Abstract
Although songbirds accumulate mercury at rates equivalent to better-studied aquatic avian species, effects of mercury bioaccumulation in songbirds remain understudied. Little is known about the effects of mercury on endocrine physiology, but recent evidence indicates that mercury may disrupt the function of the hypothalamic–pituitary–adrenal axis. Both field-based correlational studies and a recent dosing experiment suggest that mercury exposure alters levels of the primary avian stress hormone, CORT. We sampled zebra finches that had been dosed with 0, 0.5, or 1.0 ppm dietary methylmercury for baseline CORT twice; once during pairing and once after successfully fledging young. Circulating levels of CORT were not significantly affected by mercury exposure. However, our findings indicate potentially important differences in CORT responses between the sexes when exposed to environmentally relevant doses of mercury across the nesting cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury is a ubiquitous and increasing pollutant with well documented effects on the physiology of fish-eating and aquatic birds (Hoffman et al. 2005). Mounting evidence suggests that mercury exposure may be associated with reduced reproductive output in terrestrial songbirds (Brasso and Cristol 2008; Hallinger and Cristol 2011; Varian-Ramos et al. 2014); however, the mechanisms behind observed reproductive impairment remain to be fully described. Abnormal steroidogenic activity has been associated with mercury exposure in both mammals (Burton and Meikle 1980; Friedmann et al. 1998) and fish (Hontela et al. 1992; Leblond and Hontela 1999). However, few studies have investigated the endocrine effects of mercury exposure in birds. Inorganic mercury accumulates in the pituitary gland in primates (Cornett et al. 1998; Vahter et al. 1995). If mercury accumulates similarly in the avian pituitary, exposure may result in compromised function and abnormal synthesis and/or release of endocrine secretions such as corticosterone (hereafter CORT). The primary function of CORT is to facilitate glucose release during fasting and under challenging situations (Schoech et al. 2008). Mercury may act as a physiological stressor (Adams et al. 2009), resulting in an increase in baseline CORT concentrations. Chronic elevations of CORT are associated with the delay or complete arrest of physiological and behavioral reproductive activity (Schoech et al. 2008). Thus, mercury may have the potential to deregulate the hypothalamic–pituitary–adrenal (HPA) axis, inhibiting successful reproduction.
Conflicting reports have been published from field-based studies of CORT in mercury-exposed birds. Wada et al. (2009) documented reduced responsiveness of the HPA axis in 13–17 day-old tree swallow (Tachycineta bicolor) nestlings at a mercury-contaminated site in Virginia. Late-stage nestlings in the contaminated area had baseline CORT levels 103 % greater and stress-induced levels 27 % lower than nestlings of the same age from reference sites (Wada et al. 2009). Contrary to results obtained from nestlings in Virginia, both adult and nestling tree swallows in mercury-contaminated sites in Massachusetts showed a significant negative relationship between blood mercury level and baseline CORT (Franceschini et al. 2009). Similarly, Herring et al. (2012) measured baseline CORT in recently hatched (<3 days) and older (15–37 days) Forster’s terns (Sterna fosteri). Across the range of feather mercury concentrations in recently hatched chicks, there was an 81 % reduction in fecal CORT concentrations. Observed blood mercury concentrations of the older chicks were correlated with a 45 % reduction in CORT concentrations. As each of the aforementioned studies was conducted in the field, researchers were unable to determine the exact length or degree of mercury exposure. CORT concentrations exhibit significant diurnal fluctuations and levels change rapidly with the onset of stressors such as adverse weather, prolonged fasting, predator avoidance, and territorial disputes. Therefore, carefully controlled captive dosing studies are needed to better understand how exposure affects circulating hormone concentrations in wild passerines. One such study has recently been published (Moore et al. 2014) and indicates that while baseline CORT was not significantly altered in domestic zebra finches (Taeniopygia guttata) nesting in captivity, there was a trend towards an increase in baseline CORT and a significant reduction in the expected stress-response of increased CORT.
The purpose of our study was to investigate the relationship between mercury exposure, baseline (i.e., resting) CORT concentration, and reproductive condition in a model avian species, the zebra finch. Our captive study allowed for the control of dose and duration of mercury exposure, and enabled us to control subject age and most environmental variables of the housing facility. We dosed adult zebra finches with two environmentally relevant levels of dietary methylmercury and sampled for baseline CORT at two intervals during the breeding cycle. If mercury acts as a physiological stressor and increases baseline CORT, disruption of the HPA axis may be a mechanism behind the well-documented mercury-associated reproductive impairments in birds.
Materials and Methods
Zebra finches were housed in an indoor aviary at The College of William and Mary in Williamsburg, VA. Birds were assigned to one of three rooms according to treatment group; control, 0.5 or 1.0 ppm methylmercury. Twenty birds were assigned as controls and each of the treatment groups had 28 birds. Finches were given mercury-dosed food (ad libitum), which was prepared by adding an aqueous solution of methylmercury equal to 10 % of the weight of the food to obtain the appropriate concentration. The methylmercury solution was thoroughly homogenized with finch feed (ZuPreem Avian Maintenance Fruitblend Premium, Mission, KS, USA) – using a large tumbler. Food dose was confirmed to be within 10 % of the nominal dose for each batch by testing mercury concentration on a Direct Mercury Analyzer-80 (Milestone, Shelton, CT, USA).
For the first 10 weeks of dosing, birds were housed in single sex cages. Each room was maintained at 21 ± 2°C and a light:dark photoperiod of 14:10 h. Birds were sampled biweekly to monitor increases in blood mercury concentration. Blood samples were collected from the cutaneous ulnar vein, which was pricked using a 30-gauge needle. Approximately 50 μL of blood was collected from each bird in a 70 μL heparinized capillary tube and frozen until analysis.
Upon plateau of blood mercury levels at week 10, birds were randomly paired within treatment group. Breeding pairs were allowed to produce at least two clutches. The first clutch of eggs was collected for another study and the offspring from the second clutch were raised until the late fledging stage. Birds that failed to produce offspring from the second clutch were allowed to re-nest until successful.
All blood samples were analyzed for mercury using a Direct Mercury Analyzer. Average method detection limit over the study period was 0.0033–0.0042 μg/g. The DMA-80 was calibrated regularly throughout the experiment to ensure a standard reference material value within 7.5 % of certified values. A sample blank (no sample or container), methods blank (empty container), and two samples of standard reference material (DORM-3 and DOLT-4) were run before and after each batch of approximately 20 samples. Recovery of total mercury was 102.6 % ± 4.91 % for DORM-3 and 99.6 % ± 3.85 % for DOLT-4. Relative percent difference between 24 duplicate blood samples was 16.1 % ± 16.0 %.
After a 3-day mate acclimation period, birds were sampled for baseline CORT. The 3-day interval before sampling was intended to avoid effects of changes in HPA axis activity associated with pair bonding (Remage-Healey et al. 2003). Birds experienced no prior disturbance on the day that samples were taken, ensuring that the resting basal or baseline CORT was not artificially elevated. Two pairs from both sides of each treatment room were sampled for 3 consecutive days, with the final 2 pairs sampled on day 4. After the collection of pre-nesting baseline serum CORT, pairs were allowed to breed for 12–25 weeks. This time period was dependent on the number of weeks pairs needed to successfully raise offspring until independence. To control for the effect of breeding stage on stress physiology, the second baseline CORT sample was taken while offspring were in the late fledgling stage (~50 days).
Each bird was sampled within 140 s of the onset of disturbance (entrance to housing room) to minimize any disturbance-related changes in circulating CORT. Sampling began 3 h after the start of the light phase, as basal CORT in a passerine bird peaks prior to dawn, decreases with the onset of activity, and stabilizes for several hours (Breuner et al. 1999). All samples were completed between 0800 and 0830. Blood samples were collected as previously described for measurement of blood mercury concentration.
Upon sample completion, whole heparinized blood was stored on ice until returned to the laboratory, where it was centrifuged at 9,000g to separate plasma from cells. Plasma was frozen at −80°C until analysis. CORT 125I-double antibody radioimmunoassay (RIA) kits (MP Biomedicals, Salon, OH, USA) were used to measure serum CORT. The manufacturer’s protocol was followed, with the exception that 10 μL serum samples were diluted in 220 μL of steroid diluent rather than 300 μL in order to bring the sample hormone concentration into the range of the standard curve. All kit standards (range 25–1,000 ng/mL), samples, and controls were run in duplicate. Two controls (provided by the manufacturer, Control I = 63–93 ng/mL, Control II = 440–668 ng/mL) with known concentrations were used as a check on reliability criteria. For each assay, a standard curve was created using the logit of the B/B0 of the standards versus the log concentration of the standards, from which sample CORT concentrations were determined. Radioimmunoassay kit was validated using zebra finch plasma, as well as the plasma of another passerine, the European Starling Sturnus vilgaris. Validation was confirmed by demonstrating that the slope of the logit/log-transformed serial dilution of authentic corticosterone (Sigma-Alrich; chromatographed and recrystallized) reconstituted in a pool of baseline finch plasma was not significantly different from the standard curve. Intra-assay variation was calculated as the average coefficient of variation of sample duplicates within each assay. Inter-assay variation was calculated as the average coefficient of variation of Control I and II across all assays. Intra- and inter-assay coefficients of variation were 3.0 % and 21.3 %, respectively. The manufacturer reports 0.34 % cross-reactivity for deoxycorticosterone and 0.10 % for testosterone. All other reported steroid cross-reactivity is below 0.05 %.
All values for zebra finch plasma were at or above the assay limit of detection, which was determined using an assay in which a charcoal-stripped zebra finch plasma pool was spiked with a serial dilution (25.0–2.5 ng/mL) of authentic corticosterone. The lowest spiked sample that was shown to be different than the 0 ng/mL standard provided by the kit manufacturer was the 5.0 ng/mL duplicate sample. We elected to use a more conservative estimate, which was the value obtained by subtracting 2 times the standard deviation of the 0 tube duplicates from the mean value of the 0 tube (limit of detection 5.7 ng/mL).
Linear regression analysis was used to determine the relationships between time since initial disturbance and CORT, as well as the relationship between blood mercury and CORT. CORT levels were modeled using a general linear model with repeated measures where the samples at pairing and during the fledgling period were the two measures and individual sex, mercury treatment level, and their interaction were fixed effects. We used IBM SPSS (version 19.0, IBM Corp, Armonk, NY, USA) statistical software for all analyses.
Results and Discussion
CORT did not significantly increase with increasing time since onset of disturbance for pre-nesting (n = 49, R2 = 0.005, p = 0.624) or post-nesting birds (n = 49, R2 = 0.065, p = 0.077). All baseline CORT samples were completed within 140 s of the onset of disturbance. No relationship was detected between blood mercury and circulating baseline CORT levels in pre-nesting birds (n = 49, R2 = 0.009, p = 0.516) or post-nesting birds (n = 49, R2 = 0.000, p = 0.900). To control for the effect of reproductive condition on the breeding baseline CORT concentration, unsuccessful breeding pairs (n = 4) were eliminated from analyses.
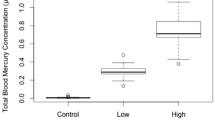
There were no statistically significant effects of mercury treatment group (F2, 43 = 1.261, p = 0.294), sex (F1, 43 = 0.742, p = 0.394), or the interaction of sex-by-treatment (F2, 43 = 0.229, p = 0.796) on the overall baseline CORT (Fig. 1). There was no effect of mercury treatment (F2, 43 = 1.089, p = 0.346) or sex (F1, 43 = 1.498, p = 0.288) on change in baseline CORT over the breeding cycle (Fig. 2). There was a significant interaction of sex-by-treatment (F2, 43 = 3.841, p = 0.029; Fig. 2) on the change in CORT between the two samples, indicating that the change in baseline CORT between the beginning and end of the breeding cycle was not the same for males and females. This was largely driven by a decrease in CORT in males on the 0.5 ppm treatment across the breeding cycle and contrasting increase in CORT in females on the 0.5 ppm treatment over the same period (Fig. 3).
Our results indicate that mercury did not drastically disrupt HPA axis function. No statistically significant relationship between mercury treatment group and baseline CORT was found for pre-nesting birds, post-nesting birds, or over the course of the breeding cycle.
Previous studies of basal CORT in mercury-exposed wild songbirds have reported conflicting effects of mercury exposure. Although results from the current study do not indicate that mercury exposure affected baseline CORT, several confounding factors should be considered. Both doses of mercury were environmentally relevant, particularly the low dose, but it is possible that exposure was not of long enough duration to produce statistically significant effects. As the organizational and activational pathways are established early in embryonic development, mercury may exert effects on the HPA axis only when birds are exposed in ovo. Wada et al. (2009) showed significant effects of mercury exposure on both basal and stress-induced CORT levels in developmentally exposed nestling tree swallows. Birds in the current study were dosed as adults, which raises the possibility that endocrine pathways that may be susceptible during development are more resistant in adults. In a recent study on captive zebra finches, adults exposed to dietary mercury from the embryonic stage to adulthood showed a statistically significant suppression of the stress response (Moore et al. 2014). Adults also had a minimal or nonexistent elevation of baseline CORT (Moore et al. 2014), a finding consistent with the present results.
Among low-dose birds there was a significant interaction between sex and mercury treatment across the breeding period, such that baseline CORT declined across the breeding cycle in males and increased in females (Figs. 2, 3). This sex-based difference in response to an environmentally relevant concentration of mercury suggests the need for further investigation. The lack of a similar response in high dose birds could be a non-linear effect of mercury, similar to responses to mercury observed in foraging behavior and hatching success (Adams and Frederick 2008; Heinz et al. 2011). The multiple biological processes involved in glucocorticoid regulation may also be targets for alteration by mercury, each with potentially variable thresholds; thus complex responses are unsurprising (Adams et al. 2009). Mercury has the potential to act via many mechanisms, including action as a hormone agonist or antagonist, alteration of hormone production at the endocrine source, interference with the release of stimulatory or inhibitory hormones from the pituitary or hypothalamus, alteration of biosynthetic pathways via disturbance of enzyme activity, and/or the disruption of serum-binding proteins (Guillette et al. 2000). Presently, there is insufficient data to determine the mechanisms behind the observed sex-based patterns in low-dose finches.
The present study is the first to our knowledge in which measurements of basal CORT have been made in a mercury-exposed songbird at two distinct intervals in the breeding cycle. Sampling birds at both pre-and post-nesting stages allowed the potential change in baseline CORT with increasing duration of mercury exposure to be quantified. There are multiple pathways by which mercury may interfere with endocrine processes, some of which may be synergistic and therefore, difficult to determine without further study. It is likely that mercury affects avian reproductive physiology and existing research indicates that endocrine function may be compromised at exposure levels lower than levels currently known to cause adverse effects in this species (Moore et al. 2014). Research that involves careful monitoring of hormone levels in mercury-dosed individuals and the establishment of a threshold level of environmental mercury exposure to protect wild passerines from endocrine disruption is a priority.
References
Adams EM, Frederick PC (2008) Effects of methylmercury and spatial complexity on foraging behavior and foraging efficiency in juvenile white ibises (Eudocimus albus). Environ Toxicol Chem 27:1708–1712. doi:10.1897/07-466.1
Adams EM, Frederick PC, Larkin ILV, Guillette LJ Jr (2009) Sublethal effects of methylmercury on fecal metabolites of testosterone, estradiol, and corticosterone in captive juvenile white ibises (Eudocimus albus). Environ Toxicol Chem 28:982–989. doi:10.1897/08-253.1
Brasso RL, Cristol DA (2008) Effects of mercury exposure on the reproductive success of tree swallows (Tachycineta bicolor). Ecotoxicology 17:133–141. doi:10.1007/s10646-007-0163-z
Breuner CW, Wingfield JC, Romero LM (1999) Diel rhythms of basal and stress-induced corticosterone in a wild, seasonal vertebrate, Gambel’s white-crowned sparrow. J Exp Zool 284:334–342
Burton GV, Meikle AW (1980) Acute and chronic methyl mercury poisoning impairs rat adrenal and testicular function. J Toxicol Environ Health 6:597–606. doi:10.1080/15287398009529877
Cornett CR, Ehmann WD, Wekstein DR, Markesbery WR (1998) Trace elements in Alzheimer’s disease pituitary glands. Biol Trace Elem Res 62:107–114. doi:10.1007/BF02820026
Franceschini MD, Lane OP, Evers DC, Reed JM, Hoskins B, Romero LM (2009) The corticosterone stress response and mercury contamination in free-living tree swallows, Tachycineta bicolor. Ecotoxicology 18:514–521. doi:10.1007/s10646-009-0309-2
Friedmann AS, Chen H, Rabuck LD, Zirkin BR (1998) Accumulation of dietary methylmercury in the testes of the adult brown Norway rat: impaired testicular and epididymal function. Environ Toxicol Chem 17:867–871. doi:10.1002/etc.5620170514
Guillette LJ, Crain DA, Gunderson MP, Kools SAE, Milnes MR, Orlando EF, Rooney AA, Woodward AR (2000) Alligators and endocrine disrupting contaminants: a current perspective. Am Zool 40:438–452. doi:10.1093/icb/40.3.438
Hallinger KK, Cristol DA (2011) The role of weather in mediating the effect of mercury exposure on reproductive success of tree swallows. Ecotoxicology 20:1368–1377. doi:10.1007/s10646-011-0694-1
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA (2011) Hormesis associated with a low dose of methylmercury injected into mallard eggs. Arch Environ Contam Toxicol 62:141–144. doi:10.1007/s00244-011-9680-0
Herring G, Ackerman JT, Herzog MP (2012) Mercury exposure may suppress baseline corticosterone levels in juvenile birds. Environ Sci Technol 46:6339–6346. doi:10.1021/es300668c
Hoffman DJ, Spalding MG, Frederick PC (2005) Subchronic effects of methylmercury on plasma and organ biochemistries in great egret nestlings. Environ Toxicol Chem 24:3078–3084. doi:10.1897/04-570.1
Hontela A, Rasmussen JB, Audet C, Chevalier G (1992) Impaired cortisol stress response in fish from environments polluted by PAHs, PCBs, and mercury. Arch Environ Contam Toxicol 22:278–283
Leblond VS, Hontela A (1999) Effects of in vitro exposures to cadmium, mercury, zinc, and 1-(2-chlorophenyl)-1-(4-chlorophenyl)-2,2-dichloroethane on steroidogenesis by dispersed interrenal cells of rainbow trout (Oncorhynchus mykiss). Toxicol Appl Pharmacol 157:16–22. doi:10.1006/taap.1999.8660
Moore CS, Cristol DA, Maddux SL, Varian-Ramos CW, Bradley EL (2014) Lifelong exposure to methylmercury disrupts stress-induced corticosterone response in zebra finches (Taeniopygia guttata). Environ Toxicol Chem 33:1072–1076. doi:10.1002/etc.2521
Remage-Healey L, Adkins-Regan E, Romero LM (2003) Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Horm Behav 43:108–114. doi:10.1016/S0018-506X(02)00012-0
Schoech SJ, Rensel MA, Bridge ES, Boughton RK, Wilcoxen TE (2008) Environment, glucocorticoids, and the timing of reproduction. Gen Comp Endocrinol 163:201–207. doi:10.1016/j.ygcen.2008.09.009
Vahter ME, Mottet NK, Friberg LT, Lind SB, Charleston JS, Burbacher TM (1995) Demethylation of methyl mercury in different brain sites of Maccaca-fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol Appl Pharmacol 134:273–284. doi:10.1006/taap.1995.1193
Varian-Ramos CW, Swaddle JP, Cristol DA (2014) Mercury reduces avian reproductive success and imposes selection: an experimental study with adult- or lifetime-exposure in zebra finch. PLoS One 9:e95674. doi:10.1371/journal.pone.0095674
Wada H, Cristol DA, McNabb FMA, Hopkins WA (2009) Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol 43:6031–6038. doi:10.1021/es803707f
Acknowledgments
Funding for this research was provided by a grant from E.I. DuPont de Nemours and Company and the Williamsburg Bird Club. Research was completed with oversight from the South River Science Team, which is a collaboration of state and federal agencies, academic institutions, and environmental interests. We thank Matthias Leu, John Swaddle, Margaret Whitney, Catherine Lewis, Kenton Buck, Amanda Bessler, Stephanie Apple, Joanna Weeks, and Eliza Spear.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maddux, S.L., Cristol, D.A., Varian-Ramos, C.W. et al. The Effect of Mercury on Baseline Corticosterone in a Breeding Songbird. Bull Environ Contam Toxicol 94, 135–139 (2015). https://doi.org/10.1007/s00128-014-1440-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1440-1