Abstract
Purpose
It has been reported the bioaccumulation of γ-ferric oxide nanoparticles (Fe2O3 NPs) or oxytetracycline (OTC) in crops. However, there have been little references investigating their uptake and bioaccumulation in crops after the combined exposure. The present study focused on Fe2O3 NPs and OTC accumulation on root surface and in the tissues of rice (Oryza sativa L.) seedlings under combined exposure. And, the interactive influence mechanism was also discussed.
Materials and methods
Hydroponic experiments were conducted to investigate the Fe and OTC accumulation on root surface and in rice tissues under individual and combined exposure of Fe2O3 NPs and OTC. The dynamic change of particulate Fe, ionic Fe, and Fe plaque concentrations on root surface was determined under the influence of OTC from Fe2O3 NPs and Fe-EDTA exposure. Fe2+ from Fe-EDTA was selected in order to compare the Fe bioaccumulation from ionic Fe and nanoparticle Fe exposure. Hydrodynamic diameter and ζ-potential of Fe2O3 NPs in solution were investigated when OTC was present or not, and the changes of OTC concentrations were also determined during hydroponic culture. SEM, XRD, and TEM were used to analyze Fe2O3 NP distribution on root surface and inside root under the influence of OTC.
Results and discussion
OTC promoted surface-Fe and shoot-Fe accumulation in Fe2O3 NPs treatments, which was just an opposite result from Fe-EDTA treatments. Upon Fe2O3 NP exposure, Fe plaque was formed through the direct adsorption of NPs on the outside root surface and then incorporated into plaque as its crystalline components. OTC elevated notably surface-Fe accumulation mainly through increasing adsorption and precipitation of Fe2O3 NPs on the root surface due to low repulsive electrostatic interaction between NPs and the root surface after adding OTC. Fe2O3 NPs increased surface-OTC and root-OTC levels. Compared to Fe-EDTA, surface-Fe from NP treatments can hold strongly OTC due to Fe2O3 particle precipitated on root surface with high specific surface area. NPs reduced shoot-OTC under 25 mg L−1 OTC, but not under 100 mg L−1 OTC.
Conclusions
This study clearly demonstrates that Fe/OTC accumulation in rice was always in the order root surface > shoot > root, whether Fe2O3 NPs/OTC was individual or combined exposure. The combined exposure will increase their root surface distribution comparing with individual exposure, and Fe2O3 NPs increased also root-OTC levels, which could pose a potential risk to food safety in subsequent growth of rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Iron oxide nanoparticles (Fe2O3 NPs) have been applied in various commercial products and applications, such as solar energy conversion, water splitting, and cement (Nhan et al. 2015). During the process of packaging, transport, usage, and disposal, the Fe2O3 NPs and their related products could eventually end up in the environment. It has also been reported that its application as available products was increasing rapidly for agriculture and phytoremediation (Martínez-Fernández et al. 2016; Sebastian et al. 2017). It is thus reasonable to predict that Fe2O3 NPs will be accumulated in the agricultural environment. When released to the agricultural environment, NPs can coexist with other contaminants, such as antibiotics. Oxytetracycline (OTC) is one of the important tetracycline antibiotics, and is widely distributed in agricultural environments due to the agricultural application of livestock manure as organic fertilizer (Bao et al. 2009; Li et al. 2016a). With the extensive use of OTC in livestock industry, more than 75% of OTC administered to animals was remained in manure with antimicrobially active form (Kulshrestha et al. 2004; Hu and Zhou 2013). Sun et al. (2017) reported up to 68% of OTC detection rate in agricultural soils in the Yangtze River delta. Generally, combined exposure is far more common than individual exposure (Zhou et al. 2005). Thus, NPs and antibiotics will coexist in agricultural environments. And, the interactions of antibiotics and NPs in agricultural ecosystems make their contamination more widespread and complex.
Crops are one of the most important components in agricultural terrestrial ecosystems and play a critical role in the transport and transformation of NPs and antibiotics in the environment through plant uptake, translocation, and bioaccumulation (Monica and Cremonini 2009; Bao et al. 2018). Rice (Oryza sativa L.) is one of the most important food crops, and also a primary food source for more than a third of the world’s population (Khush 1997). Currently, some studies have demonstrated individual Fe2O3 NPs or OTC uptake and their accumulation in different plant species. Nhan et al. (2015) reported the internalization of Fe2O3 NPs in the endodermis and vascular cylinder of the cotton roots, and Fe2O3 NPs also are deposited on root hairs and tips of tomato. Our previous studies showed OTC bioaccumulation in rice (Bao et al. 2018) and horsebean (Liu et al. 2015). Given the complex nature of agricultural systems, the likelihood of combined exposure to metal oxide nanoparticles and antibiotics is quite high. Hence, it is both appropriate and important to study the interactive effect of NPs and antibiotics on their uptake and bioaccumulation in plant. Liu et al. (2017) showed that the presence of tetracycline (one of tetracycline antibiotics) notably decreased Ti accumulation in both shoots and roots of Arabidopsis thaliana (L.) after combined exposure of TiO2 NPs and tetracycline. Ma et al. (2017) reported that TiO2 NPs significantly reduced the levels of tetracycline in rice shoots and roots, and the presence of tetracycline increased the Ti content in rice roots, which could be ascribed to the alteration of surface charges of TiO2 NPs as affected by tetracycline. These results indicate that, in combined exposure, one contaminant may influence bioaccumulation of another coexisting contaminant in plants, effectively reducing or increasing the potential health risk posed to humans through the food chain.
Root surface of plant is an important translocation part of contaminants due to its inherent contacts with environment and plants. Numerous studies have demonstrated that metal-based NPs, including CeO2 NP (Zhang et al. 2012; Ma et al. 2015), Yb2O3 NP (Zhang et al. 2011), La2O3 NP (Ma et al. 2011), Au NPs (Zhu et al. 2012), and Ag NP (Geisler-Lee et al. 2013), could be strongly adsorbed on root surface. Usually, a large amount of mucilage (Parsons et al. 2010) on root surface was secreted from root and electronegativity of plant cell walls, including epidermis cells on root surfaces (Meychik and Yermakov 2001), which can promote NP adsorption on root surface. We also showed OTC accumulation on root surface of rice (Bao et al. 2018). Limited research focused on the interactive effects of NPs and antibiotic on their accumulation in plant (Liu et al. 2017; Ma et al. 2017). On the root surface of rice, red iron (Fe) plaque is commonly found in rich Fe2+ ion environment media. This is because root can release oxygen and oxidants into the rhizosphere, which subsequently oxidize ferrous to ferric iron with the precipitation of iron oxides or hydroxides on the root surface (Armstrong 1997; Chen et al. 1980; Liu et al. 2006). It is reported that the amount of Fe plaque on the root surface increased with increasing aqueous ferrous ion (Fe2+) concentration added (Bao et al. 2018). Our study showed that Fe2+ ion addition induced Fe plaque formation, which increased OTC accumulation on root surface of root (Bao et al. 2018). Pardo et al. (2016) reported that Fe plaque was also developed on root surface of Phragmites australis as a wetland plant after Fe2O3 NP exposure. Hence, it is of great practical significance to study how the surface-Fe as Fe plaque is formed on root surface of rice upon Fe2O3 NP and how surface-Fe affect OTC uptake and bioaccumulation.
For combined exposure of contaminants, the change of their characteristics in solution will affect their uptake and accumulation by plant before the contaminants are close to root surface of plant. Figueroa and MacKay (2005) showed the strong adsorption of OTC on the surface of iron oxides. It is predicted that OTC could reduce the aggregation of Fe2O3 NPs through the adsorption in solution. Wang et al. (2016) reported that the OTC could stabilize Fe2+ through the complexation in solution and retard ferrous ion oxidation. Herein, it is predicted that OTC can stabilize Fe2+ in solution from Fe2O3 NP reductive dissolution through the complexation of Fe2+ and OTC. In the rhizosphere, some reductive substances were released from rice root, such as low-molecular-weight organic acids, which promoted Fe2+ released from Fe2O3 NPs. Additionally, Fe2O3 NPs could affect OTC elimination in solution, and then affect its uptake by rice. However, to date, it is largely unknown the interactive influence process of Fe2O3 NPs and OTC in solution before they are close to root surface.
In this study, we systematically investigated Fe2O3 NPs and OTC accumulation on root surface and in rice tissues in the treatments of their individual and combined exposure. In order to comprehensively understand the interactive influence mechanism of two contaminants’ bioaccumulation from solution to rice, the characteristics of Fe2O3 NPs and OTC in solution were investigated upon individual and combined exposure. During the Fe2O3 NP uptake, particulate Fe as nanoparticle and ionic Fe from NP dissolution were all accumulated by rice. Hence, Fe2+ from Fe-EDTA was also selected in order to compare the influence difference of OTC on Fe bioaccumulation from ionic Fe and nanoparticle Fe exposure. Under different treatments, the resulting impact on biomass, Fe, and OTC accumulation on root surface, as well as Fe and OTC uptake in rice tissues, was determined. Additionally, dynamic change of concentrations of particulate Fe, ionic Fe, and Fe plaque on root surface was determined under the influence of OTC. Scanning electron microscopy (SEM), X-ray diffraction (XRD), and TEM were used to characterize the changes of Fe2O3 NP properties on root surface and inside root from the effect of OTC. The results could help to improve the understanding of uptake and accumulation of Fe2O3 NPs and OTC in rice after their coexposure, as well as predict their potential human health risks through food crop uptake.
2 Materials and methods
2.1 Fe2O3 NPs and oxytetracycline
γFe2O3 NPs (20 nm) used in the study were obtained from Shanghai Macklin Biochemical Co., Ltd., and they were not surface modified. The NP suspensions were prepared by suspending an appropriate amount of NPs in ultrapure water. Oxytetracycline hydrochloride (95% purity, analytical grade) was bought from the Sigma Co. in St. Louis, USA.
2.2 Cultivation of rice seedling
Rice seeds (Oryza sativa L.; Jinyuan-E28) were from Tianjin Academy of Agricultural Sciences. The uniform rice seeds were surface sterilized in 3% H2O2 for 30 min and then thoroughly rinsed with deionized water. Rice seeds were soaked in deionized water at 37 °C for 24 h, and then placed in wet gauze. After growth at 25 °C for 10 days, uniform seedlings were selected and transferred into the flasks containing nutrient solution (one-fourth strength of international rice nutrient solution). The solution contains NH4NO3 114 mg L−1, NaH2PO4·2H2O 50.4 mg L−1, K2SO4 89.3 mg L−1, CaCl2 158.2 mg L−1, MgSO4·7H2O 405 mg L−1, MnCl2·4H2O 7.5 mg L−1, (NH4)6Mo7O24·2H2O 0.37 mg L−1, H3BO3 4.62 mg L−1, ZnSO4·7H2O 0.175 mg L−1, CuSO4·5H2O 0.155 mg L−1, and FeCl3 1.128 mg L−1. The pH of the nutrient solution was adjusted to 5.0. The flasks were wrapped with aluminum foil. Rice seedlings acclimatized in the above nutrient solution for 6 days as pre-culture in order to adapt the solution environment, and the solution was replaced every 3 days.
2.3 Individual and combined exposure assay
The Fe2O3 NP suspensions were sonicated for 1 h for dispersion. Then, NPs were added in flask to achieve a final concentration of 25 mg L−1 Fe in nutrient solution without FeCl3 nutrient. OTC was added to achieve the final concentrations of 0, 25, and 100 mg L−1, respectively. The selection of above OTC concentrations was based on the following. Usually, OTC concentration ranged from micrograms per kilogram to milligrams per kilogram in real soil environment (Manuel et al. 2018; Wei et al. 2019). And, the occurrence of several antibiotics was universal in soil. Herein, 25 mg L−1 OTC was set up in order to simulate high OTC contamination in agricultural soil. OTC (100 mg L−1) was set up as accidental contamination. And, Fe2+ ion from Fe(II)-EDTA was also added as ion-Fe species. Fe2+ was selected instead of Fe3+ as a control treatment due to it as main form from Fe2O3 NP dissolution in the study. Also, Fe2+ is present easily in the rhizosphere of rice due to the reductive substances released by root. Our preliminary results showed that 25 mg L−1 Fe from Fe2O3 NPs had no toxic effect on rice. Also, 25 and 100 mg L−1 OTC had also no effect on rice growth in this study. CK included no Fe2O3 NPs, OTC, and Fe-EDTA in one-fourth nutrient solution. Seven treatments were prepared, including (1) Fe2O3 NPs, (2) OTC of 25 mg L−1, (3) OTC of 100 mg L−1, (4) Fe2O3 NPs + OTC of 25 mg L−1, (5) Fe2O3 NPs + OTC of 100 mg L−1, (6) Fe-EDTA, and (7) Fe-EDTA + OTC of 25 mg L−1. All experiments were conducted in triplicate; six rice seedlings were included in each replicate. The initial pH of the nutrient solution was adjusted to 5.0. Deionized water was added in order to maintain initial solution volume every day during the entire exposure period (10 days). The plants were grown in incubator under room temperature (∼ 25 °C) and day (12 h)/night (12 h) duration. OTC remained in hydroponic solution was determined in different exposure time under different OTC treatments. At harvest, plants were collected and carefully rinsed with Milli-Q water to remove Fe2O3 particles and OTC at root surfaces. Plants were then divided into roots and shoots.
2.4 TEM, SEM imaging, and X-ray diffraction analysis of root samples
In the study, root uptake is only route for individual and combined pollutants in rice. To observe Fe2O3 NP accumulation on root surface, the roots from different treatments were imaged using a scanning electron microscope (SEM, S-3500N, High-Technologies Corporation, Tokyo, Japan). For in situ TEM analysis, samples in the treatments with 50 mg L−1 Fe2O3 NPs or Fe-EDTA were selected for TEM observation. Comparing with 25 mg L−1 Fe treatments in hydroponic culture, high Fe concentration of 50 mg L−1 from Fe2O3 NPs or Fe-EDTA was chosen in order to observe the obvious NP accumulation in rice root. Treated rice seedlings were washed thoroughly after 10-day exposure, and the root apexes were cut and fixed in 2.5% glutaraldehyde solution. Then, the tissues were dehydrated in a graded acetone series and embedded in Spurr’s resin. Ultrathin sections of 70 nm were cut by an UC7 ultramicrotome (Leica, Germany) with a diamond knife and collected on copper grids. Sections were observed under a Hitachi HT7700 (Japan) transmission electron microscope operating at 80 kV. The root samples from different treatments were extracted and examined using X-ray diffractometry (XRD; X’Pert PRO, Almelo, Netherlands) according to the method proposed by Liu et al. (2006). Also, XRD analysis of rice root treated with Fe2O3 NPs with different exposure methods can identify whether crystalline fractions of Fe plaque on root surface are from pristine Fe2O3 NPs. (1) Direct method: adding NPs in hydroponic solution for NPs and NPs + OTC treatments. (2) Indirect method: adding NPs in dialysis tube with molecular cutoff 3000 kb and then putting it into hydroponic solution for NPs1 and NPs1 + OTC treatments. Herein, NPs1 mean that we added NPs in dialysis tube. For indirect method, only Fe2+ and Fe3+ ions from Fe2O3 NP dissolution can pass through the dialysis membrane, and then, Fe2+ in hydroponic solution oxidized to form Fe plaque on the root surface.
2.5 Fe and OTC on root surface
Surface-Fe was accumulated as Fe plaque. Ammonium oxalate (0.175 M)-oxalic acid (0.10 M) buffer was firstly used to extract amorphous fraction of Fe plaque from the root surface. Then, the sodium dithionite (Na2S2O4)-sodium citrate (Na3C6O7H5)-sodium bicarbonate (NaHCO3) (DCB) mixture solution was used to extract crystalline fraction of Fe plaque (Bao et al. 2018; Hu et al. 2015). The Fe and OTC concentrations of the samples extracted by ammonium oxalate buffer and DCB mixture were separately analyzed by flame atomic absorption spectroscopy (FAAS) and high-performance liquid chromatography (HPLC), respectively. After two-step extraction, the roots were thoroughly rinsed with Milli-Q water and then were freeze-dried at − 65 °C for 24–48 h until analysis. The amount of Fe plaque and the OTC concentration in the Fe plaque are presented as the mass fraction of Fe and OTC in extractions to the dry weight of root after extraction (mg kg−1 DW), respectively.
2.6 Adsorption kinetics of ionic Fe and particulate Fe on the outside of Fe plaque
During Fe accumulation on root surface induced by Fe2O3 NP (25 mg L−1 of Fe) exposure w/ or w/o the presence of OTC (100 mg L−1), the amounts of ionic Fe, particulate Fe, and Fe plaque were determined at 15-min, 5-h, 24-h, and 72-h time points, respectively. A 0.1 mol L−1 CaCl2 solution as ion exchange extraction was used to extract ionic Fe adsorbed on the outside of Fe plaque. And, the extraction time is 2 h. Preliminary experiment showed that total Fe ion concentration was 0.920 mg L−1 and 0.921 mg L−1 released from Fe2O3 NPs and Fe2O3 NPs + CaCl2 treatments after 2 h, respectively. Thus, 0.1 M CaCl2 did not affect Fe2O3 NP dissolution. The suspension was ultracentrifuged at 10,000 rpm for 1 h, and the supernatant was filtered by a 0.22-μm filter; the filtrate was the ionic Fe. At the same time, the extraction was collected and digested with HNO3 and H2O2 for total Fe measurements. And, the difference of total Fe and ionic Fe was the particulate form of Fe. Then, DCB mixture solution was used to extract total amounts of Fe plaque, and the iron concentration in DCB solution was analyzed by FAAS. Additionally, an individual experiment was conducted with 25 mg L−1 Fe2+ from Fe-EDTA for Fe plaque formation in which ionic Fe, particulate Fe, and Fe plaque amounts were determined at different time points w/ or w/o the presence of OTC in order to investigate whether ionic Fe and particulate Fe were also adsorbed on the outside surface of root.
2.7 Fe and OTC in rice tissues
Shoot samples were freeze dried at − 65 °C for 24–48 h and then weighed up in order to determine Fe and OTC concentration. The dry samples of root and shoot were ground to fine powders, 50 mg of which was digested with a mixture of 5 mL HNO3 and 3 mL H2O2 on a heating plate. The residue was dissolved and diluted to 10 mL. The total Fe contents were determined using FAAS. In order to avoid environmental Fe contamination, deionized water without Fe was selected, and reagent blank was also set up. About 0.009 mg L−1 Fe was found in reagent blank. And all solution-Fe concentrations in the study subtracted the blank value. Samples were ground in a porcelain mortar in order to prepare plant slurry. Root and shoot samples were extracted by adding 20 mL of 0.1 mol L−1 Na2EDTA-McIlvaine (pH 4.0 ± 0.05) and following the method described by Bao and Zhou (2015). The extracts were filtered through 0.22-μm membrane; OTC in the solution was determined by HPLC. HPLC was a reverse-phase (Waters Corp.) with a Waters ODS-C18 (4.6 mm × 250 mm, 5 μm) column followed by UV detection at 355 nm. The mobile phase was a mixture of 0.01 M oxalic acid-acetonitrile (85:15, v/v) in an equilibrium system at a flow rate of 1.0 mL min−1. The column temperature was 25 °C.
2.8 Fe2O3 NP characterization and dissolution
The agglomeration state and the zeta potential (ζ) of NP suspensions (25 mg L−1) in root exudate solution at pH 5 were determined by dynamic light scattering using a Malvern Zeta Sizer (3000HS, Worcestershire, UK) w/ or w/o the presence of OTC (25 mg L−1). Root exudates were collected after rice seedling growth for 24 h. The (reductive) dissolution as Fe3+ and Fe2+ ions from Fe2O3 NPs at 25 mg L−1 of Fe was investigated in root exudate solution w/ or w/o the presence of OTC (25 mg L−1) at pH of 5. Fe2+ and Fe3+ ions in solution were determined at 5-min, 3-h, 24-h, 72-h, and 120-h time points, respectively. To separate the supernatant from the Fe2O3 NPs, samples were ultracentrifuged twice at 10,000 rpm for 1 h. In addition to centrifugation, the samples were filtered by a 0.22-μm filter (Millipore syringe filter) to ensure the removal of Fe2O3 NP aggregates. In preliminary experiment, we also used the dialysis tube with 3000 kb molecular to separate the NPs and the dissolved ions. The results showed that total Fe ion concentration was 0.880 mg L−1 and 0.861 mg L−1 released from Fe2O3 NPs after 3 h, respectively, in ultracentrifugation and dialysis tube treatments. Insignificant difference of Fe ion concentrations between two treatments indicated that ultracentrifugation can separate NPs and the dissolved ions in the study. Additionally, an individual experiment was conducted with Fe2+ (25 mg L−1) from Fe-EDTA in which Fe2+ and Fe3+ ions in solution were also determined at different time points w/ or w/o the presence of OTC in order to investigate the effects of OTC on the oxidation of Fe2+ into Fe3+. For the above experiments, the Fe2+ concentrations were determined by the phenanthroline (Phe) colorimetric method (Gupta 1968). Phenanthroline is known to react extremely rapidly and selectively with Fe2+ to form a stable orange-red complex with a maximum absorbance at 460 nm by UV-vis spectroscopy in HAc-NaAc buffer (pH 4.6), and it does not bind with Fe3+. The total iron ion concentrations containing both Fe2+ and Fe3+ were determined using FAAS. The difference between total iron ion and Fe2+ was Fe3+.
2.9 Data analysis
The figures were made by Origin 9.0, and the results were mean value of values of the triple test. And the data were analyzed using software package IBM SPSS Statistics 20 with LSD test, and the significant difference was determined at p < 0.05.
3 Results and discussion
3.1 Fe2O3 NP characteristic in hydroponic solution
During the uptake of Fe2O3 NPs by rice, NP characteristic change in solution could affect its accumulation on root surface and in tissue of rice. Hydrodynamic diameters and ζ-potential of Fe2O3 NPs were determined in root exudate solution when pH value was 5 after ultrasonication. The DLS measurements indicated that the introduction of OTC stabilized NPs against aggregation through decreasing significantly (p < 0.01) hydrodynamic diameters (Fig. 1a). It has been reported that tetracycline (one of tetracycline antibiotics) enhanced the stability of TiO2 NPs in aquatic system through its adsorption on the surface of NPs (Qi et al. 2018). In the study, the stabilization function increased with increasing OTC from 25 to 100 mg L−1. Similar study found that humic acid enhanced the colloidal stabilization of γ Fe2O3 NP through molecular chains adsorbed on NP surface (Ghosh et al. 2011). It could be assumed that Fe2O3 NP was present as a solid suspension in the presence of OTC due to the adsorption of OTC on the surface of Fe2O3 NP. The presence of OTC decreased significantly (p < 0.05) |ζ| values of NPs in solution (Fig. 1b), which could be ascribed to the adsorption of OTC on NP surface with multiple ionizable functional groups (e.g., -OH, -C=O) of OTC at pH 5. The ionization of group decreased |ζ| values of NPs, and such influence increased with increasing OTC concentrations. As shown in Fig. 1c, redox potential (Eh) was − 0.397 and − 0.433, respectively, in Fe2O3 NPs and OTC solution, which showed that OTC had weak reducibility.
The dissolution kinetics (Fe2+ and Fe3+) of Fe2O3 NP in root exudates in situ extracted from rice were investigated (Fig. 2a). In the first 5 min, the concentrations of the total Fe ions released from NPs was 1.340 mg L−1, and then, this value kept decreasing at each time point over 120 h, at which only the Fe3+ was found in the solution. Fe2O3 NPs underwent reductive dissolution in hydroponic solution in the presence of oxalate and ascorbic acid due to proton- and ligand- (oxalate-) promoting function (Lanzl et al. 2012). In the study, various root exudates containing low-molecular-weight organic acids as reducing agent could cause Fe2O3 NP reductive dissolution to release Fe2+. For Fe-EDTA treatment, total Fe ions were stably found at 25 mg L−1 over 120 h. The concentrations of the total released Fe ions ranged from 0.880 to 1.340 mg L−1, which only accounted for 3.52–6.07% of total Fe amount from NPs initially added to the exposure systems. The presence of OTC increased significantly (p < 0.05) Fe2+ ion amount from NP dissolution, but decreased Fe3+ ion amounts (Fig. 2a). Similar results that the presence of OTC notably elevated the Fe2+ concentration were found in the Fe-EDTA treatment with the addition of OTC (Fig. 2b), suggesting that OTC could retard Fe2+ oxidation into Fe3+. It was reported that the complexation constants of OTC and Fe2+ (103–104) (Wang et al. 2016) were far less than that of EDTA and Fe2+ (1014), suggesting more Fe2+ being complexed with EDTA, not OTC. Therefore, the complexation of OTC and Fe2+ from reductive dissolution of Fe2O3 NP might not be the main reason for the increase of Fe2+ amounts. Maybe, weak reducibility of OTC with low Eh value (Fig. 1c) could promote Fe2+ release in solution.
3.2 Fe2O3 NP accumulation on root surface of rice
Root is only route for Fe2O3 NP and OTC uptake by rice in the study. And, root surface is a necessary way for pollutants from the solution media to rice. Figure S1 (Electronic Supplementary Material (ESM)) shows either individual Fe2O3 NP or OTC, or combined exposure treatments had no impact on rice growth in terms of plant dry biomass relative to CK. Therefore, no negative toxicity effects from individual or combined pollution affect their uptake and accumulation in rice. Compared to CK, a reddish color of the root surface was observed as iron (Fe) plaque formation for all the treatments with an exposure of Fe2O3 NPs and Fe-EDTA (Fig. 3). The dark brown Fe plaque was found on the root surface in the Fe-EDTA treatment, while the light reddish Fe plaque was clearly visible on the root surface upon exposure to individual Fe2O3 NP treatment, and the reddish color was more obvious after adding OTC. The SEM observation (Fig. 3) of root surface showed that the CK treatment without Fe2O3 NPs and Fe-EDTA had a clean and smooth surface, with the exception of small irregular matrix, which could evidence a nutrient accumulation. However, when treated with Fe-EDTA and Fe2O3 NPs, the root surface became rougher, with more irregular structures on the surface, which resulted from Fe plaque development. According to a higher magnification SEM image, Fe plaque on root surface under Fe-EDTA treatment was more uniformly distributed than that for NP treatment, which was well consistent with nutrient element accumulation in CK. In NPs and NPs + OTC treatments, some large flakes or agglomerates of iron oxides were evident, but not for all Fe-EDTA treatments. The results indicated Fe2O3 NPs could be precipitated on root surface as a part of Fe plaque.
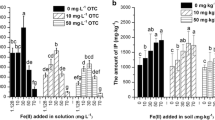
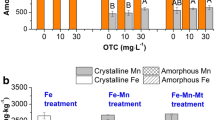
Fe-EDTA (ion-Fe) with the identical Fe concentration (25 mg L−1) as a comparative control was used to differentiate the effect of Fe2O3 NPs (particle-Fe) on Fe and OTC bioaccumulation. In the present study, limited Fe ions from Fe2O3 NP dissolution ranged from 0.880 to 1.517 mg L−1 (Fig. 2a). And, 1.128 mg L−1 Fe-EDTA was added to simulate Fe ion from NP dissolution in preliminary experiment, which found less Fe accumulation on root surface and no Fe plaque formation. We choose the same Fe concentrations from Fe2O3 NPs and Fe-EDTA treatments in order to form Fe plaque in the control (Fe-EDTA), which was also convenient to observe the difference of Fe bioaccumulation between ion-Fe and nanoparticle-Fe exposure. As shown in Fig. 4, total surface-Fe amounts were 4062–4625 mg kg−1 in all Fe-EDTA treatments, while only 1376–1779 mg kg−1 surface-Fe was found in all Fe2O3 NP treatments. Similar result was found that the efficiency of surface-Fe as plaque upon Fe2O3 NPs treatment was lower than for Fe2+ from FeSO4 (Pardo et al. 2016). Fe2+ ion addition from Fe-EDTA helped high surface-Fe accumulation as Fe plaque due to the oxidation of ferrous iron in the oxic rhizosphere and then precipitation as iron oxyhydroxides on root surface (Wu et al. 2016; Cai et al. 2016; Bao et al. 2018). Fe2O3 NPs as particle-limited Fe2+ ions release from its reductive dissolution in root exudates (Fig. 2a), which could reduce surface-Fe accumulation. In Fe-EDTA treatments, the addition of OTC notably decreased the surface-Fe concentrations. This is because OTC could have competitive adsorption and precipitation with iron oxides on root surface. However, in Fe2O3 NP treatments, OTC notably increased surface-Fe concentrations. Surface-Fe as Fe plaque mainly consists of amorphous and crystalline fractions of iron oxides or hydroxides (Armstrong 1997). In Fe2O3 NPs and Fe-EDTA treatments, OTC notably decreased the amount of amorphous fraction (p < 0.05), but increased the amount of crystalline fraction (p < 0.05). The ratios of crystalline to amorphous fractions were 0.11 and 0.20, respectively, in Fe-EDTA and Fe-EDTA + OTC treatments, which indicated amorphous fractions as main plaque components in all Fe-EDTA treatments. The ratio was 0.33 in individual Fe2O3 NP treatment. However, the presence of 25 and 100 mg L−1 OTC increased the ratios to 1.10 and 1.08, respectively. The results showed that OTC increased surface-Fe concentrations mainly through promoting crystalline fraction in Fe2O3 NP treatments. This could be a result of Fe2O3 NP taking part in Fe plaque formation by direct precipitation on root surface. Some NPs or aggregates were detected on the root surface in the NP treatments regardless of the presence of OTC by TEM imaging (Fig. S2A, ESM). However, no such aggregates were present in Fe-EDTA treatments.
Fe concentrations on root surface of rice. CK: no Fe2O3 NP or Fe(II)-EDTA exposure, NPs: 25 mg L−1 Fe from Fe2O3 NP, Fe(II)-EDTA: 25 mg L−1 Fe from Fe(II)-EDTA, OTC1: 25 mg L−1, OTC2: 100 mg L−1. Different letters on the bars indicate significant differences (p < 0.05) between different treatments. Asterisk denotes the significant differences affected by the presence of OTC. The same is below
To understand how Fe2O3 NPs were accumulated on root surface and OTC influence, XRD analysis of root was obtained (Fig. 5a). Two main peaks for Fe2O3 NP and Fe2O3 NP + OTC treatments corresponded with pristine Fe2O3 NPs. However, for Fe-EDTA treatments, no obvious peak was identified as Fe2O3 NP. The results indicated that the crystalline fraction of surface-Fe was mainly from Fe2O3 NPs. In the study, two methods of introducing Fe2O3 NP into the solution-rice system were designed: the direct NP exposure was by adding the Fe2O3 NP into the hydroponic solution directly, and the indirect NP exposure was that NP was put into a dialysis bag, which was then put into the hydroponic solution. For indirect method, only Fe2+ and Fe3+ ions from NP dissolution were close to rice root. As shown in Fig. 5b, XRD analysis of root demonstrated that two main peaks from NP treatments with or without the addition of OTC were coordinated with the pristine Fe2O3 NP, but the similar peaks were not evident in the indirect method. Thus, the crystalline fraction of surface-Fe under NP treatments was not from the oxidation of Fe2+ (as Fe2O3 NP reductive dissolution in rhizosphere) on root surface. In order to examine the adsorption of particles and ions of Fe on the outside surface of root, the adsorbed iron ion (ionic Fe) and iron oxide particle (particulate form of Fe) were firstly extracted with 0.1 mol L−1 CaCl2 solution before DCB extracting Fe plaque. The dynamic changes of the concentrations of two fractions were determined at 0–72 h, which was the initial stage of the surface-Fe accumulation. For Fe2O3 NP treatments, 77.52–90.18% of the adsorbed Fe on the root surface was particulate Fe, while ionic Fe was only 9.823–22.48% (Fig. 6a). However, for Fe-EDTA treatments, the completely opposite results were found that majority of the adsorbed Fe was ionic Fe (89.72–99.19%), and the rest part was particulate Fe, which was only 0.815–10.28% (Fig. 6b). The results suggested that, for Fe2O3 NP treatments, the NPs were adsorbed easily on the root surface, which could subsequently incorporate into the surface-Fe as one of the components. As shown in Fig. 5a, XRD analysis of root under Fe2O3 NP treatments was in accordance with pristine Fe2O3 NP. Additionally, the presence of OTC could promote significantly (p < 0.05) particulate Fe and ionic Fe concentrations on the surface of root from 5 to 72 h as compared with individual NP treatment (Fig. 6a), which would increase the amount of surface-Fe (Fig. 6c). The presence of OTC significantly increased the amounts of crystalline fraction (p < 0.05) as compared with the individual NP treatment (Fig. 4), which was consistent with the increase of particulate Fe adsorbed on the surface of rice roots (Fig. 6a). Thus, Fe2O3 NPs were adsorbed directly on the root surface and then incorporated into Fe plaque as its crystalline component. However, in Fe-EDTA treatments, OTC could decrease particulate Fe and ionic Fe concentrations on the surface of root as compared with the individual Fe-EDTA treatment (Fig. 6b), which would decrease the amount of Fe plaque (Fig. 6c). In conclusion, Fe2O3 NPs themselves could directly contribute to surface-Fe accumulation as a separate mineral phase. OTC increased surface-Fe concentrations through promoting particulate Fe and ionic Fe concentrations adsorbed on the surface of root. The promotion depended on the property change of NPs. First, after adding OTC, the decrease of hydrodynamic diameters of NPs (Fig. 1a) could stabilize NPs against aggregation and promote particulate Fe accumulation on root surface. Second, it is well known that plant cell walls, including epidermis cells on root surfaces, are negatively charged because of abundant polysaccharides containing galacturonic acid or glucuronic acid units (Meychik and Yermakov 2001). ζ-potential of NPs was − 1.513 mV (Fig. 1b), which caused strong repulsive electrostatic interactions between NPs and root surface, and then decreased particulate Fe accumulation on root surface in individual NP treatment. However, |ζ| values of NPs were to become zero (Fig. 1b) after adding OTC, which caused the less repulsion between NP and the negatively charged root surface, and then promoted particulate Fe accumulation on root surface. Third, Fe2+ ions in solution help to induce Fe plaque on root surface (Wu et al. 2016; Cai et al. 2016). In our experiment, OTC retarded Fe2+ oxidation in solution (Fig. 2a), which promoted Fe plaque formation.
XRD analysis of rice root. Conditions: 25 mg L−1 Fe added from Fe2O3 NPs or Fe(II)-EDTA, and OTC concentration added is 100 mg L−1. a XRD analysis of root under different Fe species from Fe2O3 NPs and Fe(II)-EDTA. b XRD analysis of root from different adding methods of Fe2O3 NPs. Direct method: adding NPs in hydroponic solution for NPs and NPs + OTC. Indirect method: adding NPs in dialysis tube with molecular cutoff 3000 kb and then putting it into hydroponic solution for NPs1 and NPs1 + OTC
3.3 Fe2O3 NP accumulation in rice tissues
For the Fe2O3 NP treatments, two exposure doses of OTC had no impact on the root-Fe concentrations (Fig. 7a). However, the presence of OTC significantly decreased the root-Fe accumulation (p < 0.01) as compared with the individual Fe-EDTA treatment. The distribution of Fe2O3 NPs or aggregates in whole root tissues could be affected by the presence of OTC, although root-Fe accumulation was similar among individual and combined NP exposure. According to TEM imaging (Fig. S2, ESM), there was no particle or aggregates in root under Fe-EDTA treatments. However, under Fe2O3 NP exposure, the aggregates could stick on the root surface or then enter the root cell and localize in intercellular, cytoplasm, and vacuoles. Besides root epidermis, the aggregates were also found inside the roots including the intercellular (Fig. S2B, ESM), cytoplasm (Fig. S2C, ESM), and vacuole regions (Fig. S2D, ESM). So, we speculated that Fe2O3 NPs could be taken up directly by rice. Similar result has been found by Nhan et al. (2015). In the study, OTC could increase NP aggregation in intercellular space. In cytoplasm and vacuoles, the shapes of aggregates were similar w/ or w/o the presence of OTC. For the Fe2O3 NP treatments, the presence of OTC significantly elevated the shoot-Fe accumulation (p < 0.05) (Fig. 7b), although a dose-response fashion was not evident between two OTC levels. However, the presence of OTC significantly decreased the shoot-Fe accumulation (p < 0.01) as compared with the individual Fe-EDTA exposure.
For Fe2O3 NP treatments, the order of surface-Fe (1376–1779 mg kg−1) > shoot (466–568 mg kg−1) > root (212–227 mg kg−1), regardless of the OTC presence. It was reported that Fe2O3 NPs mostly existed around the epidermis of corn root and no translocation of NPs from roots to shoots was observed (Li et al. 2016b). Although above work did not divide the root surface and root, it was still found that most Fe was accumulated in root containing root surface similar to present our study. For the Fe-EDTA treatments, when compared to its respective NP treatment, significantly high Fe concentrations were found on root surface (Fig. 4), inside roots (Fig. 7a), and shoots (Fig. 7b), suggesting that Fe-EDTA could provide more bioavailable Fe and increase Fe accumulation by plants as compared to Fe2O3 NPs. Fe distribution on root surface was 66.51–70.09% and 42.08–48.28%, respectively, for Fe2O3 NPs and Fe-EDTA treatments. However, Fe distribution inside root was only 8.352–10.95% and 7.210–14.15%, respectively. And, Fe distribution in shoot was only 21.56–23.31% and 43.77–44.51%, respectively. The results also showed that, as compared with Fe-EDTA treatment, high mass percent of surface-Fe as Fe plaque upon Fe2O3 NP exposure. The Fe ions from Fe-EDTA could be absorbed more easily by plant than Fe particles from Fe2O3 NPs. Similar results were observed for Yb accumulation in cucumber on Yb2O3 NP and YbCl3 exposure (Zhang et al. 2011). In the study, more Fe was accumulated in shoot than one in root, which showed that Fe2+ or Fe2O3 NP with less than 20 nm could be easily translocated from root to shoot.
3.4 OTC accumulation on root surface and in rice tissues
As shown in Fig. 8a, surface-OTC concentration in individual OTC treatments with 25 and 100 mg L−1 level was only 1306 and 4297 mg kg−1, respectively. In combined exposure treatments, the concentration increased to 2255 and 5801 mg kg−1, respectively. Moreover, surface-OTC concentrations were also promoted with Fe-EDTA treatment compared to individual OTC. Our previous study (Bao et al. 2018) has showed that Fe-EDTA addition induced Fe plaque formation on root surface and then increased surface-OTC accumulation. In the study, Fe plaque was formed in all Fe-EDTA and Fe2O3 NP treatments. Fe oxides (Rubert and Pederson 2006) as main Fe plaque components on root surface adsorbed and immobilized OTC, which would promote surface-OTC concentration. It was also found that Fe-EDTA and NP promoted significantly (p < 0.05) OTC accumulation in crystalline and amorphous fraction of Fe plaque simultaneously. The significant promotion (p < 0.05) from Fe-EDTA and NP treatments was also found for root-OTC concentration (Fig. 8b). Also, insignificant difference for the promotion was still found between Fe-EDTA and Fe2O3 NPs for OTC 25 mg L−1 level. However, combined exposure affected insignificantly (p > 0.05) shoot-OTC concentrations (Fig. 8c) under two OTC levels. During the exposure, the changes of OTC concentrations in hydroponic solution were determined in different treatments (Fig. S3, ESM). In the first 4-min exposure, there was insignificant difference between OTC, OTC + NPs, and OTC + Fe-EDTA treatments. Then, during 2–10-day exposure, OTC concentration in solution was in the order OTC + NPs (0.77–7.08 mg L−1) > OTC (0.54–5.85 mg L−1) > OTC + Fe-EDTA (0–0.62 mg L−1). The results indicated that OTC accumulation on root surface and in rice tissues was not consistent with OTC concentrations in solution. In a word, combined exposure cannot influence OTC accumulation in shoot although promoting OTC accumulation on root surface and inside root.
It was also found that, for OTC treatments, the order of OTC accumulation was Fe plaque of root surface (1306–5801 mg kg−1) > shoot (428.6–1220 mg kg−1) > root (158.3–657.0 mg kg−1), regardless of the NPs or Fe-EDTA presence under same OTC level. Our previous study (Bao et al. 2018) has found similar OTC bioaccumulation in rice. For the Fe-EDTA treatment, when compared to its respective NP treatment, almost equal OTC concentrations were found in Fe plaque on root surface (Fig. 8a), roots (Fig. 8b), and shoots (Fig. 8c), although high Fe concentration was found in Fe plaque, inside root and in shoot in Fe-EDTA treatments compared to NP treatment. Surface-OTC amount per kilogram of Fe plaque in NP treatment was 2.231 times of one Fe-EDTA treatment when Fe in solution was 25 mg L−1, which suggested that Fe plaque from NPs accumulated more readily OTC compared to that from Fe-EDTA. Our results from Section 3.2 have showed that, for NP treatments, Fe plaque was formed mainly through the direct adsorption of NPs on the outside root surface and then incorporated into Fe plaque as its crystalline components. We speculated that surface-Fe from NPs had large specific surface area compared to ones from Fe-EDTA due to NPs keeping the original physical properties during Fe plaque formation. As shown in Fig. 3, it was visible from SEM that there were many “small particle compositions” on the surface of Fe plaque from NP treatment; however, smooth surface was found on the surface of Fe plaque from Fe-EDTA treatment. Therefore, surface-Fe from NP treatments can hold strongly OTC due to Fe2O3 particle on root surface with high specific surface area, although less amounts of Fe plaque were formed in Fe2O3 NP treatments (1651 mg kg−1) in comparison with Fe-EDTA treatments (4062 mg kg−1).
For OTC distribution, surface-OTC distribution was 75.99–78.64% and 82.88–83.98%, respectively, for individual and combined exposure of OTC. However, root-OTC distribution was 9.211–10.35% and 10.35–11.71%, respectively. And, shoot-OTC distribution was 11.01–14.80% and 5.141–6.509%, respectively. Above results indicated that, as compared with individual OTC, high mass percent of surface-OTC remained due to Fe plaque formation and low mass percent of shoot-OTC remained upon combined exposure. Also, there was insignificant difference for the influence from NPs and Fe-EDTA treatments.
4 Conclusions
This study clearly demonstrates that the individual and combined exposure of Fe2O3 NPs and OTC in agricultural fields may result in their accumulation in rice. Whether it was individual or combined exposure, their accumulation in rice was always in the order root surface > shoot > root. The presence of OTC increased surface-Fe concentrations mainly through promoting particulate Fe and ionic Fe adsorption on the outside root surface and then taking part in Fe plaque formation through the following approach. First, OTC decreased the repulsive electrostatic interaction between NP and the negatively charged root surface. Second, OTC increases Fe2+ release in solution from NPs. OTC also promoted shoot-Fe concentrations, but not affecting root-Fe concentrations. However, in Fe-EDTA treatments, OTC decreased surface-Fe, root-Fe, and shoot-Fe concentrations, which could be caused by competitive uptake by rice root from OTC and Fe2+. It was speculated that Fe2O3 NPs could be taken up directly by rice according to TEM analysis. And, the presence of OTC affected mainly Fe2O3 NP distribution on the root surface of rice and in the intercellular space inside root. The Fe accumulation in the NP treatment is far less than that in the treatment with the identical Fe concentration in the form of Fe(II)-EDTA, which indicated particle Fe from NP-limited Fe bioaccumulation in rice due to limited bioavailable Fe ion release. The presence of Fe2O3 NPs increased surface-OTC and root-OTC levels. Surface-Fe from NP treatments can hold strongly OTC due to Fe2O3 particle on root surface with high specific surface area comparing with Fe-EDTA treatment. NPs reduced shoot-OTC under low OTC level (25 mg L−1), but not under high OTC level (100 mg L−1). From this perspective, combined exposure will increase Fe2O3 NPs and OTC bioaccumulation on the root surface comparing with individual exposure after 10-day growth of rice, which could pose a potential risk to food safety. Given the present study under a 10-day hydroponic exposure, long-term exposure of combined contaminant should be considered in the future.
References
Armstrong W (1997) The oxidising activity of roots in waterlogged soils. Physiol Plant 20:920–926
Bao YY, Zhou QX (2015) Temporal changes in horsebean bioavailability and accumulation after removing extractable oxytetracycline fractions in soils. RSC Adv 5:32572–32579
Bao YY, Zhou QX, Guan LZ, Wang YY (2009) Depletion of chlortetracycline during composting of aged and spiked manures. Waste Manag 29(4):1416–1423
Bao YY, Chen Q, Ma W, Zhou QX (2018) Influence of Fe addition on the accumulation of oxytetracycline in rice seedlings (Oryza sativa L.) growing in hydroponic and soil culture. J Soils Sediments 18:1958–1970
Cai F, Ren J, Tao S, Wang X (2016) Uptake, translocation and transformation of antimony in rice (Oryza sativa L.) seedlings. Environ Pollut 209:169–176
Chen CC, Dixon JB, Turners FT (1980) Iron coatings on rice roots morphology and models of development. Soil Sci Soc Am J 44:1113–1119
Figueroa RA, MacKay AA (2005) Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environ Sci Technol 39:6664–6671
Geisler-Lee J, Wang Q, Yao Y, Zhang W, Geisler M, Li K, Huang Y, Chen Y, Kolmakov A, Ma X (2013) Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 7:323–337
Ghosh S, Jiang W, Mcclements JD, Xing BS (2011) Colloidal stability of magnetic iron oxide nanoparticles: influence of natural organic matter and synthetic polyelectrolytes. Langmuir 27:8036–8043
Gupta UC (1968) Studies on the O-phenanthroline method for determining iron in plant materials. Plant Soil 28(2):298–305
Hu XG, Zhou QX (2013) Health and ecosystem risks of graphene. Chem Rev 113:3815–3835
Hu M, Li FB, Liu CP, Wu WJ (2015) The diversity and abundance of As(III) oxidizers on root iron plaque is critical for arsenic bioavailability to rice. Sci Rep 5:1–10
Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Oryza: from molecule to plant. Springer, Netherlands, pp 25–34
Kulshrestha P, Giese RF, Aga DS (2004) Investigating the molecular interactions of oxytetracycline in clay and organic matter: insights on factors affecting its mobility in soil. Environ Sci Technol 38(15):4097–4105
Lanzl CA, Baltrusaitis J, Cwiertny DM (2012) Dissolution of hematite nanoparticle aggregates: influence of primary particle size, dissolution mechanism, and solution pH. Langmuir 28:15797–15808
Li Y, Wang H, Liu X, Zhao G, Sun Y (2016a) Dissipation kinetics of oxytetracycline, tetracycline, and chlortetracycline residues in soil. Environ Sci Pollut Res 23:13822–13831
Li JL, Hu J, Ma CX, Wang YQ, Wu C, Huang J, Xing BS (2016b) Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 159:326–334
Liu WJ, Zhu YG, Hu Y, Williams PN, Gault AG, Meharg AA, Charnock JM, Smith FA (2006) Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza Sativa L.). Environ Sci Technol 140:5730–5736
Liu YX, Bao YY, Cai Z, Zhang ZN, Cao PL, Li XQ, Zhou QX (2015) The effect of aging on sequestration and bioaccessibility of oxytetracycline in soils. Environ Sci Pollut Res 22:10425–10433
Liu H, Ma CX, Chen GC, White JC, Wang ZH, Xing BS, Dhankher OP (2017) Titanium dioxide nanoparticles alleviate tetracycline toxicity to Arabidopsis thaliana (L.). ACS Sustain Chem Eng 5:3204–3213
Ma YH, He X, Zhang P, Zhang Z, Guo Z, Tai R, Xu Z, Zhang L, Ding Y, Zhao Y (2011) Phytoxicity and biotransformation of La2O3 nanoparticles in a terrestrial plant cucumber (Cucumissativus). Nanotoxicology 5:743–753
Ma YH, Zhang P, Zhang ZY, He X, Zhang JZ, Ding YY, Zhang J, Zheng LR, Guo Z, Zhang LJ, Chai ZF, Zhao YL (2015) Where does the transformation of precipitated ceria nanoparticles in hydroponic plants take place? Environ Sci Technol 49:10667–10674
Ma CX, Liu H, Chen GC, Zhao Q, Eitzer B, Wang ZH, Cai WJ, Newman LA, White JC, Dhankher OP (2017) Effects of titanium oxide nanoparticles on tetracycline accumulation and toxicity in Oryza sativa (L.). Environ Sci Nano 4:1827–1839
Manuel CC, Cristina AE, Remigio PN, Juan CNM, Manuel AE, Esperanza AR, María JFS, Avelino ND (2018) Occurrence of tetracyclines and sulfonamides in manures, agricultural soils and crops from different areas in Galicia (NW Spain). J Clean Prod 197:491–500
Martínez-Fernández D, Barroso D, Komárek M (2016) Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ Sci Pollut Res 23:1732–1741
Meychik NR, Yermakov IP (2001) Ion exchange properties of plant root cell walls. Plant Soil 234:181–193
Monica RC, Cremonini R (2009) Nanoparticles and higher plants. Caryologia 62:161–165
Nhan LV, Ma C, Rui Y, Cao W, Deng Y, Liu L, Xing B (2015) The effects of Fe2O3 nanoparticles on physiology and insecticide activity in non-transgenic and Bt-transgenic cotton. Front Plant Soil 6:1263
Pardo T, Martínez-Fernández D, Fuente C, Clemente R, Komáre M, Bernal MP (2016) Maghemite nanoparticles and ferrous sulfate for the stimulation of iron plaque formation and arsenic immobilization in Phragmites australis. Environ Pollut 219:296–304
Parsons JG, Lopez ML, Gonzalez CM, Peralta-Videa JR, Gardea-Torresdey JL (2010) Toxicity and biotransformation of uncoated and coated nickel hydroxide nanoparticles on mesquite plants. Environ Toxicol Chem 29:1146–1154
Qi N, Wang PF, Wang C, Ao YH (2018) Effect of a typical antibiotic (tetracycline) on the aggregation of TiO2 nanoparticles in an aquatic environment. J Hazard Mater 341:187–197
Rubert KF and Pederson JA (2006) Kinetics of Oxytetracycline Reaction with a Hydrous Manganese Oxide. Environ Sci Technol 40:7216–7221
Sebastian A, Nangia A, Prasad MNV (2017) Carbon-bound iron oxide nanoparticles prevent calcium-induced iron deficiency in Oryza sativa L. J Agric Food Chem 65:557–564
Sun JT, Zeng QT, Tsang DCW, Zhu LZ, Li XD (2017) Antibiotics in the agricultural soils from the Yangtze River Delta, China. Chemosphere 189:301–308
Wang H, Yao H, Sun P, Li D, Huang C (2016) Transformation of tetracycline antibiotics and Fe(II) and Fe(III) species induced by their complexation. Environ Sci Technol 50:145–153
Wei RC, He T, Zhang SX, Zhu L, Shang B, Li ZJ, Wang R (2019) Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China. Chemosphere 215:234–240
Wu C, Zou Q, Xue SG, Pan WS, Huang L, Hartley W, Mo JY, Wong MH (2016) The effect of silicon on iron plaque formation and arsenic accumulation in rice genotypes with different radial oxygen loss (ROL). Environ Pollut 212:27–33
Zhang P, Ma Y, Zhang Z, He X, Guo Z, Tai R, Ding Y, Zhao Y, Chai Z (2011) Comparative toxicity of nanoparticulate/bulk Yb2O3 and YbCl3 to cucumber (Cucumissativus). Environ Sci Technol 46:1834–1841
Zhang P, Ma YH, Zhang ZY, He X, Zhang J, Guo Z, Tai RZ, Zhao YL, Chai ZF (2012) Biotransformation of ceria nanoparticles in cucumber plants. ACS Nano 6:9943–9950
Zhou QX, Sun FH, Liu R (2005) Joint chemical flushing of soils contaminated with petroleum hydrocarbons. Environ Pollut 31:835–839
Zhu ZJ, Wang HH, Yan B, Zheng H, Jiang Y, Miranda OR, Rotello VM, Xing BS, Vachet RW (2012) Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ Sci Technol 46:12391–12398
Acknowledgements
Y.Y. Bao thanks the China Scholarship Council for supporting her study at the University of Massachusetts, Amherst.
Funding
This work is financially supported by the Tianjin Municipal Science and Technology Commission (Grant 16JCZDJC39200) and by National Key R&D Program of China (2018YFD0800303) from China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Dong-Mei Zhou
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The additional information for dry weight of rice, TEM imaging of rice root, as well as the elimination of OTC in hydroponic solution is presented in the supplementary file.
ESM 1
(DOCX 7127 kb)
Rights and permissions
About this article
Cite this article
Bao, Y., Ma, C., Hu, L. et al. Effect of individual and combined exposure of Fe2O3 nanoparticles and oxytetracycline on their bioaccumulation by rice (Oryza sativa L.). J Soils Sediments 19, 2459–2471 (2019). https://doi.org/10.1007/s11368-018-2216-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2216-8