Abstract
An experimental tree swallow population was established in the headwaters of the Shenandoah River, Virginia, USA to assess the accumulation and effects of mercury contamination on birds that eat emergent aquatic insects. One tributary, the South River, was contaminated with mercury before 1950. Reproductive success of swallows nesting within 50 m of this river was compared to that of three uncontaminated reference tributaries in 2005 and 2006. Female swallows on the contaminated stretch of river had significantly elevated blood and feather total mercury (blood: 3.56 ± 2.41 ppm ww vs. 0.17 ± 0.15 ppm reference; feather: 13.55 ± 6.94 ppm vs. 2.34 ± 0.87 ppm reference), possibly the highest ever reported for an insectivorous songbird. Insects collected by the swallows to be fed to nestlings averaged 0.97 ± 1.11 ppm dw total mercury, significantly higher than on reference sites. Swallows in the contaminated area produced fewer fledglings than those in reference areas. The effect of mercury contamination on productivity was detectable only for young females in the contaminated area that were breeding for the first time in 2006, a segment of the population that may already have been stressed by inexperience. Tree swallows served as practical and effective biomonitors for mercury levels and effects and have great potential as proxy biomonitors for more logistically challenging birds such as loons or eagles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tree swallow (Tachycineta bicolor), an insectivorous passerine, is found across North and Central America (Robertson et al. 1992). Adults typically forage within 400 m of their nests and remain even closer when feeding nestlings (McCarty 2001; Mengelkoch et al. 2004). Thus, most contaminants accumulated by swallows during the breeding season originate from a small area around the nest. Tree swallows have been used in many studies of environmental contaminants, including mercury, PCBs, and pesticides. As of 2006, there were at least 44 completed or on-going studies in North America on the uptake of contaminants and the effects of anthropogenic stressors in tree swallows (McCarty 2001).
Despite the growing use of this species, many studies have reported a lack of significant reproductive or survivorship responses to these environmental stressors (McCarty 2001). Reproduction has been identified as one of the most sensitive endpoints of mercury toxicity, primarily from captive dosing studies (Wolfe et al. 1998). Decreases in reproductive success of 35–50% have been observed in birds with high dietary methylmercury uptake, even in the absence of impairment in the adults (USDI 1998). In general, effects such as decreased egg weight, hatchability, and chick survival are seen at egg mercury levels of 0.5–6.0 ppm ww in laboratory studies (Thompson 1996; Burger and Gochfield 1997). Impairments and deformities of chicks occur at mercury concentrations as low as 1.0 ppm ww in laboratory-dosed eggs (Heinz and Hoffman 2003). Reproductive effects have been more elusive in field studies, but one recent example is that egg volumes in common loons (Gavia immer) decreased as mercury loading of females increased (Evers et al. 2003). Another study suggests that brain mercury residues >2.0 ppm in adult loons may have led to reduced territorial drive, decreased incubation and abandonment of territories (Barr 1986). No growth deformities or other reproductive effects related to mercury contamination have been reported in tree swallows.
Between 1929 and 1950 mercuric sulfate entered the South River (a tributary to the South Fork Shenandoah River) from an acetate fiber manufacturing plant in Waynesboro, Virginia, USA (Carter 1977). To assess the amount of mercury recently accumulated by adult and nestling tree swallows along this polluted tributary, we sampled blood from adults and nestlings during the nestling period. To eliminate the influence of atmospheric deposition or background mercury contamination, we compared mercury levels of birds nesting along the contaminated South River to the levels of birds from reference areas on three nearby uncontaminated rivers in the same watershed. Reproductive success was monitored throughout two breeding seasons to compare clutch size and date of initiation, egg volume and hatchability, and offspring survival.
Methods
Study design
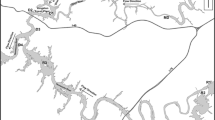
This study was carried out on the contaminated portion of the South River (approximately 40 km). The North and Middle Rivers, as well as the section of the South River upstream of Waynesboro, were the uncontaminated reference areas (Fig. 1). A pilot study was also carried out downstream of the confluence of the contaminated and reference rivers on the South Fork Shenandoah River in 2005.
Map of nest box sites along the South, Middle, North, and South Fork Shenandoah Rivers, Augusta and Rockingham counties, Virginia, USA. Black circles are nest box clusters in contaminated areas; grey circles are nest box clusters in reference areas. The source of contamination is labeled with an asterisk in Waynesboro
Beginning in February 2005, we erected 102 nest boxes in the contaminated area on the South and South Fork Shenandoah Rivers, and 89 nest boxes in reference areas on the South, Middle, and North Rivers. All nest boxes along the South Fork Shenandoah River (n = 42) were removed before the 2006 breeding season, after it was determined that mercury levels were intermediate between the contaminated and reference areas, and these were not included in any analyses. We increased the number of nest boxes to 119 in the contaminated area and 167 in the reference areas prior to the 2006 breeding season. Nest boxes were 25–50 m apart with the entrance holes oriented towards the river. We checked them weekly beginning in the first week of April. We increased checking to every 3–4 days beginning in the first week of May, and then removed nesting material from nest boxes after the chicks had fledged. We did not include data from late females initiating nests after the first round of nesting: 3 June, 2005 or 2 June, 2006. Female tree swallows maintain a brown plumage until the end of their second summer and thus can be identified in the field as second-year (hereafter, “SY”), in contrast to blue-plumaged, after-second year (hereafter, “ASY”) females.
Mercury sampling
We used a small gauge (26G ½ in.) needle to puncture the cutaneous ulnar vein, and then collected approximately 100 μl of blood in three 75 μl heparinized capillary tubes. Tubes were sealed with Crito-caps® and placed into a 10cc BD® vacutainer to prevent breakage. Samples were stored on ice for 3–6 h, after which they were frozen at −25°C until analysis.
Feather samples in this study were collected from a subsample of ASY females in 2006 to provide a longer-term estimate of body-burden during the previous breeding season. These feathers also provide comparability with other studies in which feathers, but not blood, have been sampled. When birds banded in 2005 returned to the breeding grounds in 2006, we plucked the innermost primary feather (P1) from each wing. As feather molt typically begins in July (Stutchbury and Rohwer 1990), the mercury present in P1 during the 2006 breeding season would indicate body burden from 2005.
Prey sampling
Because we captured swallows as they entered nest boxes to feed nestlings, we were usually able to collect the bolus of food in a glass jar directly from the parent’s beak. These were weighed as a whole bolus, frozen, identified to orders, freeze dried, re-weighed, homogenized and analyzed for mercury.
Laboratory and statistical analysis
Results are reported only for adult females unless otherwise noted. All mercury analysis took place at the Trace Element Research Laboratory of Texas A&M University. Samples were analyzed for total mercury with a Milestone® DMA 80 using cold vapor atomic absorption spectroscopy. Because approximately 95% of the mercury in avian blood and feathers is MeHg (Evers et al. 2005), total mercury values will accurately reflect the amount of MeHg present. Prey items, which were primarily insects in the orders Diptera and Ephemeroptera, were not analyzed for MeHg. Samples were homogenized to allow a representative aliquot to be taken for analysis and typically weighed 0.01 g.
Reproductive success parameters
We determined the date of clutch initiation by the presence of a fresh egg during our frequent nest box checks. The initiation date of the first clutch in the entire study area was denoted Julian day 1 in analyses (29 April in 2005, 18 April in 2006). The proportion of eggs that hatched was the number of hatchlings divided by the maximum clutch size. The number of nestlings that left the nest (fledglings) was our primary measure of productivity. This was determined by counting the number of nestlings alive on the last visit (day 14–16 post-hatching) and then subtracting any dead nestlings found on the post-fledging visit. Productivity was alternately expressed as proportion of hatched nestlings that fledged, to emphasize any mortality differences during the nestling period. Finally, to correct for differential female effort (i.e., clutch size variation) we estimated productivity as the proportion of eggs that survived to fledge.
In 2006 we measured 94 eggs from 16 nests in the contaminated area and 79 eggs from 14 nests from reference areas. The maximum length and width of each egg was measured to the nearest 0.1 mm. Egg volume (V) was determined by the formula,
where L = length, W = width, and 0.51 is the volume coefficient constant (Hoyt 1979).
The South River is referred to as the “contaminated” treatment throughout. Statistical analyses were done using MINITAB statistical software (version 14.2, LEAD Technologies, State College, PA). Mercury levels and nesting success were compared using an analysis of variance. We combined all data from females in both years and used treatment group, female age, and year as factors with treatment group × female age, treatment group × year, female age × year, and treatment group × female age × year as interaction terms. Tukey’s HSD test was used for multiple post-hoc comparisons. We report only those factors and interactions that were significant (P < 0.05). All means are presented ± SD, except in figures where SE is used. The proportion of eggs hatched, nestlings fledged, and eggs fledged were normalized by arcsine square-root transformation before statistical analysis, but untransformed data are shown.
Results
Mercury level
There was a significant effect of treatment on the blood mercury levels of adult females across both years (F 1,116 = 89.41, P < 0.0001). The blood mercury level of females nesting in the contaminated area (3.56 ± 2.41 ppm) was more than an order of magnitude higher than that of reference females (0.17 ± 0.15 ppm). Mercury level in females almost doubled from 2005 to 2006 (2005: 2.29 ± 1.48 ppm; 2006: 4.14 ± 2.53 ppm; F 1,116 = 4.94, P = 0.02; Fig. 2). Only in 2006 were there enough ASY females to compare the age classes. Post-hoc comparison indicated that mercury level was significantly higher in ASY than SY females (Tukey’s HSD 95% CI: −3.01, −0.01).
Adult female blood mercury levels were significantly higher in 2006 than in 2005 in contaminated areas (ANOVA, F 1,116 = 4.94, P = 0.02). There was no difference in mercury levels of adult birds in reference areas between 2005 and 2006. Error bars represent one SE of the mean. Bars with different letters differed significantly
Broods of nestling tree swallows in the contaminated area had higher mercury levels than broods in reference areas in 2005 (contaminated: 0.23 ± 0.17 ppm, reference: 0.02 ± 0.04 ppm; F 1,37 = 29.26, P < 0.0001). To allow comparisons with studies in which sexes are not separated, we also report the mercury level in all males from the contaminated area (4.22 ± 2.43 ppm), and all adult swallows in the contaminated area (3.69 ± 2.40 ppm).
The total mercury of feathers that grew during the 2005 breeding season was significantly related to the mercury level in blood sampled from the same birds (F 1,24 = 51.50, r 2 = 0.68, P < 0.0001; Fig. 3). There was a significant effect of treatment on feather mercury level (F 1,21 = 23.04, P < 0.0001); the feather mercury level of females nesting in the contaminated area (13.55 ± 6.94 ppm) was almost six fold higher than that of reference females (2.34 ± 0.87 ppm).
Relationship between feather and blood mercury for individual female tree swallows (r 2 = 0.68, P < 0.0001). Feathers grew at the end of the breeding season in which the blood was collected. Closed circles represent individuals from the contaminated area; open circles represent individuals from reference areas
Effects on eggs
There was no detectable difference between contaminated and reference females in terms of clutch initiation date (F 1,178 = 0.15, P = 0.70) or clutch size (F 1,178 = 0.49, P = 0.48; Table 1). There was a significant effect of female age on clutch initiation date (F 1,178 = 15.35, P = 0.0001), with ASY females nesting approximately 4 days earlier than SY females. There was also a significant effect of year on clutch initiation date (F 1,178 = 33.62, P < 0.0001). The nesting season began an average of five days earlier in the contaminated and seven days earlier in reference areas in 2006 than it did in 2005. ASY females also had larger clutches than SY females (F 1,178 = 7.29, P = 0.008) across both years by approximately 0.5 eggs. There was no detectable effect of treatment (F 1,178 = 2.55, P = 0.11; Table 1) or female age (F 1,178 = 2.64, P = 0.11) on the proportion of eggs that hatched across both years. However, there was a significant effect of year (F 1,178 = 5.48, P = 0.02); the mean proportion of eggs hatched per clutch was lower in 2006 than in 2005 (Table 1).
The proportion of eggs that survived to produce a fledgling was significantly lower in the contaminated area (F 1,173 = 8.00, P = 0.005). Post-hoc comparisons indicated a significant difference between the contaminated and reference areas in 2006 (Tukey’s HSD 95% CI: 0.15, 0.46; Table 1); however, no corresponding difference was detected in 2005. Among females in the contaminated area in 2006, the proportion of eggs fledged was significantly higher for ASY females than for the younger SY females (Tukey’s HSD 95% CI: −0.51, −0.10). ASY females in the contaminated area did not differ from those in reference areas in terms of the proportion of eggs producing fledglings, whereas SY females in the contaminated area were significantly lower than SY reference females (Tukey’s HSD 95% CI: 0.12, 0.54; Table 2).
In 2006, we measured volume of eggs at a random subsample of nests in the contaminated and reference areas. Reference eggs were larger than those from the contaminated area (F 1,151 = 13.57, P < 0.0001); however, there was a significant interaction of female age and treatment group (F 1,151 = 14.45, P < 0.0001) such that SY females laid smaller eggs than ASY females in the contaminated area, but not the reference areas (Fig. 4). Post-hoc comparisons indicate that SY female eggs in the contaminated area were significantly smaller than both SY (Tukey’s HSD 95% CI: 0.12, 0.51) and ASY (Tukey’s HSD 95% CI: 0.10, 0.45) eggs in reference areas. There was no difference in egg size between the age classes in the reference areas. Eggs from ASY females in the contaminated area were not significantly different in size from SY (Tukey’s HSD 95% CI: −0.09, 0.16) or ASY (Tukey’s HSD 95% CI: −0.10, 0.09) eggs in reference areas (Fig. 4). It should be noted that we did not sample an adequate number of SY females to draw anything but tentative conclusions about egg volume.
SY females in the contaminated areas laid smaller eggs than ASY females in contaminated and reference areas (ANOVA, F 1,151 = 14.45, P < 0.0001). Eggs of SY females in contaminated areas were smaller than eggs of SY females in reference areas (Tukey’s HSD 95% CI: 0.12, 0.51). Error bars represent one SE of the mean. Sample size, total number of eggs measured, and number of clutches measured (in parenthesis) provided above the error bar. Bars with different letters differed significantly
Effects on nestlings
Females in the contaminated area produced fewer fledglings (F 1,173 = 9.54, P = 0.002); there was a significant effect of female age (F 1,173 = 6.34, P = 0.01) and a significant interaction between year and female age (F 1,173 = 4.34, P = 0.04). In 2006, females nesting in the contaminated area produced fewer fledglings than reference females (Tukey’s HSD 95% CI: 0.43, 1.74); however, no corresponding difference was found in 2005 (Table 1). SY females in the contaminated area produced significantly fewer fledglings than ASY females in the contaminated area (Tukey’s HSD 95% CI: −3.05, −0.73) or either age class in reference areas (Tukey’s HSD 95% CI ASY: 1.48, 3.58; SY: 0.31, 2.73), but only in 2006 (Fig. 5).
In 2006, SY females in contaminated areas produced significantly fewer fledglings than all other females. The number of fledglings produced by ASY females in the contaminated areas did not differ significantly from SY or ASY females in reference areas. Error bars represent one SE of the mean. Bars with different letters differed significantly. See text for statistical analysis
When we measured productivity as proportion of nestlings that survived to fledge, rather than absolute number of fledglings, the results were similar. Females in the contaminated area fledged a smaller proportion of nestlings (F 1,173 = 4.22, P = 0.04) and there was again a significant interaction of female age and year (F 1,173 = 4.73, P = 0.03). Post-hoc comparisons indicated only SY females in 2006 experienced a reduction in the proportion of nestlings fledged.
Effects of individual mercury levels
We used linear regression to test for a relationship between reproductive success and the level of mercury in an individual female’s blood, using all females. The four measures related to hatching and survivorship of eggs or nestlings all had significant, negative relationships with blood mercury concentrations of female swallows (Table 3); however, the relatively low r 2 values indicate weak relationships with little explanatory power.
Mercury in prey items
Insects brought back to nestlings had significantly higher total mercury levels on contaminated sites (0.97 ± 1.11 ppm dw, n = 29 boluses) than those on reference sites (0.04 ± 0.04 ppm dw, n = 27 boluses; Mann-Whitney test P < 0.0001). Because prey was delivered in large boluses containing 1–57 invertebrates, it was not possible to pinpoint the species of invertebrates delivering the most mercury. Of the four highest boluses, two contained only Dipterans (flies, including some, but not all species with aquatic larvae), and two contained only Ephemeropterans (mayflies with aquatic larvae). Overall, 60% of the 953 individuals identified were Diptera, and 22% were Ephemeroptera, with the rest coming from at least eight other invertebrate orders, including terrestrial Aranae (spiders), Coleoptera (beetles) and Homoptera (true bugs).
Discussion
A population of tree swallows established in 2005 on a contaminated headwater tributary of the Shenandoah River had elevated mercury levels. Adult females in the contaminated area averaged more than 3.00 ppm total mercury in blood, an order of magnitude higher than that in the nearby reference areas, and perhaps the highest value ever reported for a songbird population. Mercury levels unexpectedly doubled between 2005 and 2006, perhaps due to increased methylmercury availability resulting from extreme drought. Other explanations for the increase, such as greater bioaccumulation by returning individuals, changes in nest box locations, or increased atmospheric deposition are not consistent with our findings (Brasso 2007). Mercury in nestling blood, while elevated over that of nestlings in reference areas, was an order of magnitude lower than that of adults in the contaminated area. This is consistent with the literature, as nestling birds apparently eliminate large amounts of blood mercury into their growing feathers (Spalding et al. 2000).
Females in the contaminated area produced fewer fledglings than reference females. This difference was most pronounced in females nesting for the first time (SY females) in 2006. SY females in the contaminated area fledged approximately two fewer nestlings than older (ASY) females in contaminated or reference areas, and approximately one less fledgling than SY reference females. Preliminary evidence suggests that SY females also produced smaller eggs than other females. In tree swallows, egg size and quality are directly related to offspring survival; clutches of larger eggs produce more and larger nestlings that grow faster (Ardia et al. 2006). Nesting in the contaminated area did not detectably affect clutch size, clutch initiation date or hatchability of eggs. This is consistent with at least one other study of mercury contamination in a songbird; great tits nesting along a contamination gradient in Belgium did not vary in terms of clutch initiation dates and clutch sizes (Janssens et al. 2003).
Contaminated females could suffer reduced reproductive success if they (1) under-provisioned eggs, (2) deposited enough mercury into eggs to reduce embryo viability, or (3) failed to feed nestlings properly due to behavioral problems. Our results are consistent with the first explanation, because our preliminary data indicate that eggs of two SY females in the contaminated areas were significantly undersized. Hatchability of eggs did not differ between contaminated and reference areas, suggesting that embryo viability was not affected. SY females in the contaminated area may also have been feeding their nestlings less, because survival rate was depressed, but direct examination of feeding rates is needed to test this hypothesis. We propose that the combination of inexperience and mercury contamination may have led to smaller eggs and subsequent decreased offspring survival. The fact that this was more evident in 2006 could have been due to environmental stress such as reduced food availability or to the higher mercury levels that year. Both nutritional stress and higher methylmercury availability could have resulted from the complete absence of rain during 2006, but we did not directly measure mercury or food availability. Though SY and ASY female tree swallows are easily distinguishable from each other, this is the first report of age-related differences in the effects of mercury on reproduction in this or other species.
Our finding that blood mercury concentrations among individual female swallows did not strongly predict their nesting success was not novel. Bald eagles (Haliaeetus leucocephalus) nesting at a lake with similar exposure to mercury as tree swallows in the present study did not show a strong relationship of eaglet mercury level with productivity (Weech et al. 2006). As eaglet blood and adult eagle blood were highly correlated, one can deduce that no relationship existed between adult mercury and productivity. The same variability of individual response to contaminants has been reported for tree swallows nesting on PCB-contaminated sites (Custer et al. 1998; Custer et al. 2003).
Tree swallows as biomonitors for mercury
A focus on mercury contamination as solely an aquatic problem (Weiner et al. 2003) has led to the overwhelming use of piscivores as biomonitors. Mercury accumulation in tree swallows has been documented in several studies in Canada and the northeastern United States. However, the mercury levels reported from the destructive sampling of nestling birds and eggs in those studies makes direct comparison with the non-lethal blood mercury levels reported here difficult. Evers et al. (2005) proposed a ratio relating mercury levels in different tissues of common loons. The ratio of feather:blood (6:1) accurately predicted the 6:1 feather-to-blood ratio derived from data for bald eagles reported by Weech et al. (2006), and that of tree swallows in the present study (5.8:1), and thus can be used cautiously as an approximation to compare blood mercury concentrations in our tree swallows to other published mercury data for this species (Table 4). The levels reported here for adult tree swallows of both sexes are the highest ever reported for this species. Across both years of our study only 11% of adult swallows sampled in the contaminated area had blood mercury under 1.00 ppm; only 3% were below 0.50 ppm. In fact, adult belted kingfishers (Ceryle alcyon), obligate piscivores, nesting along the South River in 2005–2006, were no higher than swallows, with blood mercury averaging 3.35 ± 2.67 ppm (n = 21, unpublished data). In this study of a riverine mercury point source, tree swallows accumulated mercury from contaminated insects, some of which had aquatic larvae, to the same level as a piscivorous species. Because we were able to quickly recruit a large breeding population of swallows to specified reference and contaminated locations, we detected subtle reproductive effects that may have been missed in a two-year study of one of the more popular species used as biomonitors for mercury. While most studies focus on piscivorous species (Scheuhammer et al. 2007), tree swallows should be considered an important part of the avian biomonitoring tool kit.
References
Ardia DR, Wasson MF, Winkler DW (2006) Individual quality and food availability determine yolk and egg mass and egg composition in tree swallows Tachycineta bicolor. J Avian Biol 37:252–259
Barr JF (1986) Population dynamics of the common loon (Gauia immer) associated with mercury-contaminated waters in northwestern Ontario. Canadian Wildlife Service, Ottawa, Ontario, Canada
Bishop CA, Koster MD, Chek AA, Hussell DJT, Jock K (1995) Chlorinated hydrocarbons and mercury in sediments, red-winged blackbirds (Agelaius phoeniceus) and tree swallows (Tachycineta bicolor) from wetlands in the Great Lakes-St. Lawerence River Basin. Environ Toxicol Chem 14:491–501
Brasso R (2007) The effects of mercury on the nesting success and return rate of tree swallows (Tachycineta bicolor). Masters thesis. The College of William and Mary, Williamsburg, VA, USA
Burger J, Gochfield M (1997) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75:160–172
Carter LJ (1977) Chemical plants leave unexpected legacy for two Virginia rivers. Science 198:1015–1020
Custer CM, Custer TW, Allen PD, Stromborg KL, Melancon, MJ (1998) Reproduction and environmental contamination in tree swallows nesting in the Fox River drainage and Green Bay, Wisconsin, USA. Environ Toxicol Chem 17:1786–1798
Custer CM, Custer TW, Dummer PM, Munney KL (2003) Exposure and effects of chemical contaminants on tree swallows nesting along the Housatonic River, Berkshire County, Massachusetts, USA, 1998–2000. Environ Toxicol Chem 22:1605–1621
Custer CM, Custer TW, Warburton D, Hoffman DJ, Bickham JW, Matson CW (2006) Trace element concentrations and bioindicator responses in tree swallows from northwestern Minnesota. Environ Monit Assess 118:247–266
Evers DC, Taylor KM, Major A, Taylor RJ, Poppenga RH, Scheuhammer AM (2003) Common loon eggs as indicators of methylmercury availability in North America. Ecotoxicology 12:69–81
Evers DC, Burgess NM, Champoux L, Hoskins B, Major A, Goodale WM, Taylor RJ, Poppenga R, Daigle T (2005) Patterns and interpretation of mercury exposure in freshwater avian communities of northeastern north America. Ecotoxicology 14:193–221
Gerrard PM, St Louis VL (2001) The effects of experimental reservoir creation on the bioaccumulation of methylmercury and reproductive success of tree swallows (Tachycineta bicolor). Environ Sci Technol 35:1329–1338
Heinz GH, Hoffman DJ (2003) Predicting mercury in mallard ducklings from mercury in chorioallantoic membranes. Bull Environ Contamin Toxicol 70:1242–1246
Hoyt DF (1979) Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77
Janssens E, Dauwe T, Pinxten R, Eens M (2003) Breeding performance of great tits (Parus major) along a gradient of heavy metal pollution. Environ Toxicol Chem 22:1140–1145
Mengelkoch JM, Niemi GJ, Regal RR (2004) Diet of the nestling tree swallow. Condor 106:423–429
McCarty JP (2001) Use of tree swallows in studies of environmental stress. Rev Toxicol 4:61–104
Robertson RJ, Stutchbury BJ, Cohen RR (1992) Tree swallow. In: Poole A, Stettenheim P, Gill F (eds) The birds of North America, no. 11. Academy of Natural Sciences, Philadelphia, and American Ornithologists’ Union, Washington DC
Scheuhammer AM, Meyer WM, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36:12–19
Spalding MG, Frederick PC, McGill HC, Bouton SN, McDowell LR (2000) Methylmercury accumulation in tissues and its effects on growth and appetite in captive great egrets. J Wildl Dis 36:411–422
Stutchbury BJ, Rohwer S (1990) Molt patterns in the tree swallow (Tachycineta bicolor). Can J Zool 68:1468–1472
Thompson DR (1996) Mercury in birds and terrestrial mammals. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife: interpreting tissue concentrations. Lewis Publishers, Boca Raton, FL, USA, pp 341–356
USDI (1998) Guidelines for the interpretation of the biological effects of selected constituents in biota, water, and sediment: mercury, Report No. 3. National irrigation water quality program information, United States Department of the Interior
Weech SA, Scheuhammer AM, Elliott JE (2006) Mercury exposure and reproduction in fish–eating birds breeding in the Pinchi Lake region, British Columbia, Canada. Environ Toxicol Chem 25:1433–1440
Weiner JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM (2003) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (eds) Handbook of ecotoxicology. Lewis Publishing, Boca Raton, FL
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160
Acknowledgements
Funding was provided by E. I. DuPont de Nemours, the National Science Foundation UBM 0436318, and the Office of the Vice Provost for Research at the College of William & Mary. We thank Anne Condon, Rachel Fovargue, Scott Friedman, Kelly Hallinger, Dana Hawley, Ravi Jefferson George, Sean Koebley, Liz Langer, Maryse Leandre, Adrian Monroe, Jack Reese, and Ariel White for assistance with fieldwork. We are also indebted to all of the cooperative landowners in the Shenandoah Valley, graduate committee members John Swaddle and Randy Chambers, and the entire South River Science Team.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brasso, R.L., Cristol, D.A. Effects of mercury exposure on the reproductive success of tree swallows (Tachycineta bicolor). Ecotoxicology 17, 133–141 (2008). https://doi.org/10.1007/s10646-007-0163-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-007-0163-z