Abstract
We evaluated whether mercury influenced survival of free-ranging American avocet (Recurvirostra americana) and black-necked stilt (Himantopus mexicanus) chicks in San Francisco Bay, California. Using radio telemetry, we radio-marked 158 avocet and 79 stilt chicks at hatching and tracked them daily until their fate was determined. We did not find strong support for an influence of in ovo mercury exposure on chick survival, despite observing a wide range of mercury concentrations in chick down feathers at hatching (0.40–44.31 μg g−1 fw). We estimated that chick survival rates were reduced by ≤3% over the range of observed mercury concentrations during the 28-day period from hatching to fledging. We also salvaged newly-hatched chicks that were found dead during routine nest monitoring. In contrast to the telemetry results, we found that mercury concentrations in down feathers of dead chicks were higher than those in randomly-sampled live chicks of similar age. However, capture site was the most important variable influencing mercury concentrations, followed by year, species, and hatching date. Although laboratory studies have demonstrated negative effects of environmentally relevant mercury concentrations on chick survival, our results concur with the small number of previous field studies that have not been able to detect reduced survival in the wild.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global increase in environmental mercury contamination over the past century has increased the risk of mercury toxicity to wildlife (Appelquist et al. 1985; U. S. EPA 1997; United Nations Environment Programme 2003). Aquatic birds are particularly susceptible because of their use of habitats where methylmercury is produced (Wiener et al. 2003a). Methylmercury is the most toxic and biologically available form of mercury and is known to have detrimental effects on birds (reviews by Scheuhammer 1987; Thompson 1996; Wolfe et al. 1998; Wiener et al. 2003a). Avian reproduction is one of the most sensitive endpoints of mercury toxicity and relatively low methylmercury exposure can reduce reproductive success (Scheuhammer et al. 1987; Thompson 1996; Wolfe et al. 1998; Wiener et al. 2003a).
Several laboratory egg-injection and controlled-feeding studies have demonstrated that environmentally relevant mercury exposure reduces egg hatchability and duckling survival (Heinz 1974; Finley and Stendell 1978; Heinz and Hoffman 1998; Heinz et al. 2006). Other laboratory studies have shown that at lower doses the neurotoxic effects of methylmercury can be manifested as subtle alterations in chick behavior, such as reduced responsiveness to parental calls and impaired movement (Heinz 1975, 1979). Such sub-lethal effects can make detecting effects of mercury toxicity on wild chicks difficult because behavioral abnormalities are particularly hard to quantify. Although lower concentrations of methylmercury may not result in overt chick mortality, behavioral impairment can affect survival by reducing a chick’s ability to forage effectively (Bouton et al. 1999; Spalding et al. 2000a) or avoid predators (Heinz 1975). Nonetheless, few field studies have been able to detect effects of mercury on chick survival in the wild (Meyer et al. 1998; Sepúlveda et al. 1999; Merrill et al. 2005).
Our objectives were to examine factors influencing mercury concentrations in shorebird chicks hatched in San Francisco Bay and, secondly, to determine if these environmental mercury concentrations impaired survival of free-ranging chicks from hatching to fledging. We used radio telemetry to estimate chick survival in relation to mercury concentrations in down feathers, which functioned as an index of a chick’s whole-body burden of mercury at hatching (Becker et al. 1993). To our knowledge, only a single previous study has examined the effect of mercury on survival of free-ranging chicks using telemetry (Sepúlveda et al. 1999), yet telemetry can be a superior methodology for estimating chick survival (Samuel and Fuller 1996; Winterstein et al. 2001). We further examined chick mortality related to mercury exposure by comparing mercury concentrations in chicks that were found dead to those in randomly-sampled live chicks of similar age.
We studied American avocet (Recurvirostra americana, hereafter avocet) and black-necked stilt (Himantopus mexicanus, hereafter stilt) chicks because they are the two most abundant breeding shorebirds in San Francisco Bay and the estuary supports the largest breeding populations of these species on the Pacific Coast (Stenzel et al. 2002; Rintoul et al. 2003). Moreover, both species have precocial offspring (Robinson et al. 1997, 1999) which may be more likely to exhibit the deleterious effects of methylmercury due to subtle behavioral impairments than those chicks of altricial species which are dependent on their parents early in life. Finally, these birds forage and nest in shallow-water wetland habitats along the Bay’s margins where methlymercury production is relatively high (Marvin-DiPasquale et al. 2003). San Francisco Bay is highly contaminated with mercury due to historic mining activities for both mercury and gold (reviews by Davis et al. 2003, Wiener et al. 2003b). Although this pollution is thought to reduce waterbird reproductive success within the estuary, few studies have investigated the influence of mercury on reproductive success within San Francisco Bay (Hothem et al. 1995; Schwarzbach et al. 2006).
Methods
Study site
We studied mercury concentrations and survival of avocet and stilt chicks in South San Francisco Bay (37.4° N, 122.0° W) where large numbers of shorebirds breed on islands and levees of former salt evaporation ponds or within managed marshes (Stenzel et al. 2002; Rintoul et al. 2003). Our study site was located at the Don Edwards San Francisco Bay National Wildlife Refuge and included former salt evaporation ponds and marshes in the eastern Alviso salt pond complex (A8, A16, Coyote Creek Lagoon, and New Chicago Marsh), Moffett salt pond complex (AB1 and AB2), and Newark salt pond complex (N4, N7, N8, and N9). Salt pond habitats were typically devoid of vegetation, except along pond edges, whereas managed marshes were dominated by pickleweed (Sarcocornia pacifica, formerly Salicornia virginica).
Capture and radio-marking chicks
We determined survival rates of avocet and stilt chicks using radio telemetry. We hand-captured avocet and stilt chicks at hatching either at nests under observation or near known nesting sites. We monitored nests weekly and floated eggs (Hays and LeCroy 1971; Alberico 1995) at each visit to determine incubation stage, and then returned to nests at their estimated hatching date to radio-mark chicks. We only radio-marked chicks that were ≤2 days old, as determined from multiple clues including presence of an egg tooth, eggshell membranes attached to feathers, the chick being found in or near the nest bowl, and chick size (<25 g for avocets and <17 g for stilts; Robinson et al. 1997, 1999). To reduce any potential for pseudoreplication, we radio-marked only one chick per clutch since mortality is often correlated within broods (Ball et al. 1975). If there were >1 hatched chicks in a brood, then we randomly selected one chick for radio-marking. We weighed chicks with a spring scale (±1.0 g with a 100-g Pesola® spring scale, Pesola Ag, Baar, Switzerland), and we measured exposed culmen length and short tarsus length with digital calipers (±0.01 mm with Fowler® electronic digital calipers, Newton, Massachusetts, USA) and flattened wing chord length with a wing board (±1.0 mm). We collected 10–15 downy feathers from the rump and mantle of each radio-marked chick for subsequent mercury analysis. We stored feathers in individually labeled Whirl-paks® (Nasco, Modesto, California, USA) until laboratory analysis.
In 2005, we used radio transmitters containing thermistor switches (Model BD-2T, Holohil Systems Ltd., Carp, Ontario, Canada (48 avocets and 29 stilts)) or without thermistor switches (Model A2410 modified, Advanced Telemetry Systems Inc., Isanti, Minnesota, USA (22 avocets and 4 stilts)). In 2006, we used only the Holohil radio transmitters that contained thermistor switches (88 avocets and 46 stilts). Transmitters with thermistor switches (89% of chicks) improved our ability to determine chick mortality because an increase or decrease in temperature given off by the chick resulted in a corresponding increase or decrease in pulse rate. The pulse rate increased from about 30 pulses per minute at 0°C to about 45 pulses per minute at 40°C. We attached radio transmitters to the dorsal midline of chicks with sutures (Ethicon® Vicryl FS-2, 3–0, Ethicon Inc., Piscataway, New Jersey, USA) through front and rear channels, and a third suture was tied in the middle and over the top of the transmitter. Each suture was tied with 2–3 knots and secured with super-glue (Loctite 422, Henkel Corp., Rocky Hill, Connecticut, USA). We used very small transmitters to reduce the potential for transmitter effects on behavior or survival (Ackerman et al. 2004). Transmitters weighed 1.1 g for avocets and 0.8 g for stilts (<6% of chick body mass), were 19 mm long × 7 mm wide (Holohil Systems Ltd.) or 18 mm long × 8 mm wide (Advanced Telemetry Systems Inc.) for avocets and 16 mm long × 6 mm wide (Holohil Systems Ltd.) or 19 mm long × 6 mm wide (Advanced Telemetry Systems Inc.) for stilts, and had a 12-cm external whip antenna. Immediately after attaching transmitters, we returned radio-marked chicks and their siblings to their nest sites or to the site of capture; radio-marked chicks were never recaptured and handled after their release.
Radio-tracking chicks
We tracked radio-marked chicks from trucks equipped with dual 4-element Yagi null-peak antenna systems (AVM Instrument Co., Livermore, California, USA), using triangulation to determine locations (e.g., Takekawa et al. 2002, Ackerman et al. 2006). We located chicks daily from the time of radio attachment until their fate (depredated, dead, fledged, or dropped transmitter) was determined. We used triangulation software (Location of a Signal, version 3.0.1, Ecological Software Solutions, Schwägalpstrasse 2, 9107 Urnäsch, Switzerland) to calculate UTM coordinates for each location and verified chick locations with visual observations (44% in 2005 and 23% in 2006) whenever possible without disturbing the chick. We tracked during the cool morning and evening hours to help differentiate the chick’s temperature from the ambient temperature and determine mortality status. Chicks that went missing were searched for daily until found (e.g., typically carried away by avian predators) or until the transmitter was estimated to have quit working (about 32 days for stilts and 40 days for avocets). If we thought the chick had died, we used hand-held Yagi antenna systems and receivers to find the transmitter and chick within 24 h.
Dead chicks
In addition to sampling downy feathers from newly-hatched live chicks, we also sampled down feathers from salvaged chicks that were found dead near nesting sites during routine nest monitoring activities. We measured the structural size of dead chicks as described above, and used culmen length as an index of age (Reed et al. 1999). We only included dead chicks that were considered to have recently hatched based on an exposed culmen length of <21.2 mm for avocet chicks and <17.6 mm for stilt chicks; these culmen lengths corresponded to the maximum measurements we observed for newly-hatched live chicks (described above). We collected about 15 downy feathers from the rump and mantle of dead chicks for mercury analysis. We captured, collected, and marked birds under California Department of Fish and Game Scientific Collection permit SC-801034-05, Federal U. S. Fish and Wildlife Service permit MB102896, and U. S. Geological Survey Bird Banding Laboratory permit 22911, and we conducted research under the guidelines of the U. S. Geological Survey, Western Ecological Research Center, Animal Care and Use Committee.
Mercury analysis
Previous research has demonstrated that >95% of mercury in avian feathers is methylmercury (Thompson and Furness 1989; Fournier et al. 2002; Evers et al. 2005; Rimmer et al. 2005). Thus, we analyzed all feather samples for total mercury. We washed each feather in a 1% Alconox solution (Alconox Inc., White Plains, New York, USA) while mechanically scrubbing each feather to remove surface debris. We then dried feathers at 60°C for 24–48 h, weighed them to the nearest 0.01 mg (Mettler Toledo, Model AT201, Greifensee, Switzerland) and transferred each feather into a quartz sample vessel. Following EPA Method 7473 (U. S. EPA 2000), we analyzed each sample for total mercury at the U. S. Geological Survey, Davis Field Station Mercury Lab on a Milestone DMA-80 Direct Mercury Analyzer (Milestone Inc., Monroe, Connecticut, USA) using an integrated sequence of drying (300°C for 60 s), thermal decomposition (850°C for 180 s), catalytic conversion, and then amalgamation, followed by atomic absorption spectroscopy. Prior to analysis, we calibrated the analyzer with dilutions of a certified mercury standard solution (SPEX CertiPrep, Metuchen, New Jersey, USA). Quality assurance measures included analysis of two certified reference materials (either dogfish muscle tissue (DORM-2; National Research Council of Canada, Ottawa, Canada), dogfish liver (DOLT-3; National Research Council of Canada, Ottawa, Canada), or lobster hepatopancreas (TORT-2; National Research Council of Canada, Ottawa, Canada)), two system and method blanks, two duplicates, one matrix spike, and one matrix spike duplicate per batch. Recoveries averaged 108.7 ± 2.83% (N = 34) and 103.2 ± 2.1% (N = 51) for certified reference materials and calibration checks, respectively. Matrix spike recoveries averaged 99.9 ± 1.3% (N = 34). Absolute relative percent difference for all duplicates and matrix spike duplicates averaged 6.4 ± 1.9%.
Statistical analysis
We conducted a three-tiered analysis to examine (1) factors influencing mercury concentrations in chick down feathers, (2) whether mercury concentrations in chicks at hatching influenced subsequent survival rates, and (3) whether mercury concentrations in dead newly-hatched chicks were higher than live newly-hatched chicks. For each analysis, we used Akaike’s Information Criterion (AIC) to select the best model from an a priori set of candidate models. AIC often performs better than restricting the selected model to those variables with statistically significant effects in hypothesis-based tests, particularly for observational data (Burnham and Anderson 1998; Anderson et al. 2000).
For the first analysis, we built a set of 15 candidate models based on potential effects of species, capture site, year, and Julian hatching date, and included a sixteenth null model (intercept and variance only) with no effects, to examine factors influencing mercury concentrations in chicks (Table 1). We calculated and compared AIC values for candidate models using ANOVA or ANCOVA with JMP® version 4.0.4 (Sall et al. 2001). We loge-transformed mercury concentrations (fresh weight, hereafter fw) to improve normality. Because our study was conducted over two years on two species, we standardized Julian hatching date for year and species using z-scores. Z-scores were calculated for each species and year by taking the difference between each individual chick’s hatching date and the mean hatching date for that species and year, and dividing that quantity by the standard deviation for that species and year. Thus, results for date should be interpreted as relative hatching date. We used six categories for the site variable including four distinct ponds (A8, A16, New Chicago Marsh, and Coyote Creek Lagoon) and two areas with multiple adjacent ponds with similar habitats that were pooled due to low sample sizes of nesting birds in these areas (Newark Ponds (includes N4, N7, N8, and N9) and Moffett Ponds (includes AB1 and AB2)). Although statistical analyses were performed using transformed data, we graphically present untransformed mercury concentrations and Julian hatching date data for easier interpretation.
For the second analysis, we examined whether mercury concentrations at hatching influenced subsequent chick survival using the known fates modeling procedure in Program MARK (White and Burnham 1999). We had two primary objectives in our survival analysis: (1) to determine whether mercury concentration had a negative effect on chick survival probability and (2) to evaluate how strong a predictor mercury concentration was for survival probability in comparison to the other variables described above (species, capture site, year, and Julian hatching date). We also included a body mass condition index in the survival analysis because it is thought to influence chick survival rates (Pelayo and Clark 2003; Traylor and Alisauskas 2006).
To calculate the body mass condition index at hatching, we controlled for the effects of structural body size (also an index of chick age) on body mass by calculating the residuals from a regression of chick body mass on the first principal component (PC) of three structural measurements (i.e., exposed culmen, short tarsus, and flattened wing chord lengths). In 2005, PC 1 accounted for 82.5%, 81.6%, and 75.2% of the variance in exposed culmen, flattened wing chord, and short tarsus, respectively, for avocets and 90.7%, 57.8%, and 94.0% of the variance for the same variables, respectively, for stilts. In 2006, PC 1 accounted for 83.4%, 71.2%, and 72.5% of the variance in exposed culmen, flattened wing chord, and short tarsus, respectively, for avocets and 85.6%, 67.1%, and 86.5% for stilts.
We restricted our analysis to a 28-day post-hatch period, as the transmitters were designed to transmit for about 32 days (stilts) and some radios had failed or been shed by that time. Chicks with radios that failed earlier were censored and we assumed that censored chicks had the same survival probability as uncensored chicks. Additionally, we used only four categories for the site variable (A8, A16, New Chicago Marsh, and Newark Ponds) in the survival analysis, and excluded chicks from Moffett Ponds (N = 1 avocet) and pooled the Coyote Creek Lagoon (N = 3 avocet and N = 2 stilt) chicks with A16 due to their close proximity and low nesting densities.
In order to limit the survival analysis candidate set to a reasonable number, we first determined the appropriate time-dependency to incorporate into our model structure. We compared three basic models wherein (1) daily survival probability differed for each of the 27 encounter opportunities (Null + t daily), (2) daily survival was constrained such that survival probability during intervals 1–7 (week1), 8–14 (week2), 15–21 (week3), and 22–27 (week4) were equivalent (Null + t weekly), or (3) daily survival was constrained to be constant throughout the study (Null + t constant). In all models, the effect of time was additive (vari + t), meaning that the logit of survival probability varied in parallel over time.
After we determined the appropriate time-dependency, we incorporated it into our candidate set of 64 models based on all possible combinations of mercury concentrations (loge-transformed), species, capture site, year, Julian hatching date (z-score transformed), and a body mass condition index, and included a final null model with no effects (Table 2). Input data were formatted for Program MARK by coding capture site as a group effect, species and year as categorical individual covariates, and mercury concentration, Julian hatching date, and body mass condition index as continuous individual covariates. The data transformations described above ensured that the continuous individual covariates were scaled such that means fell within the interval 0–1 with range −4 to +4, thus allowing numerical optimization of the parameter estimation algorithm.
In the third stage of our analyses, we evaluated whether mercury concentrations in down feathers of newly-hatched chicks found dead were higher than newly-hatched live chicks. For this analysis, we used our primary dataset of randomly-sampled chicks at hatching (i.e., radio-marked chicks) and added the additional chicks that were found dead at hatching. We then used AIC to compare the best fitting model determined by our first analysis (Table 1, model 1) to a model that included these same variables plus a fifth variable based on the chick’s fate (i.e., alive or dead when found). In this analysis, radio-marked chick data was included only when chicks were alive, and those radio-marked chicks that were later found dead were not included in the dead chick data since we did not consider them to be found randomly.
For all AIC analyses, we corrected for sample size by using AICc and considered the model with the smallest AICc to be the most parsimonious (Burnham and Anderson 1998; Anderson et al. 2000). We used the AICc differences between the best model and the other candidate models (ΔAICc i = AICc i −minimum AICc) to determine the relative ranking of each model. We considered candidate models for biological importance when ΔAICc i ≤ 2 (Anderson et al. 2001). We calculated Akaike weights (w i = exp [−ΔAICc i /2]/∑exp [−ΔAICc i /2]) to assess the weight of evidence that the selected model was the actual Kullback-Leibler best model in the set of models considered (Burnham and Anderson 1998; Anderson et al. 2000). We also calculated variable weights by summing Akaike weights across all models that incorporated the same variable to help assess the relative importance of each variable.
Unless noted otherwise, we reported model-based geometric mean ± SE mercury concentrations based on back-transformed least-squares means to indicate the expected mercury concentrations for chicks in the entire South San Francisco Bay. The model-based standard errors of the geometric mean were calculated by the delta method (Williams et al. 2002). We also used model-based geometric mean ± SE concentrations based on back-transformed least-squares means to indicate mercury concentrations for specific sites, years, and species. However, we also presented more traditional (design-based) geometric means (which do not reflect site-wide predicted mercury concentrations due to inherent biases in sampling designs such as different sample sizes between sites) for appropriate comparison to existing literature. In our case, design-based geometric means indicated higher mercury concentrations in chicks than model-based geometric means because the sites with the highest mercury levels also happened to have the largest numbers of nesting avocets and stilts.
Results
We captured and radio-marked 158 avocet and 79 stilt chicks at hatching and sampled their down feathers for mercury concentrations. Of these, 140 avocets and 75 stilts had sufficient encounter histories (i.e., >1 post-release detection) for inclusion in survival analyses. We also collected 16 avocet and 14 stilt chicks that were found dead soon after hatching during routine nest monitoring activities. Geometric mean (design-based) mercury concentrations in down feathers of newly-hatched live chicks were 4.02 ± 0.28 μg g−1 fw for avocets and 9.98 ± 0.77 μg g−1 fw for stilts.
Mercury concentrations in chicks
We found that the most parsimonious model explaining mercury concentrations in chick down was the full model incorporating effects of species, capture site, year, and Julian hatching date (Table 1). The full model had an Akaike weight of 0.98, whereas no other candidate model provided a reasonable fit to the data. Variable weights were similarly high for site (>0.99), species (>0.99), year (>0.99), and Julian hatching date (0.98). To further evaluate the relative importance of each variable, we used evidence ratios to make step-wise comparisons of Akaike weights (e.g., we compared Akaike weights of model number 4 to 3, 4 to 5, and 4 to 2; Table 1). Using this procedure, capture site was the most important variable influencing mercury concentrations in chick down feathers, followed by year, species, and Julian hatching date.
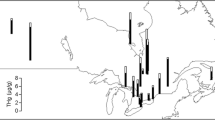
Mercury concentrations in avocet and stilt chick down feathers were highest for chicks hatching in salt pond A8 (7.19 ± 0.87 μg g−1 fw), followed by New Chicago Marsh (6.88 ± 0.58 μg g−1 fw), salt pond A16 (6.02 ± 0.86 μg g−1 fw), Coyote Creek Lagoon (3.37 ± 1.07 μg g−1 fw), Newark salt ponds (2.01 ± 0.38 μg g−1 fw), and Moffett salt ponds (1.85 ± 0.68 μg g−1 fw; Fig. 1). Mercury concentrations also were higher in 2006 (5.50 ± 0.62 μg g−1 fw) than in 2005 (2.82 ± 0.34 μg g−1 fw; Fig. 2) and higher in stilts (5.51 ± 0.83 μg g−1 fw) than in avocets (2.81 ± 0.27 μg g−1 fw; Figs. 1, 2). Furthermore, mercury concentrations in avocet and stilt chicks increased with hatching date (Fig. 3). We estimated that mercury concentrations in chicks at hatching increased by 81 ± 31% over the course of a typical 60-day nesting season.
Box plots depicting total mercury concentrations (μg g−1 fresh weight, hereafter fw) in down feathers of American avocet (filled) and black-necked stilt chicks (unfilled) at hatching differed among sites in South San Francisco Bay, California, USA during spring 2005 and 2006. Sample sizes for avocet and stilt chicks, respectively, were 53 and 1 in A8, 27 and 75 in New Chicago Marsh, 50 and 1 in A16, 3 and 2 in Coyote Creek Lagoon, 21 and 0 in Newark Ponds, and 4 and 0 in Moffett Ponds
Box plots depicting total mercury concentrations (μg g−1 fresh weight, hereafter fw) in down feathers of American avocet (filled) and black-necked stilt chicks (unfilled) at hatching differed among years in South San Francisco Bay, California, USA. Sample sizes were 70 avocet chicks and 33 stilt chicks in 2005 and 88 avocet chicks and 46 stilt chicks in 2006
Total mercury concentrations (μg g−1 fresh weight, hereafter fw) in down feathers of American avocet (circles) and black-necked stilt chicks (triangles) increased with Julian hatching date at the three main study sites in South San Francisco Bay, California, USA during 2005 (unfilled) and 2006 (filled)
Mercury’s effect on chick survival
The first step in our survival analysis was to determine the appropriate time dependency for parameter estimation. The highest ranked model among the three time dependency models we explored indicated that daily survival rate was best approximated by constraining the parameter estimates to weekly survival rates (t weekly: N = 214, K = 4, log-likelihood = −442.59, AICc = 888.21, ΔAICc = 0.00, Akaike weight = 0.94, deviance = 880.18; t daily: N = 214, K = 27, log-likelihood = −433.43, AICc = 893.74, ΔAICc = 5.54, Akaike weight = 0.06, deviance = 838.85; t constant: N = 214, K = 1, log-likelihood = −488.49, AICc = 976.97, ΔAICc = 88.77, Akaike weight = 0.00, deviance = 974.97). In the best fitting model (t weekly), daily survival rate (DSR) was lowest during the first week (DSRWeek1 = 0.862 ± 0.012 [SE]), increased considerably during the second week (DSRWeek2 = 0.962 ± 0.009 [SE]), and then leveled off to nearly 100% during the final two weeks of the 28-day monitoring period (DSRWeek3 = 0.993 ± 0.005 [SE]; DSRWeek4 = 0.993 ± 0.007 [SE]). We therefore incorporated t weekly as an additive effect in each of the 64 candidate models used to evaluate survival in relation to mercury concentration.
We found that the most parsimonious model explaining chick survival included effects of capture site, species, Julian hatching date, and the body mass condition index, and had an Akaike weight of 0.27 (Table 2). However, several other candidate models provided a reasonably good fit to the data. Five models had ΔAICc scores ≤ 2.0, all of which included species, Julian hatching date, and the body mass condition index as explanatory variables, indicating these three variables’ high importance to explaining chick survival. The best fitting model that included mercury concentration as an explanatory variable had an AICc of 2.00 and Akaike weight of 0.10 (Table 2, model 5), and, therefore, was considered for biological importance.
We used variable weights to assess the order of importance for each variable in the candidate set. Species and the body mass condition index had the strongest predictive power, with variable weights of 1.00, and Julian hatching date also was strongly supported (0.97). Capture site and year were included in several, but not all, high ranking models, and had variable weights of 0.54 and 0.43, respectively. Mercury concentration had the lowest predictive power among the six variables evaluated, and had a variable weight of 0.27.
Since mercury concentrations in down feathers at hatching appeared in a model that provided a reasonably good fit to the survival data, we further examined mercury’s potential effect on chick survival using this model’s beta estimates (Table 2, model 5). Mercury concentration’s beta estimate was −0.019 ± 0.130 (SE), indicating a negative relationship between daily survival rate and mercury concentration. However, the standard error was relatively large compared to the beta estimate, indicating the effect of mercury on chick survival was not statistically supported. In contrast, beta estimates for two other continuous covariates in the same model indicated that Julian hatching date (−0.286 ± 0.102 [SE]) and the body mass condition index (0.189 ± 0.053 [SE]) had relatively large effects on chick survival rates; chick survival rates declined with hatching date and increased with residual body mass at hatching. In the best-fitting model (Table 2, model 1), beta values indicated that there were important effects of capture site, species, Julian hatching date, body mass condition index, and time (week) period on daily survival rates.
In order to graphically depict the relationship between mercury concentration and predicted daily survival rate, we utilized the user-specified individual covariate feature in Program MARK. Using the best-fitting model that included mercury concentration as a variable (Table 2, model 5), we input mercury concentrations at the minimum (0.40 μg g−1 fw) and maximum (44.31 μg g−1 fw) observed values in chick down feathers. Mean covariate values were maintained for the other two continuous covariates included in this model (Date = −0.01, Mass = −0.07). We found a 1.5% and 0.6% difference in daily survival rates between the lowest and highest observed mercury concentrations during the first week after hatch for avocets and stilts, respectively (Fig. 4). However, for both species, this difference in survival rates diminished as chicks aged and was absent by the fourth week (0.1% for avocets and 0.0% for stilts). Overall, the cumulative 28-day survival probabilities for avocet and stilt chicks with the lowest observed mercury concentrations were 1.4% and 3.0% higher than for chicks with the highest observed mercury concentrations.
Daily survival rates of American avocet (circles) and black-necked stilt (triangles) chicks increased with chick age, but were relatively unaffected by total mercury concentrations in down feathers at hatching. Shown here are estimated daily survival rates for chicks with down feather mercury concentrations at the range of observed values in South San Francisco Bay (minimum was 0.40 μg g−1 fw (solid line) and maximum was 44.31 μg g−1 fw (dashed line)). Daily survival rates were estimated by radio-marking and tracking 158 avocet and 79 stilt chicks
Mercury concentrations in dead chicks
We compared the most parsimonious model explaining factors influencing mercury concentrations in chick down feathers (Table 1, model 1: Site + Spp + Date + Mass) to a second model incorporating the chick’s fate in addition to these same variables (Site + Spp + Date + Mass + Fate). To do so, we included the 30 additional chicks that were found dead to our main dataset of radio-marked chicks (N = 237, used in analyses one and two above) and included a fate column as either found dead or alive. The model including the chick’s fate was 4.4 times more likely than the model without the mortality information (Site + Spp + Date + Mass + Fate: N = 267, RSS = 135.10, K = 11, log-likelihood = −90.95, AICc = −158.86, ΔAICc = 0.00, Akaike weight = 0.82; Site + Spp + Date + Mass: N = 267, RSS = 137.73, K = 10, log-likelihood = −88.37, AICc = −155.89, ΔAICc = 2.98, Akaike weight = 0.18), indicating that mercury concentrations in down feathers of dead chicks at hatching (5.74 ± 0.89 μg g−1 fw) were higher than levels in randomly-sampled live chicks at hatching (4.15 ± 0.39 μg g−1 fw; Fig. 5).
Box plots depicting total mercury concentrations (μg g−1 fresh weight, hereafter fw) in down feathers of American avocet and black-necked stilt chicks found dead (filled) were higher than concentrations in chicks that were sampled while live (unfilled) just after hatching in South San Francisco Bay, California, USA. Sample sizes were 158 live and 16 dead avocet chicks and 79 live and 14 dead stilt chicks
Discussion
Mercury concentrations in avocet and stilt chick down feathers at hatching were influenced by several factors. Capture site was the most important variable influencing mercury concentrations, but year, species, and hatching date also had important effects on concentrations. Mercury concentrations were highest in the eastern Alviso salt pond complex (ponds A8, A16, and New Chicago Marsh) of the Don Edwards San Francisco Bay National Wildlife Refuge, and lower at Moffett and Newark salt pond sites (Fig. 1). We also observed similar site differences in blood mercury concentrations of adult pre-breeding avocets and stilts in San Francisco Bay (Ackerman et al. 2007b). Site differences are not surprising considering that adult avocets and stilts show strong site-fidelity to ponds during the pre-breeding season (Ackerman et al. 2007b). Presumably, high mercury concentrations in birds nesting within the eastern Alviso salt pond complex are due to their proximity to Alviso Slough, which is at the downstream end of the Guadalupe River watershed and the historic New Almaden mercury mine (Conaway et al. 2004).
Even while controlling for site effects, stilt chicks had higher mercury concentrations in down feathers than avocet chicks (Figs. 1–3). We also observed higher blood mercury concentrations in adult stilts than avocets during the pre-breeding season in San Francisco Bay (Ackerman et al. 2007b). It is unclear why stilts have higher mercury concentrations than avocets, but their exposure to mercury may differ depending on their use of micro-habitats and aquatic prey. Although they are often associated together and forage on similar foods (Hamilton 1975; Robinson et al. 1997, 1999), avocets tend to use salt ponds and tidal flats more often than stilts, whereas stilts tend to use managed marshes more often than avocets (Ackerman et al. 2007b). Moreover, even when foraging in the same pond, avocets tend to use more open water and mudflat habitats whereas stilts use more vegetated areas (Rintoul et al. 2003).
Relative hatching date also influenced mercury levels, though to a smaller degree than site, year, and species. On average, mercury concentrations increased by 81% during the typical 60-day shorebird nesting season (Fig. 3). In contrast, we did not find that date had an important effect on blood mercury concentrations of adult avocets and stilts during the pre-breeding time period in San Francisco Bay (Ackerman et al. 2007b). It is possible that adult dietary exposure to mercury may have increased over the course of the breeding season if methylation rates increased in association with rising ambient temperatures, decreasing dissolved oxygen, and anoxic conditions common during summer (Ullrich et al. 2001; Stamenkovic et al. 2005).
Despite observing a wide range of mercury concentrations in chick down feathers from 0.40 μg g−1 fw to 44.31 μg g−1 fw, we did not find strong evidence for an influence of mercury on chick survival. The best model explaining chick survival incorporated effects of capture site, species, hatching date, and the body mass condition index. Several other candidate models provided a reasonably good fit to the data, including a model that incorporated mercury concentration as an explanatory variable (Table 2). Chick survival rates tended to decline with increasing mercury concentrations at hatching, but this pattern was not statistically robust (Fig. 4). During the first week after hatching when survival was the lowest, we observed less than 2% difference in modeled chick survival rates between the minimum and maximum observed mercury concentrations. This difference diminished as chicks aged so that by the fourth week there was no difference in chick survival rates in the range of observed mercury concentrations. Furthermore, variable weights indicated that mercury concentration was by far the least important variable predicting chick survival rates. Instead, species, residual body mass at hatching, and hatching date had much more important effects on chick survival rates.
In contrast to our telemetry data, we found some evidence that mercury concentrations in down feathers of dead chicks were higher than those in live chicks sampled at similar ages (i.e., newly hatched). The addition of the fate variable (alive or dead) to the most parsimonious model explaining factors influencing mercury concentrations improved the model fit by more than 4 times. However, the fate variable still had the lowest predictive power to explain mercury concentrations in chick down feathers—similar to the telemetry results. Because chicks that we radio-marked had already survived for several hours (≤2 days) before transmitter attachment, it is possible that methylmercury had an important effect on survival during the very short time period immediately after hatching, and, if so, we may have missed sampling this subset of the population. Indeed, chicks may be most vulnerable to methylmercury shortly after hatching when their mercury concentrations are still relatively high due to high in ovo mercury exposure. Elsewhere we found that blood mercury concentrations in stilt chicks declined rapidly after hatching, presumably because chicks diluted the concentration of mercury in their bodies through growth in size and depuration of mercury into growing feathers as they aged (Ackerman et al. 2007a).
Two additional methodological considerations may have influenced our ability to detect an effect of mercury on chick survival rates. First, we used mercury concentrations in down feathers as our index of a chick’s mercury exposure at hatching. Mercury concentrations in chick down are typically correlated with whole-body mercury burdens at hatching and down contains about 38% of the whole-body’s mercury burden (Becker et al. 1993). Down feathers develop in ovo during the embryonic phase, so they represent mercury exposure in the egg (Becker et al. 1994) during a critical developmental period (Hoffman and Moore 1979). Blood or internal organ (e.g., liver) mercury concentrations may be more indicative of a chick’s mercury burden, but sampling young chicks precludes their use since avocets weighed <19 g and stilts weighed <14 g at hatching. More intrusive sampling to estimate mercury exposure would have influenced chicks’ subsequent survival rates. Dietary exposure to mercury after hatching also could influence chick survival rates. However, mercury concentrations in ovo may be more important to chick survival than mercury exposure after hatching (Heinz 1974, 1979; Kenow et al. 2003), since subsequent mercury concentrations in chicks decline as they depurate mercury into growing feathers and dilute the concentration of body mercury during growth when their size increases by nearly 10-fold before fledging (Monteiro and Furness 2001).
Second, we focused our study on newly-hatched chicks because the effects of mercury are most likely to be expressed during the 28-day flightless period from hatching to fledging when chicks must learn to forage independently and escape predation (Robinson et al. 1997, 1999). However, the post-fledgling stage could also be a sensitive period for chick survival because mercury concentrations begin increasing as feather and mass growth slows at the time of fledging (Becker et al. 1994; Fournier et al. 2002; Kenow et al. 2003). The post-fledgling stage might be especially sensitive to mercury toxicity for semi-altricial or semi-precocial species with nidicolous young (such as great egrets, Ardea alba; Bouton et al. 1999; Sepúlveda et al. 1999; Spalding et al. 2000a), but avocets and stilts have precocial young and chick mortality predominantly occurs within the first week after hatching (Fig. 4) as it does in most precocial waterbirds (Guyn and Clark 1999; Simpson et al. 2005; Traylor and Alisauskas 2006).
The geometric mean (design-based) mercury concentrations that we observed in down feathers of chicks hatching in San Francisco Bay (avocets: 4.02 μg g−1 fw; stilts: 9.98 μg g−1 fw) were somewhat higher than might be expected for shorebirds that forage at a low trophic level on aquatic invertebrates and seeds (Robinson et al. 1997, 1999). For example, arithmetic mean mercury concentrations in chick down feathers were 1.42 μg g−1 fw for herring gulls (Larus argentatus) and 0.99 μg g−1 fw for black-headed gulls (Larus ridibundus) that foraged mainly on marine invertebrates along the polluted German North Sea coast (Becker et al. 1994). In contrast, mercury concentrations in chick down feathers of more highly piscivorous birds were 19.10 μg g−1 fw (low pH lakes) and 9.95 μg g−1 fw (neutral pH lakes) for common loons in Wisconsin (K. P. Kenow, U. S. Geological Survey, unpublished data), and 5.85 μg g−1 fw for common terns (Sterna hirundo) along the German North Sea coast (Becker et al. 1994). These data indicate that chicks being raised in San Francisco Bay have relatively high levels of mercury.
Methylmercury is known to influence survival rates of captive-reared chicks. For example, feeding parents diets containing methylmercury reduces survival in mallard (Anas platyrhynchos, Heinz 1974; Heinz and Hoffman 1998; Heinz et al. 2006) and black duck ducklings (Anas rubripes, Finley and Stendell 1978), and can alter duckling behavior (Heinz 1975, 1979) and reduce duckling mass (Heinz and Hoffman 1998). Dose-response studies have also shown that methylmercury can reduce growth and alter appetite, behavior, and health of captive great egret nestlings (Bouton et al. 1999; Spalding et al. 2000a, b; Hoffman et al. 2005). In contrast, Kenow et al. (2003) conducted a dose-response laboratory study on common loon (Gavia immer) chicks but were unable to detect an effect of the methylmercury dosing treatment on chick growth or survival. Instead, natural levels of methylmercury already present in loon eggs collected from the wild may have negatively affected subsequent chick development in the lab (Kenow et al. 2003).
Field studies, on the other hand, have had less success detecting effects of methylmercury on chick survival. Using radio telemetry, Sepúlveda et al. (1999) conducted a chick survival study in relation to natural and manipulated levels of mercury, but they did not detect an effect of methylmercury concentrations on survival of great egret chicks. Using mark-resight methods, Meyer et al. (1998) found that common loon chick production was lower on Wisconsin lakes where chick blood mercury concentrations were elevated. However, using similar methods, Merrill et al. (2005) did not find that common loon chick survival was affected by mercury exposure on Wisconsin lakes. Likewise, Thompson et al. (1991) did not detect an effect of parental (feather) mercury concentrations on chick survival in great skua (Catharacta skua). Although relatively few field studies have examined the effect of mercury on chick survival, results are mostly consistent in that they have not been able to detect strong effects of mercury on survival in the wild.
References
Ackerman JT, Adams J, Takekawa JY, Carter HR, Whitworth DL, Newman SH, Golightly RT, Orthmeyer DL (2004) Effects of radio transmitters on the reproductive performance of Cassin’s auklets. Wildl Soc Bull 32:1229–1241
Ackerman JT, Eagles-Smith CA, Heinz GH, Wainwright-De La Cruz SE, Takekawa JY, Adelsbach TL, Miles AK, Hoffman DJ, Schwarzbach SE, Suchanek TH, Maurer TC (2007a) Mercury in birds of the San Francisco Bay-Delta: trophic pathways, bioaccumulation and ecotoxicological risk to avian reproduction. 2006 Annual Administrative Report to CALFED, U. S. Geological Survey, Western Ecological Research Center, and U. S. Fish and Wildlife Service, Environmental Contaminants Division, 41 pp
Ackerman JT, Eagles-Smith CA, Takekawa JY, Demers SA, Adelsbach TL, Bluso JD, Miles AK, Warnock N, Suchanek TH, Schwarzbach SE (2007b) Mercury concentrations and space use of pre-breeding American avocets and black-necked stilts in San Francisco Bay. Sci Total Environ (in press)
Ackerman JT, Takekawa JY, Orthmeyer DL, Fleskes JP, Yee JL, Kruse KL (2006) Spatial use by wintering greater white-fronted geese relative to a decade of habitat change in California’s Central Valley. J Wildl Manage 70:965–976
Alberico JAR (1995) Floating eggs to estimate incubation stage does not affect hatchability. Wildl Soc Bull 23:212–216
Anderson DR, Burnham KP, Thompson WL (2000) Null hypothesis testing: problems, prevalence, and an alternative. J Wildl Manage 64:912–923
Anderson DR, Link WA, Johnson DH, Burnham KP (2001) Suggestions for presenting the results of data analyses. J Wildl Manage 65:373–378
Appelquist H, Drabaek I, Asbirk S (1985) Variation in mercury content of guillemot feathers over 150 years. Mar Pollut Bull 16:244–248
Ball IJ, Gilmer DS, Cowardin LM, Riechmann JH (1975) Survival of wood duck and mallard broods in north-central Minnesota. J Wildl Manage 39:776–780
Becker PH, Furness RW, Henning D (1993) Mercury dynamics in young common tern (Sterna hirundo) chicks from a polluted environment. Ecotoxicology 2:33–40
Becker PH, Henning D, Furness RW (1994) Differences in mercury contamination and elimination during feather development in gull and tern broods. Arch Environ Contam Toxicol 27:162–167
Bouton SN, Frederick PC, Spalding MG, McGill H (1999) Effects of chronic, low concentrations of dietary methymercury on the behavior of juvenile great egrets. Environ Toxicol Chem 18:1934–1939
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer-Verlag, New York, USA
Conaway CH, Watson EB, Flanders JR, Flegal AR (2004) Mercury deposition in a tidal marsh of south San Francisco Bay downstream of the historic New Almaden mining district, California. Mar Chem 90:175–184
Davis JA, Yee D, Collins JN, Schwarzbach SE, Louma SN (2003) Potential for increased mercury accumulation in the estuary food web. In: Brown LR (ed) Issues in San Francisco Estuary Tidal Wetlands Restoration. San Francisco Estuary and Watershed Science, vol. 1, Issue 1 (October 2003), Article 4 < http://repositories.cdlib.org/jmie/sfews/vol1/iss1/art4>
Evers DC, Burgess NM, Champoux L, Hoskins B, Major A, Goodale WM, Taylor RJ, Poppenga R, Daigle T (2005) Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 14:193–221
Finley MT, Stendell RC (1978) Survival and reproductive success of black ducks fed methyl mercury. Environ Pollut 16:51–63
Fournier F, Karasov WH, Kenow KP, Meyer MW, Hines RK (2002) The oral bioavailability and toxicokinetics of methylmercury in common loons (Gavia immer) chicks. Comp Biochem Physiol, Part A 133:703–714
Guyn KL, Clark RG (1999) Factors affecting survival of northern pintail ducklings in Alberta. Condor 101:369–377
Hamilton RB (1975) Comparative behavior of the American avocet and the black-necked stilt (Recurvirostridae). Ornithological Monographs 17
Hays H, LeCroy M (1971) Field criteria for determining incubation stage in eggs of the common tern. Wilson Bull 83:425–429
Heinz G (1974) Effects of low dietary levels of methyl mercury on mallard reproduction. Bull Environ Contam Toxicol 11:386–392
Heinz G (1975) Effects of methylmercury on approach and avoidance behavior of mallard ducklings. Bull Environ Contam Toxicol 13:554–564
Heinz GH (1979) Methylmercury: reproductive and behavioral effects on three generations of mallard ducks. J Wildl Manage 43:394–401
Heinz GH, Hoffman DJ (1998) Methylmercury chloride and selenomethionine interactions on health and reproduction in mallards. Environ Toxicol Chem 17:139–145
Heinz GH, Hoffman DJ, Kondrad SL, Erwin CA (2006) Factors affecting the toxicity of methylmercury injected into eggs. Arch Environ Contam Toxicol 50:264–279
Hoffman DJ, Moore JM (1979) Teratogenic effects of external egg applications of methyl mercury in the mallard, Anas platyrhynchos. Teratology 20:453–462
Hoffman DJ, Spalding MG, Frederick PC (2005) Subchronic effects of methylmercury on plasma and organ biochemistries in great egret nestlings. Environ Toxicol Chem 24:3078–3084
Hothem RL, Roster DL, King KA, Keldson TJ, Marois KC, Wainwright SE (1995) Spatial and temporal trends of contaminants in eggs of wading birds from the San Francisco Bay, California. Environ Toxicol Chem 14:1319–1331
Kenow KP, Gutreuter S, Hines RK, Meyer MW, Fournier F, Karasov WH (2003) Effects of methyl mercury exposure on the growth of juvenile common loons. Ecotoxicology 12:171–182
Marvin-DiPasquale MC, Agee JL, Bouse RM, Jaffe BE (2003) Microbial cycling of mercury in contaminated pelagic and wetland sediments of San Pablo Bay, California. Environ Geol 43:260–267
Merrill EH, Hartigan JJ, Meyer MW (2005) Does prey biomass or mercury exposure affect loon chick survival in Wisconsin? J Wildl Manage 69:57–67
Meyer MW, Evers DC, Hartigan JJ, Rasmussen PS (1998) Patterns of common loon (Gavia immer) mercury exposure, reproduction, and survival in Wisconsin, USA. Environ Toxicol Chem 17:184–190
Monteiro LR, Furness RW (2001) Kinetics, dose-response, excretion, and toxicity of methylmercury in free-living Cory’s shearwater chicks. Environ Toxicol Chem 20:1816–1823
Pelayo JT, Clark RG (2003) Consequences of egg size for offspring survival: a cross-fostering experiment in ruddy ducks (Oxyura jamaicensis). Auk 120:384–393
Reed JM, Gray EM, Lewis D, Oring LW, Coleman R, Burr T, Luscomb P (1999) Growth patterns of Hawaiian stilt chicks. Wilson Bull 111:478–487
Rimmer CC, McFarland KP, Evers DC, Miller EK, Aubry Y, Busby D, Taylor RJ (2005) Mercury levels in Bicknell’s thrush and other insectivorous passerine birds in montane forests of the northeastern United States and Canada. Ecotoxicology 14:223–240
Rintoul C, Warnock N, Page GW, Hanson JT (2003) Breeding status and habitat use of black-necked stilts and American avocets in South San Francisco Bay. Western Birds 34:2–14
Robinson JA, Oring LW, Skorupa JP, Boettcher R (1997) American avocet (Recurvirostra americana). In: Poole A, Gill F (eds) The birds of North America, No. 449. The Academy of Natural Sciences, Philadelphia, Pennsylvania, The American Ornithologists’ Union, Washington, DC, USA
Robinson JA, Reed JM, Skorupa JP, Oring LW (1999) Black-necked stilt (Himantopus mexicanus). In: Poole A, Gill F (eds) The Birds of North America, No. 449. The Academy of Natural Sciences, Philadelphia, Pennsylvania, The American Ornithologists Union, Washington, DC, USA
Samuel MD, Fuller MR (1996) Wildlife radiotelemetry. In: Bookhout TA (ed) Research and management techniques for wildlife and habitats. 5th edn. revised. The Wildlife Society, Bethesda, Maryland, USA, pp 370–418
Sall J, Lehman A, Creighton L (2001) JMP Start Statistics: a Guide to statistics and data analysis using JMP and JMP IN Software. 2nd edn. Duxbury, Pacific Grove, California, USA
Scheuhammer AM (1987) The chronic toxicity of aluminum, cadmium, mercury, and lead in birds: a review. Environ Pollut 46:263–295
Schwarzbach SE, Albertson JD, Thomas CM (2006) Effects of predation, flooding, and contamination on reproductive success of California clapper rails (Rallus longirostris obsoletus) in San Francisco Bay. Auk 123:45–60
Sepúlveda MS, Williams GE Jr, Frederick PC, Spalding MG (1999) Effects of mercury on health and first-year survival of free-ranging great egrets (Ardea albus) from Southern Florida. Arch Environ Contam Toxicol 37:369–376
Simpson JW, Yerkes TJ, Smith BD, Nudds TD (2005) Mallard duckling survival in the Great Lakes region. Condor 107:898–909
Spalding MG, Frederick PC, McGill HC, Bouton SN, McDowell LR (2000a) Methylmercury accumulation in tissues and its effects on growth and appetite in captive great egrets. J Wildl Dis 36:411–422
Spalding MG, Frederick PC, McGill HC, Bouton SN, Richey LJ, Schumacher IM, Blackmore CGM, Harrison J (2000b) Histologic, neurologic, and immunologic effects of methylmercury in captive great egrets. J Wildl Dis 36:423–435
Stamenkovic J, Gustin MS, Dennett KE (2005) Net methyl mercury production versus water quality improvement in constructed wetlands: trade-offs in pollution control. Wetlands 25:748–757
Stenzel LE, Hickey CM, Kjelmyr JE, Page GW (2002) Abundance and distribution of shorebirds in the San Francisco Bay area. Western Birds 33:69–98
Takekawa JY, Warnock N, Martinelli GM, Miles AK, Tsao DC (2002) Waterbird use of bayland wetlands in the San Francisco Bay Estuary: movements of long-billed dowitchers during winter. Waterbirds 25:93–105
Thompson DR (1996) Mercury in birds and terrestrial mammals. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife, interpreting tissue concentrations. CRC Press LCC, Boca Raton, Florida, USA, pp 341–356
Thompson DR, Furness RW (1989) Comparison of the levels of total and organic mercury in seabird feathers. Mar Pollut Bull 20:577–579
Thompson DR, Hamer KC, Furness RW (1991) Mercury accumulation in great skuas Catharacta skua of known age and sex, and its effects upon breeding and survival. J Appl Ecol 28:672–684
Traylor JJ, Alisauskas RT (2006) Effects of intrinsic and extrinsic factors on survival of white-winged scoter (Melanitta fusca deglandi) ducklings. Auk 123:67–81
U. S. EPA (1997) Mercury report to congress. EPA-452/R-97–008. U. S. Environmental Protection Agency, Washington DC, USA
Ullrich SM, Tanton TW, Abdrashitova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
United Nations Environment Programme (2003) Global mercury assessment. UNEP Chemicals, Geneva, Switzerland
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46 (Supplement):120–138
Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM (2003a) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (eds) Handbook of ecotoxicology, 2nd edn. CRC Press LCC, Boca Raton, Florida, USA, pp 409–463
Wiener JG, Gilmour CC, Krabbenhoft DP (2003b) Mercury strategy for the bay-delta ecosystem: a unifying framework for science, adaptive management, and ecological restoration. Final Report to the California Bay Delta Authority, Sacramento, California, USA
Williams BK, Nichols JD, Conroy MJ (2002) Analysis and management of animal populations: modeling, estimation, and decision making. Academic Press, San Diego, California, USA
Winterstein SR, Pollock KH, Bunck CM (2001) Analysis of survival data from radiotelemetry studies. In: Millspaugh JJ, Marzluff JM (eds) Radio tracking and animal populations. Academic Press, San Diego, California, USA, pp 351–380
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160
Acknowledgments
This research was funded by the CALFED Bay-Delta Program’s Ecosystem Restoration Program (grant number ERP-02D-C12) with additional support from the USGS Western Ecological Research Center. We thank Angela Rex, Ross Wilming, Eli French, Jill Bluso, Sarah Stoner-Duncan, Carolyn Marn, Brooke Hill, Stacy Moskal, Emily Eppinger, Joe Northrup, Kristen Dybala, Lani Stinson, Scott Demers, and Terry Adelsbach for field assistance and Keith Miles, Robin Keister, and Sarah Spring for lab analyses. We also thank Clyde Morris, Joy Albertson, Mendel Stewart, Joelle Buffa, Eric Mruz, and the staff at the Don Edwards San Francisco Bay National Wildlife Refuge (Special Use Permits 11640-2005-002 and 11640-2006-006) and Nicole Athearn, Cheryl Strong, San Francisco Bay Bird Observatory, and PRBO Conservation Science for logistical support. Early versions of the manuscript were improved by discussions and comments from Mark Ricca, Nicole Athearn, and two anonymous reviewers. The use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U. S. Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ackerman, J.T., Takekawa, J.Y., Eagles-Smith, C.A. et al. Mercury contamination and effects on survival of American avocet and black-necked stilt chicks in San Francisco Bay. Ecotoxicology 17, 103–116 (2008). https://doi.org/10.1007/s10646-007-0164-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-007-0164-y