Abstract
Mercury is a ubiquitous environmental contaminant known to accumulate in, and negatively affect, fish-eating and oceanic bird species, and recently demonstrated to impact some terrestrial songbirds to a comparable extent. It can bioaccumulate to concentrations of >1 μg/g in tissues of prey organisms such as fish and insects. At high enough concentrations, exposure to mercury is lethal to birds. However, environmental exposures are usually far below the lethal concentrations established by dosing studies.
The objective of this review is to better understand the effects of sublethal exposure to mercury in birds. We restricted our survey of the literature to studies with at least some exposures >5 μg/g. The majority of sublethal effects were subtle and some studies of similar endpoints reached different conclusions. Strong support exists in the literature for the conclusion that mercury exposure reduces reproductive output, compromises immune function, and causes avoidance of high-energy behaviors. For some endpoints, notably certain measures of reproductive success, endocrine and neurological function, and body condition, there is weak or contradictory evidence of adverse effects and further study is required. There was no evidence that environmentally relevant mercury exposure affects longevity, but several of the sublethal effects identified likely do result in fitness reductions that could adversely impact populations. Overall, 72% of field studies and 91% of laboratory studies found evidence of deleterious effects of mercury on some endpoint, and thus we can conclude that mercury is harmful to birds, and the many effects on reproduction indicate that bird population declines may already be resulting from environmental mercury pollution.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Avian
- Bald eagle
- Behavior
- Bird
- Common loon
- Dosing
- Ecotoxicology
- Effects
- Endocrine function
- Forster’s tern
- Hormones
- Immune function
- Longevity
- Mallard
- Metals
- Methylmercury
- Mercury
- Neurological function
- Reproduction
- Review
- Sublethal
- Survivorship
- Tree swallow
- White ibis
- Zebra finch
1 Introduction
Environmental mercury concentrations are predicted to continue to increase worldwide, while climate change is expected to exacerbate the impact of this ubiquitous contaminant (Sunderland et al. 2009; Stern et al. 2012). The effects of mercury on wildlife have been studied extensively, but until recently the majority of birds investigated have been either piscivorous species or domesticated breeds, such as the white leghorn chicken. Recent experiments and field studies have begun to include songbirds, as it has recently been discovered that mercury is not restricted to aquatic environments but also impacts terrestrial species (Cristol et al. 2008). Additionally, recent experimental studies have tended to use lower concentrations of mercury in an effort to understand the sublethal impacts that most exposed wildlife are experiencing, such as those involving behavior. The overall number of studies on sublethal effects has increased dramatically, with only 34 published articles found during the decades before 1999, 44 identified in the first decade of the 2000s, and already 76 located with publication dates since 2010. Given the expected worsening of the mercury pollution problem, and the realization that mercury affects more types of birds than previously suspected, a review of the burgeoning literature on sublethal effects of mercury on birds is overdue.
2 Methods
To determine which level of mercury to consider “sublethal,” we searched for evidence of a lowest lethal dose and concentrations that actually occur in prey items in the environment. Domestic zebra finches (all scientific names given in Table 1, along with American Ornithological Society Alpha codes) chronically exposed to 5 μg/g dietary mercury experienced 25% mortality within 10 weeks, making it a lethal dose for some individuals (Scheuhammer 1988). Environmental mercury concentrations as high as 5 μg/g are rare in wild prey items of birds, including both fish and terrestrial arthropods; in fact, these rarely exceed 2 μg/g (Henny et al. 2002; Merrill et al. 2005; Cristol et al. 2008; Burgess and Meyer 2008). Therefore, experimental studies were included in this review of sublethal exposure only if some birds in the study were exposed to <5 μg/g methylmercury. For dosing studies, and field studies that measured mercury concentrations in prey items, we defined four categories of exposure that are referenced with each citation: trace (≤0.5 μg/g), low (0.5–1.0 μg/g), medium (1.0–2.0 μg/g), and high (>2.0 μg/g). All concentrations for exposure are on a wet weight basis unless otherwise noted as being reported in dry weight (dw). Resulting concentrations in bird tissues are presented for all studies where reported, in Table 2, and are indicated as being on a wet or dry weight basis. The form and mode of mercury exposure varied across studies reviewed, from unknown in many field studies to dietary methylmercury chloride in most dosing studies. In general, only total residues (rather than particular species of mercury) were reported from the tissues of exposed birds. We provide the mode of exposure (e.g., diet, egg injection, etc.), for each dosing study, and include the details of form of methylmercury when available. (Table 2 is organized by citation and referenced to Table 1 by AOS Alpha code for each species). This review incorporates all peer-reviewed literature discussing the effect of sublethal doses of mercury on birds of all taxa that was in English and detected by the authors using reasonable diligence on standard online search engines through May 2017. It is intended to serve as a detailed summary of the state of knowledge concerning sublethal effects of mercury on birds.
3 Reproduction
3.1 Overview
Depressed reproductive success is the most widely investigated and reported consequence of mercury exposure, but the endpoints measured have varied widely between studies, from eggshell structure to timing of breeding. Dozens of different species have been studied, both in the field and in laboratories (all experimental dosing results are denoted as such throughout the text of this review, and all unspecified studies were correlational field studies). Across a wide range of concentrations and methodologies, mercury exposure clearly has deleterious impacts on many aspects of avian reproduction. We refer to all forms of the element as “mercury” throughout this review, but we assume that impacts on wild birds were from methylmercury, which is the form most abundant in their tissues and many prey items. Although laboratory studies used a variety of forms of mercury, we are unable to ascertain which form caused any observed effects because of unstudied reactions during digestion or metabolism.
3.2 Clutch Size
The number of eggs laid in a clutch appears to be impacted by mercury in some species. Mercury contamination was associated with the reduced numbers of eggs in free-living black-legged kittiwakes (Tartu et al. 2013), as well as dosed American kestrels (Albers et al. 2007: trace, low, medium, and high in diet), dosed mallards (Heinz 1974: trace, high in diet), and dosed white leghorn chickens (Lundholm 1995: 1 mg methylmercury/day over 50 days in diet). Female eastern bluebirds with higher feather mercury, indicating long-term exposure from a nearby contaminated river, had smaller clutches (McCullagh et al. 2015). However, no differences were detected in the number of eggs laid by reference or environmentally exposed tree swallows (Brasso and Cristol 2008: low in prey (dw); Gerrard and St. Louis 2001: trace in prey (dw)), common eiders (Provencher et al. 2017), dosed black ducks (Finley and Stendell 1978: high in diet), or dosed zebra finches (Varian-Ramos et al. 2014: trace, low, medium, and high in diet; Yu et al. 2016: trace, high injected in egg). Great tits laid larger clutches in a contaminated site, but nestling blood mercury concentrations were not significantly different than in the reference site, suggesting that mercury was an unlikely cause of this difference (Costa et al. 2014). Thus, there is an equal weight of evidence, from free-living and dosed birds, supporting the hypothesis that mercury does reduce clutch size, or that it has no effect on clutch size.
3.3 Eggshells and Embryos
Eggshell thinning has been related to mercury in free-living snowy egrets (Olivero-Verbel et al. 2013) and domestic white leghorn chickens (Lundholm 1995: 1 mg methylmercury/day over 50 days in diet). Bald eagles exposed to more mercury had thinner eggshells in one study (Wiemeyer et al. 1984), but not in another (Anthony et al. 1999). Eggs of mallards maintained on a methylmercury-contaminated diet did not have thinner shells (Heinz 1974: trace, high in diet; Heinz 1976a: trace, high in diet; Heinz 1976b: trace in diet; and Heinz 1980: low, high in diet) until the third generation of exposure (Heinz 1979: trace in diet). Eggshell thinning was not related to mercury exposure for free-living great blue herons (Custer et al. 1997), common loons (Pollentier et al. 2007, note that eggshell thickness was related to lake pH, a proxy for mercury), Forster’s terns, or black skimmers (King et al. 1991), but it should be noted that these three studies reported relatively low mercury concentrations. Thus, it appears that mercury is associated with eggshell thinning, although the effect is difficult to detect under some circumstances, such as low-dose environmental exposure.
Other attributes of eggs may also be affected by mercury. Free-living egrets with higher mercury levels had wider eggs with decreased weight (Olivero-Verbel et al. 2013). Lundholm (1995: 1 mg methylmercury/day over 50 days in diet) reported eggshell defects and shorter egg length in mercury-dosed chickens, while Heinz (1974: trace, high in diet) found decreased egg weight in dosed mallards. Common loons exposed to mercury had decreased egg volume (Evers et al. 2003). Egg volume was also lower for contaminated tree swallows in one study (Brasso and Cristol 2008: low in prey, dw) but did not differ between reference and contaminated birds in a larger study on the same population (Hallinger and Cristol 2011). Egg volume was not related to mercury in Leach’s storm-petrels (Pollet et al. 2017).
Several studies indicate the effects of mercury on embryos as well. Applying mercury to the surface of mallard eggs caused teratogenicity, including skeletal defects and incomplete ossification (Hoffman and Moore 1979: trace, low, and high). When injected into eggs, mercury was teratogenic to varying degrees in 22 of 25 different species (Heinz et al. 2011: trace, low, medium, and high), including mallards and double-crested cormorants (Heinz et al. 2012b: trace, low, and medium injected in egg; Klimstra et al. 2012: trace, low, and medium injected in egg). It should be noted, however, that, injected mercury is potentially more toxic than maternally deposited mercury because an embryo is likely to encounter a larger proportion of the dose over a shorter span of time. Injection of methylmercury lengthened the necessary incubation period of common loon eggs in a dose-dependent manner (Kenow et al. 2011: trace, medium, and high). Eggs of dosed mallards experienced increased embryo mortality (Heinz 1974: trace, high in diet), with fewer viable eggs produced (Heinz 1979: trace in diet). Thick-billed murre and arctic tern eggs injected with mercury also had reduced embryo survival (Braune et al. 2012: trace, low, medium, and high). Forster’s tern eggs collected from the wild showed a positive relationship between number of malpositioned embryos and mercury concentration, but no relationship between embryo deformities and mercury. However, there was no relationship between mercury concentration and occurrence of either embryo malpositioning or deformation in free-living black-necked stilts or American avocets (Herring et al. 2010). Finally, no relationship was found between mercury and embryonic development in wild-collected eggs of white-tailed sea eagle (Helander et al. 1982) or common loons (Evers et al. 2003). A clear majority of studies from both the field and laboratory indicate that mercury is embryotoxic in a variety of ways.
3.4 Hatching and Hatchlings
Numerous studies have examined whether there is an effect of mercury exposure on survival of baby birds in the nest and around fledging time. There was a mercury-related decline in the proportion of eggs hatching in free-living tree swallows (Hallinger and Cristol 2011), as well as dosed laughing gulls (Jenko et al. 2012: trace, low, medium, and high injected in egg), zebra finches (Varian-Ramos et al. 2014: trace, low, medium, and high in diet; Yu et al. 2016: trace, high injected in egg), American kestrels (Albers et al. 2007: trace, low, medium, and high in diet), and common loons (Kenow et al. 2011: trace, medium, and high injected in egg). There was a suggestive association between paternal mercury level and hatching success in a study of three species of Arctic-nesting shorebirds (Hargreaves et al. 2010). In a set of experimental studies on mallards, hatching success declined in three studies (Hoffman and Moore 1979: applied methylmercury to eggshell resulting in egg concentrations of 0.05–0.53 μg/g; Heinz et al. 2009: trace, low, medium, and high injected in egg; Klimstra et al. 2012: trace, low, and medium injected in egg) but improved in another (Heinz et al. 2010a: trace in diet). This latter result, an apparent case of hormesis, is perhaps based on a mild antibiotic effect of mercury and was reproduced in an egg injection experiment (Heinz et al. 2012a: trace, low, medium, and high). It should be noted here that injection of mercury into eggs produces higher toxicity than the same concentration of mercury deposited by a female (Heinz et al. 2009: trace, low, medium, and high injected in egg). Blood mercury concentration in breeding female tree swallows was not associated with the hatching success of their broods (Taylor and Cristol 2015). Mercury-related changes in hatching rate were not observed for great skuas (Thompson et al. 1991), Forster’s terns, black skimmers (King et al. 1991), Leach’s storm-petrels (Pollet et al. 2017), tree swallows, or house wrens (Custer et al. 2007: trace-high in prey (dw); Custer et al. 2006: trace in prey (dw); Custer et al. 2008: trace in prey (dw); Custer et al. 2012: trace in prey (dw)), nor dosed black ducks (Finley and Stendell 1978: high in diet) or mallards (Heinz 1976b: trace in diet; Heinz et al. 2010b: low, medium, and high in diet). However, mercury concentrations were near background levels for Forster’s terns, black skimmers (King et al. 1991), and tree swallows (in 3 of the 4 tree swallow studies: Custer et al. 2006: trace in prey (dw); Custer et al. 2008: trace in prey (dw); Custer et al. 2012: trace in prey (dw)), so those negative results are not highly relevant.
In a series of landmark dosing studies on female mallards, Heinz (1974: trace, high in diet; Heinz 1976a: trace, high in diet; Heinz 1976b: trace in diet; and Heinz 1979: trace in diet) reported a reduction in the number of ducklings hatching, findings that were replicated decades later (Heinz et al. 2010b: low, medium, and high in diet). Mercury exposure also resulted in fewer hatchlings for free-living snowy egrets (Henny et al. 2002: trace, low, medium, and high in prey; Hill et al. 2008), common loons (Barr 1986; Schoch et al. 2014), and dosed black ducks (Finley and Stendell 1978: high in diet), American kestrels (Albers et al. 2007: trace, low, medium, and high in diet), and white ibises (Frederick and Jayasena 2010: trace in diet). The probability of hatching was lower for wandering albatross with higher mercury (Goutte et al. 2014a). Anthony et al. (1999) reported fewer nestlings from free-living bald eagles exposed to mercury, but Bowerman et al. (1994) and Weech et al. (2006) reported no correlations between environmental mercury exposure and the number of bald eagle nestlings. Contamination from mercury used in mining did not correlate with the number of black-crowned night-heron nestlings either (Henny et al. 2002: trace, low in prey), and Elbert and Anderson (1998) reported an unclear relationship for western grebes in the same situation. Of eggs that hatched, mercury did not reduce nestling survival in dosed zebra finches (Yu et al. 2016: trace, high injected in egg). Mercury concentrations near background levels did not reduce tree swallow nestling survival (Custer et al. 2012; trace in prey (dw)). Thus, many studies have shown that survival through the nestling period is reduced by mercury exposure beginning in ovo, but several studies failed to find this effect, and one notably found an increase in hatching rate as the result of mercury exposure.
3.5 Fledging and Fledglings
Reduction in the number of fledged or independent offspring is the effect of mercury exposure with the most robust support. This includes several reports of fewer common loon chicks in broods that had survived to late in the season (Evers et al. 2008; Burgess and Meyer 2008: trace in prey; Meyer et al. 1998), which may result in a negative population growth rate (Schoch et al. 2014). Field studies on loons are now well-established for determining the magnitude of reproductive harm that mercury may have, although studies on lakes with low pH should recognize the potential confounding impacts of reduced fish abundance and availability (Meyer et al. 1998). Reduced fledging success has been reported in free-living birds: tree swallows (Brasso and Cristol 2008: low in prey (dw); Hallinger and Cristol 2011), wandering albatross (Goutte et al. 2014a), and Acadian flycatchers (Rowse et al. 2014, trace in prey items), as well as dosed American kestrels and dosed zebra finches (Albers et al. 2007: trace, low, medium, and high in diet; Varian-Ramos et al. 2014: trace, low, medium, and high in diet). Male eastern bluebirds with higher blood mercury, indicating recent exposure from a nearby contaminated river, fledged a lower proportion of their young than males with lower blood mercury (McCullagh et al. 2015). For tree swallows hatched near a contaminated river, the feather mercury of nestlings that died in the nest was almost twice as high as that of nestlings from nests in which all nestlings fledged (Taylor and Cristol 2015). There was a nonsignificant trend of fewer fledglings among mercury-dosed white ibis in an aviary study (Frederick and Jayasena 2010: trace in diet). (Hereafter, for all nonsignificant trends reported by authors we provide sample size of the smallest treatment group, to allow assessment of one aspect of statistical power; in this case, n = 20.) There was an uncertain relationship between fledgling numbers and mercury exposure in free-living American dippers (Henny et al. 2005: trace, low, and medium in prey (dw)). The only such studies not reporting reduced numbers of offspring in birds with higher mercury were on great skuas (Thompson et al. 1991), wandering albatrosses (Bustamante et al. 2016), Leach’s storm-petrels (Pollet et al. 2017), and common loons (Barr 1986), all exposed through their natural fish diets. However, a recent study of long-term data from Antarctic colonies of two species of skua indicates an effect of tissue mercury concentration in 1 year on reproductive success the following year, an effect severe enough that it is predicted to lead to population declines (Goutte et al. 2014b). Finally, great tits fledged more offspring in a contaminated site, but nestling blood mercury concentrations did not differ significantly from the reference site so the effect is unlikely to have been due to mercury (Costa et al. 2014).
3.6 Other Measures of Reproductive Output
The literature suggests that mercury may impact a number of other reproductive endpoints, but there are too few examples of each of these to allow generalized conclusions. Nestlings from contaminated sites were more sensitive to high ambient temperatures (Hallinger and Cristol 2011), and primary sex ratios of offspring on mercury-contaminated sites were female biased in belted kingfishers, tree swallows, and eastern bluebirds, relative to reference sites (Bouland et al. 2012). No impact on sex ratio was found in dosed zebra finches (Yu et al. 2016: trace, high injected in egg).
Other metrics of reproductive success have yielded equivocal results. A model for Carolina wrens developed from field results indicated reduced nest survival, due primarily to nest abandonment, with small increases in maternal blood mercury concentration (Jackson et al. 2011). Common loons were more likely to desert nest sites in lakes contaminated with mercury (Barr 1986). However, for bald eagles, nest success, as defined by the percent of breeding territories producing at least one fledgling (Bowerman et al. 1994), did not relate to mercury contamination. Similarly, the probability of wandering albatross breeding in a given year did not change with mercury exposure (Bustamante et al. 2016). Common eiders with higher blood mercury had a higher propensity to nest, but this was not significant (n = 74) (Provencher et al. 2017).
3.7 Timing of Breeding
Studies of the effect of mercury on timing of reproductive events, such as laying and fledging, have yet to produce any consensus. The potential effect of mercury on laying date is especially unclear. Studies of dosed birds revealed increased latency to renest (zebra finches, Varian-Ramos et al. 2014: trace, low, medium, and high in diet) and delay in onset of egg laying (American kestrels, Albers et al. 2007: trace, low, medium, and high in diet), in contrast to free-living tree swallows (Hallinger and Cristol 2011) and great tits (Costa et al. 2014), where earlier onset of laying occurred on contaminated sites (although great tit nestling blood mercury concentrations did not differ from reference sites). However, the onset of laying in the same population of tree swallows was reported to be unaffected in a different study of the same mercury-contaminated sites (Brasso and Cristol 2008: low in prey (dw)). Neither great skuas (Thompson et al. 1991), black-legged kittiwakes (Tartu et al. 2013), Leach’s storm-petrels (Pollet et al. 2017), nor dosed black ducks (Finley and Stendell 1978: high in diet) exhibited a relationship between mercury concentration and onset of egg laying. Blood mercury concentration was negatively related to date of hatching in Forster’s terns (Ackerman et al. 2008a), while a positive relationship between mercury and interval from laying to hatching was observed for dosed American kestrels (Albers et al. 2007: trace, low, medium, and high in diet). No relationship between mercury and the timing of post-fledging dispersal of juvenile snowy egrets was found (Henny et al. 2017).
4 Longevity
Mercury does not appear to directly decrease longevity at environmentally relevant concentrations. No differences were found in post-fledging survival probability of Forster’s terns (Ackerman et al. 2008a) or snowy egrets (Henny et al. 2017), resight probability of dosed and released white ibises (Frederick et al. 2011: trace in diet), free-living common loons (Mitro et al. 2008), or common eiders (Provencher et al. 2017), annual adult return rate of common loons, great skuas, or Leach’s storm-petrels (Meyer et al. 1998; Thompson et al. 1991; Pollet et al. 2017), or probability of survival in great egrets (Sepúlveda et al. 1999: fed capsules for total of 3 mg methylmercury) or wandering albatross (Goutte et al. 2014a; Bustamante et al. 2016). Among yearling female tree swallows that nested in a contaminated floodplain, blood mercury level in 1 year was not a good predictor of probability of returning to breed the next year, a proxy for survivorship in this highly site-faithful species (Taylor and Cristol 2015). Survival probability of free-living American avocet and black-necked stilt chicks at more contaminated sites dropped 1.4% and 3.0%, respectively, but explanatory models specifically including mercury had low predictive power (Ackerman et al. 2008b). Similarly, predicted annual survival of tree swallows at mercury-contaminated sites dropped 1–2%, but individual mercury exposure had weak explanatory power (Hallinger et al. 2011). Mercury concentration in tissues was related to lower recapture probabilities for white-winged scoters, but not king eiders (Wayland et al. 2008). Further studies of long-lived birds observed over many years of mercury exposure may yet reveal a significant effect on survivorship, but thus far there is no evidence to this effect.
5 Behavior
5.1 Parental Behaviors
Parental behavior may be altered in a variety of ways after exposure to mercury. White ibises dosed in aviaries made fewer nesting attempts and exhibited more same-sex pairing among males than was observed in the control aviary (Frederick and Jayasena 2010: trace in diet). Both free-living common loons (Evers et al. 2008) and dosed American kestrels (Albers et al. 2007: trace, low, medium, and high in diet) spent less time incubating when exposed to dietary mercury, while mercury was also related to decreased provisioning effort in loons (Merrill et al. 2005; low, medium, and high in prey (dw)). Male snow petrels with higher mercury were more likely to neglect their egg (Tartu et al. 2015) and Carolina wrens were more likely to abandon nests when on contaminated than reference sites (Jackson et al. 2011). Male American kestrels dosed with mercury were observed cannibalizing their offspring (Fallacara et al. 2011b: trace, medium in diet). No impact on mating behavior of zebra finches that were dosed in ovo was observed (Yu et al. 2016: trace, high injected in egg; Yu et al. 2017: trace, high injected in egg).
5.2 Behavior of Dependent Young
A number of abnormal chick behaviors have also been reported. Common loon chicks with higher mercury exposure spent more time preening and less time back-riding, although they did not change their swimming or diving habits in lakes with higher mercury (Nocera and Taylor 1998). Loon chicks in lakes with low pH and higher mercury were also less capable of righting themselves after dietary exposure, and experimental in ovo mercury exposure resulted in other behavioral changes in captivity, including crossing a platform faster, spending more time on platforms and in sunlight, and exhibiting decreased responses to parental wails and frightening stimuli (Kenow et al. 2010: trace, medium in diet; Kenow et al. 2011: trace, medium, high injected in eggs already containing low maternally deposited mercury). Dosed mallard ducklings did not alter their response to maternal calls (Heinz 1975: trace, high in diet; Heinz 1976a: trace, high in diet; Heinz 1976b: trace in diet) until the third generation of exposure, when they exhibited a reduced response (Heinz 1979: trace in diet). Ducklings also ran further from frightening stimuli (Heinz 1975: trace, high in diet; Heinz 1976a: trace, high in diet; Heinz 1979: trace in diet), except in one experiment in which their response to a frightening stimulus did not change (Heinz 1976b: trace in diet). When mercury was injected into white leghorn chicken eggs, the surviving chicks did not differ in their response to frightening stimuli, but they did take longer to right themselves (Rutkiewicz et al. 2013: trace, low, medium, and high injected in egg).
5.3 Coordination and High-Energy Behaviors
Mercury appears to impact behaviors requiring a large energy input. Carolina wrens, house wrens, and song sparrows at sites with mercury contamination sang less complex, lower-frequency songs (Hallinger et al. 2010), whereas Nelson’s sparrows at marshes with higher mercury sang faster songs with higher maximum tonal frequency and shorter gaps between bouts (McKay and Maher 2012). Injection of mercury in ovo did not impact the quality of zebra finch songs (Yu et al. 2017: trace, high injected in egg). Free-living common loons with greater mercury exposure spent less time preening and swimming (Evers et al. 2008). In dosing studies that included both lethal concentrations and lowest doses of 5 μg/g, great egrets were less active (Bouton et al. 1999: trace, high force fed capsules) and were ataxic (Spalding et al. 2000a: trace, high force fed capsules), while zebra finches became lethargic and had difficulty balancing or landing on perches (Scheuhammer 1988: low, high in diet (dw)). Domestic rock pigeons dosed with mercury also were ataxic, pecked at food less accurately and at a slower rate (Evans et al. 1982: probably low, medium ingested by intubation), and made fewer and slower responses in operant conditioning tests (Laties and Evans 1980: probably medium, high ingested by intubation). Mercury also impacted American kestrel motor skills, but only when fed at concentrations above 5 μg/g (Bennett et al. 2009: medium, high in diet). Evidence of impaired cognition in dosed zebra finches included impaired spatial memory, but not inhibitory control or ability to associate color with food (Swaddle et al. 2017: medium in diet). The same colony of mercury-dosed zebra finches exhibited behavioral changes including hyperactivity and subordination to undosed finches but were not more or less neophobic. The timing of snowy egret migration (Henny et al. 2017) and the arrival date of common eiders on breeding grounds (Provencher et al. 2016) were not related to mercury.
The relationship between foraging behaviors and mercury concentration is unclear. Common loons with higher mercury exposure spent less time foraging for themselves and their chicks (Evers et al. 2008) and exhibited an increased diving frequency (Olsen et al. 2000), which may indicate that they were having difficulty foraging. Dosed zebra finches reacted more strongly to the presence of predators, waiting longer to forage after seeing a model hawk, and thus losing more mass than control birds (Kobiela et al. 2015: medium in diet). However, dosed white ibises foraged more efficiently (Adams and Frederick 2008: trace in diet) and great egrets performed as well as birds on control diets, although they had a reduced appetite (Bouton et al. 1999: trace, high force fed capsules). Food consumption of common loons dosed in captivity was unrelated to mercury concentration (Kenow et al. 2003: trace, medium in diet).
6 Neurological Function
Although fewer studies of mercury neurotoxicity in avian models have been done in recent years, there exists a solid body of evidence indicating that mercury exposure results in axonal degeneration and other neurological problems. An opportunistically collected juvenile saltmarsh sparrow from a population with high blood mercury concentrations exhibited disrupted neuronal migration, with Purkinje cells scattered through all three layers of the cerebellum and an external granule cell layer (Scoville and Lane 2013). In mallards dosed with mercury, adult axons degenerated (Pass et al. 1975: medium, high in diet), and ducklings exhibited demyelination and neuronal shrinkage (Heinz and Locke 1976: high in diet). Rock pigeons also exhibited demyelination when dosed but, in contrast to mallards, had neuronal swelling (Evans et al. 1982: low, medium ingested by intubation). Dosed American kestrels exhibited axonal degeneration but did not develop brain lesions unless fed very high concentrations above 5 μg/g (Bennett et al. 2009: medium, high in diet). Double-crested cormorants had axonal degeneration and swollen myelin sheaths when dosed (Loerzel et al. 1999: trace, high in diet). Dosed zebra finches suffered hearing impairment, with elevated auditory brainstem response thresholds, decreased amplitudes, and longer latencies for neuronal response to tones (Wolf et al. 2017: medium in diet). Dosed male zebra finches had increased telencephalon volume, but mercury had no impact on brain mass, area X, robust nucleus of the arcopallium song nuclei, or HVC (Yu et al. 2017: trace, high injected in egg). Red-tailed hawks did not show axonal degeneration unless they were fed very high concentrations (5.2 μg/g) of mercury (Fimreite and Karstad 1971: high in diet).
Several researchers have examined neurotransmitter function. Decreased binding to NMDA receptors was related to mercury concentration in free-living bald eagles and common loons (Scheuhammer et al. 2008; Rutkiewicz et al. 2011). However, no change in binding to NMDA receptors was observed for thick-billed murres or arctic terns (Braune et al. 2012: trace, low, medium, and high injected in egg), or herring gulls (Rutkiewicz et al. 2010). Domestic quail and chickens dosed in ovo did not show changes in binding to NMDA receptors in one experiment using methylmercury-chloride, but increased binding to NMDA in chickens was observed in another using methylmercury-cysteine (Rutkiewicz et al. 2013: trace, low, medium, and high injected in egg). Glutamine synthetase (GS) did not increase in dosed hatchling chickens until they were exposed to a very high dietary concentration of 6.4 μg/g, while no change in GS was found in older chicks at any concentration (Rutkiewicz et al. 2013: trace, low, medium, and high injected in egg). In free-living bald eagles, there was a positive correlation between mercury and GS (Rutkiewicz et al. 2011). Glutamic acid decarboxylase has been found to either increase or remain the same in chickens and decrease in quail with administration of mercury (Rutkiewicz et al. 2013: trace, low, medium, and high injected in egg) and was negatively correlated with inorganic mercury in bald eagles (Rutkiewicz et al. 2011). Gamma-aminobutyric acid either showed no change, for chickens or quail, increased in chickens exposed to 6.4 μg/g mercury injected in egg, or decreased in chickens exposed to 3.2 or 6.4 μg/g methylmercury-cysteine (Rutkiewicz et al. 2013: trace, low, medium, and high injected in egg). Muscarinic cholinergic (mACh) receptor density was unchanged in thick-billed murres and arctic terns (Braune et al. 2012: trace, low, medium, and high injected in egg) and herring gulls (Rutkiewicz et al. 2010), but mACh activity was related to mercury in free-living bald eagles and common loons (Scheuhammer et al. 2008). No differences were found for cholinesterase (ChE), or MAO in bald eagles or common loons. Similarly, no impacts on nicotinic cholinergic receptor density or nicotinic receptor alpha-7 mRNA expression were observed in herring gulls (Rutkiewicz et al. 2010). In another sample of common loons that died of botulism, no differences were observed for binding to NMDA receptors, mACh receptor density, MAO, or ChE, although it must be noted that these loons had relatively low mercury tissue concentrations and a molar excess of selenium in their brain tissue, which is known for mitigating the impact of mercury (Hamilton et al. 2011). Clearly, more work is necessary to sort out the potential effects of mercury on various neurochemicals, as well as the dose–response curves. Because of the well-known neurological effects of mercury, this sort of research is a priority.
7 Endocrine Function
7.1 Overview
While there is no evidence that mercury is a classic endocrine disrupting chemical that mimics or competes with specific hormones, there are data suggesting that mercury exposure is associated with alterations in profiles of several hormones. Much more work is needed in this area because the results are equivocal and no studies have been replicated with the same mercury doses, hormones, or species.
7.2 Corticosterone
Despite a considerable body of literature, the impact of mercury exposure on corticosterone (CORT) is still unclear. The expected stress-induced increase in CORT was weaker for nestling tree swallows living at contaminated sites (Wada et al. 2009) and dosed adult zebra finches (Moore et al. 2014: trace, low, medium, and high in diet) but did not relate to mercury level in free-living common eiders (Wayland et al. 2002) or snow petrels (Tartu et al. 2015). In captive juvenile common loons, stress-induced CORT was depressed, but free-living adult male loons with higher mercury had elevated stress-induced CORT and no relationship was found in females (Franceschini et al. 2017: trace, medium in diet). Baseline CORT was also elevated in free-living tree swallow nestlings exposed to environmental mercury (Wada et al. 2009) as well as in dosed juvenile white ibises, although this latter response exhibited a nonlinear relationship with dose (Adams et al. 2009: trace in diet). In adult lesser scaup ducks, baseline CORT was only related positively to mercury in individuals with larger body size, while the relationship was reversed in smaller individuals (Pollock and Machin 2009). For nestling and adult tree swallows, a nonsignificant positive relationship was reported between feather mercury concentration and baseline CORT (n = 23), but a negative relationship was found between baseline CORT and both blood (significant) and egg (nonsignificant, n = 21) mercury in the same birds (Franceschini et al. 2009). Baseline CORT was also depressed in free-living nestling Forster’s terns with higher mercury exposure (Herring et al. 2012). A nonsignificant trend of depressed baseline CORT was found in female common eiders with low blood mercury concentrations (n = 190) (Provencher et al. 2016). Finally, no significant relationship was found between mercury and baseline CORT in free-living nestling great egrets or white ibises (Herring et al. 2009, Herring et al. 2014), adult or nestling white ibises (Heath and Frederick 2005), adult snow petrels (Tartu et al. 2015), or dosed zebra finches (Moore et al. 2014: trace, low, medium, and high in diet), although the change in baseline CORT between pre- and postbreeding periods in zebra finches revealed a statistically significant interaction between sex and mercury (Maddux et al. 2014: trace, low in diet). Endocrine responses to environmental stressors are notoriously difficult to understand, given the possibility of both activational and organizational effects of stressors, and the many simultaneous confounding influences. Careful work on captive birds is needed to make progress in understanding the relationship between mercury and avian CORT responses.
7.3 Testosterone (T)
No clear patterns have yet emerged about the relationship between mercury and baseline T levels. In dosed adult white ibises, Jayasena et al. (2011: trace in diet) found no change in the baseline T of breeding males paired to females. In contrast, males paired to other males had depressed T levels while eggs were being laid in the captive colony and elevated T levels while the colony was incubating eggs. Heath and Frederick (2005) found elevated T levels associated with mercury in male white ibises incubating nests in the wild. In adult black-legged kittiwakes, baseline T was negatively related to mercury in males that skipped breeding, but not in breeding males. Gonadotropin-releasing hormone (GnRH)-induced T was not related to mercury level in breeding males or males that skipped breeding (Tartu et al. 2013). In dosed juvenile white ibises (Adams et al. 2009: trace in diet) and common loons (Franceschini et al. 2017: trace, medium in diet), no effects of mercury on T were observed. It appears that there is not a predictable relationship between mercury exposure and T, and cases with apparent relationships may be the indirect result of perturbations by mercury of other hormones (e.g., CORT) or behavior (e.g., lack of stimulus).
7.4 Other Hormones
With respect to mercury exposure, no other hormones have been studied as extensively as CORT or T. Other hormones related to reproduction have been the most studied, but like CORT and T, their levels are highly dependent on an individual’s breeding stage and thus a relationship with mercury concentration is hard to detect. The emerging relationships between mercury exposure and hormone level are correspondingly complex. A significant relationship between mercury and luteinizing hormone (LH) was found in black-legged kittiwakes that skipped breeding, but not in birds that bred. Baseline LH levels were negatively associated with mercury in skipping males but positively associated in skipping females, while LH induced by GnRH injection increased with increasing mercury levels (Tartu et al. 2013). However, both baseline and GnRH-induced LH were suppressed in male and female snow petrels with higher environmental mercury exposure (Tartu et al. 2014).
Prostaglandin synthesis declined after exposure to a high dose (5 μg/g) in a homogenate eggshell mucosa from chickens (Lundholm 1995; 1 mg methylmercury/day over 50 days in diet). White ibises had a nonsignificant increase in progesterone during incubation (n = 6) (Heath and Frederick 2005). Thyroid hormones, T3 and T4, were lower in nestling tree swallows exposed to mercury at contaminated sites (Wada et al. 2009), but T4 had no relationship to mercury in lesser scaup ducks (Pollock and Machin 2009). In great blue herons with relatively low mercury burdens, no relationship was found between mercury and total or free T3 or T4, and the hormone precursor dehydroretinol decreased with increased mercury levels in these herons, but there was no relationship to retinol (Champoux et al. 2017). Male snow petrels had depressed levels of stress-induced prolactin, but no association with mercury was found in baseline prolactin in either sex or stress-induced prolactin in female snow petrels (Tartu et al. 2015).
More information is available regarding estradiol. In female white ibises, estradiol levels were negatively related to mercury, significantly so prior to breeding, nonsignificantly during the courtship display period (n = 13) (Heath and Frederick 2005). Dosed female white ibises showed a significant decrease in estradiol in 1 year and exhibited a nonsignificant trend in the same direction the following year (n = 20). In male white ibises, estradiol levels were higher in dosed birds than controls during courtship but lower during other stages. Differences between dosed and control birds were amplified in males that paired, abnormally, with other males (Jayasena et al. 2011: trace in diet). Estradiol levels in juvenile white ibises increased in a dose-dependent manner with mercury dose (Adams et al. 2009: trace in diet). Estradiol levels were not related to mercury in dosed juvenile common loons (Franceschini et al. 2017: trace, medium in diet). Given the number of studies, it is perhaps surprising that a clearer pattern is not apparent in the relationship between mercury exposure and various hormones. There seems to be a predictable depression of the CORT response in mercury-exposed birds, but effects on the sex hormones have proven to be complex, indirect, or fleeting and cannot be generalized at this point.
8 Immunocompetence
8.1 Overview
The impact of mercury on immune function is relatively understudied. There has been little replication for most endpoints, and field investigations have been limited to nonspecific measures of immune response, such as the phytohemagglutinin (PHA) skin-swelling assay, which leave considerable room for interpretation. However, a general picture is emerging that mercury negatively affects the immune systems of birds.
8.2 Blood Cells
The most widely reported white blood cell endpoints relate to heterophils and lymphocytes. The number of heterophils increased with mercury in dosed great egrets (Spalding et al. 2000a: trace, high force fed capsules) and dosed American kestrels (Fallacara et al. 2011a: trace, medium in diet), while the percentage of heterophils increased with mercury in free-living western grebes (Elbert and Anderson 1998). Two studies of free-living egrets reported a different trend in response to higher mercury; a decrease in heterophils that was significant in two out of three years for snowy egrets (Hoffman et al. 2009), and a nonsignificant decrease in the number of heterophils in great egrets (n = 11) (Sepúlveda et al. 1999: fed capsules for total of 3 mg methylmercury). The number of lymphocytes also exhibited a nonsignificant decrease associated with mercury in that study (n = 11) (Sepúlveda et al. 1999: fed capsules for total of 3 mg methylmercury). This result corroborates other results, including a significant decrease with higher mercury in the number of lymphocytes in dosed American kestrels (Fallacara et al. 2011a: trace, medium in diet) and decreased B-cell proliferation in dosed zebra finches (Lewis et al. 2013: trace, low in diet). Dosed great egrets, however, have also exhibited an increase in the number of lymphocytes (Spalding et al. 2000a: trace, high force fed capsules), as have free-living snowy egrets (Hoffman et al. 2009). In accordance with these findings about heterophils and lymphocytes, the heterophil-to-lymphocyte ratio increased for dosed American kestrels (Fallacara et al. 2011a: trace, medium in diet) and dosed common loons (Kenow et al. 2007: trace, medium in diet). Thus, mercury exposure can increase heterophils and decrease lymphocytes, but this is not always found.
Fewer results have been published regarding other white blood cells. Eosinophils exhibited a nonsignificant decrease in number with mercury level in great egrets (n = 11) (Sepúlveda et al. 1999: fed capsules for total of 3 mg methylmercury), and a significant decrease in proportion to other blood cells in environmentally exposed western grebes (Elbert and Anderson 1998). Macrophage activity decreased with mercury level in free-living black-footed albatross (Finkelstein et al. 2007), and macrophage suppression was also observed in dosed American kestrels (Fallacara et al. 2011a: trace, medium in diet). Abundance of monocytes increased with mercury in dosed great egrets (Spalding et al. 2000a: trace, high force fed capsules) but did not change in dosed American kestrels (Fallacara et al. 2011a: trace, medium in diet).
A small amount of information is available on how mercury impacts other aspects of blood. Hematocrit decreased in response to mercury in black-crowned night herons (Hoffman et al. 2009), snowy egrets (Henny et al. 2002: trace, low, medium, and high in prey), and dosed great egrets (Spalding et al. 2000a: trace, high force fed capsules). Sepúlveda et al. (1999: fed capsules for total of 3 mg methylmercury) observed a significant increase in hematocrit with mercury exposure in great egrets during 1 year, but a nonsignificant decrease in another year (n = 11). Packed cell volume and hemoglobin were not impacted in dosed zebra finches (Yu et al. 2016: trace, high injected in egg). Plasma proteins in general may decrease, as observed in both dosed and environmentally exposed great egrets (Hoffman et al. 2005: trace, high in diet; Sepúlveda et al. 1999: fed capsules for total of 3 mg methylmercury; Spalding et al. 2000a: trace, high force fed capsules). However, the response is likely more complicated, as common loons displayed an increase in globulin and a decrease in albumin (Kenow et al. 2007: trace, medium in diet).
8.3 Immune Responsiveness
A considerable body of literature shows that mercury decreases general immune response in birds, although there are variable results from different assays. PHA-induced swelling was lower for dosed great egrets (Spalding et al. 2000a: trace, high force fed capsules), and dosed American kestrels (Fallacara et al. 2011a, b: trace, medium in diet), and environmentally exposed tree swallows (Hawley et al. 2009). Antibody response to sheep red blood cells (SRBCs) was lower in dosed American kestrels (Fallacara et al. 2011a: trace, medium in diet) and dosed common loons (Kenow et al. 2007: trace, medium in diet). However, Kenow et al. (2007: trace, medium in diet) reported no change in PHA-induced swelling in dosed common loons. In common eiders, no relationship was found between mercury and PHA-induced swelling (Wayland et al. 2002), and no difference in skin-swelling response to PHA injection was detected between dosed and control zebra finches (Caudill et al. 2015: trace, low, medium, and high in diet). Negative results were also reported for the relationship between mercury and antibody response to SRBC in a dosing study (American kestrel, Fallacara et al. 2011a: trace, medium in diet) and a field study (tree swallow, Hawley et al. 2009).
Other evidence for a generally compromised immune response includes a greater rate of bacterial infections in dosed common loons (Kenow et al. 2007: trace, medium in diet). Concentrations of heat shock protein 70 increased with mercury in great egrets, but not in white ibises (Herring et al. 2014). Finally, great white herons found dying of chronic disease (e.g., gout) had higher body burdens of mercury than birds dying of acute causes, e.g., injuries (Spalding et al. 1994). In common eiders with near-baseline mercury burdens, no correlation with immunoglobulin Y was found (Provencher et al. 2016). A concerted effort to measure the same endpoints across different mercury exposures and species might quickly resolve why results have been inconsistent across multiple studies. Specifically, more studies are needed that measure response to challenge from parasites or diseases, rather than baseline levels of various immune system components, to evaluate the effect size and potential cost of the deleterious effects of mercury on the immune system.
9 Other Physiological Endpoints
9.1 Oxidative Stress
A growing body of evidence indicates that mercury exposure induces oxidative stress. Although one study of glutathione in dosed laughing gulls failed to find evidence for changes in reduced glutathione (GSH), oxidized glutathione (GSSG), or the ratio of oxidized GSSG to reduced GSH (Jenko et al. 2012: trace, low, medium, and high injected in egg), evidence of mercury-related oxidative stress has been observed in a number of other species. GSH was negatively related to mercury level in the livers of greater scaup, surf scoters, and ruddy ducks (Hoffman et al. 1998), Forster’s terns (Hoffman et al. 2011), and great blue herons (Custer et al. 1997), and in the kidney and brain of snowy egrets (Hoffman et al. 2009), although it was not affected in livers of dosed zebra finches (Henry et al. 2014: trace, low, medium, and high in diet). One study observed the opposite relationship with mercury, elevated GSH in domestic duck brains and livers (Ji et al. 2006: trace in prey). GSSG increased in the liver, brain, and kidney of dosed common loons (Kenow et al. 2008: trace, medium in diet) and was also positively related to mercury in the livers of surf scoters and ruddy ducks (Hoffman et al. 1998), great egrets (Hoffman et al. 2005: trace, high in diet), and dosed zebra finches (Henry et al. 2014: trace, low, medium, and high in diet), and in the kidneys of snowy egrets and of Forster’s terns (Hoffman et al. 2009, 2011). Interestingly, the opposite trend was observed in the brains and livers of snowy egrets and brains of Forster’s terns (Hoffman et al. 2009, 2011).
The ratio of GSSG to GSH, which represents the ratio of unavailable to available antioxidant and may be the most relevant marker for disruption of glutathione function, increased with mercury exposure in loon brains (Kenow et al. 2008: trace, medium in diet), indicating oxidative stress. Increased GSSG:GSH was also associated with mercury in the livers of greater scaup (Hoffman et al. 1998), Forster’s tern (Hoffman et al. 2011), double-crested cormorant (Henny et al. 2002: low, medium, and high in prey), and dosed zebra finch (Henry et al. 2014: trace, low, medium, and high in diet), as well as the kidneys of free-living snowy egrets (Hoffman et al. 2009). Reports of decreased GSSG:GSH in brains and livers of snowy egrets (Hoffman et al. 2009), kidneys of great egrets (Hoffman et al. 2005: trace, high in diet), and livers of common loon (Kenow et al. 2008: trace, medium in diet) might be interpretable as compensatory responses. GSH peroxidase, which converts oxidized GSSH to reduced GSH, declined with increased mercury in great egret livers, kidneys, plasma, and brains (Hoffman et al. 2005: trace, high in diet), snowy egret blood and kidneys (Hoffman et al. 2009), cormorant livers (Henny et al. 2002: low, medium, and high in prey), and common loon brains, consistent with a link between mercury and oxidative stress. But this same bioindicator increased in loon kidney and liver (Kenow et al. 2008: trace, medium in diet), as well as in surf scoter liver (Hoffman et al. 1998) and domestic duck brain and liver (Ji et al. 2006: trace in prey). Evidence of oxidative stress was deduced from increased total thiol levels in lesser scaup (Custer et al. 2000), and wandering albatross plasma also showed evidence of oxidative damage, although no impact was observed on the inflammatory protein haptoglobin (Costantini et al. 2014). In addition to these biochemical changes, mercury exposure increased the expression of two cellular stress-related genes, glutathione peroxidase 3 and glutathione S-transferase μ3, in female double-crested cormorants (Gibson et al. 2014). In two populations of tree swallows with very low tissue mercury concentrations, a number of conflicting results were obtained. For one, protein-bound thiol (PBSH) increased with mercury and no correlation was found between mercury and GSH, thiobarbituric acid reactive substances (TBARS), GSSG, or total sulfhydryl (TSH) (Custer et al. 2006). In the other, PBSH, GSSG, and TSH decreased, GSH increased, and no correlation was observed between mercury concentration and TBARS or the ratio of GSSG:GSH (Custer et al. 2008: trace in prey).
Oxidative stress may be responsible for reports of damage to livers and other internal organs in birds with high mercury levels. Snowy egrets had liver and kidney damage (Hoffman et al. 2009), European starlings showed extensive nephritic lesions after being dosed unintentionally in captivity with an unidentified form of methylmercury in their food (Nicholson and Osborn 1984: medium in diet), black-crowned night herons, snowy egrets, and double-crested cormorants experienced hepatotoxicity and nephrotoxicity with higher exposure to mercury (Henny et al. 2002: trace, low, medium, and high in prey), and domestic ducks exhibited minor kidney damage and degeneration (Snelgrove-Hobson et al. 1988: trace, high in diet). Henny et al. (2002: trace, low, medium, and high in prey) also found that young snowy egrets had enlarged livers and kidneys (and smaller brains), and double-crested cormorants had enlarged spleens, which may have been the result of organ damage rather than growth.
9.2 Chromosomal Damage
Whether or not mercury causes chromosomal damage in birds has not been thoroughly investigated, and the issue would benefit from future research. A handful of studies have used the half-peak coefficient of variation (HPCV) of the G1 cell population as an indicator of chromosomal damage. No difference was found between the HPCV of experimentally dosed and control common loons (Kenow et al. 2008: trace, medium in diet). In free-living lesser scaup (Custer et al. 2000) and tree swallows (Custer et al. 2006: trace in prey (dw)), no evidence of chromosomal damage was found, but the mercury levels of these birds were not elevated above background concentrations and so the interpretation is difficult.
9.3 Metabolism
Very few studies have investigated changes in metabolism in response to environmentally relevant mercury contamination, and none of these have been replicated. In western grebes, blood potassium and phosphorus decreased with increasing tissue mercury concentration (Elbert and Anderson 1998), and plasma phosphate also decreased in great egrets (Hoffman et al. 2005: trace, high in diet), although plasma potassium did not change in Japanese quail fed methylmercury (Hill and Soares 1984: trace, medium, and high in diet). After dietary mercury exposure at the upper limit of what we defined as sublethal concentrations (5 μg/g), white leghorn chickens exhibited decreased calcium content in their blood plasma (Lundholm 1995: 1 mg methylmercury/day for 50 days in diet). Blood calcium and glucose levels of free-living snowy egrets also decreased with elevated mercury, as did glucose levels of black-crowned night herons (Hoffman et al. 2009). No relationship with mercury was observed in plasma triglyceride levels, an indicator of migration stopover refueling rate, in northern waterthrushes (Seewagen 2013), nor with blood glucose levels or blood reserves of lipids, protein, or minerals of lesser scaup (Anteau et al. 2007; Pollock and Machin 2009).
9.4 Growth and Condition
Mercury exposure does not appear to strongly impede overall growth but may result in some biologically significant changes in size of body components. No changes were observed in overall body mass of dosed American kestrels (Fallacara et al. 2011b: trace, medium in diet), or common loons (Kenow et al. 2003: trace, medium in diet), tarsus length of dosed American kestrels or tarsus or primary feather length of free-living tree swallows (Wada et al. 2009). A study of three species of arctic-breeding shorebirds found no association between mercury level and body condition (Hargreaves et al. 2010). Mercury-related effects were not seen in body length or asymptotic mass of common loons (Kenow et al. 2003: trace, medium in diet), nor body mass, tarsus length, or wing chord of urban red-winged blackbird nestlings (Gillet and Seewagen 2014). However, common loons from lakes with low pH, which are more susceptible to mercury bioaccumulation, did have lower asymptotic mass (Kenow et al. 2003: trace, medium in diet). Dosed great egrets reduced their food intake and had lower weight index scores (Spalding et al. 2000b: trace, high force fed capsules). Similarly, young nestling tree swallows at sites with higher mercury also had a decreased linear growth rate in grams per day, although wing and tail feather growth were not affected (Longcore et al. 2007). In contrast, female common eiders with higher blood mercury arrived at breeding grounds in better condition, as defined by mass divided by head length, although mercury levels were generally low (Provencher et al. 2016). The growth of nestling Leach’s storm-petrels, however, was not correlated with mercury burden (Pollet et al. 2017).
A multitude of other indices have been used to assess body condition after mercury exposure, ranging from size-corrected body mass to feather growth rate. These varied assays make categorizing the effect of mercury on condition difficult. Body weight, as well as liver and heart weight, decreased in surf scoters, and the liver-to-body weight ratio increased in ruddy ducks (Hoffman et al. 1998). Male American kestrels dosed with mercury also had lower body weight, but only in one treatment group (Albers et al. 2007: trace, low, medium, and high in diet). Meanwhile, no change in body or organ weight was detected in greater scaup (Hoffman et al. 1998), or in the body weight of bald eagles (Weech et al. 2006), or in body mass, body size, or organ mass of common eiders (Wayland et al. 2002). Great white herons dying of chronic disease, and with elevated mercury in tissues, had less body fat, although there was a statistical interaction with age (Spalding et al. 1994).
Studies with more complex measures of body condition provide an even more ambiguous picture. Atlantic puffins, common guillemots, razorbills, and black-legged kittiwakes with higher mercury had decreased body conditions in terms of liver-to-kidney mass (Fort et al. 2015). When defined as a ratio of mass to structural size, California clapper rails with higher mercury had lower body condition (Ackerman et al. 2012), but using the same metric, white ibises showed a nonsignificant trend of improved body condition with mercury level (n = 19) (Heath and Frederick 2005). Also, neither white ibis nor great egret chicks exhibited changes in body condition as measured by the residuals obtained from regressing mass on tarsus length (Herring et al. 2014), and similarly, using residuals of mass regressed on skull length, snow petrels did not exhibit differences in body condition that related to mercury (Tartu et al. 2015). Acadian flycatcher “frame size,” calculated using a PCA including wing chord and tarsus length regressed on mass, was not related to low level environmental mercury exposure (Rowse et al. 2014). Using body mass-to-body length and body mass-to-keel length as a measure of body condition resulted in a positive relationship between body condition and mercury in common mergansers (Kalisińska et al. 2010), but the interpretation of this result may not be straightforward given that birds acquiring more or better food might also acquire more mercury.
In terms of feather growth, common loons had increased flight feather asymmetry, but this was only the few birds with the highest environmental exposures, resulting in 40 μg/g mercury in feathers (Evers et al. 2008). Neither these loons, nor glossy ibises or double-crested cormorants, exhibited increased feather asymmetry when exposed to more moderate mercury levels (Clarkson et al. 2012). Composite fluctuating asymmetry, based on wing chord, tarsus, primary feather 10, rectrix feather 6, and, with the strongest correlation, rectrix feather 1 was related to mercury in Forster’s terns, but not Caspian terns, American avocets, or black-necked stilts (Herring et al. 2017). However, daily feather growth as a nutritional condition index, measured through ptilochronology, had a negative relationship with mercury exposure in glossy ibises (Clarkson et al. 2012). In contrast, dosed European starlings exhibited increased molt rate (Carlson et al. 2014: low, medium in diet). These starlings also exerted less energy during takeoff than birds fed control diets. Belted kingfishers with higher mercury had brighter blue feathers, indicating decreased melanin content (White and Cristol 2014), and a consistent result was found in eastern bluebirds (McCullagh et al. 2015).
10 Conclusion
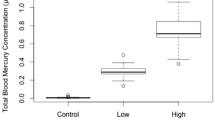
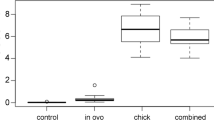
Our comprehensive review of existing studies shows that mercury can negatively impact nearly every aspect of avian physiology (Fig. 1a, b). Reproduction is by far the best-studied category of endpoints because of its immediate relation to fitness, and mercury exposure clearly reduces the number of surviving offspring in wild or captive birds. Reproductive phenology does not appear strongly altered by mercury, so the reduction in number of offspring may be a result of eggshell malformation, teratogenicity, or nestling and fledgling mortality. Meanwhile, chick behavior and parenting can be abnormal as the result of mercury exposure.
While offspring survival appears to be affected in the nest, longevity after leaving the nest does not decline detectably due to mercury exposure. Rather, exposed individuals face behavioral shifts away from higher energy activities. Hunting and foraging efficiency may be relatively resistant to the negative effects of mercury, with little consensus among published results, and similarly there is no clear pattern regarding growth and body condition. However, immune function has frequently been found to be compromised, in addition to a number of changes in white blood cell counts.
Additional endpoints that consistently reveal the deleterious effects of mercury are oxidative stress and some aspects of neurological function, including axonal degeneration. But many important endpoints remain understudied. There is currently too little information to make conclusions regarding neurotransmitter function or metabolism. Many researchers have investigated various hormones, especially CORT and T, but together the results do not provide a coherent explanation for how mercury is impacting either organizational or activational aspects of the endocrine system. For most hormones, there has been little to no investigation, particularly with respect to different stages of the reproductive cycle.
A majority of studies have detected effects of mercury exposure on myriad avian endpoints. In fact, survivorship is the only endpoint for which we can conclude that mercury has no detectable effect, and even this conclusion must be tempered by logistical issues of statistical power to detect small differences. However, it is noteworthy that for many endpoints, even those for which there is much evidence for deleterious effects of mercury, there is disagreement between studies, with some studies showing no effects. By lumping studies into categories defined by endpoint, we are necessarily glossing over other explanatory factors, such as whether exposure was experimental dosing or correlational fieldwork, or the statistical power of each study, the variable sensitivity of different species of birds to mercury, or the magnitude of the mercury exposure. Each of these factors may have influenced the findings of a particular study, but our qualitative review does not facilitate the weighing of these other explanatory variables.
Field studies, in which environmentally exposed birds were sampled and compared by site or tissue mercury concentration, may be inherently biased towards negative results because of the possibility of resistance evolving in a population that has been historically exposed to a contaminant. Strong selection by a contaminant will leave only the resistant individuals in a population. Further, sampling methods that rely on competition among individuals, such as for nest sites where eggs or tissues are sampled, will further bias results towards the strongest competitors, which may also be the most resistant individuals in the population. Thus, one could argue that field studies will underestimate the effects of a contaminant such as mercury, because only the most robust populations and individuals are present to be sampled. Experimental dosing studies, in which individuals with no history of prior exposure are assigned randomly to treatment groups, should avoid this problem and might therefore be predicted to be more likely to detect effects of contaminants. However, there is a strong argument to be made that dosing studies performed in captivity will also underestimate effects of contaminants. This is based on the observation that challenges present in the lives of wild animals, such as learning and remembering the locations of food, avoiding predators, competing for scarce resources, migrating, or choosing an appropriate mate, are generally eliminated in laboratory studies. Thus, a contaminant like mercury, with well-established neurological and cognitive effects, may still have little detectable effect on endpoints such as survival or reproduction in captive dosing studies, because most barriers to survival or reproduction that require memory and learning have been removed. In the absence of a strong basis for predicting whether field or captive studies will have greater likelihood of detecting effects of mercury, we simply note that a majority of studies detected effects of mercury on some endpoint, but laboratory studies were even more likely to detect effects of mercury than field studies (laboratory 91%, field: 72%, Fig. 1)
Another obvious difference between studies that could affect outcomes is that some had low statistical power due to small sample sizes. It is possible that endpoints with negative results in our review tended to come from the studies with smaller sample sizes. To examine this possibility, we plotted the sample sizes of each experimental dosing study to visualize the distribution of studies that detected or failed to detect effects of mercury, across all endpoints (Fig. 2). We excluded field studies because most included multiple years and study sites, complicating efforts to link sample sizes with significant results. Arbitrarily defining studies with small sample sizes as those with fewer than 20 individuals sampled in the smallest treatment group, we find that the probability of detecting some effect of mercury was similarly high across studies with small and larger sample sizes (small: 92% and larger: 80%). This suggests that the studies we reviewed generally exceeded the sample size necessary for reasonable statistical power to detect effects and thus sample size was not a determining factor in whether an effect of mercury was reported.
It is not surprising that different species, with their unique life histories and separate evolutionary paths, would differ in sensitivity to particular contaminants. To address this, we took advantage of the monumental study involving injections of methylmercury into eggs followed by artificial incubation, in which Heinz et al. (2009) categorized 23 species as having low, medium, or high sensitivity to mercury. Using our best guess, based on taxonomic similarity, we classified all species included in this review into the low, medium, and high sensitivity categories (Table 1). We then looked at all endpoints measured, whether in the same or different studies, for species in these three categories. If species sensitivity has an important effect on whether studies detected effects of mercury, there should be greater odds that a particular endpoint measured in a species with high sensitivity will show an effect of mercury exposure. Across all categories of sensitivity, approximately twice as many studied endpoints exhibited effects of mercury as were unaffected. This preponderance of results showing effects of mercury may be an effect of publication bias, or the intuition of researchers as to which endpoints are likely to be vulnerable to perturbation by mercury. But, a small effect of species sensitivity may be detectable here as well. Among 17 species with low sensitivity to mercury, the ratio of endpoints exhibiting effects of mercury to those failing to find effects was 1.80:1 (across 56 tested endpoints). For the 40 species in the medium sensitivity category, the ratio was 1.98:1 (across 61 tested endpoints), and for the 11 species with high sensitivity that increased to 2.07:1 (across 46 tested endpoints). The highest and lowest categories of species sensitivity differed by only 4% in the relative odds of detecting an effect of mercury, suggesting that species sensitivity is not a major factor in determining whether a studied endpoint will exhibit a detectable effect of mercury. However, because assignment of species sensitivity is a field still in its infancy, when considering the response to mercury of various endpoints discussed in this review, such as survival or endocrine function, the overall sensitivity of the species studied is a factor worth noting.
Finally, it seems intuitive that the intensity of exposure should be a good predictor of whether a particular study of mercury leads to a finding of deleterious effect. Unless most studies utilized exposures above the threshold for effects, or nonlinear responses are common, then studies including high exposures (dosing concentrations >2.0 μg/g) would be predicted to be more likely to produce evidence of deleterious effects of mercury than those with only trace (<0.5 μg/g), low (0.5–1.0 μg/g), or medium (1.0–2.0 μg/g) doses. In fact, 95% of the 21 studies without a high dosage produced evidence of effects of mercury, whereas only 86% of the 36 studies that included a high dose produced such results. Because there was little evidence of nonlinear effects of mercury (e.g., only 2 reports of hormesis) in the studies reviewed here, our conclusion is that in experimental studies, dosages selected were adequate to test the chosen endpoint, and thus variation in exposure is not likely to explain contrasting results for a particular endpoint.
Researchers should try to build on existing knowledge by employing previously studied biomarkers in new situations or species, or replicating previous studies with lower levels of mercury exposure. Very few studies have examined the effects of low mercury concentrations using experimental dosing, but these are likely the most relevant for understanding environmental exposures. There is a persistent gap in understanding between studies employing egg injection and those using maternal transfer. A few studies that calibrate the difference in embryotoxicity of these two means of exposure would open up vast opportunity for egg injection studies with more direct applicability in risk assessment and conservation. The most fertile frontier for research on sublethal effects of mercury will be the careful study of disruption of hormonal pathways regulating reproduction. This will be important because it relates directly to the biggest fitness effect of mercury, reduction in breeding success, but it may also provide valuable crossover knowledge for understanding human health effects of mercury. The mechanisms for many of the results reported in this review remain nearly a complete mystery, and similarly, some important traits, such as molt and migration behavior, have received disproportionately little attention. To collect meaningful data on most of the endpoints that remain inconclusive, especially neurological and endocrine function, great care must be taken to design appropriate experiments that take into account stage of the life cycle. Studies done during molt, for example, may fail to produce the same effects as those outside of molt. Disruption of endocrine pathways may occur over just a few days of the breeding season or may be apparent in one sex but not the other. Studies that provide just a snapshot of the life cycle will be less valuable and possibly misleading. Above all, researchers must remember that dosing birds in captivity may underestimate effects because of the lack of relevant challenges faced by captives. Studies on long-exposed populations in the field will also underestimate the effects of mercury if tolerance or resistance has evolved due to selection. The most effective studies will be those that examine free-living birds which have been exposed to mercury experimentally—a daunting logistical challenge but not beyond the creative abilities of the many excellent researchers featured in this review.
11 Summary
We reviewed over 150 published articles in which researchers tested the effect of methylmercury exposure (<5 μg/g) on various avian endpoints. The vast majority of both field (72%) and laboratory (91%) studies found effects of mercury, across hundreds of physiological and behavioral endpoints and almost 70 different bird species. The majority of sublethal effects were subtle and some studies of similar endpoints have reached differing conclusions. Generally, though, there was little evidence that opposing conclusions were the result of differences in sample size, species sensitivity to mercury, or intensity of methylmercury exposure. Strong support exists in the literature for the conclusion that mercury exposure reduces reproductive output, compromises immune function, and causes avoidance of high-energy behaviors. For other endpoints, notably some measures of reproductive success, endocrine function, and body condition, there is weak or contradictory evidence of adverse effects and further study is required. There was no evidence that environmentally relevant mercury exposure affects longevity, but several of the sublethal effects identified likely do result in fitness reductions that could adversely impact populations. The most definitive conclusion to be drawn from this review is that to understand how mercury is affecting birds, more experiments are required that focus on a consistent set of physiological endpoints. Despite some knowledge gaps, research on the sublethal effects of mercury has produced an overwhelming case that mercury harms individual birds in many ways, with effects on reproduction that could be responsible for population declines.
References
Ackerman JT, Eagles-Smith CA, Takekawa JY, Iverson SA (2008a) Survival of postfledging Forster’s terns in relation to mercury exposure in San Francisco Bay. Ecotoxicology 17:789–801. doi:10.1007/s10646-008-0237-6
Ackerman JT, Takekawa JY, Eagles-Smith CA, Iverson SA (2008b) Mercury contamination and effects on survival of American avocet and black-necked stilt chicks in San Francisco Bay. Ecotoxicology 17:103–116. doi:10.1007/s10646-007-0164-y
Ackerman JT, Overton CT, Casazza ML, Takekawa JY, Eagles-Smith CA, Keister RA, Herzog MP (2012) Does mercury contamination reduce body condition of endangered California clapper rails? Environ Pollut 162:439–448. doi:10.1016/j.envpol.2011.12.004
Adams EM, Frederick PC (2008) Effects of methylmercury and spatial complexity on foraging behavior and foraging efficiency in juvenile white ibises (Eudocimus albus). Environ Toxicol Chem 27:1708–1712
Adams EM, Frederick PC, Larkin ILV, Guillette LJ Jr (2009) Sublethal effects of methylmercury on fecal metabolites of testosterone, estradiol, and corticosterone in captive juvenile white ibises (Eudocimus albus). Environ Toxicol Chem 28:982–989. doi:10.1897/08-253.1
Albers PH, Koterba MT, Rossmann R, Link WA, French JB, Bennett RS, Bauer WC (2007) Effects of methylmercury on reproduction in American kestrels. Environ Toxicol Chem 26:1856–1866. doi:10.1897/06-592R.1
Anteau MJ, Afton AD, Custer CM, Custer TW (2007) Relationships of cadmium, mercury, and selenium with nutrient reserves of female lesser scaup (Aythya affinis) during winter and spring migration. Environ Toxicol Chem 26:515–520
Anthony RG, Miles AK, Estes JA, Isaacs FB (1999) Productivity, diets, and environmental contaminants in nesting bald eagles from the Aleutian archipelago. Environ Toxicol Chem 18:2054–2062. doi:10.1002/etc.5620180925
Barr JF, Canadian Wildlife Service (1986) Population dynamics of the common loon (Gavia immer) associated with mercury-contaminated waters in Northwestern Ontario. Environment Canada, Canadian Wildlife Service, Hull, QC
Bennett RS, French JBJ, Rossmann R, Haebler R (2009) Dietary toxicity and tissue accumulation of methylmercury in American kestrels. Arch Environ Contam Toxicol 56:149–156. doi:10.1007/s00244-008-9168-8
Bouland AJ, White AE, Lonabaugh KP, Varian-Ramos CW, Cristol DA (2012) Female-biased offspring sex ratios in birds at a mercury-contaminated river. J Avian Biol 43:244–251. doi:10.1111/j.1600-048X.2012.05612.x
Bouton SN, Frederick PC, Spalding MG, McGill H (1999) Effects of chronic, low concentrations of dietary methylmercury on the behavior of juvenile great egrets. Environ Toxicol Chem 18:1934–1939
Bowerman WW IV, Evans ED, Giesy JP, Postupalsky S (1994) Using feathers to assess risk of mercury and selenium to bald eagle reproduction in the Great Lakes region. Arch Environ Contam Toxicol 27:294–298. doi:10.1007/BF00213162
Brasso RL, Cristol DA (2008) Effects of mercury exposure on the reproductive success of tree swallows (Tachycineta bicolor). Ecotoxicology 17:133–141
Braune BM, Scheuhammer AM, Crump D, Jones S, Porter E, Bond D (2012) Toxicity of methylmercury injected into eggs of thick-billed murres and arctic terns. Ecotoxicology 21:2143–2152. doi:10.1007/s10646-012-0967-3
Burgess NM, Meyer MW (2008) Methylmercury exposure associated with reduced productivity in common loons. Ecotoxicology 17:83–91. doi:10.1007/s10646-007-0167-8
Bustamante P, Carravieri A, Goutte A, Barbraud C, Delord K, Chastel O, Weimerskirch H, Cherel Y (2016) High feather mercury concentrations in the wandering albatross are related to sex, breeding status and trophic ecology with no demographic consequences. Environ Res 144:1–10. doi:10.1016/j.envres.2015.10.024
Carlson JR, Cristol D, Swaddle JP (2014) Dietary mercury exposure causes decreased escape takeoff flight performance and increased molt rate in European starlings (Sturnus vulgaris). Ecotoxicology 23:1464–1473. doi:10.1007/s10646-014-1288-5
Caudill MT, Spear EL, Varian-Ramos CW, Cristol DA (2015) PHA-stimulated immune-responsiveness in mercury-dosed zebra finches does not match results from environmentally exposed songbirds. Bull Environ Contam Toxicol 94:407–411. doi:10.1007/s00128-015-1472-1
Champoux L, Boily M, Fitzgerald G (2017) Thyroid hormones, retinol and clinical parameters in relation to mercury and organohalogen contaminants in great blue heron (Ardea herodias) nestlings from the St. Lawrence River, Québec, Canada. Arch Environ Contam Toxicol 72:200–214. doi:10.1007/s00244-017-0364-2
Clarkson CE, Erwin RM, Riscassi A (2012) The use of novel biomarkers to determine dietary mercury accumulation in nestling waterbirds. Environ Toxicol Chem 31:1143–1148. doi:10.1002/etc.1767
Costa RA, Eeva T, Eira C, Vaqueiro J, Medina P, Vingada JV (2014) Great tits breeding performance and mercury contamination from the paper and pulp industry in the west coast of Portugal. Chem Ecol 30:206–215. doi:10.1080/02757540.2013.856891
Costantini D, Meillère A, Carravieri A, Lecomte V, Sorci G, Faivre B, Weimerskirch H, Bustamante P, Labadie P, Budzinski H, Chastel O (2014) Oxidative stress in relation to reproduction, contaminants, gender and age in a long-lived seabird. Oecologia 175:1107–1116. doi:10.1007/s00442-014-2975-x
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, White AE (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335
Custer TW, Hines RK, Melancon MJ, Hoffman DJ, Wickliffe JK, Bickham JW, Martin JW, Henshel DS (1997) Contaminant concentrations and biomarker response in great blue heron eggs from 10 colonies on the upper Mississippi River, USA. Environ Toxicol Chem 16:260–271. doi:10.1002/etc.5620160223
Custer TW, Custer CM, Hines RK, Sparks DW, Melancon MJ, Hoffman DJ, Bickham JW, Wickliffe JK (2000) Mixed-function oxygenases, oxidative stress, and chromosomal damage measured in lesser scaup wintering on the Indiana Harbor Canal. Arch Environ Contam Toxicol 38:522–529
Custer CM, Custer TW, Warburton D, Hoffman DJ, Bickham JW, Matson CW (2006) Trace element concentrations and bioindicator responses in tree swallows from northwestern Minnesota. Environ Monit Assess 118:247–266. doi:10.1007/s10661-006-1499-1
Custer CM, Custer TW, Hill EF (2007) Mercury exposure and effects on cavity-nesting birds from the Carson River, Nevada. Arch Environ Contam Toxicol 52:129–136. doi:10.1007/s00244-006-0103-6
Custer TW, Custer CA, Johnson KM, Hoffman DJ (2008) Mercury and other element exposure to tree swallows (Tachycineta bicolor) nesting on Lostwood National Wildlife Refuge, North Dakota. Environ Pollut 155:217–226. doi:10.1016/j.envpol.2007.12.003
Custer TW, Custer CM, Thogmartin WE, Dummer PM, Rossmann R, Kenow KP, Meyer MW (2012) Mercury and other element exposure in tree swallows nesting at low pH and neutral pH lakes in northern Wisconsin USA. Environ Pollut 163:68–76. doi:10.1016/j.envpol.2011.12.017
Elbert RA, Anderson DW (1998) Mercury levels, reproduction, and hematology in western grebes from three California Lakes, USA. Environ Toxicol Chem 17:210–213. doi:10.1002/etc.5620170212
Evans HL, Garman RH, Laties VG (1982) Neurotoxicity of methylmercury in the pigeon. Neurotoxicology 3:21–36
Evers DC, Taylor KM, Major A, Taylor RJ, Poppenga RH, Scheuhammer AM (2003) Common loon eggs as indicators of methylmercury availability in North America. Ecotoxicology 12:69–81. doi:10.1023/A:1022593030009
Evers DC, Savoy LJ, DeSorbo CR et al (2008) Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology 17:69–81. doi:10.1007/s10646-007-0168-7
Fallacara DM, Halbrook RS, French JB (2011a) Toxic effects of dietary methylmercury on immune function and hematology in American kestrels (Falco sparverius). Environ Toxicol Chem 30:1320–1327. doi:10.1002/etc.494
Fallacara DM, Halbrook RS, French JB (2011b) Toxic effects of dietary methylmercury on immune system development in nestling American kestrels (Falco sparverius). Environ Toxicol Chem 30:1328–1337. doi:10.1002/etc.519
Fimreite N, Karstad L (1971) Effects of dietary methyl mercury on red-tailed hawks. J Wildl Manage 35:293–300
Finkelstein ME, Grasman KA, Croll DA, Tershy BR, Keitt BS, Jarman WM, Smith DR (2007) Contaminant-associated alteration of immune function in black-footed albatross (Phoebastria nigripes), a North Pacific predator. Environ Sci Technol 26:1896–1903. doi:10.1897/06-505R.1
Finley MT, Stendell RC (1978) Survival and reproductive success of black ducks fed methyl mercury. Environ Pollut 16:51–64. doi:10.1016/0013-9327(78)90137-4
Fort J, Lacoue-Labarthe T, Nguyen HL, Boue A, Spitz J, Bustamante P (2015) Mercury in wintering seabirds, an aggravating factor to winter wrecks? Sci Total Environ 527:448–454. doi:10.1016/j.scitotenv.2015.05.018
Franceschini MD, Lane OP, Evers DC, Reed JM, Hoskins B, Romero LM (2009) The corticosterone stress response and mercury contamination in free-living tree swallows, Tachycineta bicolor. Ecotoxicology 18:514–521. doi:10.1007/s10646-009-0309-2
Franceschini MD, Evers DC, Kenow KP, Meyer MW, Pokras M, Romero LM (2017) Mercury correlates with altered corticosterone but not testosterone or estradiol concentrations in common loons. Ecotoxicol Environ Saf 142:348–354. doi:10.1016/j.ecoenv.2017.04.030
Frederick P, Jayasena N (2010) Altered pairing behaviour and reproductive success in white ibises exposed to environmentally relevant concentrations of methylmercury. Proc Roy Soc B. doi: 10.1098/rspb.2010.2189
Frederick P, Campbell A, Jayasena N, Borkhataria R (2011) Survival of white ibises (Eudocimus albus) in response to chronic experimental methylmercury exposure. Ecotoxicology 20:358–364
Gerrard PM, St. Louis VL (2001) The effects of experimental reservoir creation on the bioaccumulation of methylmercury and reproductive success of tree swallows (Tachycineta bicolor). Environ Sci Technol 35:1329–1338. doi:10.1021/es001537f
Gibson LA, Lavoie RA, Bissegger S, Campbell LM, Langlois VS (2014) A positive correlation between mercury and oxidative stress-related gene expression (GPX3 and GSTM3) is measured in female double-crested cormorant blood. Ecotoxicology 23:1004–1014. doi:10.1007/s10646-014-1243-5
Gillet A-MTY, Seewagen CL (2014) Mercury exposure of a wetland songbird, Agelaius phoeniceus, in the New York metropolitan area and its effect on nestling growth rate. Environ Monit Assess 186:4029–4036. doi:10.1007/s10661-014-3677-x
Goutte A, Barbraud C, Meillère A, Carravieri A, Bustamante P, Labadie P, Budzinski H, Delord K, Cherel Y, Weimerskirch H, Chastel O (2014a) Demographic consequences of heavy metals and persistent organic pollutants in a vulnerable long-lived bird, the wandering albatross. Proc R Soc B 281:20133313. doi:10.1098/rspb.2013.3313
Goutte A, Bustamante P, Barbraud C, Delord K, Weimerskirch H, Chastel O (2014b) Demographic responses to mercury exposure in two closely related Antarctic top predators. Ecology 95:1075–1086. doi:10.1890/13-1229.1
Hallinger KK, Cristol DA (2011) The role of weather in mediating the effect of mercury exposure on reproductive success in tree swallows. Ecotoxicology 20:1368–1377. doi:10.1007/s10646-011-0694-1
Hallinger KK, Zabransky DJ, Kazmer KA, Cristol DA (2010) Birdsong differs between mercury-polluted and reference sites. Auk 127:156–161. doi:10.1525/auk.2009.09058
Hallinger KK, Cornell KL, Brasso RL, Cristol DA (2011) Mercury exposure and survival in free-living tree swallows (Tachycineta bicolor). Ecotoxicology 20:39–46. doi:10.1007/s10646-010-0554-4
Hamilton M, Scheuhammer A, Basu N (2011) Mercury, selenium and neurochemical biomarkers in different brain regions of migrating common loons from Lake Erie, Canada. Ecotoxicology 20:1677–1683. doi:10.1007/s10646-011-0754-6
Hargreaves AL, Whiteside DP, Gilchrist G (2010) Concentrations of 17 elements, including mercury, and their relationship to fitness measures in arctic shorebirds and their eggs. Sci Total Environ 408:3153–3161. doi:10.1016/j.scitotenv.2010.03.027
Hawley DM, Hallinger KK, Cristol DA (2009) Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology 18:499–503
Heath JA, Frederick PC (2005) Relationships among mercury concentrations, hormones, and nesting effort of white ibises (Eudocimus albus) in the Florida Everglades. Auk 255–267. doi: 10.1642/0004-8038(2005)122[0255:RAMCHA]2.0.CO;2
Heinz G (1974) Effects of low dietary levels of methyl mercury on mallard reproduction. Bull Environ Contam Toxicol 11:386–392. doi:10.1007/BF01684947
Heinz G (1975) Effects of methylmercury on approach and avoidance-behavior of mallard ducklings. Bull Environ Contam Toxicol 13:554–564
Heinz GH (1976a) Methylmercury: second-year feeding effects on mallard reproduction and duckling behavior. J Wildl Manag 40:82–90. doi:10.2307/3800158
Heinz GH (1976b) Methylmercury: second-generation reproductive and behavioral effects on mallard ducks. J Wildl Manag 40:710–715. doi:10.2307/3800567
Heinz GH (1979) Methylmercury: reproductive and behavioral effects on three generations of mallard ducks. J Wildl Manag 43:394–401. doi:10.2307/3800348
Heinz G (1980) Eggshell thickness in mallards fed methylmercury. Bull Environ Contam Toxicol 25:498–502
Heinz GH, Locke LN (1976) Brain lesions in mallard ducklings from parents fed methylmercury. Avian Dis 20:9–17
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA (2009) Species differences in the sensitivity of avian embryos to methylmercury. Arch Environ Contam Toxicol 56:129–138. doi:10.1007/s00244-008-9160-3
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR (2010a) Enhanced reproduction in mallards fed a low level of methylmercury: an apparent case of hormesis. Environ Toxicol Chem 29:650–653. doi:10.1002/etc.64
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR (2010b) Reproduction in mallards exposed to dietary concentrations of methylmercury. Ecotoxicology 19:977–982. doi:10.1007/s10646-010-0479-y
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA (2011) Teratogenic effects of injected methylmercury on avian embryos. Environ Toxicol Chem 30:1593–1598. doi:10.1002/etc.530
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA (2012a) Hormesis associated with a low dose of methylmercury injected into mallard eggs. Arch Environ Contam Toxicol 62:141–144. doi:10.1007/s00244-011-9680-0
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR (2012b) A comparison of the teratogenicity of methylmercury and selenomethionine injected into bird eggs. Arch Environ Contam Toxicol 62:519–528. doi:10.1007/s00244-011-9717-4
Helander B, Olsson M, Reutergårdh L (1982) Residue levels of organochlorine and mercury compounds in unhatched eggs and the relationships to breeding success in white-tailed sea eagles Haliaeetus albicilla in Sweden. Ecography 5:349–366. doi:10.1111/j.1600-0587.1982.tb01049.x
Henny CJ, Hill EF, Hoffman DJ, Spalding MG, Grove RA (2002) Nineteenth century mercury: hazard to wading birds and cormorants of the Carson River, Nevada. Ecotoxicology 11:213–231
Henny CJ, Kaiser JL, Packard HA, Grove RA, Taft MR (2005) Assessing mercury exposure and effects to American dippers in headwater streams near mining sites. Ecotoxicology 14:709–725. doi:10.1007/s10646-005-0023-7
Henny CJ, Hill EF, Grove RA, Chelgren ND, Haggerty PK (2017) Mercury and drought along the lower Carson River, Nevada: IV. Snowy egret post-fledging dispersal, timing of migration and survival, 2002-2004. Ecotoxicol Environ Saf 135:358–367. doi:10.1016/j.ecoenv.2016.10.002
Henry KA, Cristol DA, Varian-Ramos CW, Bradley EL (2014) Oxidative stress in songbirds exposed to dietary methylmercury. Ecotoxicology 1–7. doi: 10.1007/s10646-014-1400-x
Herring G, Gawlik DE, Rumbold DG (2009) Feather mercury concentrations and physiological condition of great egret and white ibis nestlings in the Florida Everglades. Sci Total Environ 407:2641–2649. doi:10.1016/j.scitotenv.2008.12.043
Herring G, Ackerman JT, Eagles-Smith CA (2010) Embryo malposition as a potential mechanism for mercury-induced hatching failure in bird eggs. Environ Toxicol Chem 29:1788–1794. doi:10.1002/etc.208
Herring G, Ackerman JT, Herzog MP (2012) Mercury exposure may suppress baseline corticosterone levels in juvenile birds. Environ Sci Technol 46:6339–6346. doi:10.1021/es300668c
Herring G, Eagles-Smith CA, Gawlik DE, Beerens JM, Ackerman JT (2014) Physiological condition of juvenile wading birds in relation to multiple landscape stressors in the Florida Everglades: effects of hydrology, prey availability, and mercury bioaccumulation. PLoS One 9:e106447. doi:10.1371/journal.pone.0106447
Herring G, Eagles-Smith CA, Ackerman JT (2017) Mercury exposure may influence fluctuating asymmetry in waterbirds. Environ Toxicol Chem 36:1599–1605. doi:10.1002/etc.3688
Hill EF, Soares JH (1984) Subchronic mercury exposure in coturnix and a method of hazard evaluation. Environ Toxicol Chem 3:489–502. doi:10.1002/etc.5620030311
Hill EF, Henny CJ, Grove RA (2008) Mercury and drought along the lower Carson River, Nevada: II. Snowy egret and black-crowned night-heron reproduction on Lahontan Reservoir, 1997-2006. Ecotoxicology 17:117–131. doi:10.1007/s10646-007-0180-y
Hoffman D, Moore J (1979) Teratogenic effects of external egg applications of methyl mercury in the mallard, Anas platyrhynchos. Teratology 20:453–461. doi:10.1002/tera.1420200315
Hoffman DJ, Ohlendorf HM, Marn CM, Pendleton GWP (1998) Association of mercury and selenium with altered glutathione metabolism and oxidative stress in diving ducks from the San Francisco Bay region, USA. Environ Toxicol Chem 17:167–172. doi:10.1002/etc.5620170205
Hoffman DJ, Spalding MG, Frederick PC (2005) Subchronic effects of methylmercury on plasma and organ biochemistries in great egret nestlings. Environ Toxicol Chem 24:3078–3084
Hoffman DJ, Henny CJ, Hill EF, Grove RA, Kaiser JL, Stebbins KR (2009) Mercury and drought along the lower Carson River, Nevada: III. Effects on blood and organ biochemistry and histopathology of snowy egrets and black-crowned night-herons on Lahontan reservoir, 2002-2006. J Toxicol Environ Health Part A 72:1223–1241. doi:10.1080/15287390903129218
Hoffman DJ, Eagles-Smith CA, Ackerman JT, Adelsbach TL, Stebbins KR (2011) Oxidative stress response of Forster’s terns (Sterna forsteri) and Caspian terns (Hydroprogne caspia) to mercury and selenium bioaccumulation in liver, kidney, and brain. Environ Toxicol Chem 30:920–929. doi:10.1002/etc.459
Jackson AK, Evers DC, Etterson MA, Condon AM, Folsom SB, Detweiler J, Schmerfeld J, Cristol DA (2011) Mercury exposure affects the reproductive success of a free-living terrestrial songbird, the Carolina wren (Thryothorus ludovicianus). Auk 128:759–769. doi:10.1525/auk.2011.11106
Jayasena N, Frederick PC, Larkin ILV (2011) Endocrine disruption in white ibises (Eudocimus albus) caused by exposure to environmentally relevant levels of methylmercury. Aquat Toxicol 105:321–327
Jenko K, Karouna-Renier NK, Hoffman DJ (2012) Gene expression, glutathione status, and indicators of hepatic oxidative stress in laughing gull (Larus atricilla) hatchlings exposed to methylmercury. Environ Toxicol Chem 31:2588–2596. doi:10.1002/etc.1985
Ji X, Hu W, Cheng J, Yuan T, Xu F, Qu L, Wang W (2006) Oxidative stress on domestic ducks (Shaoxing duck) chronically exposed in a mercury-selenium coexisting mining area in China. Ecotoxicol Environ Saf 64:171–177. doi:10.1016/j.ecoenv.2005.03.009
Kalisińska E, Budis H, Podlasińska J, Łanocha N, Kavetska KM (2010) Body condition and mercury concentration in apparently healthy goosander (Mergus merganser) wintering in the Odra estuary, Poland. Ecotoxicology 19:1382–1399. doi:10.1007/s10646-010-0524-x
Kenow KP, Gutreuter S, Hines RK, Meyer MW, Fournier F, Karasov WH (2003) Effects of methyl mercury exposure on the growth of juvenile common loons. Ecotoxicology 12:171–181. doi:10.1023/A:1022598525891
Kenow KP, Grasman KA, Hines RK, Meyer MW, Gendron-Fitzpatrick A, Spalding MG, Gray BR (2007) Effects of methylmercury exposure on the immune function of juvenile common loons (Gavia immer). Environ Toxicol Chem 26:1460–1469
Kenow KP, Hoffman DJ, Hines RK, Meyer MW, Bickham JW, Matson CW, Stebbins KR, Montagna P, Elfessi A (2008) Effects of methylmercury exposure on glutathione metabolism, oxidative stress, and chromosomal damage in captive-reared common loon (Gavia immer) chicks. Environ Pollut 156:732–738. doi:10.1016/j.envpol.2008.06.009
Kenow KP, Hines RK, Meyer MW, Suarez SA, Gray BR (2010) Effects of methylmercury exposure on the behavior of captive-reared common loon (Gavia immer) chicks. Ecotoxicology 19:933–944. doi:10.1007/s10646-010-0475-2
Kenow KP, Meyer MW, Rossmann R, Gendron-Fitzpatrick A, Gray BR (2011) Effects of injected methylmercury on the hatching of common loon (Gavia immer) eggs. Ecotoxicology 20:1684–1693. doi:10.1007/s10646-011-0743-9
King K, Custer T, Quinn J (1991) Effects of mercury, selenium, and organochlorine contaminants on reproduction of Forster terns and black skimmers nesting in a contaminated Texas bay. Arch Environ Contam Toxicol 20:32–40
Klimstra JD, Yee JL, Heinz GH, Hoffman DJ, Stebbins KR (2012) Interactions between methylmercury and selenomethionine injected into mallard eggs. Environ Toxicol Chem 31:579–584. doi:10.1002/etc.1708
Kobiela ME, Cristol DA, Swaddle JP (2015) Risk-taking behaviours in zebra finches affected by mercury exposure. Anim Behav 103:153–160. doi:10.1016/j.anbehav.2015.02.024
Laties VG, Evans HL (1980) Methylmercury-induced changes in operant discrimination by the pigeon. J Pharmacol Exp Ther 214:620–628
Lewis CA, Cristol DA, Swaddle JP, Varian-Ramos CW, Zwollo P (2013) Decreased immune response in zebra finches exposed to sublethal doses of mercury. Arch Environ Contam Toxicol 64:327–336. doi:10.1007/s00244-012-9830-z
Loerzel SM, Samuelson DA, Szabo N (1999) Ocular effects of methylmercury in juvenile double-crested cormorants (Phalacrocorax auritus). Invest Ophthalmol Vis Sci 40:S445
Longcore JR, Dineli R, Haines TA (2007) Mercury and growth of tree swallows at Acadia National Park, and at Orono, Maine, USA. Environ Monit Assess 126:117–127. doi:10.1007/s10661-006-9325-3
Lundholm C (1995) Effects of methyl mercury at different dose regimes on eggshell formation and some biochemical characteristics of the eggshell gland mucosa of the domestic fowl. Comp Biochem Physiol C-Pharmacol Toxicol Endocrinol 110:23–28. doi:10.1016/0742-8413(94)00081-K
Maddux SL, Cristol DA, Varian-Ramos CW, Bradley EL (2014) The effect of mercury on baseline corticosterone in a breeding songbird. Bull Environ Contam Toxicol. doi: 10.1007/s00128-014-1440-1
McCullagh EA, Cristol DC, Phillips JB (2015) Plumage color and reproductive output of eastern bluebirds (Sialia sialis) nesting near a mercury-contaminated river. J Environ Sci Health A 50:1020–1028. doi:10.1080/10934529.2015.1038168
McKay JL, Maher CR (2012) Relationship between blood mercury levels and components of male song in Nelson’s sparrows (Ammodramus nelsoni). Ecotoxicology 21:2391–2397. doi:10.1007/s10646-012-0994-0
Merrill EH, Hartigan JJ, Meyer MW (2005) Does prey biomass or mercury exposure affect loon chick survival in Wisconsin? J Wildl Manag 69:57–67. doi:10.2193/0022-541X(2005)069<0057:DPBOME>2.0.CO;2
Meyer MW, Evers DC, Hartigan JJ, Rasmussen PS (1998) Patterns of common loon (Gavia immer) mercury exposure, reproduction, and survival in Wisconsin, USA. Environ Toxicol Chem 17:184–190. doi:10.1897/1551-5028(1998)017<0184:POCLGI>2.3.CO;2
Mitro MG, Evers DC, Meyer MW, Piper WH (2008) Common loon survival rates and mercury in New England and Wisconsin. J Wildl Manag 72:665–673. doi:10.2193/2006-551
Moore CS, Cristol DA, Maddux SL, Varian-Ramos CW, Bradley EL (2014) Lifelong exposure to methylmercury disrupts stress-induced corticosterone response in zebra finches (Taeniopygia guttata). Environ Toxicol Chem 33:1072–1076. doi:10.1002/etc.2521
Nicholson JK, Osborn D (1984) Kidney lesions in juvenile starlings Sturnus vulgaris fed on a mercury-contaminated synthetic diet. Environ Pollut Series A Ecol Biol 33:195–206. doi:10.1016/0143-1471(84)90010-2
Nocera JJ, Taylor PD (1998) In situ behavioral response of common loons associated with elevated mercury (Hg) exposure. Ecol Soc 2:10. http://www.consecol.org/vol2/iss2/art10/
Olivero-Verbel J, Agudelo-Frias D, Caballero-Gallardo K (2013) Morphometric parameters and total mercury in eggs of snowy egret (Egretta thula) from Cartagena Bay and Totumo Marsh, north of Colombia. Mar Pollut Bull 69:105–109. doi:10.1016/j.marpolbul.2013.01.013
Olsen B, Evers D, DeSorbo C (2000) Effect of methylated mercury on the diving frequency of the common loon. J Ecol Res 2:67–72
Pass DA, Little PB, Karstad LH (1975) The pathology of subacute and chronic methyl mercury poisoning of the Mallard duck (Anas platyrhynchos). J Comp Pathol 85:7–21. doi:10.1016/0021-9975(75)90079-1
Pollentier CD, Kenow KP, Meyer MW (2007) Common loon (Gavia immer) eggshell thickness and egg volume vary with acidity of nest lake in northern Wisconsin. Waterbirds 30:367–374. doi:10.1675/1524-4695(2007)030[0367:CLGIET]2.0.CO;2
Pollet IL, Leonard ML, O’Driscoll NJ, Burgess NM, Shutler D (2017) Relationships between blood mercury levels, reproduction, and return rate in a small seabird. Ecotoxicology 26:97–103. doi:10.1007/s10646-016-1745-4
Pollock B, Machin KL (2009) Corticosterone in relation to tissue cadmium, mercury and selenium concentrations and social status of male lesser scaup (Aythya affinis). Ecotoxicology 18:5–14. doi:10.1007/s10646-008-0250-9
Provencher JF, Forbes MR, Hennin HL, Love OP, Braune BM, Mallory ML, Gilchrist HG (2016) Implications of mercury and lead concentrations on breeding physiology and phenology in an Arctic bird. Environ Pollut 218:1014–1022. doi:10.1016/j.envpol.2016.08.052
Provencher JF, Forbes MR, Mallory ML, Wilson S, Gilchrist HG (2017) Anti-parasite treatment, but not mercury burdens, influence nesting propensity dependent on arrival time or body condition in a marine bird. Sci Total Environ 575:849–857. doi:10.1016/j.scitotenv.2016.09.130
Rowse LM, Rodewald AD, Sullivan SMP (2014) Pathways and consequences of contaminant flux to Acadian flycatchers (Empidonax virescens) in urbanizing landscapes of Ohio, USA. Sci Total Environ 485:461–467. doi:10.1016/j.scitotenv.2014.03.095
Rutkiewicz J, Scheuhammer A, Crump D, Jagla M, Basu N (2010) Investigation of spatial trends and neurochemical impacts of mercury in herring gulls across the Laurentian Great Lakes. Environ Pollut 158:2733–2737. doi:10.1016/j.envpol.2010.04.018
Rutkiewicz J, Nam D-H, Cooley T, Neumann K, Padilla IB, Route W, Strom S, Basu N (2011) Mercury exposure and neurochemical impacts in bald eagles across several Great Lakes states. Ecotoxicology 20:1669–1676. doi:10.1007/s10646-011-0730-1
Rutkiewicz J, Bradley M, Mittal K, Basu N (2013) Methylmercury egg injections: part 2—pathology, neurochemistry, and behavior in the avian embryo and hatchling. Ecotox Environ Safety 93:77–86. doi:10.1016/j.ecoenv.2013.04.007
Scheuhammer A (1988) Chronic dietary toxicity of methylmercury in the zebra finch, Poephila guttata. Bull Environ Contam Toxicol 40:123–130
Scheuhammer AM, Basu N, Burgess NM, Elliott JE, Campbell GD, Wayland M, Champoux L, Rodrigue J (2008) Relationships among mercury, selenium, and neurochemical parameters in common loons (Gavia immer) and bald eagles (Haliaeetus leucocephalus). Ecotoxicology 17:93–101. doi:10.1007/s10646-007-0170-0
Schoch N, Glennon MJ, Evers DC, Duron M, Jackson AK, Driscoll CT, Ozard JW, Sauer AK (2014) The impact of mercury exposure on the common loon (Gavia immer) population in the Adirondack Park, New York, USA. Waterbirds 37:133–146
Scoville SA, Lane OP (2013) Cerebellar abnormalities typical of methylmercury poisoning in a fledged saltmarsh sparrow, Ammodramus caudacutus. Bull Environ Contam Toxicol 90:616–620. doi:10.1007/s00128-013-0974-y
Seewagen CL (2013) Blood mercury levels and the stopover refueling performance of a long-distance migratory songbird. Can J Zool 91:41–45. doi:10.1139/cjz-2012-0199
Sepúlveda MS, Williams GE Jr, Frederick PC, Spalding MG (1999) Effects of mercury on health and first-year survival of free-ranging great egrets (Ardea albus) from southern Florida. Arch Environ Contam Toxicol 37:369–376
Snelgrove-Hobson SM, Rao PV, Bhatnagar MK (1988) Ultrastructural alterations in the kidneys of Pekin ducks fed methylmercury. Can J Vet Res 52:89–98
Spalding MG, Bjork RD, Powell GVN, Sundlof SF (1994) Mercury and cause of death in great white herons. J Wildl Manag 58:735–739. doi:10.2307/3809688
Spalding MG, Frederick PC, McGill HC, Bouton SN, Richey LJ, Schumacher IM, Blackmore CG, Harrison J (2000a) Histologic, neurologic, and immunologic effects of methylmercury in captive great egrets. J Wildl Dis 36:423–435. doi:10.7589/0090-3558-36.3.423
Spalding MG, Frederick PC, McGill HC, Bouton SN, McDowell LR (2000b) Methylmercury accumulation in tissues and its effects on growth and appetite in captive great egrets. J Wildl Dis 36:411–422
Stern GA, Macdonald RW, Outridge PM, Wilson S, Chetelat J, Cole A, Hintelmann H, Loseto LL, Steffen A, Wang F, Zdanowicz C (2012) How does climate change influence arctic mercury? Sci Total Environ 414:22–42. doi:10.1016/j.scitotenv.2011.10.039
Sunderland EM, Krabbenhoft DP, Moreau JW, Strode SA, Landing WM (2009) Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Global Biogeochm Cycles 23:GB2010
Swaddle JP, Diehl TR, Taylor CE, Fanaee AS, Benson JL, Huckstep NR, Cristol DA (2017) Exposure to dietary mercury alters cognition and behavior of zebra finches. Curr Zool 63:213–219. doi:10.1093/cz/zox007
Tartu S, Goutte A, Bustamante P, Angelier F, Moe B, Clément-Chastel C, Bech C, Gabrielsen GW, Bustnes JO, Chastel O (2013) To breed or not to breed: endocrine response to mercury contamination by an Arctic seabird. Biol Lett 9:20130317. doi:10.1098/rsbl.2013.0317
Tartu S, Bustamante P, Goutte A, Cherel Y, Weimerskirch H, Bustnes JO, Chastel O (2014) Age-related mercury contamination and relationship with luteinizing hormone in a long-lived Antarctic bird. PLoS One 9:e103642. doi:10.1371/journal.pone.0103642
Tartu S, Angelier F, Wingfield JC, Bustamante P, Labadie P, Budzinski H, Weimerskirch H, Bustnes JO, Chastel O (2015) Corticosterone, prolactin and egg neglect behavior in relation to mercury and legacy POPs in a long-lived Antarctic bird. Sci Total Environ 505:180–188. doi:10.1016/j.scitotenv.2014.10.008
Taylor CE, Cristol DA (2015) Tissue mercury concentrations and survival of tree swallow embryos, nestlings and young adult females on a contaminated site. Bull Environ Contam Toxicol 95:459–464. doi:10.1007/s00128-015-1643-0
Thompson DR, Hamer KC, Furness RW (1991) Mercury accumulation in great skuas Catharacta skua of known age and sex, and its effects upon breeding and survival. J Appl Ecol 28:672–684. doi:10.2307/2404575
Varian-Ramos CW, Swaddle JP, Cristol DA (2014) Mercury reduces avian reproductive success and imposes selection: an experimental study with adult- or lifetime-exposure in zebra finch. PLoS One 9:e95674. doi:10.1371/journal.pone.0095674
Wada H, Cristol DA, McNabb FMA, Hopkins WA (2009) Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol 43:6031–6038. doi:10.1021/es803707f
Wayland M, Gilchrist HG, Marchant T, Keating J, Smits JE (2002) Immune function, stress response, and body condition in Arctic-breeding common eiders in relation to cadmium, mercury, and selenium concentrations. Environ Res 90:47–60. doi:10.1006/enrs.2002.4384
Wayland M, Drake KL, Alisauskas RT, Kellett DK, Traylor J, Swoboda C, Mehl K (2008) Survival rates and blood metal concentrations in two species of free-ranging North American sea ducks. Environ Toxicol Chem 27:698–704. doi:10.1897/07-321
Weech SA, Scheuhammer AM, Elliott JE (2006) Mercury exposure and reproduction in fish-eating birds breeding in the Pinchi Lake region, British Columbia, Canada. Environ Toxicol Chem 25:1433–1440
White AE, Cristol DA (2014) Plumage coloration in belted kingfishers (Megaceryle alcyon) at a mercury-contaminated river. Waterbirds 37:144–152. doi:10.1675/063.037.0203
Wiemeyer SN, Lamont TG, Bunck CM, Sindelar CR, Gramlich FJ, Fraser JD, Byrd MA (1984) Organochlorine pesticide, polychlorobiphenyl, and mercury residues in bald eagle eggs—1969–79—and their relationships to shell thinning and reproduction. Arch Environ Contam Toxicol 13:529–549. doi:10.1007/BF01056332
Wolf SE, Swaddle JP, Cristol DA, Buchser WJ (2017) Methylmercury exposure reduces the auditory brainstem response of zebra finches (Taeniopygia guttata). J Assoc Res Otolaryngol. doi: 10.1007/s10162-017-0619-7
Yu MS, Eng ML, Williams TD, Basu N, Elliott JE (2016) Acute embryotoxic effects but no long-term reproductive effects of in ovo methylmercury exposure in zebra finches (Taeniopygia guttata). Environ Toxicol Chem 35:1534–1540. doi:10.1002/etc.3307
Yu MS, Eng ML, Williams TD, Guigueno MF, Elliott JE (2017) Assessment of neuroanatomical and behavioural effects of in ovo methylmercury exposure in zebra finches (Taeniopygia guttata). Neurotoxicology 59:33–39. doi:10.1016/j.neuro.2017.01.001
Acknowledgments
This work was supported by National Science Foundation (IOS-1257590) as well as the American Ornithologists’ Union, Virginia Academy of Science, Williamsburg Bird Club, and College of William and Mary Graduate School. An early draft of this manuscript was sent to many active researchers in the field for their feedback, and we thank J. Ackerman, R. Brasso, B. Braune, A. Condon, T. and C. Custer, C. Henny, G. Heinz, A. Jackson, K. Kenow, J. Rutkiewicz, and C. Seewagen for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Whitney, M.C., Cristol, D.A. (2017). Impacts of Sublethal Mercury Exposure on Birds: A Detailed Review. In: de Voogt, P. (eds) Reviews of Environmental Contamination and Toxicology Volume 244. Reviews of Environmental Contamination and Toxicology, vol 244. Springer, Cham. https://doi.org/10.1007/398_2017_4
Download citation
DOI: https://doi.org/10.1007/398_2017_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-66874-1
Online ISBN: 978-3-319-66875-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)