Abstract
Although methylated mercury (MeHg) is known to have neurological, immunological, reproductive, and endocrine effects on vertebrates at low environmental exposure levels, effects on survival of exposed birds have not been demonstrated in the wild. Here, we report on survival of the same group of White Ibises (Eudocimus albus) during exposure to 4 levels of dietary MeHg in captivity and later as depurated free-ranging animals. Ibises were chronically exposed in captivity to dietary MeHg in groups at 0 (control), 0.05 (Low), 0.1 (Medium) and 0.3 (High) ppm MeHg ww for 43 months. No differences in annualized survival among captive MeHg groups were seen within age classes. Survival of all ages taken together was significantly lower for Control birds than for Low or Medium dosed birds, but was not different from High dosed birds. While this might be evidence of a hormetic effect, none of the captive results support the prediction that MeHg impairs survival. Using a mark-recapture analysis we found no effects of dose group or of Hg exposure on survival or resight probabilities during the first 99 days post-release to the wild. The latter results suggest that there is no lasting, post-depuration effect of even high MeHg exposure (0.3 ppm ww dietary) on survival. While these results agree with a variety of studies of survival of free-ranging birds, we suggest many survival studies have been confounded by seasonal depuration through molt, and variation in exposure rates. We suggest future studies concentrate on evaluating survival effects during nonmolting periods in species for which methylmercury exposure is relatively constant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exposure to mercury in its methylated form (CH3Hg, hereafter MeHg) is known to have a number of detrimental effects on vertebrates, including decreased motor control, neurological degeneration, endocrine disruption, impaired immunity, and behavioral and developmental abnormalities (Scheuhammer 1987; Fitzgerald and Clarkson 1991; Wolfe et al. 1996; Evers et al. 2008; Tan et al. 2009; Crump and Trudeau 2009) and ultimately, adverse reproductive impacts (Burgess and Meyer 2008; Evers et al. 2008; Frederick and Jayasena 2010). Carnivorous birds are generally at high risk of exposure because of their high metabolism and trophic position, and carnivorous birds in aquatic environments may be at further risk because of high bioaccumulative potential of aquatic (Evers et al. 2005) and terrestrial (Cristol et al. 2008) foodwebs.
Although detrimental effects of MeHg exposure in birds have typically been studied separately on different physiological endpoints, there is clearly the potential for a cumulative effect of MeHg on survival of individuals, particularly in the context of the multiple physiological and life history challenges typical of the wild (Wingfield 2004). Relatively few studies have examined effects of MeHg on survival in natural environments in part because survival is difficult to monitor and controlled dosing difficult to achieve in a natural setting. Tissue concentrations in fish-eating birds have been related to both susceptibility to disease (Spalding et al. 1994) and survival during adverse weather (Van der Molen et al. 1982), and a relationship has been demonstrated between exposure and decreasing immunocompetence (Hawley et al. 2009). Interannual return rates of adult Great Skuas (Catharacta skua) showed no relationship with methylmercury exposure at mean adult feather mercury values of 7.0 ug/g dw (Thompson et al. 1991). Survival rates of Forsters Terns (Sterna forsteri) during the 35-days following fledging were not related to blood THg levels, and young found dead did not have higher THg in feathers than live chicks (Ackerman et al. 2008a). Similarly, only weak evidence of Hg exposure was found on survival of fledgling American Avocets (Recurvirostra americana) and Black-necked Stilts (Himantopus mexicanus, Ackerman et al. 2008b). No effects of mercury exposure were found on survival of Common Loons (Gavia immer, Meyer et al. 1998; Mitro et al. 2008) despite the ability to detect annual survival differences of 3%. Similarly, despite high exposure levels, only a 1% difference was found in overwinter survival rates of exposed and unexposed female Tree Swallows (Tachycineta bicolor Hallinger et al. 2010). However, survival is an integrative endpoint influenced by numerous factors, and many of the studies to date have been hampered by an inability to include true controls for mercury exposure, and to control for variable survival challenges across exposure classes.
Mercury and especially MeHg can act directly on developmental processes (Heinz and Locke 1976; Fitzgerald and Clarkson 1991; Ottinger et al. 2008), and so may cause impairment that lasts well beyond the period of exposure. In contrast, studies of animals exposed only as adults also shows a number of strong physiological and behavioral effects (Heinz 1979; Wolfe et al. 1996), indicating that some effects of contamination may be dependent upon current circulating concentrations in the bloodstream. Both exposure and depuration in birds can vary hugely with location and season (Spalding et al. 2000; Ackerman et al. 2008b), and effects of current and past exposure are probably being mixed in measurements of many of the avian survival studies. There has been little work aimed at separating the mix of effects that derive from prior and current exposure. The resolution of this question has practical value, since it is unclear whether mitigative actions like depuration can resolve impairment of animals that results from prior exposure.
Here, we report on survival of captive White Ibises (Eudocimus albus) while they were being exposed chronically to known MeHg doses from 90 days to 3 years of age in captivity. Any effects of MeHg during this time could be attributed to some combination of effects due to exposure during the developmental period, as well as continuing exposure. We also followed the fates of these birds after they were released to the wild at the end of the captive study period. At the time of release, ibises had been depurated for 2 months. This part of the study asked whether prior chronic exposure alone could result in lasting effects that impact the survival of individuals.
Methods
The White Ibis is a wetland bird that typically feeds on crustaceans and small fishes in shallow fresh and estuarine marshes (Heath et al. 2009). White Ibises achieve sexual maturity in their second spring (18–24 months) and breed annually thereafter. In the wild, ibises may be exposed to MeHg contamination (Frederick et al. 2004) to the extent that both endocrine function and reproductive effort are affected (Heath and Frederick 2005; Jayasena 2010; Frederick and Jayasena 2010).
White Ibises were collected from wild breeding colonies in Florida as nestlings in April and May of 2005, and raised in a free-flight aviary to an age of 40 months on ad libitum diets containing 0, 0.05, 0.1 and 0.3 ppm MeHg ww (Jayasena 2010). These food concentrations span the range of MeHg concentrations in prey items that ibises might encounter in the Everglades (Loftus 2000). The food ration was a custom blended pelletized diet (Mazuri Flamingo, modified), whose primary constituents were fishmeal, alfalfa, corn gluten meal, rice flour, brewers dried yeast, shrimp meal, ground soft wheat, and fish oil, resulting in a diet containing 28% protein, 9% fat.
MeHg was introduced into pelletized food by spraying MeHg (Ch3HgCl, 99.9%, Alfa Aesar, Ward Hill, MA) dissolved in a corn oil vehicle onto a mass of pellets rotating in a small cement mixer. Concentrations of CH3Hg in corn oil were derived empirically, adjusting for Hg content, and resulting concentrations in food was checked via direct measurement of THg in pelletized food samples. Control doses contained the corn oil vehicle only. Researchers mixing the feed, feeding birds, and measuring survival were blind to the concentrations of food being fed to different groups. We also checked actual exposure in birds by measuring THg concentrations in 2–5 scapular feathers collected from all individuals in January of 2006, 2007 and 2008. All THg determinations were done using nitric acid digestion of feathers followed by cold vapor atomic absorbtion spectrometry. All determinations were performed by the Florida Department of Environmental Protection Chemistry Section, Tallahassee, FL (detection limit 0.1 mg/l).
Exposure to diets containing MeHg was begun at approximately 90 days of age (±18 days), and stopped in fall of 2008 (40 months of age for most individuals). All birds were banded with individually recognizable alpha-numeric colored aluminum bands, and all birds were sexed using DNA (Animal Genetics Inc, Tallahassee FL). During this time, the birds matured to adulthood and most individuals had bred up to three times.
We monitored survival in the aviary daily from June 2005 through November 2008. Since all birds were individually recognizable and confined in the aviary, we express survival exactly, as number or proportion of birds surviving.
Ibises were released to the wild in south Florida in December 2008 using a soft-release approach. The birds were transported in cages by vehicle from the aviary in Gainesville, FL to Flamingo Gardens in suburban Davie, Broward County, FL (FG). The FG site has an open-topped aviary in which flighted birds could come and go, but have a safe roosting location and continuous access to ad libitum food. FG was located 0.5 km from an existing nocturnal roost site of several hundred wild ibises and other ciconiform birds (Long Key Natural Area, “LK”). FG is also located 15–25 km from the nearby Everglades, a core breeding area for White Ibises.
We released 157 ibises on 21 December 2008 at the FG site (see Table 1). We monitored post-release survival using a mark-resight approach. We looked for banded birds daily between December 22 2008 and 31 March 2009 at FG and the LK roost site. We also searched once every 2–4 days for banded birds at 2 other known roosting sites within 5 km of FG. Between 22 December 2008 and 31 March 2009 we performed 78 h of resight effort on 72 days, or a resight effort once every 1.39 days (sd 0.6205 days). Not all sites were visited on each day, however, and we organized the 72 days of resight effort into 26 consecutive sampling periods, such that each period contained at least one sample from each of the observation sites.
We modeled post-release survival and resight probabilities using Program Mark (White and Burnham 1997). Since the number of juveniles was small (Table 1), we analyzed sightings only from adult birds. We constructed a priori models that included the effects of sex, MeHg exposure group, and time as potential covariates (singly and in all additive combinations) on both survival and resight probability. We used an information-theoretic approach (Akaike’s Information Criterion) for selecting models that balanced accuracy and parsimony, and accepted models with QAIC differences from the best model of up to 2 as being likely to have additional explanatory power. When constructing models that used all 26 periods, we frequently found unrealistically high or low estimates for survival and resight, as well as very high variability in estimates among periods. We then estimated resight and survival probabilities on intervals that spanned every two sequential resight periods (13 intervals total). Resight information was therefore preserved from all periods, but fewer parameters were estimated.
Capture and later release of ibises, and protocols while in captivity were performed under permits from the US Fish and Wildlife Service (MB 720214-0), Florida Fish and Wildlife Conservation Commission (WX 03527 and LSSC-09-0065), and University of Florida Institutional Animal Care and Use Committee (D424).
Results
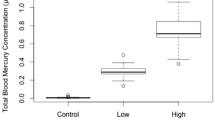
Feather total mercury (THg) concentrations varied by dose group and generally mimicked the range of THg concentrations found in wild birds (Table 2, Frederick et al. 2004). Concentrations in feathers of Control birds were similar to those found in pre-contamination samples from the Everglades (Control: 0.47–0.74 ppm, pre-contamination mean 1.04 sd 0.77 ppm, Frederick et al. 2004). Mean concentrations of ibis feathers from the post-contamination period averaged 7.47 (sd 4.58) ppm (Frederick et al. 2004), which was similar to the range we found for Low and Medium dose groups.
We found high annualized survival rates of both juveniles (92%) and adults (92%) in captivity, and found no significant differences due to MeHg dose group in annual survival rates of either age class (Table 3). For all age classes combined during the entire study period, we found survival in controls was marginally significantly different from both Low (p = 0.052, Fishers Exact test) and Medium (p = 0.051) groups, but was not significantly different from the High dose group.
With the birds released to the wild, we found no evidence of an effect of MeHg dosing level or sex on survival or resight probabilities when using all 4 groups as separate treatments. The top model was one which included only time effects for both survival and resight probabilities (Table 4). The next most parsimonious model was one that differed by over 14 QAIC points. In addition, for models that included treatment effects, survival and resight estimates for the three dosed groups were encompassed by the confidence interval for survival of control birds.
We also constructed models that compared the control group with all MeHg dosed birds combined (Table 5). A single model with effects of time on both survival and resight probability had the lowest QAIC (Table 5). Several other models included effects of treatment as a main effect, but all were much less parsimonious (>10 AIC units larger) at explaining survival and resight probability than the model with time effects alone. In addition, for models that included treatment effects, survival and resight estimates for the dosed grouping were encompassed by the confidence interval for control birds.
The most parsimonious model was therefore the same, regardless of whether we used all three dosed groups separately or lumped into a single “dosed” group. In the most parsimonious model, survival estimates during the 13 periods varied between 0.897 and 0.997. Overall survival using data from all groups for the entire period of observation (99 days) was estimated to be 0.679, or a daily survival probability of 0.996. Resight probability varied across intervals between 0.918 and 0.384 (\( \overline{X} \) = 0.537, n = 12 intervals, sd = 0.1488).
Discussion
While the birds were in captivity, we found a general lack of support for the prediction that chronic exposure to low/medium mercury exposure should be associated with reduced survival. While there were no significant differences by dose group in annualized survival, there were differences between control and both low and medium dose groups in total survival. However, there was no difference between control and high dose groups, where we might have expected to find the greatest difference. In addition, the differences we did find were in the opposite direction to those predicted—control birds survived less well than dosed birds. The effect at low and medium doses might have been due to a weak protective effect of MeHg against diseases (Heinz et al. 2010), which were then counterbalanced by somewhat stronger negative effects at the high dose level. Since there were relatively few deaths during the study (n = 14, or 8% of the population over 44 months) it may be that these marginally significant differences were a statistical artifact of small sample size. It is also true that the stressors that most likely affect survival were all removed or attenuated in the captive situation, making the captive situation one in which we might least expect to see survival differences due to MeHg exposure.
In contrast, the birds released to the wild would be expected to be challenged much more by stressors that might affect survival. This test of effects of exposure to low/medium MeHg exposure on survival had several characteristics that allowed good power of detecting an effect of prior long-term MeHg exposure. We had relatively high resight probabilities overall (0.54), so that if individuals from one of the treatment groups had experienced lower resight probability or survival, we had reasonable ability to detect that change. This also suggests that our birds were for the most part not leaving the area. Daily survival was relatively high (0.996 overall), suggesting that any effects of MeHg were unlikely to have been masked by high mortality overall. Finally, survival and resight probabilities remained relatively constant across the study period, suggesting that there was no particular event that may have introduced sudden changes in these parameters.
The lack of an effect of MeHg in this study suggests that prior chronic MeHg exposure at low to medium dietary concentrations did not alter survival probabilities, nor the propensity to emigrate or be detected by our surveys. However, the soft-release conditions that the ibises were exposed to might also have dampened effects of previous MeHg exposure, at least by comparison with truly wild ibises. The main sources of stressors for birds newly released after a life spent in captivity would probably be exposure to predation, exposure to disease agents, and interruptions in food availability. During the study period, the released ibises had continuous access at the FG site to pelletized food, which could have reduced any effect of food stress. However, the study ibises also routinely roosted and fed gregariously with large numbers of wild ibises and other waterbirds both at the FG aviary, and at the LK roost site, presenting an excellent mode for transmission of diseases and parasites novel to the captive birds. Densities of birds at roosting and feeding sites were high, and the concentration of wild, captive and released birds of several species at the FG feed station was very different from the wild condition. Ibises at the release site may also have been exposed to more predation pressure than in the wild. Ibises at FG and in surrounding suburban neighborhoods were often seen foraging on lawns and in residential yards. Typically ibises in the wild choose open wetland habitats where they are unlikely to be exposed to mammalian predators (Heath et al. 2009). We suspect that ibises foraging in and near the FG site would be exposed to elevated densities of mammalian predators such as domestic dogs, cats, and raccoons (Procyon lotor). Thus, while food stress was probably reduced by comparison with the wild condition, exposure to disease and predation was likely to have been typical or even elevated in this study.
We believe our results should be interpreted to mean that long term prior exposure to MeHg at 0.05–0.3 ppm ww in diet does not have permanent measurable effects on survival ability of adults. The lack of permanent effects from prior exposure does not preclude the possibility that survival deficits might derive from high circulating levels of MeHg at the time survival is being measured. The only measurements of current exposure effects we were able to carry out involved captive animals, where survival challenges were minimal. We therefore believe that predictions about survival and current exposure in wild avian populations have yet to be evaluated fully.
The results presented here mirror the lack of effects found in loons, skuas, terns and swallows and to date we are not aware of studies that demonstrate decreased survival in birds in the wild due to MeHg exposure. However, it should be recognized that this conclusion is based almost entirely on nonexperimental studies. Further, the lack of findings may partially be an artifact of periodic depuration. In the case of loons and swallows, the primary source of exposure for both species was on the breeding grounds, and the majority of the year when survival is being measured is spent away from those sources. In addition, both species undergo complete body molt during the course of the nonbreeding season, during which there is an opportunity to shed a significant portion of the body burden into newly grown feathers (Braune and Gaskin 1987; Spalding et al. 2000; Condon and Cristol 2009). The likely decrease in exposure, and depuration during full molt during the survival period being measured are arguably quite similar in effect to the period of pre-release depuration that ibises underwent in this study.
Taken together, these studies suggest that ambient MeHg exposure at low to medium concentrations does not result in a severe or obvious reduction in annual survival of wild adult birds, or at least that exposure may be confounded by seasonal depuration. While we feel that this idea requires more examples before being generally accepted, at this stage we suggest it as a working hypothesis for future research. We suggest that the most dramatic test of an effect of MeHg exposure on survival would be in birds that are undergoing constant exposure, and that are not molting at the time survival is being measured.
References
Ackerman JT, Eagles-Smith CA, Takekawa JY, Iverson SA (2008a) Survival of postfledging Forster’s terns in relation to mercury exposure in San Francisco Bay. Ecotoxicology 17(8):789–801
Ackerman JT, Takekawa JY, Eagles-Smith CA, Iverson SA (2008b) Mercury contamination and effects on survival of American avocet and black-necked stilt chicks in San Francisco Bay. Ecotoxicology 17:103–116
Braune BM, Gaskin PE (1987) A mercury budget for the Bonaparte’s gull during autumn moult. Ornis Scand 18:244–250
Burgess NM, Meyer MW (2008) Methylmercury exposure associated with reduced productivity in common loons. Ecotoxicology 17:83–91
Condon AM, Cristol DA (2009) Feather growth influences blood mercury level of young songbirds. Environ Toxicol Chem 28(2):395–401
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger LK, Monroe AP, White AE (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335
Crump KL, Trudeau VL (2009) Mercury-induced reproductive impairment in fish. Environ Toxicol Chem 28(5):895–907
Evers DC, Burgess N, Champoux L, Hoskins B, Major A, Goodale W, Taylor R, Poppenga R, Daigle T (2005) Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 14:193–222
Evers DC, Savoy LJ, DeSorbo CR, Yates DE, Hanson KM, Taylor J, Siegel LS, Cooley JH Jr, Bank MS, Major A, Munney K, Mower BF, Vogel HS, Schoch N, Pokras M, Goodale MW, Fair J (2008) Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology 17:69–81
Fitzgerald WF, Clarkson TW (1991) Mercury and monomethylmercury: present and future concerns. Environ Health Perspect 96:159–166
Frederick PC, Jayasena, NUA (2010) Altered pairing behavior and reproductive success in White Ibises exposed to environmentally relevant concentrations of methylmercury. Proc Roy Soc B. doi:10.1098/rspb.2010.2189
Frederick PC, Hylton BA, Heath JA, Spalding MG (2004) A historical record of mercury contamination in southern Florida as inferred from avian feather tissue. Environ Toxicol Chem 23:1474–1478
Hallinger KK, Cornell KL, Brasso RL, Cristol DL (2010) Mercury exposure and survival in free-living tree swallows (Tachycineta bicolor). Ecotoxicology. doi:10.1007/s10646-010-0554-4
Hawley DM, Hallinger KK, Cristol DA (2009) Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology 18(5):499–503
Heath JA, Frederick PC (2005) Relationships among mercury concentrations, hormones, and nesting effort of White Ibises (Eudocimus albus) in the Florida Everglades. The Auk 122:255–267
Heath JA, Frederick PC, Kushlan JA, Bildstein KL (2009) In: Poole A (ed) White ibis (Eudocimus albus), the birds of North America online. Cornell Lab of Ornithology, Ithaca. doi:10.2173/bna.9. http://bna.birds.cornell.edu/bna/species/009. Accessed 26 Jul 2010
Heinz GH (1979) Methylmercury: reproductive and behavioral effects on three generations of mallard ducks. J Wildl Manag 43(2):394–401
Heinz GH, Locke LN (1976) Brain lesions in mallard ducklings from parents fed methylmercury. Avian Dis 20(1):9–17
Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR (2010) Enhanced reproduction in mallards fed a low level of methylmercury: an apparent case of hormesis. Environ Toxicol Chem 29(3):650–653
Jayasena NUA (2010) Effects of chronic methylmercury exposure on reproductive success, behavior, and steroid hormones of the white ibis (Eudocimus albus). PhD Dissertation, University of Florida, Gainesville
Loftus WF (2000) Accumulation and fate of mercury in an Everglades aquatic food web. PhD dissertation, Florida International University, Miami, 295 p
Meyer MW, Evers DC, Hartigan JJ, Rasmussen PS (1998) Patterns of common loon (Gavia immer) mercury exposure reproduction and survival in Wisconsin, U.S.A. Environ Toxicol Chem 17(2):184–190
Mitro MG, Evers DC, Meyer MW, Piper WH (2008) Common loon survival rates and mercury in New England and Wisconsin. J Wildl Manag 72:665–673
Ottinger MA, Lavoiea E, Thompson N, Barton A, Whitehouse K, Barton M, Abdelnabi M, Quinn M Jr, Panzica G, Viglietti-Panzica C (2008) Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res Rev 57:376–385
Scheuhammer AM (1987) The chronic toxicity of aluminum, cadmium, mercury, and lead in birds: a review. Environ Pollut 46:263–295
Spalding MG, Bjork RD, Powell GVN, Sundlof SF (1994) Mercury and cause of death in Great White Herons. J Wildl Manag 8(4):735–739
Spalding MG, Frederick PC, McGill HC, Bouton SN, McDowell LR (2000) Methylmercury accumulation in tissues and its effects on growth and appetite in captive great egrets. J Wildl Dis 36:411–422
Tan SW, Meiller JC, Mahaffey KR (2009) The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol 39:228–269
Thompson DR, Hamer KC, Furness RW (1991) Mercury accumulation in great skuas Catharacta skua of known age and sex, and its effects upon breeding and survival. J Appl Ecol 28(2):672–684
Van der Molen EJ, Blok AA, De Graaf GJ (1982) Winter starvation and mercury intoxication in grey herons (Ardea cinerea) in the Netherlands. Ardea 70:173–184
White GC, Burnham KP (1997) Program MARK: survival estimation from populations of marked animals. http://epa.gov/waterscience/fish/advisories/2006/tech.html
Wingfield JC (2004) Allostatic load and life cycles: implications for neuroendocrine control mechanisms. In: Schulkin J (ed) Allostasis homeostasis and the costs of physiological adaptation. Cambridge University Press, Cambridge, pp 303–342
Wolfe MF, Sulaiman RS, Schwarzbach SE, Hofius J (1996) Mercury effects on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160
Acknowledgments
This work was supported by funding from the Florida Department of Environmental Protection, the U.S. Army Corps of Engineers, the U.S. Fish and Wildlife Service, and the U.S. Geological Service. We thank the owners and staff of Flamingo Gardens for allowing us to use the facility, and for their cooperation and expertise.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frederick, P., Campbell, A., Jayasena, N. et al. Survival of White Ibises (Eudocimus albus) in response to chronic experimental methylmercury exposure. Ecotoxicology 20, 358–364 (2011). https://doi.org/10.1007/s10646-010-0586-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0586-9