitigate phytotoxicity in tomato plants. Environmental Science and Pollution Research, 29, 34147–34163.
Haisel, D., Cyrusova, T., Vanek, T., & Podlipna, R. (2019). The effect of nanoparticles on the photosynthetic pigments in cadmium-zinc interactions. Environmental Science and Pollution Research, 26, 4147–4151.
Helaly, M. N., El-Metwally, M. E. A., El-Hoseiny, H., Omar, S. A., & Elsheery, N. I. (2014). Effect of nanoparticles on biological contamination of in vitro cultures and organogenic regeneration of banana. Australian Journal of Crop Science, 8, 612–624.
Huang, Y., Zhao, L., & Keller, A. A. (2017). Interactions, transformations, and bioavailability of nano-copper exposed to root exudates. Environmental Science & Technology, 51, 9774–9783.
Hussain, A., Ali, S., Rizwan, M., ur Rehman, M. Z., Javed, M. R., Imran, M., Chatha, S. A. S., & Nazir, R. (2018). Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environmental Pollution, 242, 1518–1526.
Hussain, A., Ali, S., Rizwan, M., Rehman, M. Z. U., Qayyum, M. F., Wang, H., & Rinklebe, J. (2019). Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicology and Environmental Safety, 173, 156–164.
Hussain, S., Shafiq, I., Skalicky, M., Brestic, M., Rastogi, A., Mumtaz, M., Hussain, M., Iqbal, N., Raza, M. A., Manzoor, S., Liu, W., & Yang, W. (2021). Titanium application increases phosphorus uptake through changes in auxin content and root architecture in soybean (Glycine Max L.). Frontiers in Plant Science, 12, 743618.
Jan, N., Majeed, N., Ahmad, M., Ahmad Lone, W., & John, R. (2022). Nano-pollution: Why it should worry us. Chemosphere, 302, 134746.
Ji, Y., Zhou, Y., Ma, C., Feng, Y., Hao, Y., Rui, Y., Wu, W., Gui, X., Le, V. N., Han, Y., Wang, Y., Xing, B., Liu, L., & Cao, W. (2017). Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiology and Biochemistry, 110, 82–93.
Jośko, I., Oleszczuk, P., & Skwarek, E. (2017). Toxicity of combined mixtures of nanoparticles to plants. Journal of Hazardous Materials, 331, 200–209.
Jośko, I., Dobrzyńska, J., Dobrowolski, R., Kusiak, M., & Terpiłowski, K. (2020). The effect of pH and ageing on the fate of CuO and ZnO nanoparticles in soils. Science of the Total Environment, 721, 137771.
Jośko, I., Kusiak, M., Oleszczuk, P., Swieca, M., Konczak, M., & Sikora, M. (2021a). Transcriptional and biochemical response of barley to co-exposure of metal-based nanoparticles. Science of the Total Environment, 782, 146883.
Jośko, I., Kusiak, M., Xing, B., & Oleszczuk, P. (2021b). Combined effect of nano-CuO and nano-ZnO in plant-related system: From bioavailability in soil to transcriptional regulation of metal homeostasis in barley. Journal of Hazardous Materials, 416, 126230.
Ju-Nam, Y., & Lead, J. (2016). Properties, sources, pathways, and fate of nanoparticles in the environment. In B. Xing, C. D. Vecitis, & N. Senesi (Eds.), Engineered nanoparticles and the environment: Biophysicochemical processes and toxicity (pp. 93–117). John Wiley & Sons, Inc..
Kalwani, M., Chakdar, H., Srivastava, A., Pabbi, S., & Shukla, P. (2022). Effects of nanofertilizers on soil and plant-associated microbial communities: Emerging trends and perspectives. Chemosphere, 287, 132107.
Kamali-Andani, N., Fallah, S., Peralta-Videa, J. R., & Golkar, P. (2022). A comprehensive study of selenium and cerium oxide nanoparticles on mung bean: Individual and synergistic effect on photosynthesis pigments, antioxidants, and dry matter accumulation. Science of the Total Environment, 830, 154837.
Katarína, K., Masarovičová, E., & Jampílek, J. (2021). Risks and benefits of metal-based nanoparticles for vascular plants. In M. Pessarakli (Ed.), Handbook of plant and crop physiology (pp. 923–963). Taylor & Francis Group.
Khan, M. R., Ahamad, F., & Rizvi, T. F. (2019a). Effect of nanoparticles on plant pathogens. In M. Ghorbanpour & S. H. Wani (Eds.), Advances in phytonanotechnology (pp. 215–240). Academic Press.
Khan, Z. S., Rizwan, M., Hafeez, M., Ali, S., Javed, M. R., & Adrees, M. (2019b). The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environmental Science and Pollution Research, 26, 19859–19870.
Khan, I., Raza, M. A., Awan, S. A., Shah, G. A., Rizwan, M., Ali, B., Tariq, R., Hassan, M. J., Alyemeni, M. N., Brestic, M., Zhang, X., Ali, S., & Huang, L. (2020). Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiology and Biochemistry, 156, 221–232.
Kolenčík, M., Ernst, D., Urík, M., Ďurišová, Ľ., Bujdoš, M., Šebesta, M., Dobročka, E., Kšiňan, S., Illa, R., Qian, Y., Feng, H., Černý, I., Holišová, V., & Kratošová, G. (2020). Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common sunflower under field conditions. Nanomaterials, 10, 1619.
Kornarzyński, K., Sujak, A., Czernel, G., & Wiącek, D. (2020). Effect of Fe3O4 nanoparticles on germination of seeds and concentration of elements in Helianthus annuus L. under constant magnetic field. Scientific Reports, 10, 8068.
Labeeb, M., Badr, A., Haroun, S. A., Mattar, M. Z., El-Kholy, A. S., & El-Mehasseb, I. M. (2020). Ecofriendly synthesis of silver nanoparticles and their effects on early growth and cell division in roots of green pea (Pisum sativum L.). Gesunde Pflanzen, 72, 113–127.
Lian, J., Zhao, L., Wu, J., Xiong, H., Bao, Y., Zeb, A., Tang, J., & Liu, W. (2020). Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere, 239, 124794.
Liu, Y., Pan, B., Li, H., Lang, D., Zhao, Q., Zhang, D., Wu, M., Steinberg, C. E. W., & Xing, B. (2020). Can the properties of engineered nanoparticles be indicative of their functions and effects in plants? Ecotoxicology and Environmental Safety, 205, 111128.
Liu, Y., Xiao, Z., Chen, F., Yue, L., Zou, H., Lyu, J., & Wang, Z. (2021). Metallic oxide nanomaterials act as antioxidant nanozymes in higher plants: Trends, meta-analysis, and prospect. Science of the Total Environment, 780, 146578.
Lizzi, D., Mattiello, A., Piani, B., Gava, E., Fellet, G., & Marchiol, L. (2021). Single and repeated applications of cerium oxide nanoparticles differently affect the growth and biomass accumulation of silene flos-cuculi L. (Caryophyllaceae). Nanomaterials, 11, 229.
Lv, J., Christie, P., & Zhang, S. (2019). Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environmental Science: Nano, 6, 41–59.
Ma, C., Liu, H., Chen, G., Zhao, Q., Eitzer, B., Wang, Z., Cai, W., Newman, L. A., White, J. C., Dhankher, O. P., & Xing, B. (2017). Effects of titanium oxide nanoparticles on tetracycline accumulation and toxicity in Oryza sativa (L.). Environmental Science: Nano, 4, 1827–1839.
Ma, Y., Xie, C., He, X., Zhang, B., Yang, J., Sun, M., Luo, W., Feng, S., Zhang, J., Wang, G., & Zhang, Z. (2020). Effects of ceria nanoparticles and CeCl3 on plant growth, biological and physiological parameters, and nutritional value of soil grown common bean (Phaseolus vulgaris). Small, 16, 1907435.
Madzokere, T. C., Murombo, L. T., & Chiririwa, H. (2021). Nano-based slow releasing fertilizers for enhanced agricultural productivity. Materials Today: Proceedings, 45, 3709–3715.
Mirzajani, F., Askari, H., Hamzelou, S., Farzaneh, M., & Ghassempour, A. (2013). Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicology and Environmental Safety, 88, 48–54.
Molina, L., Wittich, R.-M., van Dillewijn, P., & Segura, A. (2021). Plant-bacteria interactions for the elimination of atmospheric contaminants in cities. Agronomy, 11, 493.
Morales, M. I., Rico, C. M., Hernandez-Viezcas, J. A., Nunez, J. E., Barrios, A. C., Flores-Marges, J. P., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2013). Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. Journal of Agricultural and Food Chemistry, 61, 6224–6230.
Naasz, S., Altenburger, R., & Kuehnel, D. (2018). Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Science of the Total Environment, 635, 1170–1181.
Nhan, L. V., Yukui, R., Weidong, C., Jianying, S., Shutong, L., Trung, N. Q., & Liming, L. (2016). Toxicity and bio-effects of CuO nanoparticles on transgenic Ipt-cotton. Journal of Plant Interactions, 11, 108–116.
Obrador, A., González, D., Almendros, P., García-Gómez, C., & Fernández, M. D. (2022). Assessment of phytotoxicity and behavior of 1-year-aged Zn in soil from ZnO nanoparticles, bulk ZnO, and Zn sulfate in different soil-plant cropping systems: From biofortification to toxicity. Journal of Soil Science and Plant Nutrition, 22, 150–164.
Osbourn, A. E. (2000). Plant secondary metabolites – A primary resource: Biochemistry of plant secondary metabolism and functions of plant secondary metabolites and their exploitation in biotechnology, edited by M. Wink. Trends in Biotechnology, 18, 321–322.
Pagano, L., Pasquali, F., Majumdar, S., De la Torre-Roche, R., Zuverza-Mena, N., Villani, M., Zappettini, A., Marra, R. E., Isch, S. M., Marmiroli, M., Maestri, E., Dhankher, O. P., White, J. C., & Marmiroli, N. (2017). Exposure of Cucurbita pepo to binary combinations of engineered nanomaterials: Physiological and molecular response. Environmental Science: Nano, 4, 1579–1590.
Pallavi, C., Mehta, M., Srivastava, R., Arora, S., & Sharma, A. K. (2016). Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech, 6, 254.
Palmqvist, N. G. M., Seisenbaeva, G. A., Svedlindh, P., & Kessler, V. G. (2017). Maghemite nanoparticles acts as nanozymes, improving growth and abiotic stress tolerance in Brassica napus. Nanoscale Research Letters, 12, 631.
Peng, C., Tong, H., Yuan, P., Sun, L. J., Jiang, L., & Shi, J. Y. (2019). Aggregation, sedimentation, and dissolution of copper oxide nanoparticles: Influence of low-molecular-weight organic acids from root exudates. Nanomaterials, 9, 841.
Pérez-de-Luque, A. (2017). Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Frontiers in Environmental Science, 5, 12.
Predoi, D., Ghita, R. V., Liliana Iconaru, S., Laura Cimpeanu, C., & Mariana Raita, S. (2020). Application of nanotechnology solutions in plants fertilization. In Urban horticulture – Necessity of the future. IntechOpen.
Priester, J. H., Moritz, S. C., Espinosa, K., Ge, Y., Wang, Y., Nisbet, R. M., Schimel, J. P., Susana Goggi, A., Gardea-Torresdey, J. L., & Holden, P. A. (2017). Damage assessment for soybean cultivated in soil with either CeO2 or ZnO manufactured nanomaterials. Science of the Total Environment, 579, 1756–1768.
Qiu, H., & Smolders, E. (2017). Nanospecific phytotoxicity of CuO nanoparticles in soils disappeared when bioavailability factors were considered. Environmental Science & Technology, 51, 11976–11985.
Rafique, R., Zahra, Z., Virk, N., Shahid, M., Pinelli, E., Kallerhoff, J., Park, T. J., & Arshad, M. (2018). Data on rhizosphere pH, phosphorus uptake and wheat growth responses upon TiO2 nanoparticles application. Data in Brief, 17, 890–896.
Raghib, F., Naikoo, M. I., Khan, F. A., Alyemeni, M. N., & Ahmad, P. (2020). Interaction of ZnO nanoparticle and AM fungi mitigates Pb toxicity in wheat by upregulating antioxidants and restricted uptake of Pb. Journal of Biotechnology, 323, 254–263.
Rajput, V. D., Minkina, T., Kumari, A., Harish, V., Singh, K., Verma, K. K., Mandzhieva, S., Sushkova, S., Srivastava, S., & Keswani, C. (2021). Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants, 10, 1221.
Ramadan, T., Sayed, S. A., Abd-Elaal, A. K. A., & Amro, A. (2022). The combined effect of water deficit stress and TiO2 nanoparticles on cell membrane and antioxidant enzymes in Helianthus annuus L. Physiology and Molecular Biology of Plants, 28, 391–409.
Rana, R. A., Siddiqui, M. N., Skalicky, M., Brestic, M., Hossain, A., Kayesh, E., Popov, M., Hejnak, V., Gupta, D. R., Mahmud, N. U., & Islam, T. (2021). Prospects of nanotechnology in improving the productivity and quality of horticultural crops. Horticulturae, 7, 332.
Rawat, S., Pullagurala, V. L. R., Adisa, I. O., Wang, Y., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2018). Factors affecting fate and transport of engineered nanomaterials in terrestrial environments. Current Opinion in Environmental Science & Health, 6, 47–53.
Rizwan, M., Ali, S., Ali, B., Adrees, M., Arshad, M., Hussain, A., ur Rehman, M. Z., & Waris, A. A. (2019a). Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere, 214, 269–277.
Rizwan, M., Ali, S., Rehman, M. Z. U., Adrees, M., Arshad, M., Qayyum, M. F., Ali, L., Hussain, A., Chatha, S. A. S., & Imran, M. (2019b). Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environmental Pollution, 248, 358–367.
Romero-Freire, A., Lofts, S., Martín Peinado, F. J., & van Gestel, C. A. (2017). Effects of aging and soil properties on zinc oxide nanoparticle availability and its ecotoxicological effects to the earthworm Eisenia andrei. Environmental Toxicology and Chemistry, 36, 137–146.
Rui, M., Ma, C., Hao, Y., Guo, J., Rui, Y., Tang, X., Zhao, Q., Fan, X., Zhang, Z., Hou, T., & Zhu, S. (2016). Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Frontiers in Plant Science, 7, 815.
Rui, M., Ma, C., White Jason, C., Hao, Y., Wang, Y., Tang, X., Yang, J., Jiang, F., Ali, A., Rui, Y., & Cao, W. (2018). Metal oxide nanoparticles alter peanut (Arachis hypogaea L.) physiological response and reduce nutritional quality: A life cycle study. Environmental Science: Nano, 5, 20188–22102.
Ruttkay-Nedecky, B., Krystofova, O., Nejdl, L., & Adam, V. (2017). Nanoparticles based on essential metals and their phytotoxicity. Journal of Nanobiotechnology, 15, 33.
Sarraf, M., Vishwakarma, K., Kumar, V., Arif, N., Das, S., Johnson, R., Janeeshma, E., Puthur, J. T., Aliniaeifard, S., Chauhan, D. K., Fujita, M., & Hasanuzzaman, M. (2022). Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: An overview of the mechanisms. Plants, 11, 316.
Seleiman, M. F., Almutairi, K. F., Alotaibi, M., Shami, A., Alhammad, B. A., & Battaglia, M. L. (2020). Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants, 10, 2.
Servin, A. D., De la Torre-Roche, R., Castillo-Michel, H., Pagano, L., Hawthorne, J., Musante, C., Pignatello, J., Uchimiya, M., & White, J. C. (2017a). Exposure of agricultural crops to nanoparticle CeO2 in biochar-amended soil. Plant Physiology and Biochemistry, 110, 147–157.
Servin, A. D., Pagano, L., Castillo-Michel, H., De la Torre-Roche, R., Hawthorne, J., Hernandez-Viezcas, J. A., Loredo-Portales, R., Majumdar, S., Gardea-Torresday, J., Dhankher, O. P., & White, J. C. (2017b). Weathering in soil increases nanoparticle CuO bioaccumulation within a terrestrial food chain. Nanotoxicology, 11, 98–111.
Shah, V., Collins, D., Walker, V. K., & Shah, S. (2014). The impact of engineered cobalt, iron, nickel and silver nanoparticles on soil bacterial diversity under field conditions. Environmental Research Letters, 9, 024001.
Shah, T., Latif, S., Saeed, F., Ali, I., Ullah, S., Abdullah Alsahli, A., Jan, S., & Ahmad, P. (2021). Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. Journal of King Saud University – Science, 33, 101207.
Shang, Y., Hasan, M. K., Ahammed, G. J., Li, M., Yin, H., & Zhou, J. (2019). Applications of nanotechnology in plant growth and crop protection: A review. Molecules, 24, 2558.
Sharifan, H., Moore, J., & Ma, X. (2020). Zinc oxide (ZnO) nanoparticles elevated iron and copper contents and mitigated the bioavailability of lead and cadmium in different leafy greens. Ecotoxicology and Environmental Safety, 191, 110177.
Skiba, E., Michlewska, S., Pietrzak, M., & Wolf, W. M. (2020). Additive interactions of nanoparticulate ZnO with copper, manganese and iron in Pisum sativum L., a hydroponic study. Scientific Reports, 10, 13574.
Sturikova, H., Krystofova, O., Húska, D., & Adam, V. (2018). Zinc, zinc nanoparticles and plants. Journal of Hazardous Materials, 349, 101–110.
Sun, L., Song, F., Guo, J., Zhu, X., Liu, S., Liu, F., & Li, X. (2020). Nano-ZnO-induced drought tolerance is associated with melatonin synthesis and metabolism in maize. International Journal of Molecular Sciences, 21, 782.
Suriyaprabha, R., Karunakaran, G., Yuvakkumar, R., Rajendran, V., & Kannan, N. (2012). Silica nanoparticles for increased silica availability in maize (Zea mays L.) seeds under hydroponic conditions. Current Nanoscience, 8, 902–908.
Sweet, M. J., & Singleton, I. (2015). Soil contamination with silver nanoparticles reduces Bishop pine growth and ectomycorrhizal diversity on pine roots. Journal of Nanoparticle Research, 17, 448.
Thakur, S., Asthir, B., Kaur, G., Kalia, A., & Sharma, A. (2021). Zinc oxide and titanium dioxide nanoparticles influence heat stress tolerance mediated by antioxidant defense system in wheat. Cereal Research Communications, 50, 385–396.
Thiruvengadam, M., Gurunathan, S., & Chung, I.-M. (2015). Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma, 252, 1031–1046.
Tripathi, D. K., Shweta, S., Singh, S., Singh, R., Pandey, V., Singh, P., Sharma, N. C., Prasad, S. M., Dubey, N. K., & Chauhan, D. K. (2017a). An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiology and Biochemistry, 110, 2–12.
Tripathi, D. K., Singh, S., Singh, S., Srivastava, P. K., Singh, V. P., Singh, S., Prasad, S. M., Singh, P. K., Dubey, N. K., Pandey, A. C., & Chauhan, D. K. (2017b). Nitric oxide alleviates silver nanoparticles (AgNPs)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiology and Biochemistry, 110, 167–177.
Uwizeyimana, H., Wang, M., Chen, W., & Khan, K. (2017). The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environmental Toxicology and Pharmacology, 55, 20–29.
Vannini, C., Domingo, G., Onelli, E., De Mattia, F., Bruni, I., Marsoni, M., & Bracale, M. (2014). Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. Journal of Plant Physiology, 171, 1142–1148.
Venkatachalam, P., Jayaraj, M., Manikandan, R., Geetha, N., Rene, E. R., Sharma, N. C., & Sahi, S. V. (2017). Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: A physiochemical analysis. Plant Physiology and Biochemistry, 110, 59–69.
Verma, A., & Khanam, Z. (2020). Phyto-nanotechnology and agriculture. In Phytonanotechnology (pp. 289–301). Elsevier.
Wang, F., Adams, C. A., Shi, Z., & Sun, Y. (2018a). Combined effects of ZnO NPs and Cd on sweet sorghum as influenced by an arbuscular mycorrhizal fungus. Chemosphere, 209, 421–429.
Wang, X. P., Li, Q. Q., Pei, Z. M., & Wang, S. C. (2018b). Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biologia Plantarum, 62, 801–808.
Wang, X., Sun, W., Zhang, S., Sharifan, H., & Ma, X. (2018c). Elucidating the effects of cerium oxide nanoparticles and zinc oxide nanoparticles on arsenic uptake and speciation in rice (Oryza sativa) in a hydroponic system. Environmental Science & Technology, 52, 10040–10047.
Watson, J.-L., Fang, T., Dimkpa, C. O., Britt, D. W., McLean, J. E., Jacobson, A., & Anderson, A. J. (2015). The phytotoxicity of ZnO nanoparticles on wheat varies with soil properties. Biometals, 28, 101–112.
Wei, X., Cao, P., Wang, G., Liu, Y., Song, J., & Han, J. (2021). CuO, ZnO, and γ-Fe2O3 nanoparticles modified the underground biomass and rhizosphere microbial community of Salvia miltiorrhiza (Bge.) after 165-day exposure. Ecotoxicology and Environmental Safety, 217, 112232.
Worrall, E. A., Hamid, A., Mody, K. T., Mitter, N., & Pappu, H. R. (2018). Nanotechnology for plant disease management. Agronomy, 8, 285.
Xiao, Y., Du, Y., Xiao, Y., Zhang, X., Wu, J., Yang, G., He, Y., Zhou, Y., Peijnenburg, W. J. G. M., & Luo, L. (2021). Elucidating the effects of TiO2 nanoparticles on the toxicity and accumulation of Cu in soybean plants (Glycine max L.). Ecotoxicology and Environmental Safety, 219, 112312.
Xiao, Y. M., Li, Y., Shi, Y., Li, Z. Q., Zhang, X. Y., Liu, T., Farooq, T. H., Pan, Y. L., Chen, X. Y., & Yan, W. D. (2022). Combined toxicity of zinc oxide nanoparticles and cadmium inducing root damage in Phytolacca americana L. Science of the Total Environment, 806, 151211.
Yang, J., Cao, W., & Rui, Y. (2017). Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. Journal of Plant Interactions, 12, 158–169.
Yang, L., Luo, X.-B., & Luo, S.-L. (2019). Assessment on toxicity of nanomaterials. In X. Luo & F. Deng (Eds.), Nanomaterials for the removal of pollutants and resource reutilization (pp. 273–292). Elsevier.
Yasmeen, F., Raja, N. I., Razzaq, A., & Komatsu, S. (2017). Proteomic and physiological analyses of wheat seeds exposed to copper and iron nanoparticles. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics, 1865, 28–42.
Yuan, P., Peng, C., Shi, J. Y., Liu, J. S., Cai, D. Q., Wang, D. F., & Shen, Y. H. (2021). Ferrous ions inhibit Cu uptake and accumulation via inducing iron plaque and regulating the metabolism of rice plants exposed to CuO nanoparticles. Environmental Science: Nano, 8, 1456–1468.
Yusefi-Tanha, E., Fallah, S., Rostamnejadi, A., & Pokhrel, L. R. (2020a). Particle size and concentration dependent toxicity of copper oxide nanoparticles (CuONPs) on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Science of the Total Environment, 715, 136994.
Yusefi-Tanha, E., Fallah, S., Rostamnejadi, A., & Pokhrel, L. R. (2020b). Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Science of the Total Environment, 738, 140240.
Zahra, Z., Arshad, M., Rafique, R., Mahmood, A., Habib, A., Qazi, I. A., & Khan, S. A. (2015). Metallic nanoparticle (TiO2 and Fe3O4) application modifies rhizosphere phosphorus availability and uptake by Lactuca sativa. Journal of Agricultural and Food Chemistry, 63, 6876–6882.
Zeng, W., Nyapete, C., Benziger, A., Jelliss, P., & Buckner, S. (2019). Encapsulation of reactive nanoparticles of aluminum, magnesium, zinc, titanium, or boron within polymers for energetic applications. Current Applied Polymer Science, 3, 3–13.
Zhang, H., & Zhang, Y. (2020). Effects of iron oxide nanoparticles on Fe and heavy metal accumulation in castor (Ricinus communis L.) plants and the soil aggregate. Ecotoxicology and Environmental Safety, 200, 110728.
Zhang, W., Dan, Y., Shi, H., & Ma, X. (2017). Elucidating the mechanisms for plant uptake and in-planta speciation of cerium in radish (Raphanus sativus L.) treated with cerium oxide nanoparticles. Journal of Environmental Chemical Engineering, 5, 572–577.
Zhang, W., Long, J., Li, J., Zhang, M., Xiao, G., Ye, X., Chang, W., & Zeng, H. (2019). Impact of ZnO nanoparticles on Cd toxicity and bioaccumulation in rice (Oryza sativa L.). Environmental Science and Pollution Research, 26, 23119–23128.
Zhang, R., Bai, X., Shao, J., Chen, A., Wu, H., & Luo, S. (2020). Effects of zero-valent iron nanoparticles and quinclorac coexposure on the growth and antioxidant system of rice (Oryza sativa L.). Ecotoxicology and Environmental Safety, 203, 111054.
Zhang, Y., Qi, G., Yao, L., Huang, L., Wang, J., & Gao, W. (2022). Effects of metal nanoparticles and other preparative materials in the environment on plants: From the perspective of improving secondary metabolites. Journal of Agricultural and Food Chemistry, 70, 916–933.
Zhao, L., Lu, L., Wang, A., Zhang, H., Huang, M., Wu, H., Xing, B., Wang, Z., & Ji, R. (2020). Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. Journal of Agricultural and Food Chemistry, 68, 1935–1947.
Zhu, J. H., Zou, Z. H., Shen, Y., Li, J. F., Shi, S. N., Han, S. W., & Zhan, X. H. (2019). Increased ZnO nanoparticle toxicity to wheat upon co-exposure to phenanthrene. Environmental Pollution, 247, 108–117.
Zuverza-Mena, N., Medina-Velo, I. A., Barrios, A. C., Tan, W., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2015). Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environmental Science: Processes & Impacts, 17, 1783–1793.
Funding
This chapter was supported by the Community of Madrid, project AGRISOST-S2018/BAA-4330.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fernández, M.D., García-Gómez, C. (2023). Impact of Emerging Metal-Based NPs on Plants and Their Influence on the Phytotoxicity of Other Pollutants. In: Aftab, T. (eds) Emerging Contaminants and Plants. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-031-22269-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-22269-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22268-9
Online ISBN: 978-3-031-22269-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)