Abstract
Titanium dioxide (TiO2) is one of the most widely used pigments in the world. Due to its heavy use in industry and daily life, such as food additives, cosmetics, pharmaceuticals, and paints, many residues are released into the environment and currently TiO2 nanoparticles are considered an emerging environmental contaminant. Although several studies have shown the effect of TiO2 nanoparticles on a wide range of organisms including bacteria, algae, plankton, fish, mice, and rats, little research has been performed on land plants. In this study, we investigated the effect of TiO2 nanoparticles on the growth, development, and gene expression of tobacco, an important economic and agricultural crop in the southeastern USA as well as around the world. We found that TiO2 nanoparticles significantly inhibited the germination rates, root lengths, and biomasses of tobacco seedlings after 3 weeks of exposure to 0.1, 1, 2.5, and 5 % TiO2 nanoparticles and that overall growth and development of the tobacco seedlings significantly decreased as TiO2 nanoparticle concentrations increased. Overall, tobacco roots were the most sensitive to TiO2 nanoparticle exposure. Nano-TiO2 also significantly influenced the expression profiles of microRNAs (miRNAs), a recently discovered class of small endogenous noncoding RNAs (∼20–22 nt) that are considered important gene regulators and have been shown to play an important role in plant development as well as plant tolerance to abiotic stresses such as drought, salinity, cold, and heavy metal. Low concentrations (0.1 and 1 %) of TiO2 nanoparticles dramatically induced miRNA expression in tobacco seedlings with miR395 and miR399 exhibiting the greatest fold changes of 285-fold and 143-fold, respectively. The results of this study show that TiO2 nanoparticles have a negative impact on tobacco growth and development and that miRNAs may play an important role in tobacco response to heavy metals/nanoparticles by regulating gene expression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium dioxide (TiO2) is a common whitening agent used in the production of foods, sunscreens, cosmetics, plastics, medicines, papers, pharmaceuticals, and paints. Estimates have shown that approximately 4 million metric tons of bulk TiO2 is produced annually around the globe with the USA leading production with 1.3 million metric tons (Lubick 2009). TiO2 production also accounts for 70 % of the total amount of manufactured pigments worldwide (Baan et al. 2006; Trouiller et al. 2009). TiO2 is becoming increasingly used in the industry due to its white pigment, ability to block ultraviolet light, and photocatalytic properties that are commonly used to destroy pollutants in soil, air, and water/wastewater treatment (Aarthi and Madras 2007; Higarashi and Jardim 2002).
Due to its heavy use in the industry and daily life, many TiO2 residues are released into the environment, and currently, TiO2 nanoparticles are being considered an emerging environmental contaminant. Studies analyzing the health effects of TiO2 nanoparticle exposure have led it to be classified by the International Agency for Research on Cancer as a group 2B carcinogen, meaning that it is potentially harmful to humans (Baan et al. 2006). Accordingly, mounting evidence has shown that TiO2 nanoparticles create lipid peroxidation products and reactive oxygen species that induce epithelial cell injury and oxidative DNA damage in cell lines, mice, and rats (Falck et al. 2009; Trouiller et al. 2009). Since TiO2 nanoparticles are a potential hazard to human health, studies in rat models have shown that lung cancer can be induced after exposure to nano-TiO2 (Pott and Roller 2005). It should be noted, however, that no studies have shown a correlation between TiO2 nanoparticle exposure and the development of lung cancer in industry workers (Boffetta et al. 2004; Fryzek et al. 2003).
As the manufacturing and industrial use of TiO2 increases, the amount of TiO2 nanoparticles released into the environment is also likely to increase. This is due in part to the high use of TiO2 and the ability of TiO2 nanoparticles to travel freely through wastewater treatment plants and enter neighboring soils and streams (Kiser et al. 2009; Lubick 2009). Therefore, TiO2 is also being investigated as a potential environmental contaminant and the effect of TiO2 in water (Adams et al. 2006; Kaegi et al. 2008) and on soils (Higarashi and Jardim 2002; Mattigod et al. 2005), bacteria (Kubo et al. 2005; Wang et al. 2009), algae (Hartmann et al. 2009; Hund-Rinke and Simon 2006), fungi (Sichel et al. 2007), plankton (Hund-Rinke and Simon 2006; Lovern and Klaper 2006; Lovern et al. 2007), and fish (Federici et al. 2007; Reeves et al. 2008) have been analyzed. A recent study has also observed that TiO2 nanoparticles cause genetic toxicity to Salmonella typhimurium (Pan et al. 2010). It is surprising, given the increasing concern of TiO2 nanoparticles to the environment, that only two studies have analyzed the effect of TiO2 nanoparticles on plants (Asli and Neumann 2009; Zheng et al. 2005), and no study has been performed on understanding the effect of nanoparticles on regulatory RNAs.
microRNAs (miRNAs) are a recently discovered class of small regulatory endogenous RNAs (∼20–22 nt) that do not code for proteins (Bartel 2004; Zhang et al. 2006). miRNAs play an important role in plant growth and development as they have been shown to aid in leaf development (Juarez et al. 2004), stem and root growth (Laufs et al. 2004; Mallory et al. 2004), organ maturation (Achard et al. 2004; Aukerman and Sakai 2003; Chen et al. 2004; Mlotshwa et al. 2006), and the plant’s ability to withstand abiotic stresses (Jones-Rhoades and Bartel 2004; Sunkar and Zhu 2004). miRNAs target messenger RNAs (mRNAs) and regulate gene expression by marking these mRNAs for degradation or repressing protein translation (Bartel 2004; Vaucheret 2006). Recent studies have shown that miRNAs mediate the expression of more than 30 % of genes coding for protein (Lewis et al. 2005; Xie et al. 2005), and this number is expected to rise as more miRNAs and their target sequences are identified.
miRNAs have been shown to play an important role in many biological and metabolic processes. Specifically, certain miRNAs have been identified in plant responses to abiotic stresses. For example, miR156, miR169, miR395, miR399, and miR399* (miR399* is the opposite strand of miR399 in the pre-miRNA stem-looped structure) expression levels are altered during the response of Arabidopsis to phosphate starvation (Fujii et al. 2005; Hsieh et al. 2009). miR398 and miR408 have been shown to be upregulated in drought-exposed Medicago truncatula plants (Trindade et al. 2010), and miR398 has been implicated in plant response to copper and oxidative stress (Abdel-Ghany and Pilon 2008; Sunkar et al. 2006). miRNA expression has also been shown to be altered under both cold (Zhang et al. 2009; Zhou et al. 2008a) and salt (Ding et al. 2009; Jia et al. 2009) stresses.
In this study, we used tobacco as a model species to analyze the effect of TiO2 nanoparticles on the growth, development, and miRNA expression levels of an agricultural plant. Tobacco is an important economic and agricultural crop in the southern USA as well as around the world. Although much research has been dedicated to this crop, no experiments have shown the effects of TiO2 nanoparticles on tobacco growth and development. We found that as the concentration of nano-TiO2 increased, the germination rates, root lengths, biomasses, and leaf counts of 3-week-old tobacco seedlings decreased in a concentration dependent manner. We also found that the expression levels of miRNAs were either up- or downregulated in response to increasing TiO2 nanoparticle concentrations. Ultimately, we were able to conclude that TiO2 nanoparticles negatively affect tobacco growth and that miRNAs may play an important role in tobacco plants response to heavy metal toxicity, including nanoparticles.
Materials and methods
Tobacco treatment
Tobacco (Nicotiana tabacum) seeds were sterilized in 70 % ethanol for 2 min followed by 10 % bleach for 15 min. The seeds were then rinsed with sterile distilled water until no bleach odor remained. Sterilized seeds were sowed on Petri dishes containing Murashige and Skoog (MS) medium containing Gamborg’s B5 vitamins (0.44 % salts, 1 % sucrose, 0.8 % agar, pH 5.8) and 0, 0.1, 1, 2.5, or 5 % TiO2 nanoparticles. TiO2 nanoparticles were purchased from Sigma. The part size is less than 25 nm with more than 99.5 % purity. A total number of 25 seeds were sowed per plate, and there were a total number of 5 plates for the control and each treatment with different TiO2 nanoparticle concentrations. The plates were inverted at a 75° angle to promote downward root growth and allowed to grow for 3 weeks on a 16-h day/8 h night cycle.
Measuring germination rate, leaf count, root length, and biomass
After 3 weeks of growth, the germination rate of all five plates for the control and each concentration of nano-TiO2 was recorded. We followed the procedure as described in Lin and Xing (2007), in which we recorded a seed germinated if the radical or cotyledon was observed protruding from the seed coat. The number of leaves per seedling per plate for the control and different concentrations of TiO2 was also recorded. Seedlings were removed from the media, and the root lengths of the control, 0.1 % TiO2, and 1 % TiO2 plates were measured (in centimeters) using a standard ruler. The root lengths of 2.5 and 5 % seedlings were not measured because no roots were formed. In the case where more than one root was formed, all roots were measured, but only the largest root measurement was used in calculating average root length. Once root lengths were recorded, seedlings were then placed on a weigh boat and the weight of biomass measured using a Discovery 214C balance (OHaus, Pine Brook, NJ). After the biomass of each plate was recorded, the seedling tissue was immediately placed in liquid nitrogen and stored at −80 °C until RNA extraction.
Total RNA extraction
Total RNA was isolated from the 3-week-old seedlings using the mirVana miRNA Isolation Kit (Ambion, Austin, TX) according to the manufacturer’s protocol. Total RNA was then quantified and assessed for quality using a Nanodrop ND-1000 (Nanodrop Technologies, Wilmington, DE). RNA samples were stored at −80 °C until further analysis.
Analyzing microRNA expression changes using RT-PCR and qRT-PCR
Quantitative real-time PCR (qRT-PCR) has become one of the most mature techniques to detect the miRNA expression in different organisms; this is because it can be used to distinguish one single nucleotide change during the miRNA sequence, and it is highly sensitive to miRNA expression (Chen et al. 2005). This technique has been used to measure and validate miRNA expression in many plant species. Thus, in this study, Applied Biosystems TaqMan microRNA Assays were employed to detect and quantify tobacco miRNAs using stem loop real-time PCR according to the manufacturer’s instructions. There were two steps in the TaqMan miRNA Assays: (a) reverse transcription of the mature miRNA to a longer single-stranded cDNA sequence using a miRNA-specific stem-looped primer and (b) quantitative real-time PCR. Briefly, a single-stranded miRNA cDNA was generated from 1 μg of the total RNA from the control, 0.1 %, and 1 % TiO2 nanoparticle tissue samples by reverse transcription using the Applied Biosystems TaqMan microRNA Reverse Transcription Kit and miRNA-specific stem-looped RT primers. Each reaction was repeated in triplicate. In total, we investigated changes in the expression levels of 11 different miRNAs (miR156, miR159, miR162, miR167, miR169, miR172, miR393, miR395, miR396, miR398, and miR399) and 2 stress-related genes (alcohol dehydrogenase (ADH) and alcohol peroxidase (APX)). The reason for selecting these 11 miRNAs is because these 11 miRNAs were aberrantly expressed during abiotic stresses in model plant species or play important role in plant growth and development (Khraiwesh et al. 2012; Zhang et al. 2006). Two housekeeping genes (elongation factor 1 (EF1) and tubulin) were used as reference genes to normalized expression values. All reactions were performed in triplicate, and the results were analyzed using the ΔΔCT method.
Results
Nano-TiO2 inhibited tobacco seed germination and plant growth
After 3 days of planting, seeds started to germinate. At low concentrations (0.1 and 1.0 %), nano-TiO2 did not affect tobacco seed germination (Fig. 1a). However, nano-TiO2 significantly affected tobacco seed germination at higher concentrations (p = 0.016). When 2.5 % TiO2 nanoparticles were added into the MS medium, the germination rate of seeds decreased from 98–100 to 93.60 ± 3.58 %. Also, the germination of tobacco seeds was completely inhibited by exposure to 5.0 % nano-TiO2. Although tobacco seeds germinated with a higher percentage after exposure to 2.5 % nano-TiO2, it was hard for these seeds to produce roots and fully developed cotyledons. Therefore, the germinated seedlings quickly died within about 1 week.
After 3 weeks, there was a significant obvious decrease in plant growth and development after exposure to elevated TiO2 nanoparticle concentrations as evidenced by decreases in root lengths (p < 0.001), leaf numbers (p < 0.001) as well as plant biomasses (p < 0.001) (Figs. 1 and 2). Roots were the most sensitive to TiO2 nanoparticle exposure (Fig. 1b). Under normal conditions, the root lengths reached to 1.43 ± 0.15 cm after 3 weeks of culture; however, after exposure to 0.1 and 1 % nano-TiO2, the root lengths were significantly reduced to 0.74 ± 0.05 and 0.21 ± 0.04 cm (p < 0.001), respectively. A 2.5 % nano-TiO2 completely inhibited the appearance and elongation of roots. An interesting phenomenon is that TiO2 nanoparticle exposure influenced differential root patterns. Tobacco is a dicot; generally speaking, one seed only produces one major root. However, after exposure to 1.0 % TiO2, a majority of tobacco seedlings produced two roots, although both were very short with an average of 0.21 ± 0.04 cm after 3 weeks of growth. One of the potential reasons is that nano-TiO2 exposure can damage the major root apical meristem and therefore allow the seedlings to produce an additional root from the damaged location (Fig. 2c).
Effect of nano-TiO2 on tobacco growth and development after 3 weeks of treatment. A MS media (control). B MS + 0.1 % nano-TiO2. C MS + 1 % nano- TiO2. D MS + 2.5 % nano-TiO2. Figure 2c shows that multiple roots were generated from the damaged sites
Exposure to nano-TiO2 also influenced tobacco leaf development as demonstrated by a reduction in the total number of leaves and the leaf size. After exposure to nano-TiO2, tobacco leaves grew much smaller compared to the untreated control (Fig. 2). The averages for total leaf number were 4.07 ± 0.19, 3.92 ± 0.06, 4.19 ± 0.24, and 1.72 ± 0.63 for the control, 0.1 % nano-TiO2, 1 % nano-TiO2, and 2.5 % nano-TiO2 groups, respectively (Fig. 1c). Although TiO2 nanoparticle exposure significantly reduced the total number of seedling leaves at higher concentrations (2.5 %; p < 0.001), no significant differences were observed between the untreated control and low nano-TiO2 treatments (p = 0.536 for 0.1 % and p = 0.626 for 1 %).
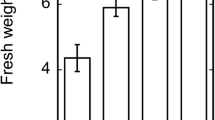
TiO2 nanoparticle exposure significantly reduced plant growth and biomass even at very low concentrations (Figs. 1d and 2; p < 0.001). For the untreated control, the average biomass was 146 ± 49 mg. Nano-TiO2 significantly inhibited tobacco plant growth, and plant biomass was reduced to 81 ± 21 mg after exposure to 0.1 % nano-TiO2 (p < 0.001). However, there was no significant difference in plant biomass between the 0.1 and 1 % TiO2 nanoparticle treatments (p = 0.431). When 2.5 % nano-TiO2 was added into the medium, almost all seedlings died within 1 week of germination and minimal plant biomass was harvested (Fig. 1d).
TiO2 nanoparticles affect miRNA expression in tobacco
TiO2 nanoparticle exposure significantly affected miRNA expression (Fig. 3). Among the 11 miRNAs investigated in this study, the miRNA expression levels were upregulated at both concentrations (0.1 and 1.0 %) after nano-TiO2 exposure for all miRNAs except miR156 at high concentration (1.0 %). According to their response to nano-TiO2 exposure, these 11 miRNAs could be classified into three groups: (1) miRNAs whose expression levels were increased as TiO2 nanoparticle concentrations increased, the majority of tested miRNAs belonged to this group, which included miR169, miR393, miR395, miR399, miR172, and miR396; (2) miRNAs whose expression levels decreased as TiO2 nanoparticle concentrations increased, there are two miRNAs (miR159 and miR156) that belonged to this group; and (3) miRNAs whose expression levels were not significantly changed as TiO2 nanoparticle concentrations increased, there are three miRNAs (miR162, miR167, and miR398) that belonged to this group.
Among the 11 tested miRNAs, miR395 and miR399 were the two most sensitive to nano-TiO2 exposure, exhibiting the greatest fold changes to 285-fold and 143-fold, respectively. This suggests that these two miRNAs play an important function in tobacco seedlings facing nanoparticle or metal stress. It was also observed that these two miRNAs were upregulated under different environmental abiotic stresses, such as drought and salinity stress in other plant species (Jones-Rhoades and Bartel 2004; Zhao et al. 2007). Six miRNAs (miR169, miR159, miR393, miR398, miR396, and miR172) are moderately sensitive to nano-TiO2 exposure, exhibiting at least a 10-fold change after 3 weeks of growth on the medium containing 0.1 or 1.0 % of TiO2. However, miR156 and miR162 were less sensitive to nano-TiO2 exposure as they exhibited only less than a 2-fold change after 3 weeks of treatment. According to previous studies, miR156 regulates plant leaf development (Schwab et al. 2005; Zhang et al. 2006), and no study has reported that miR156 plays a function in stress response. In this study, we observed that miR156 expression is upregulated by 0.1 % nano-TiO2 but inhibited by 1.0 % nano-TiO2. One potential reason for this change is that nano-TiO2 inhibited tobacco seedling development and therefore the decrease in expression was due to a decrease in overall plant growth (Figs. 1 and 2).
TiO2 nanoparticles affect the expression of stress-related genes in tobacco
In this study, we also observed that TiO2 nanoparticle treatment affected the expression levels of stress-related genes. Both APX and ADH were significantly upregulated by both 0.1 and 1 % nano-TiO2 concentrations after 3 weeks of exposure. ADH and APX showed relatively the same fold increase in expression in 0.1 % TiO2 nanoparticle-exposed seedlings with ADH increasing 3.9-fold and APX increasing 3.6-fold. At a higher concentration (1.0 %), TiO2 nanoparticles increased the expression of ADH to 5.73-fold, while the expression of APX was kept around a 3.5-fold change. This suggests that nanoparticle treatment also induces abnormal expression of stress-related genes, and plants may have similar mechanisms to handle both nanoparticle toxicity as well as other abiotic stresses.
Discussion
Nanoparticles are used in a variety of applications (paints, papers, manufactured products, etc.) and are interesting to study because their large surface area to volume ratio often times leads to an increase in reactivity (Gao and Zhang 2001). As nanotechnologies become increasingly used, nanoparticles will continue to be an environmental concern, and the phytotoxicity of these nanoparticles on terrestrial plants must be elucidated. In this study, we analyzed the effect of TiO2 nanoparticles on the growth and development of tobacco seedlings. We found that as TiO2 nanoparticle concentrations increased, the germination rates, leaf counts, root lengths, and overall biomasses of the tobacco seedlings decreased. We also found that TiO2 nanoparticles influenced miRNA expression levels in tobacco.
The results of our study correspond to the results of other studies in which germination rates of ryegrass and corn were inhibited by Zn and ZnO nanoparticles (Lin and Xing 2007), and black cumin and wheat germination rates were inhibited by Zn2+ (El-Ghamery et al. 2003). Our results are slightly different from those found by Zheng et al. (2005), in which they found that low concentrations of nano-sized TiO2 enhanced the germination rates of naturally aged spinach seeds (Zheng et al. 2005). While TiO2 nanoparticles did not enhance the germination rate of the tobacco seeds, we did not observe a linear decrease in germination rate either, and germination rates rather decreased as TiO2 nanoparticle concentrations reached 2.5 % and above. One potential reason is that Zheng et al. only used TiO2 to treat spinach seeds, while we added TiO2 into the medium for longer exposure time. The seed coat has been shown to play a vital role in protecting the embryo from dangerous environmental factors (Wierzbicka and Obidzinksa 1998). Lin and Xing (2007) offered the explanation that even though seeds are exposed to nanoparticles, the nanoparticles cannot invade the seed coat and effect germination (Lin and Xing 2007). Therefore, it is only after the seed begins to germinate that it comes into contact with the nanoparticles. In this study, we noticed a corresponding decrease in overall tobacco growth with an increase in TiO2 nanoparticle concentration, suggesting that the nanoparticles have a negative impact on tobacco growth after seed germination has begun.
In this study, we also observed a decrease in average root length of tobacco seedlings as TiO2 nanoparticle concentrations increased. Seedlings grown in 0.1 % nano-TiO2 exhibited a decrease in average root growth by 48 % as compared to the control. An even greater inhibition of root growth was observed in 1 % nano-TiO2 tobacco seedlings as the average root growth was reduced by 85 %. At 2.5 % nano-TiO2, no root growth was observed. Therefore, we were able to show that TiO2 nanoparticles have a negative impact on the development of tobacco roots.
The phytotoxicity of other metal oxide nanoparticles on the root growth of a variety of plant species has also been evaluated. Results have shown that nano-Al2O3 negatively impacts root development in corn (Lin and Xing 2007) and that ZnO nanoparticles damage epidermal and cortical cells in the roots of ryegrass (Lin and Xing 2008), leading to an overall decrease in plant growth. The mechanism by which TiO2 nanoparticles affect root growth in tobacco still needs to be investigated; however, a recent study by Asli and Neumann (2009) showed that TiO2 nanoparticles affected the water transport ability of maize roots. The authors were able to show that TiO2 nanoparticles formed aggregates along the root cell wall that blocked the absorption of water, ultimately leading to reduced shoot and leaf development (Asli and Neumann 2009). Therefore, TiO2 nanoparticles might inhibit tobacco root development by interfering with the pore on the root cell wall and reducing water uptake.
It should be noted that tobacco seedlings grown on 1 % nano-TiO2 produced more than one root. If TiO2 nanoparticles do adhere to the root cell walls of tobacco seedlings, the growth of more than one root would be necessary in order to increase water uptake and promote plant development.
Overall biomass of tobacco seedlings decreased as TiO2 nanoparticle concentration increased. A 47 % decrease in biomass, as compared to the control, was observed for 0.1 % nano-TiO2 seedlings. Interestingly, seedlings grown on 1 % nano-TiO2 showed only a 37 % reduction in overall biomass. This result is surprising since a decrease in root development and an overall decline in plant health was noticed in these plants. The increase in biomass could be attributed to an intake of TiO2 nanoparticles. A recent study has shown that carbon-coated iron nanoparticles can be transported through the xylem in pumpkin plants (Corredor et al. 2009), and alternate studies have demonstrated that copper (Lee et al. 2008) along with Zn and ZnO (Lin and Xing 2008) nanoparticles can also be taken in by plant roots. It is possible that, over the 3-week course of this experiment, TiO2 nanoparticles entered into the tobacco seedlings through pores in the root cell wall. This would cause the slight increase in biomass that was observed for tobacco seedlings exposed to 1 % nano-TiO2.
As the TiO2 nanoparticle concentration increased, a visible reduction in plant health occurred. Tobacco seedlings exposed to increasing levels of nano-TiO2 exhibited yellowing, wilting, reduced leaf sizes and leaf counts, reduced root growth, and a decrease in shoots. In the case of tobacco seeds grown on 2.5 % nano-TiO2, no roots were formed. The results from our study do not agree with those by Zheng et al. (2005), in which they concluded that nano-TiO2 improved the growth of spinach plants (Zheng et al. 2005). Our results, however, do agree with others and appear to be symptoms of heavy metal toxicity (El-Ghamery et al. 2003; Lin and Xing 2008) and indicate cell death (Abraham 1997). Since TiO2 nanoparticles have been shown to induce the formation of reactive oxygen species leading to oxidative DNA damage and lipid peroxidation (Falck et al. 2009; Federici et al. 2007; Trouiller et al. 2009), a future study is needed to determine the mechanisms of TiO2 nanoparticle toxicity on tobacco. Alternate studies, as suggested by Murashov (2006), are also needed to determine if disassociation of the titanium ion is a cause of phytotoxicity rather than the TiO2 nanoparticles themselves (Murashov 2006).
miRNAs are a newly discovered class of small regulatory RNAs (Bartel 2004) that have been shown to play a role in plant response to many environmental stresses such as drought (Zhao et al. 2007), salinity (Jia et al. 2009), and cold (Zhang et al. 2009). miRNAs have also been identified in plant response to heavy metals. For instance, Sunkar et al. (2006) have shown that miR398 targets two related superoxide dismutase genes in Arabidopsis and by downregulating this miRNA, the plant has a higher tolerance to copper and zinc metals (Sunkar et al. 2006). Other investigations have analyzed the effect of cadmium, aluminum, and mercury on miRNA expression levels in Medicago truncatula and rice and have found that miRNA expression levels are altered after exposure to heavy metals (Huang et al. 2009; Zhou et al. 2008b).
In this study, we analyzed changes in expression levels of 11 miRNAs in response to TiO2 nanoparticles. We found that all 11 miRNAs were upregulated and, in comparison to the control plants, miR399b and miR395a in tobacco seedlings exposed to 1 % nano-TiO2 exhibited the greatest fold changes of 143 and 285, respectively. The expression levels of two stress-related genes, ADH and APX, were also upregulated after exposure to TiO2 nanoparticles. Since miRNAs do play a significant role in negatively regulating gene expression, we can assume that the upregulation of these miRNAs is affecting the expression levels of many important developmental genes. This would explain, in part, the observed decrease in overall plant growth.
In conclusion, TiO2 nanoparticles had a negative impact on the growth and development of 3-week-old tobacco seedlings. An overall reduction in germination rates, leaf counts, root lengths, and biomasses were observed in seedlings exposed to 0.1, 1, 2.5, and 5 % nano-TiO2. This result could be due to the interference of TiO2 clusters with the root cell wall, ultimately inhibiting water uptake, or the induction of reactive oxygen species leading to oxidative DNA damage and lipid peroxidation of the cell membrane in tobacco cells. More research needs to be performed in order to elucidate the mechanisms of TiO2 nanoparticle toxicity and to determine if phytotoxicity is caused by dissociation of the titanium ion. As the manufacturing of TiO2 continues to increase, TiO2 nanoparticles will continue to be released into the environment. Studies have shown that TiO2 nanoparticles can reach depths of between 40 and 370 cm in soil suspensions (Fang et al. 2009). Therefore, the phytotoxicity of TiO2 on important crop plants, such as tobacco, must be investigated.
microRNA expression levels in tobacco were also altered after exposure to nano-TiO2. In particular, miR399 and miR395 exhibited the greatest fold increases of 143 and 285, respectively. Our study analyzed changes in expression levels of 11 conserved miRNAs. In order to identify novel tobacco miRNAs that may play a role in plant tolerance to heavy metal stress, other experiments such as high throughput deep sequencing or direct cloning will be needed. We found that TiO2 nanoparticles affected miRNA expression levels in tobacco and since they function as important gene regulators, miRNAs may play a vital role in tobacco response to heavy metal stress.
References
Aarthi T, Madras G (2007) Photocatalytic degradation of rhodamine dyes with nano-TiO2. Ind Eng Chem Res 46(1):7–14
Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression response to low copper availability in Arabidopsis. J Biol Chem 283:15932–15945
Abraham S (1997) Studies on cytological changes induced by muriate of potatsh in Allium cepa. Cytologia 62(3):291–294
Achard P, Herr A, Baulcombe DC, Harberd NP (2004) Modulation of floral development by a gibberellin-regulated microRNA. Development 131(14):3357–3365. doi:10.1242/dev.01206
Adams LK, Lyon DY, Alvarez PJJ (2006) Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40(19):3527–3532
Asli S, Neumann PM (2009) Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ 32:577–584
Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15(11):2730–2741. doi:10.1105/tpc.016238
Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE, Cogliano V (2006) Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol 7(4):295–296
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Boffetta P, Soutar A, Cherrie JW et al (2004) Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control 15(7):697–706
Chen CZ, Li L, Lodish HF, Bartel DP (2004) MicroRNAs modulate hematopoietic lineage differentiation. Science 303(5654):83–86. doi:10.1126/science.1091903
Chen CF, Ridzon DA, Broomer AJ et al (2005) Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33(20):e179. doi:10.1093/nar/gni178
Corredor E, Testillano PS, Coronado M-J et al (2009) Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol 9:45. doi:10.1186/1471-2229-9-45
Ding D, Zhang LF, Wang H, Liu ZJ, Zhang ZX, Zheng YL (2009) Differential expression of miRNAs in response to salt stress in maize roots. Ann Bot 103(1):29–38. doi:10.1093/aob/mcn205
El-Ghamery AA, El-Kholy MA, El-Yousser MA (2003) Evaluation of cytological effects of Zn2+ in relation to germination and root growth of Nigella sativa L. and Triticum aestivum L. Mutat Res 537:29–41
Falck G, Lindberg H, Suhonen S et al (2009) Genotoxic effects of nanosized and fine TiO2. Hum Exp Toxicol 28:339–352
Fang J, X-q S, Wen B, J-m L, Owens G (2009) Stability of titania nanoparticles in soil suspensions and transport in saturated homogenous soil columns. Environ Pollut 157:1101–1109
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhyncus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84(4):415–430
Fryzek JP, Chadda B, Marano D et al (2003) A cohort mortality study among titanium dioxide manufacturing workers in the United States. J Occup Environ Med 45(4):400–409
Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK (2005) A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol 15(22):2038–2043. doi:10.1016/j.cub.2005.10.016
Gao L, Zhang Q (2001) Effects of amorphous contents and particle size on the photocatalytic properties of TiO2 nanoparticles. Scr Mater 44(8–9):1195–1198
Hartmann NB, Von der Kammer F, Hofmann T, Baalousha M, Ottofuelling S, Baun A (2009) Algal testing of titanium dioxide nanoparticles—testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology. doi:10.1016/j.tox.2009.08.008
Higarashi MM, Jardim WF (2002) Remediation of pesticide contaminated soil using TiO2 mediated by solar light. Catal Today 76(2–4):201–207
Hsieh L-C, Lin S-I, Shih AC-C et al (2009) Unconvering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151:2120–2132
Huang SQ, Peng J, Qiu CX, Yang ZM (2009) Heavy metal-regulated new microRNAs from rice. J Inorg Biochem 103(2):282–287. doi:10.1016/j.jinorgbio.2008.10.019
Hund-Rinke K, Simon M (2006) Ecotoxic. Effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ Sci Pollut Res 13(4):225–232
Jia XY, Wang W, Ren L et al (2009) Differential and dynamic regulation of miR398 in response to ABA and salt stress in Populus tremula and Arabidopsis thaliana. Plant Mol Biol 71(1–2):51–59
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant MicroRNAs and their targets, including a stress-induced miRNA. Mol Cell 14(6):787–799
Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MCP (2004) microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428(6978):84–88. doi:10.1038/nature02363
Kaegi R, Ulrich A, Sinnet B et al (2008) Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ Pollut 156(2):233–239
Khraiwesh B, Zhu JK, Zhu JH (2012) Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta Genet Regul Mech 1819(2):137–148. doi:10.1016/j.bbagrm.2011.05.001
Kiser MA, Westerhoff P, Benn T, Wang Y, Perez-Rivera J, Hristovski K (2009) Titanium nanomaterial removal and release from wastewater treatment plants. Environ Sci Technol 43(17):6757–6763
Kubo M, Onodera R, Shibasaki-Kitakawa N, Tsumoto K, Yonemoto T (2005) Kinetics of ultrasonic disinfection of Escherichia coli in the presence of titanium dioxide particles. Biotechnol Prog 21(3):897–901
Laufs P, Peaucelle A, Morin H, Traas J (2004) MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 131(17):4311–4322. doi:10.1242/dev.01320
Lee W-M, An Y-J, Yoon H, Kweon H-S (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluable nanoparticles environmental. Toxicol Chem 27(9):1915–1921
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120(1):15–20. doi:10.1016/j.cell.2004.12.035
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42(15):5580–5585
Lovern SB, Klaper R (2006) Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem 25(4):1132–1137
Lovern SB, Strickler JR, Klaper R (2007) Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C60, and C60HxC70Hx). Environ Sci Technol 41(12):4465–4470
Lubick N (2009) Hunting for engineered nanomaterials in the environment. Environ Sci Technol 43:6446–6447. doi:10.1021/es902174z
Mallory AC, Dugas DV, Bartel DP, Bartel B (2004) MicroRNA regulation of NAC-domain targets is required for proper formation and separation of adjacent embryonic, vegetative, and floral organs. Curr Biol 14(12):1035–1046. doi:10.1016/j.cub.2004.06.022
Mattigod SV, Fryxell GE, Alford K et al (2005) Functionalized TiO2 nanoparticles for use for in situ anion immobilization. Environ Sci Technol 39(18):7306–7310
Mlotshwa S, Yang ZY, Kim YJ, Chen XM (2006) Floral patterning defects induced by Arabidopsis APETALA2 and microRNA172 expression in Nicotiana benthamiana. Plant Mol Biol 61(4–5):781–793. doi:10.1007/s11103-006-0049-0
Murashov V (2006) Comments on "Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles" by Yang, L., Watts D.J., Toxicology Letters, 2005, 158, 122–132. Toxicology Lett 164(2):185–187
Pan XP, Redding JE, Wiley PA, Wen L, McConnell JS, Zhang BH (2010) Mutagenicity evaluation of metal oxide nanoparticles by the bacterial reverse mutation assay. Chemosphere 79:113–116. doi:10.1016/j.chemosphere.2009.12.056
Pott F, Roller M (2005) Carcinogenicity study with nineteen granular dusts in rats. Eur J Oncol 10(4):249–282
Reeves JF, Davies SJ, Dodd NJF, Jha AN (2008) Hydroxyl radicals (.OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res 640(1–2):113–122
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005) Specific effects of MicroRNAs on the plant transcriptome. Dev Cell 8(4):517–527
Sichel C, de Cara M, Tello J, Bianco J, Fernandez-Ibanez P (2007) Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl Catal B Environ 74(1–2):152–160
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16(8):2001–2019. doi:10.1105/tpc.104.022830
Sunkar R, Kapoor A, Zhu J-K (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18(8):2051–2065
Trindade I, Capitao C, Dalmay T, Fevereiro MP, Santos DM (2010) miR398 and miR408 are up-regulated in response to water deficit in Medicago truncatula. Planta 231(3):705–16
Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH (2009) Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 69(22):8784–8789
Vaucheret H (2006) Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev 20(7):759–771. doi:10.1101/gad.1410506
Wang S, Chang L-Y, Wang Y-J, Wang Q, Yang C-H, Mei R-H (2009) Nanoparticles affect the survival of bacteria on leaf surfaces. FEMS Microbiol Ecol 68:182–191
Wierzbicka M, Obidzinksa J (1998) The effect of lead on seed imbibition and germination in different plant species. Plant Sci 137:155–171
Xie XH, Lu J, Kulbokas EJ et al (2005) Systematic discovery of regulatory motifs in human promoters and 3 ' UTRs by comparison of several mammals. Nature 434(7031):338–345. doi:10.1038/nature03441
Zhang BH, Pan XP, Cobb GP, Anderson TA (2006) Plant microRNA: a small regulatory molecule with big impact. Dev Biol 289(1):3–16. doi:10.1016/j.ydbio.2005.10.036
Zhang J, Xu Y, Huan Q, Chong K (2009) Deep sequencing of Brachypodium small RNAs at the global genome level identifies microRNAs involved in cold stress response. BMC Genomics 10
Zhao BT, Liang RQ, Ge LF et al (2007) Identification of drought-induced microRNAs in rice. Biochem Biophys Res Commun 354(2):585–590. doi:10.1016/j.bbrc.2007.01.022
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Zhou XF, Wang GD, Sutoh K, Zhu JK, Zhang WX (2008a) Identification of cold-inducible microRNAs in plants by transcriptome analysis. Biochim Biophys Acta Genet Regul Mech 1779(11):780–788. doi:10.1016/j.bbagrm.2008.04.005
Zhou ZS, Huang SQ, Yang ZM (2008b) Bioinformatic identification and expression analysis of new microRNAs from Medicago truncatula. Biochem Biophys Res Commun 374(3):538–542
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frazier, T.P., Burklew, C.E. & Zhang, B. Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum). Funct Integr Genomics 14, 75–83 (2014). https://doi.org/10.1007/s10142-013-0341-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-013-0341-4