Abstract
Bioprinting has emerged in recent years as an attractive method for creating 3-D tissues and organs in the laboratory, and therefore is a promising technology in a number of regenerative medicine applications. It has the potential to (i) create fully functional replacements for damaged tissues in patients, and (ii) rapidly fabricate small-sized human-based tissue models, or organoids, for diagnostics, pathology modeling, and drug development. A number of bioprinting modalities have been explored, including cellular inkjet printing, extrusion-based technologies, soft lithography, and laser-induced forward transfer. Despite the innovation of each of these technologies, successful implementation of bioprinting relies heavily on integration with compatible biomaterials that are responsible for supporting the cellular components during and after biofabrication, and that are compatible with the bioprinting device requirements. In this review, we will evaluate a variety of biomaterials, such as curable synthetic polymers, synthetic gels, and naturally derived hydrogels. Specifically we will describe how they are integrated with the bioprinting technologies above to generate bioprinted constructs with practical application in medicine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioprinting has emerged as a flexible tool in regenerative medicine with potential in a variety of applications. Bioprinting is a relatively new field within biotechnology that can be described as robotic additive biofabrication that has the potential to build or pattern viable organ-like or tissue structures in 3 dimensions.82 In general, bioprinting uses a computer-controlled 3-D printing device to accurately deposit cells and biomaterials into precise geometries with the goal being the creation of anatomically-correct biological structures. Generally, bioprinting devices have the ability to print cell aggregates, cells encapsulated in hydrogels or viscous fluids, or cell-seeded microcarriers—all of which can be referred to as “bioink”—as well as cell-free polymers that provide mechanical structure or act as placeholders.21,53 Biologically-inspired, physiologically relevant computer-assisted designs can be used to design and guide the placement of specific types of cells and materials into precise, planned geometries that mimic the architecture of actual tissue construction,17 which can subsequently be matured into functional tissue constructs or organs.9,54 One of the most important features of bioprinting devices is the capability to reliably and accurately place small volumes of materials and cells in specific locations repeatedly, which allows bioprinting to be implemented in a high throughput fashion. To date, a complete fully functional human-sized organ has not been printed, but it remains the primary long-term goal of bioprinting research and development.17 However, small-scale “organoids” are currently being implemented in a number of applications, including pathology modeling, drug development, and toxicology screening.
In order to create either human-sized tissues and organs for implantation, or large quantities of bioprinted constructs or organoids for high-throughput screening drug development, cellularized structures must be organized into 3-D architectures. However, it is not a trivial task to do this while also maintaining viability and function. Maintenance of viability and function in large constructs has been difficult due to the need for constant nutrient and oxygen supply. Diffusion itself cannot supply enough nutrients and oxygen within constructs more the a few hundred microns thick, thus necessitating the need for incorporation of complex vascular architectures or perfusion systems, which are difficult to fabricate at the current level of resolution available in most bioprinting platforms. To effectively provide large numbers of bioprinted tissues, fabrication (including preparation, actual deposition rates, and transfer into supportive post-printing environments) needs to occur rapidly. Failure to do so may result in deteriorating viability and function of the resulting constructs, limiting their effectiveness regardless of their end application. To better facilitate implementation of bioprinting techniques and application of bioprinted tissues, there are a number of considerations that need to be understood. In particular, the choice of biomaterials, which facilitates the printing process by integration with the bioprinting devices and supplies structural and biochemical support to the cellular components, is especially important. This review will discuss a number of commonly implemented biomaterial types, their characteristics, pros and cons, and applications, as well as how they are integrated to work with various bioprinting technologies.
Bioprinting Modalities
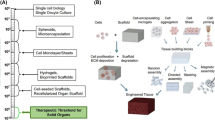
A number of bioprinting approaches have been recently explored, encompassing use of inkjet-like printers, extrusion devices, laser-assisted bioprinters, and photocuring-based devices.22 To better understand the concept and realm of bioprinting technologies and the biofabrication of viable tissue and organ structures in 3-D, here we will provide some background specific to some of the most common printing modalities currently being employed, of which conceptual illustrations are depicted in Fig. 1.
Common printing modalities for bioprinting applications. (a) Inkjet droplet printing; (b) extrusion printing; (c) stereolithography; and (d) laser-induced forward transfer (LIFT)-based printing. (b-i) A syringe-based extrusion printer; (b-ii) Extruded cell-hydrogel tubes that (b-iii) mature over time into cellularized ECM-containing tubes
Inkjet Printing
Inkjet printing, also refereed to as drop-by-drop bioprinting, is one bioprinting approach that is being explored for creating 3-D biological structures, which is closely related to technologies used for cell patterning.10,72 Where basic cell patterning creates a 2-D pattern comprised of cells on a surface, by incorporating a hydrogel or other cell-supportive biomaterial, with inkjet bioprinting, 3-D cellularized structures can be fabricated drop by drop.12,28 These types of bioprinters often use cartridge-based delivery systems mounted to XYZ plotting devices. The cartridge systems have often been similar to those used in traditional inkjet printing, in that cells and biomaterials can be loaded into individual cartridges and subsequently deposited under computer control. Examples of this implementation for tissue construct engineering include collagen-encapsulated smooth muscle cells that were printed drop-by-drop to create muscular patches,59 and the use of alginate and fibrin gel droplets for creating structures such as fibers and multilayered cell sheets.64 Our laboratory has recently demonstrated that this approach to bioprinting is also effective for in situ skin printing to accelerate wound healing.76 To successfully accomplish 3-D fabrication, the drop-by-drop approach relies on being able to quickly polymerize or stabilize the printed material in place, so that subsequent droplets can be added to the growing structure. Gelation rates of the printed materials are a direct product of the various crosslinking chemistries innate to materials used, and are essential for inkjet printing to function correctly. This requirement for a fast-gelling material unfortunately places a limitation on the types of materials that can be applied in this manner, and as the printable droplets are typically small volumes, scaling up to fabricate large tissue structures can be difficult. On the other hand, small droplet print volumes support high-resolution printing of intricate structures, like many of the tissue architectures present in the body.
Extrusion Bioprinting
Extrusion-based deposition, generally from syringe-like pieces of equipment housed on XYZ-mobile carriages, is an additional approach for 3-D bioprinting that primarily relies on the mechanical and temporal properties of the polymer materials being printed. In this modality, the properties of the printed polymer or hydrogel facilitate extrusion through a syringe tip, commonly driven by pneumatic pressure or mechanical pistons controlled by the computer. The reliance on the material properties for printing, means that the material must be soft or nearly fluid-like enough to facilitate extrusion through the small diameter tip or nozzle, but must also support itself mechanically after deposition. The rheological properties of the material can be tailored in such a manner that the materials have a high enough elastic modulus, or the solid component of its mechanical properties, such that extrusions maintain their shape. Simultaneously, the material may maintain a sufficient loss modulus, or the fluid-like component, such that extrusion is possible.78,79 Regardless, this method of printing very much relies on the mechanical properties of the bioink. One common approach is to employ melt-curable polymers such as polycaprolactone, which when heated can be deformed and printed at relatively high resolutions, but can cool down to a solid material. These materials can be used to build rather large and intricate structures, capable of mimicking physiological structures. However, melt-cure printing cannot be done with cells at this time. Cells must be seeded at a later time, or other cell-supportive materials can be incorporated, such as hydrogels, where the cells are encapsulated in the printing process. Printing with hydrogels via extrusion techniques can be difficult when working with materials that rely on time for gelation to occur. Mistiming the deposition process can result in either a structure that collapses because crosslinks have not formed quickly enough, or conversely, clogging of the bioprinting device due as a result of polymerization that was too fast. However, numerous studies have implemented novel crosslinking chemistries, photopolymerization techniques, and methods to facilitate spatial and temporal control over material properties that can contain encapsulated cells for printing 3-D structures.68,78,79
Another approach to extrusion-based printing is what has been coined by some as “scaffold-free” bioprinting, which is based on the principles of tissue liquidity and tissue fusion of multi-cellular components.65 In this approach, aggregates, rods, or tissue fragments comprised primarily of cells that are bound to one another are printed in geometric patterns or shaped and allowed to fuse over time to form larger constructs.38 Multiple layers of aggregates or rods can be printed, and after fusing together during a post-print maturation period, singular 3-D structures remain. In this work, an approach was used to build branched vascular structures,65 and more recently nerve grafts.50 Despite the name, it should be noted that this type of bioprinting often does rely on biomaterials. In most cases, the cell aggregates or cell rods are either printed into a cell-free biopolymer substrate or additively stacked using space-holding biomaterials to preserve the appropriate structures during the tissue fusion and maturation processes. These space-holders are generally removed when the construct is sufficiently fused and possesses sufficient mechanical properties to exist without the supporting spaceholders. The strength of this method lies within its high cell density, which allows for rapid fusion between discretely printed pieces. This method has been explored extensively and is the basis for the current commercial entity Organovo, one of the first bioprinting-based companies. Additionally, our laboratory previously explored a hydrogel-based approach that mimicked this technique, by printing hydrogel and cell-hydrogel rods that fused over time to create tubular constructs in vitro,80 and Bertassoni et al. used this approach to print HepG2 liver cells within methacrylated gelatin rods.5
Stereolithography and Projection Patterning
Stereolithography is a long-used solid freeform fabrication technique that employs a reservoir containing photocurable polymer solution or resin, a laser with X–Y control, and a stage or fabrication platform with vertical control. Fabrication occurs at the resin surface and the stages lowers incrementally, allowing layers to be polymerized on top of each other, thus creating 3-D structures in a bottom-up fassion. There also exists a top-down stereolithography approach, which is less common, but is employed for some applications. Resolution can be modulated by the focus of the laser and the energy of the laser, and has the capacity for very high resolution. Traditionally stereolithography has been used to create cell-free scaffolds, but with the development of polymers and proteins with bioactive and cell-adherent properties that can be photopolymerized on demand, the potential for stereolithography to be used for tissue engineering has dramatically increased. Some examples of biomaterials that are compatible with this technique are methacrylated or acrylated materials such as gelatin-methacrylate, hyaluronic acid-methacrylate, polyethylene glycol diacrylate (PEGDA), and polyethylene glycol dimethacrylate (PEGDMA).7,34,81 Recent developments have also led to projection stereolithography that uses visible light as a curing source for cell-laden materials, thus minimizing potential for cell damage from UV light sources and lasers.47 These techniques have also been multiplexed using digital mirror device, which allows UV light to be applied to polymer solutions as projections of millions of individual points or pixels at once. This facilitates curing of entire layers of the 3-D at one time, greatly increasing fabrication speed.89 This approach was recently employed to fabricate PEGDA-based liver architecture-inspired micro-devices containing nanoparticles that collect pore-forming toxin molecules for detoxification.26
Laser-Induced Forward Transfer (LIFT)
Laser-induced forward transfer (LIFT)-based bioprinting is a method that has recently been adopted from other fields of manufacturing by researchers pursuing bioprinting4,8 LIFT technology was initially developed for high resolution patterning of metals for use in commercial manufacturing areas such as computer chip fabrication. More recently it has been employed to create micropatterned peptide, DNA, and cell arrays. LIFT technology is comprised of a laser beam that is pulsed at desired time durations and a donor “ribbon” comprised of the printable material. The ribbon is supported on a transport layer such as gold or titanium that absorbs the laser energy and transfers it to the ribbon. When the laser pulse is transferred to the ribbon, the focused energy generates an incredibly small, high-pressure bubble that induces propulsion of a droplet of the donor material onto a collecting substrate. By moving the substrate stage or the laser in relation to the ribbon, the material can be patterned on the collecting substrate.13,15,18 In the case of LIFT-based bioprinting, the ribbon may be comprised of a biopolymer or protein, and can contain cells within. In this scenario, the laser pulse-driven ribbon droplets containing cells are deposited in a pattern on the substrate to create cellular structures and patterns. As with inkjet printing, this approach can be performed in multiple layers, resulting in 3-D structures. The lack of a nozzle in LIFT is a departure from other printing modalities, and does away with clogging issues. This results in increased flexibility in the printing materials, as long as they can be sufficiently transferred by the energy supplied by the laser. Studies have shown little to no negative effects on cell viability27,32,43 and the ability to print nearly a single cell per droplet,29 positioning LIFT as a bioprinted modality with much potential in the future. However, there are also some challenges. The high resolution and subsequently small printing volume per laser pulse requires fast gelation kinetics of the printable material and a fast moving stage for fabrication. In current LIFT methods, preparation times of the ribbon, especially when containing cells and thus cell-supportive biomaterials, can be time consuming. Furthermore, to create structures of size, multiple ribbons are often employed, requiring reloading during the printing process.
Biomaterials as “Bioinks”
The term biomaterials comprises a vast range of materials that is constantly evolving. Biomaterials range from cell supportive soft hydrogels, to stiff metal or ceramic implants; from nanoparticles and quantum dots for drug delivery and imaging, to complex functioning medical devices such as left ventricular assist devices and artificial hearts. As research in materials chemistry and biological sciences continues to expand, so will the number classifications of biomaterial types.83,84 In the context of bioprinting and biofabrication, biomaterials generally are limited to 2 primary categories. The first category is that of curable polymers that result in mechanically robust and durable materials that provide structure and scaffolding to printed constructs. Many such materials typically require high temperatures or toxic solvents to facilitate printing, and therefore are not appropriate for printing together with cells. Because of this, cells are usually seeded onto the scaffolds created from these materials after fabrication, thus avoiding conditions harmful to the cells. The second category of biomaterials is that of soft biomaterials such as hydrogels, generally with a high water content, inside of which cells are capable of residing. These can be comprised of synthetic or natural polymers, and do not possess the same levels of mechanical properties as curable support polymers. The inherent characteristics of these different printing materials, including mechanical properties, melting points, and available chemistries for crosslinking and functionalization make up the factors responsible for successful bioprinting.
One of the major problems that the field of bioprinting currently faces is the lack of biomaterials that are designed specifically for use in bioprinting. Much work has focused on adapting more traditional materials to bioprinting processes and hardware, instead of using bioprinting parameters as blueprints from which new materials are developed. In this section, we provide an overview of some of the traditional materials used in bioprinting and variations of these materials that have been developed with bioprinting in mind (Table 1). Importantly, some of the biomaterial customization approaches described below represent potential strategies for achieving versatile materials to support successful of bioprinting of viable and functional living structures.
Melt-Cure Polymers
Three-dimensional bioprinting technology originally stems from earlier applications requiring fabrication of intricate 3-D structures comprised of thermoplastics and metals that employ melting and curing fabrication techniques. Often, techniques associated with these kinds of materials involve high temperatures, toxic organic solvents or crosslinking agents, rendering them incompatible with living cells and biological materials such as growth factors and proteins that aid in achieving cellular function. However, due to the robust mechanical properties associated with such materials when fully cured, they have been implemented extensively in the realm of bioprinting as the structural scaffolding components of biological constructs, often printed prior to addition of biological components.
Polycaprolactone (PCL) is an example of a synthetic polymer material that is commonly employed in bioprinting as a scaffolding component. PCL is a polyester based material that due to its ability to be biodegraded by the body over time and its relatively low melting temperature of 60 °C is commonly used as a structural printing component. PCL is well established for long-term implantable devices and constructs, but other than non-specific binding of cells to hydrophobic PCL material, it lacks any natural peptide sequence motifs that provide specific binding sites for cells, a key factor that influences tissue integration. Due to this limitation, PCL is often combined with other functionalized materials or naturally derived materials such as the hydrogels that do contain these binding motifs to create more complex hybrid structures. This integrative bioprinting approach, which concurrently prints high resolution PCL, which cools quickly, and hydrogels based on gelatin, hyaluronic acid, or fibrin is currently being implemented in our laboratory to create numerous types of tissue constructs and organs of various sizes.61,86 Other melt-curable polymers such as polystyrene-based materials and resins such as epoxy can be using in printing applications, however the high temperatures associated with reaching a flowing melt state that can be extruded and toxic curing agents has limited their use in biomedical applications to primarily structural components.
Cell-Supportive Hydrogels
Here we focus specifically on the category of biomaterials consisting of hydrogels and their implementation in regenerative medicine applications such as cell therapy and tissue engineering. To be considered cell-supportive, these hydrogels must not induce toxicity in cells during and should provide cell-binding motifs to allow for cell adherence, be they innate to the base materials or through chemical modification steps. Hydrogels are comprised of polymer or peptide chains, which can be synthetically synthesized or derived from natural sources, which are crosslinked to form a macromolecular network. Normally, the polymers within are hydrophilic in nature, and as such the polymer chain network swells with water, hence the name “hydrogel”. With the exception of the stiffest tissue types such as bone and teeth, hydrogels can recapitulate a range of elastic modulus (E′) values through manipulation of chemistry, crosslinking density, and polymer concentration, thus mimicking the elastic moduli of most the soft tissues in the body. Processing techniques to generate crosslinking reactions can be designed to be non-cytotoxic, allowing 3-D encapsulation of cells within the hydrogel polymer networks at time of gelation. This is becoming exceedingly important as there is an increasing movement from 2- to 3-D tissue culture in tissue engineering and regenerative medicine research and application.69 In particular, within bioprinting applications, nearly all fabricated constructs are 3-D in nature. Indeed, as early as 2004 at the First International Workshop on Bioprinting and Biopatterning, it was determined that printing of living tissues and organs requires a 3-D approach.57 Hydrogels that support encapsulation procedures are significantly more efficient for 3-D uses than rigid scaffold seeding approaches of the past. It is important to note that in general, 3-D applications in tissue engineering provide cellular environments more like those in the body compared to traditional 2-D models. Conceptual illustrations of the 2-D vs. 3-D environments, and the ability of hydrogels to better mimic 3-D in vivo niches are described in Fig. 2.
Two-dimensional vs. three-dimensional environments. (a) In traditional cell culture environments, cells sit on a non-permeable, artificial surface, and experience gravity in a manner unlike that in the body. (b) A representation of a cell niche within a tissue in the body. Cells are suspended in 3-D by multiple contacts with the surrounding extracellular matrix, decreasing the role of stress by gravity. (c) A representation of a hydrogel network that acts as a simplified version of the extracellular matrix. The balance between accurate recapitulation of in vivo conditions and need for simplicity and efficiency for bioprinting is important in scaling up tissue engineered construct fabrication
Hydrogel biomaterials fall into one of two major categories: synthetic hydrogels, which employ polymers that are synthesized in the laboratory, or naturally derived hydrogels, which employ polymers, often polysaccharides, but can also be comprised of peptides or proteins, purified from natural sources and are often further manipulated in the laboratory. Common examples of synthetic hydrogels include polyethylene glycol (PEG)-based materials, such as PEG diacrylate (PEGDA), as well as polyacrylamide (PAAm)-based gels. Examples of naturally derived materials that are commonly used in the laboratory include collagen, hyaluronic acid, alginate, and fibrin. Typically, with synthetic materials, one can have more control over molecular weights and molecular weight distributions, as well as crosslinking densities, allowing for precise control of specific mechanical properties such as elastic modulus E′. On the other hand, the polymers employed within naturally derived hydrogels may be more difficult to manipulate into specific ranges of physical properties, but often have an innate bioactivity through naturally occurring peptide sequences or conformational motifs that cells can interact with, aiding with cell and tissue integration and biocompatibility. Below, we will discuss the use of some common synthetic hydrogels, but primarily focus on naturally derived hydrogel biomaterials, as they are more efficient at mimicking the biological nature of the native ECM environment.
Synthetic Polymer Hydrogels
A variety of synthetic materials have been implemented as hydrogels for applications in regenerative medicine. Synthetic polymers are advantageous for one primary reason—as indicated above, they allow for precise control over their chemical and physical properties. Researchers can maintain precise chemical control over molecular weight, functional groups, and hydrophobicity/hydrophilicity at a monomer level. As a result, crosslinking rates and mechanical properties can be precisely controlled. Polyethylene glycol (PEG) and polyacrylamide are examples of commonly used synthetic polymers in biomedical applications. PEG, which is perhaps most common, has long been used as medical device coatings to control host immune responses or appended to drug constructs to reduce degradation in vivo. It can also be manipulated to form a variety of hydrogels for cell culture and stem cell differentiation. PEG is often chemically modified with acrylate groups to create a photopolymerizable polyethylene glycol diacrylate (PEGDA) in which cells can quickly be encapsulated. The same features that allow such precise control over the chemical and mechanical properties also translate into an inherent drawback. Since synthetic polymer chains typically do not contain natural attachment sites that can interact with cells, all biological activity must be artificially preprogrammed into the material. PEG requires chemical immobilization of cell adhesion motifs in order to support cell adherence. Alternatively, many hydrogels derived from natural polymers and peptides retain some, if not all, of their original biological activity.
Collagen
Collagen is arguably the most commonly employed natural material for cell and tissue culture applications, since it is the most abundant component of the ECM in most tissues.31 Isolation and purification processes are well established, particularly for collagen type I, so using collagen materials to create surface coatings and gels as cell culture environments has become a commonplace practice industry-wide. Inherent in the structure of collagen fibers are important arginine-glycine-aspartic acid (RGD) amino acid sequences, which form the motifs that allow cells to adhere and proliferate via integrin-RGD binding. In normal tissue and ECM, collagen is one of many components, and as such hybrid materials consisting of collagen and other ECM components are being explored. Collagen matrices are indeed useful and have yielded numerous important biological advances, but matrices comprised of 100% collagen may not be entirely optimal. The lack of other common ECM components such as elastin, glycosaminoglycans (GAGs), fibrinogen, and laminin, may result in biological signaling that can induce undesirable cellular changes, perhaps through higher than normal integrin binding-mediated signaling. Furthermore, collagen fibers and gels primarily contain hydrophobic peptide motifs. As such, when used as implants or cell delivery agents, collagen gels can exclude water and contract, potentially resulting in decreased function, decreased diffusion of nutrients and gases, and cell death. Despite this limitation, collagen is still used extensively in tissue culture. However its future application might be improved with development of new hybrid biomaterials consisting of combinations of collagen and other ECM components with superior properties.
Hyaluronic Acid
Hyaluronic acid (HA), or hyaluronan, is a hydrophilic non-sulfated GAG consisting of repeating disaccharide units that is present in tissues as a major component of the ECM and has shown great potential in regenerative medicine applications.2,42 Unmodified HA has been used in the clinical for several decades, in applications such as alleviation therapy for damaged joints and arthritis.24,75 However, by employing chemical modification of HA, its usability has been extended significantly, resulting in a robust biomaterial that can be crosslinked into a hydrogel or loaded with other bioactive factors.70
HA hydrogels are commonly formed by modification with photocrosslinkable methacrylate groups appended to the HA chains that can undergo free radical polymerization when exposed to ultraviolet (UV) light. These result in soft hydrogels, referred to here as MA-HA hydrogels. Photocrosslinkable MA-HA hydrogels have been used in many applications, from cutaneous and corneal wound healing52 to prototype vessel structure bioprinting.79 Thiol-modification of HA yields a material by which hydrogels can be formed through Michael-type addition crosslinking with PEGDA crosslinkers. Like the MA-HA variety of HA, thiol-modified HA, particularly a thiolated carboxymethyl HA (CMHA-S), has also been employed in a number of applications in regenerative medicine, including wound healing,41 tumor modeling,48 and bioprinting of cellularized structures.78 Limitations of HA as a biomaterial for bioprinting encountered by our laboratory were that HA has typically been used to form very soft hydrogels that are not structurally robust, and the crosslinking methods employed did not facilitate effective extrusion bioprinting. As described above, this can be a limitation in terms of scalability. To address these problems, we investigated various crosslinking techniques using HA-based hydrogels. First, we discovered that gold nanoparticles (AuNPs) could serve as thiophilic crosslinking agents when paired with thiolated HA and gelatin solutions. The gold-thiol interactions resulted in a hydrogel that gelled slowly, increasing in elastic modulus over the course of 96 h. This slow reaction produced a large window during which the material was extrudable for bioprinting (at about 24 h of crosslinking). We printed cellularized tubular structures that after layer-by-layer deposition, fused in culture during the next several days. After 4 weeks in culture the constructs had become opaque with proliferating cells and cell-secreted ECM as they remodeled the construct. This crosslinking strategy was also reversible, allowing us to use cell-free AuNP gels as structural supports and space holders that could be washed away by interrupting the gold-thiol bonds, resulting a flexible system for building constructs.78 We also explored the use of photocrosslinkable methacrylated HA and gelatin for continuous bioprinting deposition. This crosslinking strategy allowed an initial partial gelation step which left the gel in a soft and extrudable, but structurally sound state during which cellularized tubular constructs were fabricated. After layer-by-layer deposition, the individual segments were fused and stiffened with a secondary photocrosslinking step. Like the previous example, these constructs were remodeled as the cells proliferated and deposited ECM.79 More recently we have been further exploring HA hydrogels mixed with tissue-specific growth factors and ECM components77 and multiple crosslinking chemistries to created flexible bioinks for bioprinting tissue organoids of varying structural stiffness.
Gelatin
Gelatin is a mixture of peptide sequences derived from collagen that has undergone partial hydrolysis. Unlike native collagen, this degraded product can be dissolved in pH neutral aqueous solutions, while still maintaining the ability to form simple gels when solutions are brought to low temperatures through hydrophobic crosslinking. However, because the melt temperature of gelatin gels typically lies between 30 and 35 °C, this form of gelatin is limited to applications that are below physiological temperatures. Due to this limitation, gelatin often requires additional chemical modification, alternative crosslinking techniques, or combination with other proteins or polymers for implementation in living systems.
Gelatin–fibrinogen crosslinked with glutaraldehyde have been used in in vitro studies together with dermal fibroblasts to develop dermal matrices to be used in wound repair. These matrices showed characteristics such as collagen production, cellular infiltration, and eventual biodegradation, all of which are important in wound healing treatments.16 Other in vitro studies explored electrospun gelatin-PCL nanofibers as scaffolds for human dermal fibroblasts, keratinocytes, and mesenchymal stem cells. These scaffolds and gelatin-only nano-spun scaffolds were then implemented in vivo, resulting in accelerated wound closure and epithelialization compared to gauze treatments.20 Gelatin has been combined with its precursor, collagen, to form a hybrid sponge from which loaded bFGF could be released sustainably. In a pressure-induced diabetic ulcer mouse model, these bFGF-loaded scaffolds achieved accelerated dermis-like tissue formation, wound closure, and new blood vessel formation in comparison to control scaffolds loaded with saline.40 Gelatin-based materials have also been used for cell delivery to wounds. In one study, gelatin-polyethylene glycol matrices were used to encapsulate MSCs through thiol-ene crosslinking and applied to full thickness wounds in rats. This combinatorial treatment decreased the overall immune response, reducing immune cell infiltration and foreign giant cell formation, while accelerating wound closure, re-epithelialization, and neovascularization.87 For use in bioprinting, gelatin has been employed in several ways, including the use of the temperature sensitivity characteristic of gelatin to facilitate extrusion, and by covalent addition of functional groups to induce additional crosslinking approaches.5,81,85
Alginate
Alginate is a naturally occurring polysaccharide that is derived from algae or seaweed. It has been widely used in regenerative medicine applications due to the ease at which it can form a hydrogel through an almost instantaneous sodium–calcium ion exchange reaction. This has made alginate the material of choice for microencapsulation of cells, in which easily available and inexpensive sodium alginate, which is unmodified, is quickly rearranged into calcium alginate hydrogel microspheres, held together through ionic interactions.73 These constructs have been extensively used for creating hydrogel capsules containing trapped liver cells or pancreatic islets.66 However, without chemical modification, alginate, like PEG, is mostly inert, and its use for cell and tissue culture is limited without incorporating cell-adherent motifs. Additionally, the reagents commonly used for creating cell-laden hydrogel microspheres, such as CaCl2, the crosslinking reagent, as well as sodium citrate and ethylenediaminetetraacetic acid (EDTA), commonly used chelators, can have a detrimental effect on cell viability during the encapsulation process.14 However, due to the ease with which alginate gels can be formed, it remains a popular and effective choice as a material in applications requiring cell encapsulation. If handled correctly, this same crosslinking mechanism can be employed for bioprinting purposes.39
Fibrin
Another natural-sourced material for generating hydrogels is fibrin, which has been implemented for culture of various tissues types. Fibrin is comprised of fibrinogen monomers that are joined by thrombin-mediated cleavage crosslinking. In the body, it has an important role in blood clotting, wound healing and tumor growth. In a concentrated glue-like form, it has been used clinically as a hemostatic agent and sealant in surgery. More recently, less concentrated fibrin gels have been used as a scaffold for regenerative medicine due to its quick crosslinking rates and robust mechanical properties.1 In the context of bioprinting, our laboratory has used a fibrin-collagen blend to bioprint hydrogels containing stem cells to accelerate skin regeneration.76 In this work, full thickness wounds were created surgically in nu/nu mice. In situ printing of fibrin-collagen gels with amniotic fluid-derived stem cells induced increased wound closure rates as well as increased vascularization of the regenerating tissue.
The Role of Biomaterials in Bioprinting Integration
With the tissue construct building blocks chosen—cells, biomaterials, biochemical signals—for the given target tissue construct to be fabricated, an appropriate device is necessary for the physical fabrication steps. Depending on the type of device or printing modality, as discussed above, specific concerns arise based on deposition methodology and are often also biomaterial-specific, as printing and fabrication depends on the crosslinking or curing kinetics of the material and the native or chemically or environmentally-induced material properties of the material. Some of these general types of curing and crosslinking approaches are described in Fig. 3. In the end, the biomaterial needs to have (1) the appropriate mechanical properties to allow deposition (be it extrusion through a nozzle as a gel or an inket as a droplet), (2) the ability to hold its shape as a component of a 3-D structure after deposition, (3) the capability for user control of the 2 prior characteristics, and (4) a cell friendly and supportive environment at all phases of the bioprinting procedure. In general, manipulations of external pressure, shear stress, temperature or the chemical nature of the materials allows achievement of these requirements (Fig. 4).
(a) Integration with hardware platforms for successful bioprinting requires control parameters such as pressure, shear stress, temperature, and chemistries that change the material mechanical properties, thereby facilitating material deposition. (b) Maintenance of hospitable environmental conditions during material preparation, construct biofabrication, and post-printing maintenance and maturation periods are each significant factors in bioprinting viable and functional constructs
Pressure and Shear Stress Influence and Control
The physical driving force behind most bioprinting modalities is pneumatic pressure or mechanical force (an exception is stereolithography). Both of these driving forces result in pressure being translated to the material being extruded. In the case of melt-curable polymers, the pressure required to perform efficient extrusion can be quite high. The interplay between nozzle size and driving pressure determines the shear stress that the material experiences. Importantly, when cells are being printed within biocompatible hydrogels, they also experience these forces, which can significantly impact cell viability. For example, in one study, the effects of dispensing pressures on cell viability and death were evaluated. This work demonstrated that when tissue constructs were printed at 40 psi viability decreased by nearly 40% in comparison to tissue constructs printed at 5 psi, due to differences in shear stress.63 Similarly, shear stress placed on cells as they move through the printing device—for example, against the walls of a syringe or syringe needle tip—can impact cells as well. The same study described above found that shear stress as an effect of nozzle size had less of an effect on viability than overall pressure did.63 However, shear stress is related to both the shear rate experienced when cells and surrounding material move through the bioprinter as well as the material viscosity.11 Therefore, one must weigh the value of fast printing vs. maintenance of cell viability.
Fortunately, materials can be designed to employ applied pressure or shear stress as a control parameter that facilitates bioprinting. In general, such materials are characterized as thixotropic, or in some cases shear thinning, and exist in a semi-gelled state in the absence of external forces. Upon applying mechanical force, the bonds maintaining the gel structure fail. The material elastic modulus then decreases, and the material effectively transitions to a viscous fluid. This phenomenon is incredibly useful for applications employing extrusion through nozzles or syringes. In one such example, a gel comprised of polypeptide DNA was used to direct write various 3-D structures.45 To date, this material has not been combined with cells for true bioprinting, but holds great potential nonetheless. A number of other thixotropic hydrogels have been developed, but have yet to be employed for 3-D printing. Rather, these materials have been investigated as delivery vehicles of drugs or cells for in situ injection.46,67,88
Temperature Influence and Control
Temperature can come into play during bioprinting in several ways. First and foremost, as described above, some biomaterials require elevated temperatures in order to be printed. These conditions are almost always incompatible with printing methodologies in which cells are to be printed also. Because of this, when cells are printed within materials such as hydrogels, care should be taken to maintain physiological temperature for the duration of the printing. Alternatively, if temperatures must change, the magnitude and duration of temperature changes should be minimized. Indeed, in our experience, we observed that if we bioprinted primary hepatocytes at ambient room temperature during a 30–60 min protocol, viability was not as high as we had hoped. By incorporating environmental controls into the bioprinter and maintaining the bioprinter stage and cell-bioink reservoirs at 37 °C, we were able to significantly improve cell viability within the bioprinted tissue constructs and increase the functional output of the constructs.
Temperature can be used as a control parameter for facilitating the bioprinting process by employing temperature-sensitive or thermo-reversible materials. Temperature-sensitive polymers have been used extensively for preparation of cell sheets, but less so in the context of bioprinting.51 However, some polymers and proteins possess innate properties allowing rapid transitions from liquid to solid states, and vice versa, positioning them as promising bioinks. These materials can be printed as a liquid or viscous fluid under one temperature onto a stage or into an environment of a different temperature that causes a sol–gel transition, allowing for smooth extrusion and fabrication of 3-D structures. For example, an alginate-gelatin blend was printed as a fluid using a heated syringe device onto a cold stage, which initiates hydrophobic crosslinking of the gelatin component. After deposition and formation of 3-D structures, the alginate component could be more permanently crosslinked by introduction of calcium chloride. This approach was used to bioprint MSC and hydroxyapatite-containing constructs and may be a promising technique for biofabrication of bone constructs.85 A similar approach using a N-isopropylamid and poly oxyethylene solution was used to build intricate structures by deposition through a cooled nozzle onto a warm stage. Notably, the same nozzle could be used to reverse the sol–gel transition, thus removing portions of printed structure, creating voids.35 By this multi-step method, one can envision the ability to create customized complex 3-D organ structures complete with spaces that could be used as vasculature or other ductile structures. The ability to create such voids has also been accomplished by bioprinting thermo-reversible polymers as sacrificial structures. For example, poloxamer 407 solutions exist as a fluid at 4 °C and a gel above 20 °C. This material was printed within agarose and later washed out to form channels within the 3-D agarose structure.60
Chemical Reaction-Based Control
In addition to using the driving pressure and temperature sensitivity of biomaterials for integration with bioprinting devices, often the chemistries employed for crosslinking are chosen in order to specifically tailor the biomaterials to the bioprinting process. These chemistries are broad and include not only covalent crosslinking techniques, but also chemically or pH induced crosslinking by ionic and physical mechanisms. Importantly, examples of chemical customization of materials exist for various printing modalities. For example, pH-sensitive polyurethane (PU) solutions were formulated that are in a fluid state and can be printed by inkjet devices, but polymerize upon deposition into a basic PU environment, allowing fabrication of high precision 3-D scaffolds and preventing inkjet clogging.90 Our laboratory has focused on developing hydrogel bioinks using thiolated HA and thiolated gelatin as base materials. In its native form, the mixture of these materials is crosslinked with PEGDA, and require 15–30 min to polymerize, which is unsuitable for the fast deposition and crosslinking speeds required in applications such as 3-D bioprinting. To overcome that limitation, we have explored several variations of the gel using different crosslinking approaches. We developed a 4-arm PEG-based crosslinker comprised of a symmetrical and compact core from which 4 functionalized PEG chains extend. Use of this crosslinker in place of linear PEG crosslinkers resulted in a stiffer hydrogel, allowing stacking of extruded rods in increased aspect ratios into a tubular structure.80 We implemented methacrylate-based photopolymerization to employ a 2-step photocrosslinking protocol allowing extrusion through a syringe or printing head, and subsequent increases in elastic modulus to print stable cellular tube structures.79 We also exploited the thiophilic nature of gold nanoparticles as crosslinkers to make slow-forming gels. This property allowed extrusion of partially-gelled materials into 3-D orientations that would later fuse together into single seamless structures.78 Recently, we observed that by adding the Irgacure 2959 photoinitiator to a solution comprised of the aforementioned base materials (thiolated HA, thiolated gelatin, and PEGDA), we could use UV irradiation to achieve near instantaneous photopolymerization through thiol-ene chemistry. This hydrogel was tested against a panel of other commonly used materials in characteristics such as gelation times, ease of use, biocompatibility, and immunogenicity, demonstrating that it was suitable for use in bioprinting devices,62 and subsequently we have implemented it in both 3-D organoid biofabrication as well as in situ bioprinting for wound healing (unpublished). Methacrylated gelatin, polymerized by UV light, has been used in several bioprinting applications, including direct write printing and as a bulk material in which perfusion channels were patterned.5,6 New chemistries and novel combinations of common chemistries will continue to provide alternatives that can improve bioprinting technology in the future. For example, in our laboratory, we are currently combining multiple discrete crosslinking chemistries to allow control over mechanical properties for effective extrusion, and additional crosslinking to tailor the printed construct elastic modulus to that of the target tissue type being generated.
Cell Support and Construct Maturation
During time period in which the printing process occurs, the cells that will be printed are often encapsulated in a material or are suspended in hydrogel precursors that will be crosslinked during or after deposition. During this time, it is important that the potential requirement of nutrients and oxygen that a cell may need is considered. The sensitivity of the cells to stresses and the duration of the printing procedure are the determining factors here. For example, if the printing procedure is short and the cell component is comprised of robust cells lines, cell viability may not be impacted by the printing. However, if the print time (including preparation) is long, and the cells in question are fragile and sensitive primary cells, then without supplying an extra nutritional component to the procedure, viability may decrease severely. For example, our laboratory has employed the HepG2 hepatoma cell line, which has been used extensively as a liver cell surrogate, in proof-of-concept bioprinting experiments. These cells are quite hardy, and remain viable through bioprinting protocols that last several hours. Conversely, primary human hepatocytes are much more sensitive to their environment, and as such are more difficult to maintain viable during bioprinting protocols. We aim to reduce preparation and biporinting times to under an hour. In recent work under these latter conditions we have also observed that by incorporating both tissue derived growth factors, as described above, and cell culture media into the bioprintable materials, we can significantly improve viability of primary cells within bioprinted constructs.
After biofabrication is finalized and a structure containing viable cells is complete, there often remains significant work to be done to ensure that the tissue or organ construct is not only viable for an extended period of time, but also is able to function as intended. In this post-fabrication stage, the cells and tissue must be maintained in such a manner that the cells are able to form the appropriate connections with one another for communication, mobilize if necessary, have the opportunity to reorganize or secrete their own matrix components, and in some cases can be conditioned physically so that they can function as they would in the body.
Provided that cells are in an appropriate environment, they possess the ability to use the surrounding matrix to move around and interact with one another. Over time cells reach equilibrium states between cell–matrix adhesions, such as integrins, and cell–cell adhesions, such as tight junctions and adherens junctions. This equilibrium state is variable depending both on the cell type or types, as well as the matrix. For example, in many cases epithelial lineages require cell–cell adhesions, expressing increased ZO-1 or occludin (tight junction markers) and increased E-cadherin and Ep-CAM, in order to form leak-free layers.3,33 Conversely, cells of mesenchymal phenotypes may have an increased expression level of cell–matrix adhesions vs. cell–cell adhesions, resulting in a more mobile-capably phenotype.74 These preferential interactions between like cells or between certain types of lineages supports the ability of cell populations to spontaneously reorganize within a 3-D environment, a property known as tissue liquidity.36,37 This spontaneous self-organization happens during development in vivo, but has been recapitulated in numerous in vitro applications. For example, cell spheroids can be placed into geometric architectures, which over time fuse together into seamless structures. This has been performed to make rings, tubes, and branches of vasculature.58,65 In a more complex example, when a cell spheroid comprised of mixed smooth muscle and endothelial cell spheroids is bioprinted into a hydrogel together, the cells naturally self-organize into a new architecture in which endothelial cells form a lumen-like structure inside a smooth muscle-based layer. If multiple uniluminal spheroids are then placed adjacently, they can fuse together, and reorganize into one larger multilayered luminal spheroid.23 By manipulating the host biomaterial environment composition, self-organization can be controlled. Migration of cells in 3-D can be expedited or minimized depending on the ratios of matrix components such as collagens and glycosaminoglycans.56 This external manipulation can also be used to spatially organize uniluminal spheroid and harness their fusion to create tubular structures, rather than fusion into one larger spheroid.58
Cells also have the ability to reorganize their surrounding matrix. In particular, cells of mesenchymal phenotype, such as fibroblasts, can break up or modify the matrix material used in the biofabrication stages, and secrete their own matrix materials during the maturation process. This will often provide increased strength to the construct. In fact, we observed this behavior in several studies. Using different bioprinting techniques we fabricated tubular vessel-like prototype tissue constructs using hydrogels and either 3T3 fibroblasts or HEPG2 cells. In both studies, the bioprinted constructs were maintained in culture for several weeks after which the constructs had transitioned from translucent to completely opaque; an effect of both cell growth and cell secretion of additional extracellular matrix proteins. This secretion activity was verified by Masson’s trichrome staining, revealing presence of collagen in the bioprinted constructs. Furthermore, immunostaining for cytoplasmic procollagen indicated that the cells were actively producing the internal precursor to collagen fibers.78,79 Supplying a supportive environment that allows the cellular components of biofabricated constructs to freely reorganize themselves and their environment is ultimately important for long-term development of mature functional tissues.
The phenomena involved in maturation that were discussed above can sometimes be accelerated using techniques such as mechanical conditioning. Periodic stretching, pulsing, or compression of a tissue construct that mimics the physical forces its corresponding tissue or organ experiences in vivo can increase strength and flexibility, as well as increase matrix reorganization and maturation of the construct.25 This concept has been explored extensively with biofabricated blood vessels and skeletal muscle constructs, typically employing perfusion bioreactors and tensile conditioning bioreactors.55 While mechanical properties can be modulated through the biomaterial composition,44 often additional conditioning is required. It is well documented that pulsatile flow through blood vessel constructs increases production of collagen within the construct walls. This increases the stiffness of the tissue,30,71 which in turn increases the pressure of flow that the construct can withstand. In the case of skeletal muscle, material manipulation such as fiber alignment can induce muscle cell organization, but to achieve a functional contracting tissue, cyclic mechanical preconditioning is often required. Applying unidirectional tensile loading to muscle constructs aids in achieving cellular alignment, muscle fiber formation, and increases the force which the constructs can generate during contraction.19 These improvements in function due to preconditioning are crucial for applications where fabricated constructs are implanted in vivo and are expected to integrate with surrounding tissue and function appropriately.49 Other examples exist beyond vascular and muscular structures, and for those constructs, the appropriate type of bioreactor (perfusion, tensile, compression, rotating, air–liquid interface, etc.) must be chosen based on the particular mechanical forces that correspond to the type of tissue being matured.
Conclusion
As viability, function, as well as safety concerns are demonstrated to be sufficient from a regulatory standpoint, demand for bioprinted tissue constructs as viable options for transplants in patients and screening tools for drug candidates will likely increase. To meet this eventual demand, not only will the supply of tissue-engineered constructs need to be expanded, but so will the capabilities to maintain and mature the constructs, both of which can be aided by implementation of optimal biomaterials. Biomaterials play an integral role in many variations of bioprinting technology, as in some applications, biomaterials act as the “glue”, figuratively and literally, that connect the fabrication approaches and the biological cellular components. Currently, few biomaterials exist that both integrate seamlessly with bioprinting hardware and are optimally compatible with living cells. Instead, a large focus of biomaterial work for bioprinting applications has been the adaptation of traditional materials to printing procedures. Instead, for bioprinting to become the source from which tissue engineered products are fabricated on a bulk scale, there will need to be an increased focus on developing novel biomaterials specifically for use in bioprinting and other biofabrication techniques. Biomaterial development and implementation will have a substantial impact on the practical realization of successful applications of viable and functional tissue engineered constructs and entire organ structures, in both clinical and research settings. If successful, this set of tools, which together comprises bioprinting, can be used to create living structures and customized environments that have the potential to change the way medicine is practiced.
References
Ahmed, T. A., E. V. Dare, and M. Hincke. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 14:199–215, 2008.
Allison, D. D., and K. J. Grande-Allen. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 12:2131–2140, 2006.
Balzar, M., F. A. Prins, H. A. Bakker, G. J. Fleuren, S. O. Warnaar, and S. V. Litvinov. The structural analysis of adhesions mediated by Ep-CAM. Exp. Cell Res. 246:108–121, 1999.
Barron, J. A., B. R. Ringeisen, H. Kim, B. J. Spargo, and D. B. Chrisey. Application of laser printing to mammalian cells. Thin Solid Films. 453:383–387, 2004.
Bertassoni, L. E., J. C. Cardoso, V. Manoharan, A. L. Cristino, N. S. Bhise, W. A. Araujo, P. Zorlutuna, N. E. Vrana, A. M. Ghaemmaghami, M. R. Dokmeci, and A. Khademhosseini. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 6:024105, 2014.
Bertassoni, L. E., M. Cecconi, V. Manoharan, M. Nikkhah, J. Hjortnaes, A. L. Cristino, G. Barabaschi, D. Demarchi, M. R. Dokmeci, Y. Yang, and A. Khademhosseini. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 14:2202–2211, 2014.
Billiet, T., M. Vandenhaute, J. Schelfhout, S. Van Vlierberghe, and P. Dubruel. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 33:6020–6041, 2012.
Bohandy, J., B. Kim, and F. Adrian. Metal deposition from a supported metal film using an excimer laser. J. Appl. Phys. 60:1538, 1986.
Boland, T., V. Mironov, A. Gutowska, E. A. Roth, and R. R. Markwald. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 272:497–502, 2003.
Boland, T., T. Xu, B. Damon, and X. Cui. Application of inkjet printing to tissue engineering. Biotechnol. J. 1:910–917, 2006.
Brodkey, B. S. Non-Newtonian Phenomena. The Phenomena of Fluid Motions. Reading: Addison-Welsley, 1967.
Catros, S., J. C. Fricain, B. Guillotin, B. Pippenger, R. Bareille, M. Remy, E. Lebraud, B. Desbat, J. Amedee, and F. Guillemot. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication. 3:025001, 2011.
Chrisey, D. B. Materials processing: the power of direct writing. Science. 289:879–881, 2000.
Cohen, J., K. L. Zaleski, G. Nourissat, T. P. Julien, M. A. Randolph, and M. J. Yaremchuk. Survival of porcine mesenchymal stem cells over the alginate recovered cellular method. J. Biomed. Mater. Res. A. 96:93–99, 2011.
Colina, M., P. Serra, J. M. Fernandez-Pradas, L. Sevilla, and J. L. Morenza. DNA deposition through laser induced forward transfer. Biosens. Bioelectron. 20:1638–1642, 2005.
Dainiak, M. B., I. U. Allan, I. N. Savina, L. Cornelio, E. S. James, S. L. James, S. V. Mikhalovsky, H. Jungvid, and I. Y. Galaev. Gelatin-fibrinogen cryogel dermal matrices for wound repair: preparation, optimisation and in vitro study. Biomaterials. 31:67–76, 2010.
Derby, B. Printing and prototyping of tissues and scaffolds. Science. 338:921–926, 2012.
Dinca, V., E. Kasotakis, J. Catherine, A. Mourka, A. Ranella, A. Ovsianikov, B. N. Chichkov, M. Farsari, A. Mitraki, and C. Fotakis. Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett. 8:538–543, 2008.
du Moon, G., G. Christ, J. D. Stitzel, A. Atala, and J. J. Yoo. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng. Part A. 14:473–482, 2008.
Dubsky, M., S. Kubinova, J. Sirc, L. Voska, R. Zajicek, A. Zajicova, P. Lesny, A. Jirkovska, J. Michalek, M. Munzarova, V. Holan, and E. Sykova. Nanofibers prepared by needleless electrospinning technology as scaffolds for wound healing. J. Mater. Sci. Mater. Med. 23:931–941, 2012.
Fedorovich, N. E., J. Alblas, J. R. de Wijn, W. E. Hennink, A. J. Verbout, and W. J. Dhert. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 13:1905–1925, 2007.
Ferris, C. J., K. G. Gilmore, and G. G. Wallace. Biofabrication: an overview of the approaches used for printing of living cells. Appl. Microbiol. Biotechnol. 97:4243–4258, 2013.
Fleming, P. A., W. S. Argraves, C. Gentile, A. Neagu, G. Forgacs, and C. J. Drake. Fusion of uniluminal vascular spheroids: a model for assembly of blood vessels. Dev. Dyn. 239:398–406, 2010.
Galus, R., M. Antiszko, and P. Wlodarski. Clinical applications of hyaluronic acid. Pol. Merkur Lekarski. 20:606–608, 2006.
Goldstein, A. S., and G. Christ. Functional tissue engineering requires bioreactor strategies. Tissue Eng. Part A. 15:739–740, 2009.
Gou, M., X. Qu, W. Zhu, M. Xiang, J. Yang, K. Zhang, Y. Wei, and S. Chen. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat. Commun. 5:3774, 2014.
Gruene, M., A. Deiwick, L. Koch, S. Schlie, C. Unger, N. Hofmann, I. Bernemann, B. Glasmacher, and B. Chichkov. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C 17:79–87, 2010.
Guillotin, B., and F. Guillemot. Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol. 29:183–190, 2011.
Guillotin, B., A. Souquet, S. Catros, M. Duocastella, B. Pippenger, S. Bellance, R. Bareille, M. Remy, L. Bordenave, J. Amedee, and F. Guillemot. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 31:7250–7256, 2010.
Hahn, M. S., M. K. McHale, E. Wang, R. H. Schmedlen, and J. L. West. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann. Biomed. Eng. 35:190–200, 2007.
Hesse, E., T. E. Hefferan, J. E. Tarara, C. Haasper, R. Meller, C. Krettek, L. Lu, and M. J. Yaszemski. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J. Biomed. Mater. Res. A. 94:442–449, 2010.
Hopp, B., T. Smausz, N. Kresz, N. Barna, Z. Bor, L. Kolozsvari, D. B. Chrisey, A. Szabo, and A. Nogradi. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 11:1817–1823, 2005.
Howarth, A. G., and B. R. Stevenson. Molecular environment of ZO-1 in epithelial and non-epithelial cells. Cell Motil. Cytoskelet. 31:323–332, 1995.
Hribar, K. C., P. Soman, J. Warner, P. Chung, and S. Chen. Light-assisted direct-write of 3D functional biomaterials. Lab. Chip. 14:268–275, 2014.
Iwami, K., T. Noda, K. Ishida, K. Morishima, M. Nakamura, and N. Umeda. Bio rapid prototyping by extruding/aspirating/refilling thermoreversible hydrogel. Biofabrication. 2:014108, 2010.
Jakab, K., B. Damon, F. Marga, O. Doaga, V. Mironov, I. Kosztin, R. Markwald, and G. Forgacs. Relating cell and tissue mechanics: implications and applications. Dev. Dyn. 237:2438–2449, 2008.
Jakab, K., A. Neagu, V. Mironov, R. R. Markwald, and G. Forgacs. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc. Natl. Acad. Sci. USA. 101:2864–2869, 2004.
Jakab, K., C. Norotte, B. Damon, F. Marga, A. Neagu, C. L. Besch-Williford, A. Kachurin, K. H. Church, H. Park, V. Mironov, R. Markwald, G. Vunjak-Novakovic, and G. Forgacs. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng. Part A. 14:413–421, 2008.
Jia, J., D. J. Richards, S. Pollard, Y. Tan, J. Rodriguez, R. P. Visconti, T. C. Trusk, M. J. Yost, H. Yao, R. R. Markwald, and Y. Mei. Engineering alginate as bioink for bioprinting. Acta Biomater. 10:4323–4331, 2014.
Kanda, N., N. Morimoto, A. A. Ayvazyan, S. Takemoto, K. Kawai, Y. Nakamura, Y. Sakamoto, T. Taira, and S. Suzuki. Evaluation of a novel collagen-gelatin scaffold for achieving the sustained release of basic fibroblast growth factor in a diabetic mouse model. J. Tissue Eng. Regen. Med. 8:29–40, 2014.
Kirker, K. R., Y. Luo, S. E. Morris, J. Shelby, and G. D. Prestwich. Glycosaminoglycan hydrogels as supplemental wound dressings for donor sites. J. Burn. Care Rehabil. 25:276–286, 2004.
Knudson, C. B., and W. Knudson. Cartilage proteoglycans. Semin. Cell Dev. Biol. 12:69–78, 2001.
Koch, L., S. Kuhn, H. Sorg, M. Gruene, S. Schlie, R. Gaebel, B. Polchow, K. Reimers, S. Stoelting, N. Ma, P. M. Vogt, G. Steinhoff, and B. Chichkov. Laser printing of skin cells and human stem cells. Tissue Eng. Part C 16:847–854, 2010.
Lee, S. J., J. Liu, S. H. Oh, S. Soker, A. Atala, and J. J. Yoo. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials. 29:2891–2898, 2008.
Li, C., P. Chen, Y. Shao, X. Zhou, Y. Wu, Z. Yang, Z. Li, T. Weil, and D. Liu. A writable polypeptide-DNA hydrogel with rationally designed multi-modification sites. Small, 2014. doi:10.1002/smll.201401906.
Li, J., X. Li, X. Ni, X. Wang, H. Li, and K. W. Leong. Self-assembled supramolecular hydrogels formed by biodegradable PEO-PHB-PEO triblock copolymers and alpha-cyclodextrin for controlled drug delivery. Biomaterials. 27:4132–4140, 2006.
Lin, H., D. Zhang, P. G. Alexander, G. Yang, J. Tan, A. W. Cheng, and R. S. Tuan. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials. 34:331–339, 2013.
Liu, Y., X. Z. Shu, and G. D. Prestwich. Tumor engineering: orthotopic cancer models in mice using cell-loaded, injectable, cross-linked hyaluronan-derived hydrogels. Tissue Eng. 13:1091–1101, 2007.
Machingal, M. A., B. T. Corona, T. J. Walters, V. Kesireddy, C. N. Koval, A. Dannahower, W. Zhao, J. J. Yoo, and G. J. Christ. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng. Part A. 17:2291–2303, 2011.
Marga, F., K. Jakab, C. Khatiwala, B. Shepherd, S. Dorfman, B. Hubbard, S. Colbert, and F. Gabor. Toward engineering functional organ modules by additive manufacturing. Biofabrication. 4:022001, 2012.
Matsuura, K., R. Utoh, K. Nagase, and T. Okano. Cell sheet approach for tissue engineering and regenerative medicine. J. Control Release. 190C:228–239, 2014.
Miki, D., K. Dastgheib, T. Kim, A. Pfister-Serres, K. A. Smeds, M. Inoue, D. L. Hatchell, and M. W. Grinstaff. A photopolymerized sealant for corneal lacerations. Cornea. 21:393–399, 2002.
Mironov, V., T. Boland, T. Trusk, G. Forgacs, and R. R. Markwald. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 21:157–161, 2003.
Mironov, V., V. Kasyanov, C. Drake, and R. R. Markwald. Organ printing: promises and challenges. Regen. Med. 3:93–103, 2008.
Mironov, V., V. Kasyanov, K. McAllister, S. Oliver, J. Sistino, and R. Markwald. Perfusion bioreactor for vascular tissue engineering with capacities for longitudinal stretch. J. Craniofac. Surg. 14:340–347, 2003.
Mironov, V., G. Prestwich, and G. Forgacs. Bioprinting living structures. J. Mater. Chem. 17:2054–2060, 2007.
Mironov, V., N. Reis, and B. Derby. Review: bioprinting: a beginning. Tissue Eng. 12:631–634, 2006.
Mironov, V., R. P. Visconti, V. Kasyanov, G. Forgacs, C. J. Drake, and R. R. Markwald. Organ printing: tissue spheroids as building blocks. Biomaterials. 30:2164–2174, 2009.
Moon, S., S. K. Hasan, Y. S. Song, F. Xu, H. O. Keles, F. Manzur, S. Mikkilineni, J. W. Hong, J. Nagatomi, E. Haeggstrom, A. Khademhosseini, and U. Demirci. Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng. Part C Methods. 16:157–166, 2010.
Muller, M., J. Becher, M. Schnabelrauch, and M. Zenobi-Wong. Printing thermoresponsive reverse molds for the creation of patterned two-component hydrogels for 3D cell culture. J. Vis. Exp. 77:e50632, 2013.
Murphy, S. V., and A. Atala. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32:773–785, 2014.
Murphy, S. V., A. Skardal, and A. Atala. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. A. 101:272–284, 2013.
Nair, K., M. Gandhi, S. Khalil, K. C. Yan, M. Marcolongo, K. Barbee, and W. Sun. Characterization of cell viability during bioprinting processes. Biotechnol. J. 4:1168–1177, 2009.
Nakamura, M., S. Iwanaga, C. Henmi, K. Arai, and Y. Nishiyama. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication. 2:014110, 2010.
Norotte, C., F. S. Marga, L. E. Niklason, and G. Forgacs. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 30:5910–5917, 2009.
Opara, E. C., S. H. Mirmalek-Sani, O. Khanna, M. L. Moya, and E. M. Brey. Design of a bioartificial pancreas(+). J. Investig. Med. 58:831–837, 2010.
Parisi-Amon, A., W. Mulyasasmita, C. Chung, and S. C. Heilshorn. Protein-engineered injectable hydrogel to improve retention of transplanted adipose-derived stem cells. Adv. Healthc. Mater. 2:428–432, 2013.
Pescosolido, L., W. Schuurman, J. Malda, P. Matricardi, F. Alhaique, T. Coviello, P. R. van Weeren, W. J. Dhert, W. E. Hennink, and T. Vermonden. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules. 12:1831–1838, 2011.
Prestwich, G. D. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc. Chem. Res. 41:139–148, 2008.
Prestwich, G. D., and J. W. Kuo. Chemically-modified HA for therapy and regenerative medicine. Curr. Pharm. Biotechnol. 9:242–245, 2008.
Rashid, S. T., B. Fuller, G. Hamilton, and A. M. Seifalian. Tissue engineering of a hybrid bypass graft for coronary and lower limb bypass surgery. FASEB J. 22:2084–2089, 2008.
Roth, E. A., T. Xu, M. Das, C. Gregory, J. J. Hickman, and T. Boland. Inkjet printing for high-throughput cell patterning. Biomaterials. 25:3707–3715, 2004.
Santos, E., J. Zarate, G. Orive, R. M. Hernandez, and J. L. Pedraz. Biomaterials in cell microencapsulation. Adv. Exp. Med. Biol. 670:5–21, 2010.
Scanlon, C. S., E. A. Van Tubergen, R. C. Inglehart, and N. J. D’Silva. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J. Dent. Res. 92:114–121, 2013.
Schiavinato, A., M. Finesso, R. Cortivo, and G. Abatangelo. Comparison of the effects of intra-articular injections of Hyaluronan and its chemically cross-linked derivative (Hylan G-F20) in normal rabbit knee joints. Clin. Exp. Rheumatol. 20:445–454, 2002.
Skardal, A., D. Mack, E. Kapetanovic, A. Atala, J. D. Jackson, J. J. Yoo, and S. Soker. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 1:792–802, 2012.
Skardal, A., L. Smith, S. Bharadwaj, A. Atala, S. Soker, and Y. Zhang. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials. 33:4565–4575, 2012.
Skardal, A., J. Zhang, L. McCoard, S. Oottamasathien, and G. D. Prestwich. Dynamically crosslinked gold nanoparticle—hyaluronan hydrogels. Adv. Mater. 22:4736–4740, 2010.
Skardal, A., J. Zhang, L. McCoard, X. Xu, S. Oottamasathien, and G. D. Prestwich. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. Part A. 16:2675–2685, 2010.
Skardal, A., J. Zhang, and G. D. Prestwich. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 31:6173–6181, 2010.
Soman, P., P. H. Chung, A. P. Zhang, and S. Chen. Digital microfabrication of user-defined 3D microstructures in cell-laden hydrogels. Biotechnol. Bioeng. 110:3038–3047, 2013.
Visconti, R. P., V. Kasyanov, C. Gentile, J. Zhang, R. R. Markwald, and V. Mironov. Towards organ printing: engineering an intra-organ branched vascular tree. Expert. Opin. Biol. Ther. 10:409–420, 2010.
Williams, D. F. On the nature of biomaterials. Biomaterials. 30:5897–5909, 2009.
Williams, D. The continuing evolution of biomaterials. Biomaterials. 32:1–2, 2011.
Wust, S., M. E. Godla, R. Muller, and S. Hofmann. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 10:630–640, 2014.
Xu, T., K. W. Binder, M. Z. Albanna, D. Dice, W. Zhao, J. J. Yoo, and A. Atala. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 5:015001, 2013.
Xu, K., D. A. Cantu, Y. Fu, J. Kim, X. Zheng, P. Hematti, and W. J. Kao. Thiol-ene Michael-type formation of gelatin/poly(ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds. Acta Biomater. 9:8802–8814, 2013.
Zawaneh, P. N., S. P. Singh, R. F. Padera, P. W. Henderson, J. A. Spector, and D. Putnam. Design of an injectable synthetic and biodegradable surgical biomaterial. Proc. Natl. Acad. Sci. USA. 107:11014–11019, 2010.
Zhang, A. P., X. Qu, P. Soman, K. C. Hribar, J. W. Lee, S. Chen, and S. He. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv. Mater. 24:4266–4270, 2012.
Zhang, C., X. Wen, N. R. Vyavahare, and T. Boland. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials. 29:3781–3791, 2008.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Rosemarie Hunziker oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Skardal, A., Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann Biomed Eng 43, 730–746 (2015). https://doi.org/10.1007/s10439-014-1207-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1207-1