Abstract
Three-dimensional (3D) bioprinting is an emerging field that holds promise for creating functional living tissues and organs. Bioprinting enables to fabricate structurally complex 3D tissue constructs by precise positioning and spatially separated patterns of multiple types of cells, biomaterials, and bioactive molecules within a single construct. With recent advances in bioprinting strategies, 3D bioprinting has been applied in various research areas, including tissue engineering and regenerative medicine, biology, physiology, drug discovery, and cancer/stem cell research. In tissue engineering and regenerative medicine, many types of 3D tissue constructs have been bioprinted to generate functional tissues for implantation, with the ultimate goal of clinical use. In addition, 3D bioprinting has been used as a tool to create in vitro tissue/organ models for drug discovery and cancer research, enabling deeper understanding of physiological phenomena of specific tissues/organs and more accurate prediction of drug or toxicity responses. In this chapter, we discuss recent applications of 3D bioprinting; first to create tissues and organs for the purposes of tissue engineering and regenerative medicine and then as platforms for in vitro tissue/organ models in drug discovery/toxicity testing and cancer research. We also discuss current challenges and future perspectives for practical applications of 3D bioprinting.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Biofabrication
- Bioprinting
- Application
- Translation
- Transplantation
- In vitro tissue/organ model
- Tissue engineering
- Regenerative medicine
- Drug discovery
- Toxicity test
- Drug screening
- Cancer research
1 Introduction
Bioprinting technology enables the creation of three-dimensional (3D) living tissue and organ constructs with potential use in a variety of applications. Bioprinting allows for precise positioning of different tissue elements, such as living cells, biomaterials, and bioactive molecules, in a spatially organized pattern within a single structure through computer-aided design and manufacturing (CAD/CAM) (Arslan-Yildiz et al. 2016; Mandrycky et al. 2015; Ozbolat 2015). 3D living tissue constructs are fabricated during the printing process by direct patterning and stacking of cell-laden bioinks, one layer at a time (Murphy and Atala 2014). Because of its detailed nature, 3D bioprinted structures have the potential to accurately mimic the complex structure and function of native tissues, as compared to traditional fabrication methods of 3D scaffolds (Arslan-Yildiz et al. 2016).
To create 3D living tissue constructs with the desired structural and functional mimicry of target tissues, many factors need to be considered, including the type of bioprinter, cells, and bioinks. Commonly used bioprinting methods are inkjet-, extrusion-, and laser-based bioprinting. The features, advantages, and drawbacks of each printing modality are summarized in Table 1 (Arslan-Yildiz et al. 2016; Mandrycky et al. 2015; Murphy and Atala 2014; Sears et al. 2016). Recently, bioprinting systems have been combined with other fabrication technologies to enhance outcomes (Chang et al. 2010; Kolesky et al. 2016). To achieve the biological functions of target tissues, selection of cells is critical. A variety of primary/immortalized cells and progenitor/stem cells have been used for bioprinting (Murphy and Atala 2014). Heterogeneous tissue constructs with spatially organized patterns of multiple cell types have been created to engineer more complex and functional tissue constructs (Duan et al. 2013; Fedorovich et al. 2011; Kolesky et al. 2014; Xu et al. 2013b). Bioinks also have been refined in recent years. Naturally derived hydrogels, such as fibrinogen, collagen, hyaluronic acid (HA), and gelatin, are mainly used because they can provide superior cell survival and proliferation (Stanton et al. 2015). In addition, synthetic polymers such as polyethylene glycol (PEG), polycaprolactone (PCL), and polyurethane (PU) have been used as bioinks (Cui et al. 2012), or have been patterned as a supporting architecture within the tissue constructs to enhance their mechanical properties (Kang et al. 2016; Merceron et al. 2015).
3D bioprinting technology has been used in various fields, including tissue engineering and regenerative medicine, pharmaceutical, drug discovery, cancer, and personalized medicine research (Arslan-Yildiz et al. 2016; Knowlton et al. 2015; Ozbolat et al. 2016). Several types of tissue constructs have been bioprinted with tissue-specific cells or stem cells and successfully implanted in vivo. In addition, 3D bioprinted in vitro tissue/organ models offer new opportunities for drug discovery and toxicity testing, as well as for cancer research (Pati et al. 2016). As such, 3D bioprinting technology presents with an enormous potential to change the way science and medicine is practiced.
In this chapter, the current development efforts and utility of 3D bioprinting technology will be discussed. These include bioprinting of tissue and organ constructs for reconstruction and development of in vitro tissue and organ models for drug discovery and screening. Furthermore, we discuss current challenges and future perspectives in advancing 3D bioprinting technology for translational applications.
2 Bioprinting of Tissues and Organs for Implantation In Vivo
The ability of 3D bioprinting to create living tissues and organs with complex geometry and function has led to translational applications in tissue engineering and regenerative medicine. With 3D bioprinting technology, investigators have created tissue constructs such as the bone (Tang et al. 2016), cartilage (Kang et al. 2016), skin (Skardal et al. 2012), nerve tissue (Owens et al. 2013), cardiac tissue (Duan 2016) and heart valve (Jana and Lerman 2015), and blood vessels (Hoch et al. 2014) for structural and functional repair of damaged tissues (Table 2). Currently, 3D bioprinted tissue constructs have been applied to various animal models, which show potential for functional tissue regeneration (Box 1) (Arslan-Yildiz et al. 2016; Ozbolat et al. 2016; Seol et al. 2014). By combining medical imaging and CAD/CAM technology, investigators are able to engineer anatomically accurate, patient-specific tissue constructs for reconstructive procedures (Kang et al. 2016; Murphy and Atala 2014).
Despite great progress, current applications of 3D bioprinted tissues are mainly limited to implantation in small animals (Ozbolat et al. 2016). To build clinically relevant sized tissue constructs for application, many challenges have to be addressed (see Sect. 4). In this section, we review recent applications of 3D bioprinting in tissue engineering and regenerative medicine and discuss 3D bioprinting strategies for mimicking tissue-specific properties of structure and function and their biological outcomes.
Box 1
Advantages of 3D bioprinting for transplantation:
-
Engineers 3D living tissue/organ constructs that mimic natural structure and function
-
Forms complex tissues by direct patterning and precise placement of living cells on specific locations
-
Creates anatomically shaped, patient-specific tissue constructs by combining medical imaging and CAD/CAM technologies
-
Rapidly produces tissues and organs compared with traditional tissue engineering scaffold fabrication methods
2.1 Bone
The bone supports the body structure, controls movement, and protects other organs (Cohen 2006). The bone is known to possess relatively good regenerative and self-repair capacity when defects are small. However, when bone is subjected to injury from trauma, cancer, pathological fractures, infection, or arthritis and other rheumatic diseases, its ability to fully regenerate is impaired and can lead to loss of function. Current surgical treatment options for bony defects include autograft, allograft, or prosthetic procedures. Autografts are considered as the best option for treating extensive bone defects, but it is expected that approximately 60% of them will fail within 10 years (Cancedda et al. 2007). As an alternative, engineered bone scaffolds have been actively explored in the tissue engineering and regenerative medicine areas. Most of these scaffold are composed of homogeneous mixtures of biomaterials, bioactive molecules (e.g., osteogenic factors), and cells. Cell-free scaffolds have been translated into the clinic, but are limited by inadequate integration into native bone and bone tissue formation (Gibbs et al. 2014). Recently, cell-based bone tissue constructs have been engineered, but it remains difficult to create clinically relevant tissue constructs, in terms of shape and size with conventional fabrication techniques (Amini et al. 2012).
Bioprinting technology has become an emerging method for bone graft generation. Grafts created via 3D bioprinting contain cellular and noncellular components in a single construct, thereby mimicking the complex structure and functionality of native bone tissue. Bioprinted bone constructs with cell-laden biomaterials have been successfully implanted into animal models of bony defects (Tang et al. 2016). In a recent report, bone tissue constructs were fabricated using an integrated tissue-organ printer (ITOP) system consisting of multi-dispensing modules; bone tissue formation was successful in their animal model (Kang et al. 2016). Calvarial bone constructs (8 mm diameter × 1.2 mm thickness) were fabricated with PCL/tricalcium phosphate (TCP) mixture and human amniotic fluid-derived stem cells (hAFSCs) (Fig. 1a–b). In this study, composite hydrogels consisting of fibrinogen, gelatin, HA, and glycerol were used to deliver hAFSCs. To mimic the mechanical properties and structural stability of 3D bone constructs, multiple layers of PCL/TCP patterns were placed in between hAFSC patterns. By patterning Pluronic F-127 in outer layers of the constructs as a sacrificial material, the complex 3D structure was maintained during the entire printing process. The Pluronic F-127 was then dissolved out after cross-linking of fibrinogen with thrombin. Micro-channels (500 × 300 um2) created within the construct were essential to maintain viability of cells by supplying adequate oxygen and nutrients supplement to the volumetric construct through diffusion. The printed bone constructs were then implanted to a calvarial bone defect region in Sprague Dawley rats. Five months later, new bone formation was observed in the implanted constructs with adequate vascular integration, demonstrating the feasibility of using 3D bioprinted bone constructs for calvarial bone tissue reconstruction.
Bioprinting of the bone. (a–b) Calvarial bone reconstruction (Kang et al. 2016). (a) Visualized motion program for bioprinting calvarial bone construct (top) and 3D bioprinted calvarial bone construct (bottom). (b) Photographs of implanted calvarial bone constructs in calvarial bone defect model in rats at day 0 and month 5. (c–f) Mandible bone reconstruction (Kang et al. 2016). (c) 3D CAD model of human mandible bone defect. (d) 3D human mandible bone construct generated by visualized motion program. (e) 3D bioprinted mandible bone construct after 28 days of in vitro culture. (f) Osteogenic differentiation of hAFSCs in the mandible bone constructs as evidenced by calcium deposition with Alizarin Red S staining (red color) (Figures reprinted with permission from Kang et al. (2016))
For bone tissue reconstruction and regeneration, customized and personalized tissue constructions to fit patient-specific defects in size and shape would be desirable. Unlike other tissues, the bone is relatively hard, so a mismatch in shape and size between defects and implants may lead to failure of implantation or unfavorable outcomes (Shafiee and Atala 2016). 3D bioprinting enables to fabricate anatomically accurate patient-specific tissue constructs. Kang et al. fabricated a customized mandible bone construct based on a CT scan of a human mandible defect for mandible bone reconstruction (Fig. 1c–f) (Kang et al. 2016). The mandible bone construct was fabricated using hAFSC-laden composite hydrogels (final dimensions 3.6 cm × 3.0 cm × 1.6 cm), and osteogenic differentiation of hAFSCs was observed in vitro.
Small bone constructs containing cells (mm scale) have been fabricated via 3D bioprinting technologies and successfully implanted in animal models. However, larger-scale bone constructs for translational applications in large animal models and eventual clinical translation are still challenged by poor cell survival due to limited vascularization. To prevent cell death and achieve successful bone formation of 3D bioprinted bone constructs, adequate supply of oxygen and nutrients is necessary; thus, one approach is to fabricate pre-vascularized bone constructs (Liu et al. 2013). Fedorovich et al. printed heterogeneous constructs containing two different cell types with a BioScaffolderTM pneumatic dispensing system (Fedorovich et al. 2011). Porous constructs containing spatially organized mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs) were printed. The constructs were implanted subcutaneously, and tissue formation by each type of cell was evaluated. In their study, the printed constructs maintained cellular heterogeneity after implantation; bone formation and mature blood vessel formation were observed in constructs containing MSCs and EPCs at 6 weeks post-implantation. This study illustrates that functional vascularized bone grafts can be created by bioprinting, which may eventually be used for treating larger bone defects in patients.
2.2 Cartilage
With the increasing numbers of aging people in our population, and the greater numbers of obese individuals, more people have cartilage damage and osteoarthritis than ever before (Li et al. 2013). Current treatments for articular cartilage repair include mosaicplasty, microfracture, autologous chondrocyte transplantation, and osteochondral allograft transplantation. Unfortunately, these treatments often result in structural and mechanical mismatches between regenerated tissues and surrounding native cartilage (Jeong and Atala 2015; Schuurman et al. 2015).
Articular cartilage is heterogeneous and composed of zonally differentiated cells and extracellular matrix (ECM); each zone has different biological and mechanical properties. Tissue engineering approaches aim to mimic the zonal structure and function of the articular cartilage to recapitulate normal cartilage; however, recreating zonally stratified articular cartilage tissue remains a challenge. To this end, 3D bioprinting technology has been utilized to engineer articular cartilage constructs with zonally different cells and ECMs composition. Schuurman et al. designed a 3D zonal architecture of cartilage tissues composed of different cells contained in hydrogel and thermoplastic fibers in patterns with different mechanical stiffnesses, with the goal of improving clinical outcomes following implantation (Schuurman et al. 2011, 2015).
Current tissue engineering approaches for osteochondral implants to regenerate cartilage and subchondral bone are limited by insufficient tissue formation and poor integration between layers of cartilage and bone. In conventional strategies for engineering osteochondral composite tissues, two different types of scaffolds, one for cartilage and the other for bone, were created and then combined physically or chemically (Nooeaid et al. 2012). However, the two scaffolds were easily separated following implantation, leading to insufficient osteochondral tissue formation (Schaefer et al. 2000).
Bioprinting technology is expected to address these issues because it allows for controlled spatial organization of multiple cell types and biomaterials in a single contiguous construct. Fedorovich et al. fabricated a cell-laden, heterogeneous osteochondral graft by introducing a 3D fiber deposition technique (Fedorovich et al. 2012). Alginate bioinks combined with chondrocytes and osteogenic progenitor cells were deposited directly adjacent to each other, with dimensions of 1 cm × 2 cm. The spatially organized cells remained in the initially printed region during in vitro culture. Six weeks after subcutaneous implantation in mice, heterogeneous tissue formation with a specific organization and matrix composition was observed, as evidenced by osteocalcin and collagen production. This study demonstrates the feasibility of engineering centimeter-scaled, porous, heterogeneous constructs using 3D fiber deposition technology to regenerate osteochondral defects.
Naturally derived biomaterials are widely used as cell-carrier materials in bioprinting due to their structural and biological resemblance to native tissues. However, these biomaterials have low to moderate mechanical properties that limit their utility in bioprinting of load-bearing tissues such as the bone and cartilage (Schuurman et al. 2011). Therefore, Xu et al. introduced a hybrid inkjet/electrospinning system to generate durable tissue constructs (Fig. 2a) (Xu et al. 2013a). They constructed electrospun PCL fibers and printed rabbit elastic chondrocytes in fibrin-collagen hydrogels deposited in alternating layers of 1 mm thickness. The hybrid constructs demonstrated enhanced mechanical properties compared to the constructs fabricated using inkjet printing alone, and the constructs formed cartilage-like tissue after subcutaneous implantation in mice.
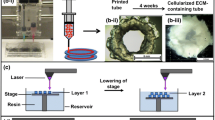
Bioprinting of the cartilage. (a) Bioprinting of hybrid cartilage tissue construct by combining inkjet printing and electrospinning technologies (Xu et al. 2013a). (b–c) Human ear-shaped cartilage reconstruction (Kang et al. 2016). (b) 3D bioprinting of human ear by using the ITOP system and (c) cartilage formation in vivo (Figures reprinted with permission from Xu et al. (2013a) and Kang et al. (2016))
3D printing also has been used to fabricate human auricular prostheses, and clinical applications for patients have been reported (Watson and Hatamleh 2014). However, 3D bioprinting of cell-laden ear structures remains challenging because it is difficult to print mechanically stable, human-scale tissue constructs with 3D complex architecture by using cell-laden hydrogel platforms. Very recently, Kang et al. successfully fabricated a human-sized external ear construct by using the ITOP system guided by CT images of the human ear (Fig. 2b) (Kang et al. 2016). The system contains multi-dispensing modules that can print chondrocyte-laden composite hydrogel and PCL layers in a single construct. The multiple PCL patterns prevented the collapse of cell-laden hydrogel patterns, resulting in construction of a structurally stable human ear (3.2 cm × 1.6 cm × 0.9 cm) with cartilaginous matrix formation in vitro. The anatomically shaped human ear was implanted in dorsal subcutaneous tissues in mice for 2 months; cartilage formation in the implants and structural maintenance were observed (Fig. 2c). This work brings the creation of 3D bioprinted biological ear cartilage constructs closer to human applications.
2.3 Skin
The skin is the largest organ in the body and plays an important role in the protection from the external environment and maintaining homeostasis (Bouwstra et al. 2003). Current treatment methods for skin injuries mainly caused by extensive burn injuries and full-thickness skin wounds include autologous split-thickness skin grafts, allografts, and xenografts. Despite some successful results in clinical applications, these treatments are still limited by the size of donor sites, immune rejection, and poor cosmetic outcomes (Sheridan and Greenhalgh 2014; Skardal and Atala 2015). One alternative is the use of acellular dermal skin substitutes such as Integra® and MatriDerm®. Although these substitutes can overcome some limitations and achieve efficient re-epithelialization and revascularization of the damaged skin, regeneration of damaged skin is a lengthy process (Pereira et al. 2007; Pham et al. 2007). Thus, cell-based skin substitutes, such as Dermagraft®, Apligraf®, and TransCyteTM, have been used to accelerate wound repair of large skin defects. These products require several weeks to culture cells in vitro prior to clinical use (Pham et al. 2007; Skardal et al. 2012). An alternative strategy, cell spraying, delivers cells directly to the wound, which is effective for superficial and partial-thickness burns, but further advances are needed (Gerlach et al. 2011).
One of the promising methods for cell-based therapy of skin injuries is skin bioprinting. Skin bioprinting can mimic the proper anatomic configuration of skin, which composed of multiple layers containing different cell types. The skin is comprised of two layers of dermis and epidermis, of which the main cell type is fibroblasts and keratinocytes (Bouwstra et al. 2003). To mimic normal human skin, Yoon et al. developed 3D skin grafts by modifying rapid prototyping methods and cell printing techniques (Fig. 3a) (Yoon et al. 2016). The bioprinted skin graft was composed of four layers of cell-laden collagen hydrogels. The top layer was printed with keratinocytes as an epidermis, and the other three layers of the bottom were fabricated with fibroblasts as a dermis. The printed scaffolds with dimensions of 5 × 5 mm2 were transplanted to the full-thickness excision model of mice and demonstrated human skin-like tissue reconstruction and effective proliferation and migration of keratinocytes and fibroblasts. Another study by Michael et al. created a multilayered, cellularized skin substrate via a laser-assisted bioprinting technique (Fig. 3b–d) (Koch et al. 2012). The skin substrates were fabricated by depositing 20 layers each of fibroblast- and keratinocyte-embedded collagen matrices onto a sheet of Matriderm® (2.3 cm × 2.3 cm), a commercialized acellular skin graft. The skin substitutes were fully connected to the host skin tissue and formed a stratified epidermis with differentiation and formation of the stratum corneum when they were transplanted to the full-thickness skin wounds on dorsa of mice (Michael et al. 2013).
Bioprinting of the skin. (a) Multilayered, cell-laden 3D skin scaffold composed of one layer of keratinocytes and three layers of fibroblasts (top) and transplantation of the bioprinted skin scaffold in full-thickness skin excision model in a mouse (bottom) (Yoon et al. 2016). (b–d) Bi-layered skin tissue generated by laser cell printing (Koch et al. 2012). (b) Micropatterning capacity of the laser printing. (c) Hematoxylin and eosin stained images and (d) fluorescent images of skin mimicking a bi-layered construct composed of 20 layers of fibroblasts (red) and two layers of human keratinocytes (green) (Figures reprinted with permission from Yoon et al. (2016) and Koch et al. (2012))
Recently, in situ bioprinting of stratified skin substitutes has been proposed as a technique to apply skin cells directly onto the wound surface. In situ skin bioprinting technology can rapidly and uniformly cover wounds with different composition of cells and ECMs and geometry, depending on the wound sites. Skardal et al. used a skin bioprinter for in situ regeneration of large-scale wounds and burns (Skardal et al. 2012). Using in situ 3D bioprinting, skin substitutes composed of two layers of AFSCs-laden fibrin-collagen hydrogels were bioprinted directly onto full-thickness wounds in mice. Wound closure with re-epithelialization and microvascularization was achieved. For cross-linking, two layers of fibrin-collagen gel, used as a skin substitute, were printed by alternatively depositing thrombin and fibrinogen/collagen layers. AFSCs have a high proliferation rate, can differentiate to multiple lineages, and are nonimmunogenic.
A skin printer is currently being developed and optimized (Atala and Yoo 2015). The skin printer is built into a portable frame that is easily accessible for patients in the operating room. A scanning system is incorporated into the skin printer, scans the topography and dimensions of the wound, and guides the printer to deposit cells and extracellular matrix in layers to approximate the anatomic skin configuration. By introducing multiple-dispensing modules, skin substitutes composed of fibroblast- and keratinocyte-laden fibrin/collagen gel layers can be bioprinted in situ (Atala and Yoo 2015). Wound healing capacity of the in situ bioprinted skin substitutes is being investigated in full-thickness wound models in pigs before being tested in clinical applications.
2.4 Nerve
Clinically, the goal of surgery of damaged or severed nerve is to minimize loss of function by suturing the ends of the nerves. If this is not possible, a nerve guide is required to bridge the severed nerve ends to regrow axons and restore motor and sensory function. The gold standard for repair of nerve tissue is an autologous graft, but it is limited by donor site morbidity and mismatches between diameters and mechanical properties of the nerve (Wolford and Stevao 2003). Autologous vein grafts, as a non-neural autologous tissue, have been used clinically and show axonal regeneration with functional repair, but these effects are not proven for gaps larger than 3 cm (Wolford and Stevao 2003). Most grafts currently in clinical use are noncellular grafts composed of synthetic polymers (e.g., PEG, PCL) or collagen (Gu et al. 2014). These materials allow control of graft size and address the shortage of the autologous grafts. However, noncellular grafts could not fully regenerate severed nerve tissues without cellular components. Thus, developing a cellular nerve graft is required for nerve repair, but it is still a challenge for tissue engineering and regenerative medicine.
Current neural tissue engineering has been focused on constructing straight tubular structures to guide nerve and cell growth. In particular, 3D bioprinting techniques enable control of the diameter and length of the nerve grafts as well as geometrical parameters. By applying 3D bioprinting techniques, Marga et al. developed a fully cellular bioprinted nerve graft for repair of peripheral nerve injury (Fig. 4) (Marga et al. 2012; Owens et al. 2013). They used a scaffold-free, self-assembly-based method to construct 3D nerve grafts, which had structures with multi-lumen channels. To create cellular cylinders, cellular spheroids (~500 μm) composed of Schwann cells and bone marrow stem cells (BMSCs) were printed, together with agarose support rods, by an extrusion-based bioprinting system (Fig. 4a–b). The printed cellular spheroids fused over time, resulting in formation of continuous tubes. After maturation for 7 days, the supporting agarose rods were removed for implantation (Fig. 4c). Histologic and functional repair of the fully biological nerve grafts was evaluated in a rat model of sciatic nerve injury model. The injury model was created by excision of 1 cm of sciatic nerve and then bridging the resulting gap with the bioprinted nerve graft. After 3 weeks of follow-up, about 40% of axons crossed the implanted nerve graft (Marga et al. 2012). This model showed functional repair of motor and sensory neurons at 40 weeks post-implantation, demonstrating the potential of a fully cellular bioprinted nerve graft for regenerating damaged or severed nerves (Owens et al. 2013).
Bioprinting of fully cellular nerve grafts (Marga et al. 2012; Owens et al. 2013). (a) Schematic diagram to create tubular structures with cellular spheroids and arrangement. (b) Schematics of bioprinting strategy for multicellular, cylinder-shaped nerve grafts (left) and photographs of bioprinted nerve grafts (right). (c) Bielschowsky’s staining of histological sections of a bioprinted nerve graft, which was transplanted in the sciatic nerve damage model in a rat, showing axonal regeneration in the graft at week 40 (Figures reprinted with permission from Marga et al. (2012) and Owens et al. (2013))
2.5 Blood Vessels and Vascular Networks
Blood vessels and microvascular networks are essential to transfer oxygen and nutrients and remove metabolic wastes in the body (Novosel et al. 2011). In the field of tissue engineering and regenerative medicine, the ability to engineer functional blood vessels and vascular networks is required for viable tissues (Novosel et al. 2011). Any types of cell cannot survive in large volumetric tissue constructs for a long time without vascular networks, since the maximum diffusion distance of oxygen and nutrients is only about 200 μm (Novosel et al. 2011). Success in transplantation of bioprinted scale-up tissues and organs also may depend on vascularization which provides oxygen and nutrient supply to the cells for their survival and thereby exerting normal function. However, engineering of the vascular structure within 3D constructs remains a significant challenge in 3D tissues and organs. Several methods have been developed for fabricating vascular and microvascular structures by using 3D bioprinting technology (Hoch et al. 2014). Even though only a few have been tested in vivo, several approaches have potential use for in vivo applications on their own, or for creating vascularized complex tissue constructs.
A scaffold-free, self-assembly-based bioprinting approach similar to that used for nerves (mentioned in the previous section) was also used to engineer vascular grafts (Fig. 5a, b) (Marga et al. 2012; Norotte et al. 2009). To fabricate a cylindrical vascular graft, cell spheroids including human aortic smooth muscle cells, human aortic endothelial cells, and human dermal fibroblasts were printed in the agarose templates (Fig. 5a). The printed multicellular cell spheroids were fused and self-assembled into tubular structures at the post-printing stages (Fig. 5b, c), and the vascular graft was perfused in the bioreactor for vessel conditioning and maturation up to 3 weeks. This study yielded fully functional perfused and clinically relevant vascular grafts, which are applicable in vivo (Fig. 5d). A scaffold-free vascular tissue was generated by combining needle-assay technology and 3D bioprinting by Itoh et al. (Itoh et al. 2015). The 3D bioprinter placed multicellular spheroids in the needle-array system to have a 3D tube-shaped structure, and then it was matured in a perfusion system. The 3D printed vascular tissues of 1.5 mm in diameter and 7 mm in length were implanted into abdominal aortas of rats showing remodeling and endothelialization. In another study by Lee et al., a functional vascular channel with a perfused open lumen was created by combining 3D bioprinting with a flow chamber perfusion system (Lee et al. 2014). To fabricate the vascular channel, a mixture of gelatin and human umbilical vein endothelial cells (HUVECs) was printed within a thick collagen matrix, and the printed constructs were then transferred to a custom-designed flow chamber. The resulting vascular grafts had a lumen structure covered with viable, aligned endothelial lining. The structure was maintained for up to 2 weeks under dynamic flow conditions (Fig. 5e, f).
Bioprinting of blood vessels and microvascular networks. (a–d) Bioprinting scaffold-free tubular structures for engineering blood vessels (Marga et al. 2012; Norotte et al. 2009). (a) The printed construct by layer-by-layer deposition of agarose rods and cells spheroids. (b) Photographs of bioprinted blood vessels resulted after 3 days of post-printed fusion. (c) The branched tubular construct after 6 days of post-printed fusion. (d) Masson’s trichrome staining of bioprinted blood vessels at day 21 of in vitro culture. (e–f) Perfused functional vascular channels (Lee et al. 2014). (e) Schematics of the bioprinted vascular channel construction connected to a custom-designed flow chamber. (f) Fluorescent images of vascular channel system on in vitro dynamic flow culture indicating the creation of perfused open lumen. (g) 3D printed vascularized, heterogeneous cell-laden tissue constructs (Kolesky et al. 2014). (h) 3D vascularized thick tissue housed within a perfusion chamber (Kolesky et al. 2016) (Figures reprinted with permission from Marga et al. (2012), Norotte et al. (2009), Lee et al. (2014), and Kolesky et al.(2016))
Very recently, Kolesky et al. bioprinted a 3D cell-laden, vascularized thick tissue (>1 cm) that could be perfused for over 6 weeks (Kolesky et al. 2016). They also demonstrated the ability to create 3D bioprinted microvascular networks by using extrusion printing (Fig. 5g) (Kolesky et al. 2014). In that study, they integrated parenchyma, stroma, and endothelium into a single engineered 3D tissue model by co-printing multiple bioinks composed of human MSCs (hMSCs) and human neonatal dermal fibroblasts in which vasculature was embedded (Fig. 5h). To create vascular networks within the customized and multicellular perfusion chip, acellular Pluronic F127 was used as a fugitive ink. After washing out the ink using cold cell media, HUVECs were injected to fill the vascular network. This 3D microvasculature perfused growth factors, which promoted differentiation of hMSCs toward an osteogenic lineage in situ. This study proposed a strategy to fabricate a physiologically relevant 3D vascularized thick tissue model using biofabrication technology with potential use for in vivo applications.
2.6 Cardiac Tissue and Heart Valve
Engineered cardiac tissue constructs for damaged cardiac muscles have been developed in tissue engineering and regenerative medicine, but very little success has been achieved in clinical trials due to significant biologic and physiologic challenges (Buikema et al. 2013; Jawad et al. 2008). In addition, traditional fabrication methods are inadequate to create cardiac constructs that mimic the complex anatomy of myocardial organization and beating property. Therefore, 3D bioprinting of cardiac tissue is promising in cardiac tissue reconstruction and repair (Duan 2016). For instance, Gaebel et al. fabricated a geometrically patterned cardiac patch by using a laser-based printer for treatment of myocardial infarction (Gaebel et al. 2011). The cardiac patch was patterned with hMSCs and HUVECs on polyester urethane urea (PEUU) and transplanted to the infarcted area in a rat after ligation of left anterior descending coronary artery (Fig. 6a). This bioprinted cardiac patch enhanced vessel formation and preservation of cardiac function. This group also showed the printability of human cardiac-derived cardiomyocyte progenitor cells (hCMPCs) by using a BioScaffolder (Gaetani et al. 2012). Printed hCMPCs were highly viable after at least 7 days in vitro culture and showed cardiac lineage phenotypes, as evidenced by gene expression of early cardiac transcription factors.
Bioprinting of the cardiac tissue and heart valve. (a) Bioprinted cardiac patch with HUVECs and hMSCs in a defined pattern and cardiac repair evidenced by increased infarct wall thickness (Fast Green FCF/Sirius Red staining images) (Gaebel et al. 2011). (b) 3D bioprinted heterogeneous aortic valve conduit (Duan et al. 2013). (c) 3D bioprinted trileaflet valve conduit (Duan et al. 2014) (Figures reprinted with permission from Gaebel et al. (2011) and Duan et al. (2013))
Heart valves are complex, with very limited regeneration capacity. Mechanical or biological prostheses have been used to replace damaged heart valves, but have been plagued by thrombogenicity and calcification (Jana and Lerman 2015). By contrast, 3D bioprinting technology can fabricate functional and cellular heart valves that mimic the native anatomic complexity. Duan et al. printed anatomically complex, heterogeneous aortic valve conduits using extrusion-based, dual-nozzle bioprinters (Duan et al. 2013). The valve root and leaflet were patterned with aortic root sinus smooth muscle cells and aortic valve leaflet interstitial cells, encapsulated on alginate/gelatin composite hydrogels, respectively (Fig. 6b). Printed cells were over 80% viable at 7 days in vitro culture within the 3D structure, with phenotypic retention. This group later fabricated a trileaflet valve conduit using human aortic valvular interstitial cells and encapsulated hybrid hydrogels (Fig. 6c) (Duan et al. 2014). By controlling the concentration and ratio of methacrylated hyaluronic acid and methacrylated gelatin in the hybrid hydrogel, they could fabricate anatomically accurate trileaflet valves that were highly viable and had remodeling potential.
3 Biofabrication of In Vitro Tissue/Organ Models
In vitro tissue/organ models can provide platforms for development of drugs and specific therapeutics and for understanding biological phenomena. Current 2D culture models cannot provide the tissue-specific, physiologically relevant functions of 3D models due to lack of cell-cell and cell-microenvironment interactions (Griffith and Swartz 2006). With the marked advances in 3D tissue fabrication/culture methods within the last decade, 3D in vitro tissue/organ models can now provide more realistic environments that mimic spatial and chemical complexity of living tissues and tissue-specific functions (Pampaloni et al. 2007). Various types of 3D tissue/organ models have been developed, such as randomly distributed or spatially separated cell-encapsulated hydrogel constructs, cellular spheroids, and mini-organs or organoids (tissue-like masses mimicking parts of an organ’s functions). In addition, microfluidic organs on chips have been developed by combining 3D culture techniques with microfluidic systems (Bhatia and Ingber 2014). However, most in vitro 3D tissue/organ models are still unable to create highly controllable, multicellular, spatially and functionally complex microscale architecture (Pati et al. 2016). Therefore, 3D bioprinting techniques have been introduced to develop 3D in vitro tissue/organ models (Arslan-Yildiz et al. 2016; Cancedda et al. 2007; Pati et al. 2016). Although 3D bioprinted in vitro tissue/organ models are relatively new, they offer platforms for deeper understanding of physiological phenomena of tissues/organs and more accurate prediction of therapeutic/toxic responses. Advantages of 3D bioprinted in vitro tissue/organ models are described in Box 2 (Arslan-Yildiz et al. 2016; Mandrycky et al. 2015; Ozbolat et al. 2016; Pati et al. 2016). In the next section, we introduce and discuss 3D bioprinted tissue/organ models for drug discovery and toxicity testing and cancer research (Table 3).
Box 2
Advantages of 3D bioprinted in vitro tissue/organ models:
-
Realistic 3D mimicry of complex morphological, pathological, and physiological structure and functions of living tissue in vitro
-
Multiple cell types and biomaterials at targeted locations with high precision
-
Creates cell-cell interactions, cell-extracellular matrix environments, and tissue-tissue interfaces
-
Rapid production and high-throughput screening
-
Accurately predictable and cost-effective preclinical drug discovery/screening tools
-
Elucidates basic mechanisms of tissue/organ physiology, pathophysiology, and tumorigenesis, reducing need for animal studies and facilitating translation of drugs into clinics
-
Possible customization and personalization of specific tissue models for drug discovery and therapeutics
3.1 Drug Discovery and Toxicity Testing
The use of 3D bioprinted in vitro tissue/organ models is promising in drug discovery and toxicity test studies with various advantages. These models can be more realistic than traditional 2D culture and 3D tissue models. 3D bioprinting can fabricate complex tissues/organs, either normal or diseased, by controlling specific locations of several cell types and materials, which could improve accuracy of drug response. The development of in vitro human tissue/organ models with high precision can reduce the numbers of preclinical animal studies needed for drug testing, thereby reducing time and costs and accelerating translation of drugs to clinical applications (Pati et al. 2016). Therefore, 3D bioprinted in vitro tissue/organ models can offer an accurate, reproducible, highly controllable, and cost-effective tool for drug discovery.
Liver tissue/organ models are increasingly of interest in drug testing and high-throughput screening because the liver plays a primary role in drug metabolism in the body (Bernal and Wendon 2013). For instance, Chang et al. developed a 3D liver micro-organ device as an in vitro model for drug discovery and metabolism test (Chang et al. 2008). They combined 3D bioprinting with microfluidics to apply continued perfusion flow to the bioprinted liver micro-organ. For creating a liver micro-organ chamber, hepatocyte-laden alginate hydrogels were directly bioprinted within the microfluidic chamber of the polydimethylsiloxane substrate. The 3D bioprinted liver micro-organ device showed predictable cell viability and proliferation outcomes and enhanced liver cell-specific function, such as urea synthesis, compared to traditional 2D culture method. In addition, they showed effective drug metabolic function compared to the static culture condition. These results demonstrated feasibility of the 3D bioprinted liver micro-organ device for a drug-testing platform.
A single-tissue model sometimes yields false-positive or false-negative results for drug tests, because some drugs are metabolized and converted to the active or inactive forms before reaching the target tissues. Therefore, to facilitate cell-cell interaction and more closely mimic downstream effects of metabolism on the target liver tissue, a multiple-tissue model has been used. Amifostine, for example, is an anti-radiation drug which is converted to an active form by epithelial cells. To model pathogenesis in vivo, Snyder et al. (Snyder et al. 2011) created a dual-tissue microfluidic chip (Fig. 7a) to test the conversion and radioprotective effects of amifostine on the target liver tissue. The dual-tissue microfluidic chip was created by direct printing of epithelial cell- and hepatocyte-encapsulated Matrigel within each microfluidic chamber. After radiation exposure, the injected drug passed the epithelial cell-laden chamber before reaching the hepatocyte-laden chamber in this chip, as the drug would pass endothelial cells from the blood stream to the target tissue in vivo. Enhanced radioprotective effects of the testing drug were reported compared to the single-tissue system.
Bioprinting of 3D in vitro tissue/organ model. (a) Dual-tissue microfluidic chip (Snyder et al. 2011). (b) Fabrication of 3D skin wound model (Lee et al. 2009). 3D PDMS mold having non-planner surface for printing multiple layers of skin cells (left). Immunofluorescent images of multilayered printing of keratinocytes and fibroblasts (Keratin: green, β-tubulin: red). (c) Bioprinted cervical cancer model. Cell spheroids formation in 3D constructs at day 8 (Zhao et al. 2014) (Figures reprinted with permission from Snyder et al. (2011), Lee et al. (2009), and Zhao et al. (2014))
Recently, the 3D printed exVive3DTM platform was tested for use in drug toxicity tests for liver tissue (Vaidya 2015). A scaffold-free liver tissue model consisting of pellets of human hepatocytes, hepatic stellate and endothelial cells was printed using a NovoGen MMX BioprinterTM. It had a liver-like structure and long-term function, as shown by secretion of liver-specific albumin protein up to 42 days. This model has now been used in clinical trials for drug toxicity testing, making a breakthrough toward commercialization of a 3D bioprinted model of liver.
Faulkner-Jones et al. bioprinted 3D mini-livers for the first time by using human-induced pluripotent stem cells (hiPSCs) (Faulkner-Jones et al. 2015). By using alginate hydrogel as a cell-encapsulating material and a dual-head valve-based inkjet printer, the hiPSCs can be bioprinted, maintaining cell viability and pluripotency. In this study, post-printing differentiation of printed hiPSCs into hepatocyte-like cells was reported, as shown by expression of hepatocyte markers, including hepatocyte nuclear factor 4 alpha (HNF4α) and albumin secretion. Their work showed the potential to bioprint tissues or organs containing patient-specific cells for animal-free drug discovery and eventually for personalized medicine.
Bioprinting of lung tissue is relatively new, and a realistic 3D in vitro alveolar model is not yet available. An advanced 3D lung model was created using BioFactory® (regenHU) for drug testing (Horvath et al. 2015). To mimic the microenvironment of the native tissue, an in vitro human air-blood barrier architecture was fabricated composed of zonally stratified endothelial cells, basement membrane, and epithelial cell layers. By using 3D bioprinting, very thin layers of Matrigel were used as a basement membrane and uniform cell layers were generated compared to the conventional manual seeding method. This bioprinted 3D lung tissue model is expected to provide an excellent tool for high-throughput screening for drug screening and toxicity tests.
In the pharmaceutical and cosmetics industries, 3D bioprinting technology has been applied to engineer human skin tissue substitutes for testing specific drugs or products. For instance, to create a more realistic 3D skin wound model, Lee et al. printed multilayered skin tissues on the PDMS mold having non-planner surface (Fig. 7b) (Lee et al. 2009). The 3D bioprinted skin tissue model had dermal/epidermal-like distinctive layers consisting of human skin fibroblasts and keratinocytes, showing that biologically comparable skin tissue printing is possible. This study showed the potential to create a tailored skin tissue model in wound shape for disease models and drug tests. Recently, cosmetic companies began to introduce 3D bioprinted skin tissue models to test cosmetics. L’Oreal USA announced a partnership with Organovo to develop 3D bioprinted skin tissue (Markin 2016). Their collaboration is expected to provide more advanced skin tissue models for prescription drug and toxicity tests.
3.2 Cancer Research
2D models have been used for cancer research in vitro, but they provide very limited information due to lack of biomimicry of natural tumor environments. Therefore, a 3D tissue model that can mimic/represent complex tumor environments of cell-cell and cell-matrix interactions in vivo is needed for better understanding of cancer biology before effective treatments can be developed (Kim 2005; Padron et al. 2000). Bioprinting holds promise to fabricate 3D in vitro cancer tissue models that more closely imitate the complex and physiological environments of tumors. 3D bioprinting to create cancer tissue models in vitro is an emerging field, and only a few studies have been reported so far.
In one example, a 3D cervical tumor model was recently created by 3D bioprinting (Zhao et al. 2014). The in vitro cervical tumor model (dimensions of 10 × 10 × 2 mm3) was extrusion printed by patterning HeLa cells derived from cervical cancer tissues. To mimic the extracellular matrix of cervical cancer tissue, a composite hydrogel composed of gelatin, alginate, and fibrinogen was used as a bioink. The printed HeLa cells showed viability of over 90% and formed spheroids in the 3D environment (Fig. 7c). This is promising because 3D cellular spheroids are commonly used in vitro as 3D tumor models for antitumor therapy (Friedrich et al. 2007). The possibility of 3D bioprinting of in vitro cancer model was further demonstrated by increased cell proliferation, indicating factors of tumor metastasis and chemoresistance compared to a 2D culture model.
Physiologically relevant 3D co-culture models of fibroblasts and ovarian cancer cells are needed because fibroblasts are closely related to the growth and progression of ovarian cancer (Kenny et al. 2007). However, precise control and patterning of each cell type are difficult in conventional 3D tissue models. Therefore, an in vitro 3D ovarian cancer co-culture model was developed with a bioprinting system (Xu et al. 2011). This group printed human ovarian cancer cells and normal fibroblasts on Matrigel platforms. Cell density per droplet, droplet size, and spatial distance between droplets of each cell types were controlled. Spontaneous multicellular acini formations of printed cells were observed; these proliferated within the 3D patterned co-culture model. This study demonstrated the ability to create an in vitro ovarian cancer co-culture model via bioprinting technology for future cancer research and high-throughput screening.
A scaffold-free human breast cancer model (the NovoGen BioprintingTM Platform) was created for therapeutic drug screening (King et al. 2014). To mimic the tumor microenvironment, the models were composed of breast cancer cells in the core as well as breast stromal cells such as mammary fibroblasts, endothelial cells and adipose cells. The 3D breast neo-tissue was then directly printed into multi-well plates for high-throughput screening of chemotherapeutic drugs. The bioprinted breast cancer neo-tissue was viable for 2 weeks and less susceptible to chemotherapeutic agents than breast cancer cells in 2D culture. The therapeutic effect of anticancer drugs is frequently overestimated in the 2D cancer tissue models because cells in 2D react quickly and are more sensitive than cells in the complex 3D microenvironments. Therefore, the results demonstrated that this 3D bioprinted breast cancer model could be an effective tool for development of anticancer therapeutics and drug screening.
4 Current Challenges and Future Perspectives
3D bioprinting has shown great progress and promise for creating 3D living tissues and organs. Several types of 3D bioprinted tissues and organs that mimic structural and functional characteristics of natural tissues and organs have been created by spatial patterning of cell-laden bioinks with inkjet-, extrusion-, or laser-based bioprinters. In tissue engineering and regenerative medicine, 3D bioprinted living tissues (including bone, cartilage, skin, nerve, and cardiac tissue, heart valve, blood vessel and microvascular networks) have been constructed. Some of them have successfully been transplanted to animals. Furthermore, 3D bioprinting has been widely applied in areas such as physiology, oncology, and pathology and in the cosmetics and pharmaceutical industries. The 3D bioprinted in vitro tissues/organ models offer a drug discovery and screening platform, enabling an accurate prediction of preclinical effects of drugs and a tool for understanding basic mechanisms of tissue/organ physiology and tumorigenesis. However, despite the great progress and broad applications of 3D bioprinting, there remain many challenges to be addressed for further applications and eventual translation to clinical use (Box 3).
Current applications for implantation of 3D bioprinted tissues in tissue engineering and regenerative medicine are mainly limited to small animals, and none of the bioprinted tissues has been used clinically. The ability to scale up tissues and organs via bioprinting techniques is needed for rapid clinical transplantation. To do so, issues related to cell viability and long-term structural stability should be addressed. In large-sized tissues, supplying oxygen and nutrient supplies to the cells are difficult. To overcome this limitation, vascularization of bioprinted tissues is a possible strategy. Incorporation of vascular patterns such as bioprinted blood vessels or microvascular networks into bioprinted tissue constructs can be a potential solution for maintaining cell survival. Incorporation of biological factors for promoting angiogenesis within the bioprinted tissues can be an alternative approach. In addition, porosity and pore size of scaffolds could be redesigned to provide sufficient space for formation of vascular networks and accelerate angiogenesis.
To fabricate tissues large enough for clinical use, biologically functional and mechanically robust bioinks are needed. Current printable bioinks are mainly natural hydrogel-based biomaterials and their composites. The hydrogel-based bioinks provide 3D microenvironments for the cells to survive and proliferate long-term within the bioprinted tissue constructs. However, they have weak mechanical properties after cross-linking (Stanton et al. 2015). Synthetic biomaterials used as bioinks are mechanically stable and easily controllable compared to the natural-derived hydrogels, but they have relatively long degradation times and low cytocompatibility that results in delayed tissue formation in vitro and in vivo (Skardal and Atala 2015). Therefore, optimizing bioinks that can support cell functions and mechanical properties may be a key factor for advancement in 3D bioprinting technologies.
Cell source and quality also need to be addressed for translation of 3D bioprinted tissues and organs into clinical uses and pharmaceutical applications. In the tissue engineering and regenerative medicine fields, the usage of patient-specific primary cells isolated has been recommended for implantation to reduce immunogenic risks. In vitro 3D tissue/organ models using patient-specific cells would enable diagnostics and development of patient-specific drugs/therapies in personalized medicine. For further application of 3D bioprinted tissues and organs, clinically relevant and reproducible cell sources are needed that can be expanded in large quantities and are well characterized. Although stem cells such as hAFSCs and hiPSCs have currently been used as patient-specific cell sources (Skardal et al. 2012; Yoshida and Yamanaka 2010), more refinements are needed to better control differentiation of stem cells with specific lineages or functions within the 3D bioprinted tissues and organs. In addition, multiple cell types are needed to more closely mimic the highly complex anatomy and function of native tissues.
Although the 3D bioprinters such as extrusion-, inkjet-, and laser-based bioprinters have seen significant improvements for fabricating 3D living tissues and organs, several issues remain to be addressed. To fabricate more complex tissue constructs, high-resolution bioprinters (<10 μm) is needed. In addition, integration of multi-dispensing systems into the bioprinter facilitates a simultaneous bioprinting of multiple cell types and biomaterials to fabricate complex tissues with desired function (Kang et al. 2016). For fabricating tissues large enough for clinical use, high-resolution bioprinters are required to increase the speed of the printing process. Since cells are exposed to stressful conditions (e.g., limited oxygen and nutrient supply) in the cartilage, and high pressure and shear stress during the printing process, a longer printing time leads to more cell death. In addition, mechanical properties of bioinks, especially hydrogels, can change during long printing process, impairing printing resolution. Therefore, a real-time monitoring/controlling system of the printing environment is needed in future 3D bioprinting technologies. New types of bioprinters in which several types of bioprinting modalities are integrated or other biofabrication technologies are combined (i.e., microfluidics, electrospinning, microfabrication) are among the potential solutions to overcome current technical limitations of 3D bioprinting (Kolesky et al. 2016; Snyder et al. 2011; Visser et al. 2015; Xu et al. 2013a).
Challenges facing the 3D bioprinting technologies related to technical, mechanical, biological, and biomaterial issues can be addressed through close collaborations among engineers, scientists, and clinicians. This multidisciplinary approach can offer solutions for advancements in 3D bioprinting and accelerate translation and applications.
Box 3
Future perspectives of 3D bioprinting tissues and organs:
-
Bioprinting of vascular networks for scaling up 3D tissues or organs for clinical translation
-
Development of biologically functional and mechanically robust bioinks for use in bioprinting
-
Clinically relevant, well-characterized, reproducible cell sources
-
Bioprinting of 3D tissues and organs with multiple cell types to mimic highly complex anatomy and function of native tissues
-
Advanced bioprinting technologies with increased resolution and speed and automatic monitoring/controlling system
-
Combinations with other biofabrication technologies to overcome current technical challenges
-
Standardization and optimization of bioprinting processes to enable manufacturing and commercialization
-
Collaboration with engineers, basic scientists, clinicians, and manufacturers
5 Conclusions
3D bioprinting is an advanced technology for fabricating 3D living tissues and organs with great potential in a variety of applications. This technique can fabricate several types of complex and functional tissue and organ constructs and has shown great regenerative capacity in animal transplantation. It also provides a tool for drug discovery, toxicity tests, and cancer research. The 3D bioprinted in vitro tissue/organ models can facilitate future investigations of physiological phenomena of normal/diseased/tumorigenic tissues and enables accurate prediction and fast screening of drugs/therapies. Even though 3D bioprinting technology is still in its infancy and faces many challenges to be addressed for clinical translation, multidisciplinary research and close collaboration between engineer, scientists, and clinicians will be able to overcome the challenges and realize the potential of 3D printing in translation and applications to a variety of areas.
References
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40:363–408
Arslan-Yildiz A, Assal RE, Chen P, Guven S, Inci F, Demirci U (2016) Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 8:014103
Atala A, Yoo J (2015) Essentials of 3D biofabrication and translation. Elsevier, London
Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R (2014) 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 16:247–276
Ballyns JJ, Gleghorn JP, Niebrzydowski V, Rawlinson JJ, Potter HG, Maher SA, Wright TM, Bonassar LJ (2008) Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng Part A 14:1195–1202
Bernal W, Wendon J (2013) Acute liver failure. N Engl J Med 369:2525–2534
Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32:760–772
Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M (2003) Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res 42:1–36
Buikema JW, Van Der Meer P, Sluijter JP, Domian IJ (2013) Concise review: engineering myocardial tissue: the convergence of stem cells biology and tissue engineering technology. Stem Cells 31:2587–2598
Campbell PG, Miller ED, Fisher GW, Walker LM, Weiss LE (2005) Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials 26:6762–6770
Cancedda R, Giannoni P, Mastrogiacomo M (2007) A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28:4240–4250
Chang R, Nam J, Sun W (2008) Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng Part C Methods 14:157–166
Chang R, Emami K, Wu H, Sun W (2010) Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2:045004
Chang CC, Boland ED, Williams SK, Hoying JB (2011) Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater 98:160–170
Cohen MM Jr (2006) The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A 140:2646–2706
Cui XF, Breitenkamp K, Finn MG, Lotz M, D’Lima DD (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18:1304–1312
Demirci U, Montesano G (2007) Single cell epitaxy by acoustic picolitre droplets. Lab Chip 7:1139–1145
Duan B (2016) State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann Biomed Eng. https://doi.org/10.1007/s10439-016-1607-5
Duan B, Hockaday LA, Kang KH, Butcher JT (2013) 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A 101:1255–1264
Duan B, Kapetanovic E, Hockaday LA, Butcher JT (2014) Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater 10:1836–1846
Faulkner-Jones A, Fyfe C, Cornelissen DJ, Gardner J, King J, Courtney A, Shu W (2015) Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 7:044102
Fedorovich NE, Wijnberg HM, Dhert WJ, Alblas J (2011) Distinct tissue formation by heterogeneous printing of osteo- and endothelial progenitor cells. Tissue Eng Part A 17:2113–2121
Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, van Weeren PR, Malda J, Alblas J, Dhert WJ (2012) Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods 18:33–44
Friedrich J, Ebner R, Kunz-Schughart LA (2007) Experimental anti-tumor therapy in 3-D: spheroids – old hat or new challenge? Int J Radiat Biol 83:849–871
Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, Gruene M, Toelk A, Wang W, Mark P et al (2011) Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 32:9218–9230
Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, Sluijter JP (2012) Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 33:1782–1790
Gerlach JC, Johnen C, McCoy E, Brautigam K, Plettig J, Corcos A (2011) Autologous skin cell spray-transplantation for a deep dermal burn patient in an ambulant treatment room setting. Burns 37:e19–e23
Gibbs DM, Vaezi M, Yang S, Oreffo RO (2014) Hope versus hype: what can additive manufacturing realistically offer trauma and orthopedic surgery? Regen Med 9:535–549
Griffith LG, Swartz MA (2006) Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7:211–224
Gruene M, Pflaum M, Hess C, Diamantouros S, Schlie S, Deiwick A, Koch L, Wilhelmi M, Jockenhoevel S, Haverich A et al (2011) Laser printing of three-dimensional multicellular arrays for studies of cell-cell and cell-environment interactions. Tissue Eng Part C Methods 17:973–982
Gu X, Ding F, Williams DF (2014) Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 35:6143–6156
Guillemot F, Souquet A, Catros S, Guillotin B, Lopez J, Faucon M, Pippenger B, Bareille R, Remy M, Bellance S et al (2010) High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater 6:2494–2500
Guillotin B, Guillemot F (2011) Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol 29:183–190
Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, Bareille R, Remy M, Bordenave L, Amedee J et al (2010) Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 31:7250–7256
Hoch E, Tovar GE, Borchers K (2014) Bioprinting of artificial blood vessels: current approaches towards a demanding goal. Eur J Cardiothorac Surg 46:767–778
Horvath L, Umehara Y, Jud C, Blank F, Petri-Fink A, Rothen-Rutishauser B (2015) Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep 5:7974
Itoh M, Nakayama K, Noguchi R, Kamohara K, Furukawa K, Uchihashi K, Toda S, Oyama J, Node K, Morita S (2015) Correction: scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS One 10:e0145971
Jana S, Lerman A (2015) Bioprinting a cardiac valve. Biotechnol Adv 33:1503–1521
Jawad H, Lyon AR, Harding SE, Ali NN, Boccaccini AR (2008) Myocardial tissue engineering. Br Med Bull 87:31–47
Jeong CG, Atala A (2015) 3D printing and biofabrication for load bearing tissue engineering. Adv Exp Med Biol 881:3–14
Jiao A, Trosper NE, Yang HS, Kim J, Tsui JH, Frankel SD, Murry CE, Kim DH (2014) Thermoresponsive nanofabricated substratum for the engineering of three-dimensional tissues with layer-by-layer architectural control. ACS Nano 8:4430–4439
Jones N (2012) Science in three dimensions: the print revolution. Nature 487:22–23
Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34:312–319
Kenny HA, Krausz T, Yamada SD, Lengyel E (2007) Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer 121:1463–1472
Kim JB (2005) Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 15:365–377
Kim JD, Choi JS, Kim BS, Choi YC, Cho YW (2010) Piezoelectric inkjet printing of polymers: stem cell patterning on polymer substrates. Polymer 51:2147–2154
King SM, Presnell SC, Nguyen DG (2014) Development of 3D bioprinted human breast cancer for in vitro drug screening. Cancer Res 74:2034
Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S (2015) Bioprinting for cancer research. Trends Biotechnol 33:504–513
Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R, Polchow B, Reimers K, Stoelting S, Ma N et al (2010) Laser printing of skin cells and human stem cells. Tissue Eng Part C Methods 16:847–854
Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, Zychlinski D, Schambach A, Reimers K, Vogt PM (2012) Skin tissue generation by laser cell printing. Biotechnol Bioeng 109:1855–1863
Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA (2014) 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26:3124–3130
Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA (2016) Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A 113:3179–3184
Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, Park JK, Yoo SS (2009) Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 30:1587–1595
Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo SS, Vincent PA, Dai G (2014) Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 35:8092–8102
Li Y, Wei X, Zhou J, Wei L (2013) The age-related changes in cartilage and osteoarthritis. Biomed Res Int 2013:916530
Liu Y, Lim J, Teoh SH (2013) Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv 31:688–705
Lu T, Li Y, Chen T (2013) Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int J Nanomedicine 8:337–350
Mandrycky C, Wang Z, Kim K, Kim DH (2015) 3D bioprinting for engineering complex tissues. Biotechnol Adv 34:422
Marga F, Jakab K, Khatiwala C, Shepherd B, Dorfman S, Hubbard B, Colbert S, Gabor F (2012) Toward engineering functional organ modules by additive manufacturing. Biofabrication 4:022001
Markin CA (2016) News: recognitions, societies, and academia. Regen Eng Transl Med 2:51–52
Merceron TK, Burt M, Seol YJ, Kang HW, Lee SJ, Yoo JJ, Atala A (2015) A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 7:035003
Michael S, Sorg H, Peck CT, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K (2013) Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One 8:e57741
Mironov V, Kasyanov V, Markwald RR (2011) Organ printing: from bioprinter to organ biofabrication line. Curr Opin Biotechnol 22:667–673
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32:773–785
Murphy SV, Skardal A, Atala A (2013) Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A 101:272–284
Nair K, Gandhi M, Khalil S, Yan KC, Marcolongo M, Barbee K, Sun W (2009) Characterization of cell viability during bioprinting processes. Biotechnol J 4:1168–1177
Nooeaid P, Salih V, Beier JP, Boccaccini AR (2012) Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med 16:2247–2270
Norotte C, Marga FS, Niklason LE, Forgacs G (2009) Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30:5910–5917
Novosel EC, Kleinhans C, Kluger PJ (2011) Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 63:300–311
Owens CM, Marga F, Forgacs G, Heesch CM (2013) Biofabrication and testing of a fully cellular nerve graft. Biofabrication 5:045007
Ozbolat IT (2015) Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol 33:395–400
Ozbolat IT, Yu Y (2013) Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng 60:691–699
Ozbolat IT, Peng W, Ozbolat V (2016) Application areas of 3D bioprinting. Drug Discov Today 21:1257
Padron JM, van der Wilt CL, Smid K, Smitskamp-Wilms E, Backus HH, Pizao PE, Giaccone G, Peters GJ (2000) The multilayered postconfluent cell culture as a model for drug screening. Crit Rev Oncol Hematol 36:141–157
Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839–845
Pati F, Gantelius J, Svahn HA (2016) 3D bioprinting of tissue/organ models. Angew Chem Int Ed Engl 55:4650–4665
Pereira C, Gold W, Herndon D (2007) Review paper: burn coverage technologies: current concepts and future directions. J Biomater Appl 22:101–121
Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G (2007) Bioengineered skin substitutes for the management of burns: a systematic review. Burns 33:946–957
Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P (2008) Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells 26:127–134
Schaefer D, Martin I, Shastri P, Padera RF, Langer R, Freed LE, Vunjak-Novakovic G (2000) In vitro generation of osteochondral composites. Biomaterials 21:2599–2606
Schuurman W, Khristov V, Pot MW, van Weeren PR, Dhert WJ, Malda J (2011) Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 3:021001
Schuurman W, Klein TJ, Dhert WJ, van Weeren PR, Hutmacher DW, Malda J (2015) Cartilage regeneration using zonal chondrocyte subpopulations: a promising approach or an overcomplicated strategy? J Tissue Eng Regen Med 9:669–678
Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E (2016) A review of three-dimensional printing in tissue engineering. Tissue Eng Part B Rev 22:298
Seol YJ, Kang HW, Lee SJ, Atala A, Yoo JJ (2014) Bioprinting technology and its applications. Eur J Cardiothorac Surg 46:342–348
Shafiee A, Atala A (2016) Printing technologies for medical applications. Trends Mol Med 22:254–265
Sheridan RL, Greenhalgh D (2014) Special problems in burns. Surg Clin North Am 94:781–791
Skardal A, Atala A (2015) Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng 43:730–746
Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S (2012) Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 1:792–802
Smith CM, Stone AL, Parkhill RL, Stewart RL, Simpkins MW, Kachurin AM, Warren WL, Williams SK (2004) Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng 10:1566–1576
Smith CM, Christian JJ, Warren WL, Williams SK (2007) Characterizing environmental factors that impact the viability of tissue-engineered constructs fabricated by a direct-write bioassembly tool. Tissue Eng 13:373–383
Snyder JE, Hamid Q, Wang C, Chang R, Emami K, Wu H, Sun W (2011) Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 3:034112
Stanton MM, Samitier J, Sanchez S (2015) Bioprinting of 3D hydrogels. Lab Chip 15:3111–3115
Tang D, Tare RS, Yang LY, Williams DF, Ou KL, Oreffo RO (2016) Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials 83:363–382
Vaidya M (2015) Startups tout commercially 3D-printed tissue for drug screening. Nat Med 21:2
Visser J, Melchels FP, Jeon JE, van Bussel EM, Kimpton LS, Byrne HM, Dhert WJ, Dalton PD, Hutmacher DW, Malda J (2015) Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun 6:6933
Wang Z, Abdulla R, Parker B, Samanipour R, Ghosh S, Kim K (2015) A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 7:045009
Watson J, Hatamleh MM (2014) Complete integration of technology for improved reproduction of auricular prostheses. J Prosthet Dent 111:430–436
Wolford LM, Stevao EL (2003) Considerations in nerve repair. Proc (Bayl Univ Med Cent) 16:152–156
Xu T, Jin J, Gregory C, Hickman JJ, Boland T (2005) Inkjet printing of viable mammalian cells. Biomaterials 26:93–99
Xu F, Celli J, Rizvi I, Moon S, Hasan T, Demirci U (2011) A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol J 6:204–212
Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, Atala A (2013a) Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 5:015001
Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A (2013b) Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 34:130–139
Yanez M, Rincon J, Dones A, De Maria C, Gonzales R, Boland T (2015) In vivo assessment of printed microvasculature in a bilayer skin graft to treat full-thickness wounds. Tissue Eng Part A 21:224–233
Yoon H, Lee JS, Yim H, Kim G, Chun W (2016) Development of cell-laden 3D scaffolds for efficient engineered skin substitutes by collagen gelation. Rsc Adv 6:21439–21447
Yoshida Y, Yamanaka S (2010) Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation 122:80–87
Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, Cheng S, Sun W (2014) Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 6:035001
Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA et al (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 109:9342–9347
Acknowledgments
This research was supported, in part, by the Basic Science Research Program through the Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2012R1A6A3A03040684). The authors thank Karen Klein, MA, at the Wake Forest Clinical and Translational Science Institute (UL1 TR001420; PI: Li) for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this entry
Cite this entry
Kim, J.H., Atala, A., Yoo, J. (2018). Translation and Applications of Biofabrication. In: Ovsianikov, A., Yoo, J., Mironov, V. (eds) 3D Printing and Biofabrication. Reference Series in Biomedical Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-45444-3_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-45444-3_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45443-6
Online ISBN: 978-3-319-45444-3
eBook Packages: EngineeringReference Module Computer Science and Engineering