Abstract
3D bioprinting is the most popular additive manufacturing method, in which solid objects are constructed by depositing several layers in sequence for the fabrication of biostructures. Bioprinted structures, like tissues, organs, and Organ-on-chip, in 3D, are now widely used to study and understand the functions of the human body. The 3D bioprinted structures are closely similar to naturally occurring biologics systems, and studies performed on 3D biostructures can be more biologically relevant than in vitro studies conducted in 2D. 3D bioprinting has advanced over the years and is commonly used in tissue engineering and regenerative medicine. The fabrication of biomaterials like cells, tissues, and organs using 3D bioprinting is becoming an alternative and favorable tissue and organ transplantation approach. Bioprinting is a relatively novel method and holds great promise but has several challenges and limitations. To this end, this chapter summarizes the concept of 3D bioprinting, bioinks and their classification, different methods of bioprinting, and their applications in areas of health, pharmaceutical science, and biomedical engineering. The chapter also highlights the challenges associated with the clinical utilization of 3D bioprinting. 3D bioprinting and its applications in personalized medicine and tissue bioengineering are continuously growing. In the future, this technology could provide advanced tools to the researchers to develop targeted treatment and improve patient outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- 3D bioprinting
- Tissue engineering
- Regenerative medicine
- Bioinks
- Pharmaceuticals
- Medical devices
- Organ-on-chip
15.1 Introduction
3D bioprinting (3DBP) technology is defined as “the process of obtaining a three-dimensional object in a layer-by-layer fashion with computer-controlled designs” (Eshkalak et al. 2020).The ultimate aim of this technology is to design tissues and organs similar to naturally occurring biologics systems. In the conventional method, non-advanced tools or non-standard geometries are used to produce objects from the bulk substances, resulting in low-quality production (Campbell et al. 2011; Hwang et al. 2018). In contrast to the conventional method, 3DBP technology is more rapid and convenient to handle and automate customized processes (Peterson et al. 2014; Anciaux et al. 2016; Wang et al. 2016a). 3D bioprinting technology is based on 3D printing principles that provide an opportunity to obtain biological materials which include cells, tissues, and organs from biocompatible and biomimetic biomaterials. There are several kinds of 3D printing methods such as inkjet bioprinting, laser-based and extrusion-based bioprinting, and stereolithography bioprinting that are currently in use. Apart from organ printing, it is also utilized in preclinical studies to generate in vitro/in vivo models for developing and screening drug delivery systems. For pharmaceutical research, it offers faster means of drug testing at a low cost and can also have better biological relevance to humans than animal studies. It is a robust tool technology and offers several advantages such as enhanced safety profile and R and D productivity with high efficacy (Ngo et al. 2018). Tailored medication, personalization, quick disintegration, and mini dispenser unit are some of the advantages of 3D printing. However, there are certain limitations of 3DBP. Indeed, bioink formulation does not possess adequate self-binding property, precise viscosity of ink, and exact object printability during the end of the process (Ali et al. 2020). Technology has undoubtedly enhanced patient compliance with the development of scaffolds, tissues, organs, and novel drug carrier systems which are suitable for patient anatomy and specificity by optional and rapid production. The novel technology of 3D bioprinting holds great promise; it is a cost-effective and time-saving technique and is attracting immense attention, especially in healthcare, pharmaceutical sciences, and biomedical engineering (Koçak et al. 2020; Yan et al. 2018).
15.2 History
For the first time, Dr. Kodama from Japan discovered 3D printing technology in his name during 1980. In 1984, the stereolithography apparatus was invented, representing the birth of 3D printing. Moreover, the first use of laser technology illustrating 2D patterning of live cells was reported in 1999. In 2002, the first extrusion-based bioprinting method, which was known as “3D Bioplotter,” was launched. Wilson and Boland developed the first inkjet bioprinter in 2003. In the year 2006, living cells were deposited by the application of an electrodynamic jetting. Scaffold-free vascular tissue was fabricated by using bioprinting in 2009. The coming years saw the rise of numerous new bioprinting products, for example, artificial liver and articular cartilage in 2012 and tissue integration with the vascular system in 2014. In 2016, the cartilage model was manufactured by the Integrated Tissue and Organ Printing (ITOP) system. In the same year, Spritam (levetiracetam) tablets for oral use were manufactured using an inkjet printing method; the first 3D-printed drug approved by the Food and Drug Administration (FDA) in 2016 was developed by Aprecia Pharmaceuticals (Jose and Christoper 2018). In 2019, a cardioid structure was bioprinted in Tel Aviv University; the collagen human heart at various scales was engineered using FRESH technology (Gu et al. 2019).
15.3 3D Bioprinting Procedure
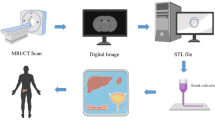
The procedure of 3D bioprinting is generally relied upon the principle of the exact layering of biomaterials. The initial step in 3D bioprinting is to generate a 3D model design using CAD/CAM software. Selection of biomaterial or bioink is also included in this step (Zhang et al. 2018). The second step involves converting the digital model into the STL file format, which stands for standard tessellation language or stereolithography that can be readable to the printer (Agarwal et al. 2020). The STL file contains polygons or triangles, which provide instructions about the designed surface for introducing a model to the printer. In the third step, the data is converted into G-code that encloses information for printing the material layer by layer with the help of slicer software that is placed in the 3D printer (Gross et al. 2014). After that, the extra printed parts are removed before the final step. Step five is a post-processing step, and it relies on different technologies for the maturation of the fabricated construct in a bioreaction to create a complete 3D object (Jamroz et al. 2018; Highley 2019; Papaioannou et al. 2019) (Fig. 15.1).
15.4 Bioinks
The most crucial step in the bioprinting strategy is to select and design the ideal bioinks. Bioinks are “a formulation of cells suitable for processing by an automated biofabrication technology that may also contain biologically active contents and biomaterials” (Groll et al. 2018). Characteristics of ideal bioinks include high mechanical, chemical, and rheological properties. Moreover, it should have good biocompatibility, biodegradability, nontoxicity, and non-immunogenicity, which aid in both cell viability and printability.
Two kinds of bioinks that are adopted in 3D bioprinting are as follows:
-
1.
Scaffold-free bioinks.
-
2.
Scaffold-based bioinks.
Scaffold-free bioinks are the ones in which living cells are directly printed into 3D constructs (Achilli et al. 2012; Mironov et al. 2009; Cui et al. 2017). Firstly, clusters or aggregates are produced from cells (like spheroids) and placed into suitable patterns, which further go for fusion and maturation into broad-scale functional tissues (Rezende et al. 2013). In the case of scaffold-based bioinks, polymeric hydrogels are used to fix the cells, and then the scaffolds are employed for printing with tissue-specific 3D architectures (Liu et al. 2017). In this case, when the degradation of the hydrogel scaffold occurs, the encapsulated cells begin to proliferate and grow into wide-scale tissues. The later bioink strategies are beneficial in manufacturing biomimetic tissues. However, it also exhibits certain drawbacks due to the immobilization of cells in the scaffold, which induce hindrance in cell proliferation, migration, and colonization. Furthermore, scaffold-free bioinks show close biomimicry of the generated tissue and improve functionality for a longer duration (Yipeng et al. 2017). Bioprinting technology consists of three essential components: polymer solution (natural or synthetic), viable cells, and 3D printers (Aljohani et al. 2018).
15.5 Hydrogels for Bioprinting
Hydrogel is a class of 3D network polymers formed through crosslinking methods to form gel-like structures and are essential components of bioink. It functions as a cell carrier, maintains its viability, and serves as substrate or scaffolds supporting its growth and proliferation. The hydrogel can swell in water but does not dissolve in it. Moreover, a few hydrogels exhibit permeability that is similar to the natural extracellular matrix (ECM). The hydrogels are considered as an ideal material for cell bioprinting due to biocompatibility, high water content, and biodegradability (Wang et al. 2020). Electrospun fibers were utilized as scaffolds to replace the blood vessels, bone, cartilage, and other organs in earlier times. However, nowadays, it is substituted with many polymers as collagen, chitosan, polyanhydride, alginate, agarose, Matrigel, and fibronectin (Gu et al. 2015; Jia et al. 2014). There are two types of hydrogels applied for bioprinting of biomaterials: a natural polymer, synthetic polymer, or a combination of both natural and synthetic polymer-based biomaterials. Natural polymers are widely available from sources like animals, plants, and microbes and are the main component of extracellular matrices (ECMs). Before printing, natural polymers enclose the viable cells and biologically active agents and make them safe from printing stress during the process. After printing, semipermeable substrates are formed, and these substrates are permeable to nutrients and metabolites of a cell; through this, cells can exchange gases (e.g., oxygen). The natural polymers commonly used include hyaluronic acid (HA), gelatin, fibrin, laminin, collagen, and fibronectin. Apart from this, various other biopolymers such as chitosan, alginate, starch, and cellulose are also used as hydrogels (Liu et al. 2018a).
On the contrary, synthetic polymers are produced by several chemical synthesis processes. It is known to mimic the extracellular environment with the help of various native components of ECM. Natural polymers are usually modified to ameliorate the biodegradability, biocompatibility, thermal, physico-mechanical, biological, chemical, and conductive properties through synthesis of composites (Xu et al. 2012). Derivations in cross-linkable structures provide a better opportunity in tissue engineering and regenerative medicines for application purposes. Poly(ethylene glycol) (PEG), PEG-dimethacrylate (PEG-DMA) (Cui et al. 2012), poly(ethylene glycol) diacrylate (PEGDA), poly(ɛ-caprolactone) (PCL), polymethylmethacrylate (PMMA) poly(vinyl alcohol) (PVA), polyurethane (PU), poly(hydroxypropylmethacrylamide) (PHMMA), GelMA/gellan gum, and alginate hydrogel (Visser et al. 2013), and poly lactic-co-glycolic acid (PLGA) biomaterials are implemented to engineer desired organ through the scaffold-free cell printing technology (Shim et al. 2012; Guo et al. 2014). Among these polymers, PEG, PLGA, and PU are known as synthetic polymers, which exhibit excellent 3D printability, tissue compatibility, and stability properties (Zhang and Wang 2019).
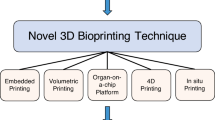
15.6 Methods of 3D Bioprinting
15.6.1 Inkjet-Based Bioprinting
It is a conventional and commonly used method of printing. In this method, droplets acquiring cells and biomaterials are patterned layer-by-layer on a substrate (Shafiee and Atala 2016). Generally, bioink is filled into the cartridge, and then droplets are placed on the substrate with the assistance of computer-controlled thermal or piezoelectric (acoustic) actuators to build a 3D model (Zhang et al. 2016). The heat required to generate bubbles is up to 300 °C for a few microseconds, and then picoliter-sized ink droplets from the nozzle spread onto the substrate (Arslan et al. 2016). Generally, the high temperature encompasses a minor impact on the viability of the cellular material (Xu et al. 2005). The piezoelectric crystal actuator generates electrical pulses to eject nanoliter or picoliter droplets at regular intervals (Park et al. 2017). Furthermore, this method offers high resolution and allows printing with high cell viability (Demirci and Montesano 2007). The benefits of inkjet bioprinting include the high printing speed (up to 10,000 droplets per second), cost-effectiveness, and wide availability. In addition, the repairment of skin and cartilage tissues is a common application of inkjet bioprinting where the high speed of printer enables direct ejection of cells and biomaterials onto skin and cartilage lesions. However, the major drawback is that the cells can sometimes form aggregation in between the cartridges and block the nozzles (Arslan et al. 2016).
15.6.2 Laser-Based Bioprinting
Laser-based bioprinting or laser-induced forward transfer bioprinting (LIFT bioprinting) is a method applied for printing living cells in the engineering of tissue structures. In this method, the high-energy light source is utilized for printing design (Moroni et al. 2018; Hospodiuk et al. 2017). During the process, repeated laser beam pulse is received by substrate at a controlled rate for printing the cells (Badylak and Gilbert 2008). This device is comprised of a focusing system, pulsed laser beam, bioink, laser absorbing ribbon, and a receiving substrate (Nguyen et al. 2017; Lorson et al. 2017). The resolution of laser bioprinting relies on various parameters such as surface tension, type of laser used, the wettability (substrate), viscosity (organic layer), and the air voids between the substrate and the ribbon (Liu et al. 2018b). Unlike inkjet printing, it has no nozzle, and as such high density of cells and viscous bioinks can be loaded without causing clogging. It has been used to print cellularized skin constructs, cells of the human dermal fibroblasts, and pulmonary artery endothelial cells. However, expensive installment and selection of bioinks make this method less preferable (Arslan et al. 2016).
15.6.3 Extrusion Bioprinting/Direct Writing
Extrusion bioprinting (EBB) is very common additive manufacturing method that is applied in the area of tissue engineering and biofabrication (Ji and Guvendiren 2017). This technique is broadly used on a large scale for bioprinting the cells in which the continuous filaments from the automatic nozzle extrudes the bioink on to the substrate (Ozbolat and Gudapati 2016; Hospodiuk et al. 2016). The biological materials are extruded and regulated by pneumatic or mechanical dispensing techniques. The viscosity ranges of hydrogel solutions for supporting cell materials lie from 30 to > 6 × 107 mPa/s for extrusion-based bioprinting (Murphy and Atala 2014). This device has a dispenser (single ejector or multiple ejectors), an automatic robotic system, and a stage controller. The significant advantages of EBB are its high printability speed, reduced risk of clogging, compatibility for printing materials, and high-density cell deposition (Wang et al. 2017; Yeo and Kim 2017).This technology is commonly used to manufacture scaffolds that mimic soft tissues and bone structures, which provide an opportunity for possible implants. Nevertheless, the pressure and shear stress influence deposited cells’ viability in viscous fluids forming a structure with low fabrication resolution (∼200 μm) by extrusion (Xiong et al. 2017). These drawbacks can be overcome by optimization and modification in the nozzle, syringe, or motor control systems to some extent (Yu et al. 2013; Dababneh and Ozbolat 2014).
15.6.4 Bioplotting
Bioplotting is type of 3D printing technique employed for tissue engineering and regeneration. Its adaptability provides new opportunities to extrude spheroids or tubes of materials from the syringe (Gloria et al. 2009). Generally, this printer system has multiple syringes and can be applied to numerous cell types enabling the fabrication of soft tissues. In this mechanism, deposition of materials is arranged in a bottom-up approach (layer-by-layer) and cured via UV radiation. Despite these advantages, challenges in selecting the bioprinting materials are known to exist (Mobaraki et al. 2020).
15.6.5 Fused-Deposition Modeling (FDM)
Among additive manufacturing techniques, FDM is one of the oldest processes involved in 3D printing. In the FDM printer, the head resembles an inkjet printer (Salentijn et al. 2017). However, during the heating process, beads are liberated from the print head, as it then constructs the object in fine layers. The procedure undergoes several repetitions until the complete structure of each layer is formed precisely. After that, each layer becomes hard, gradually developing into a solid object. FDM printers can use several polymeric materials, but it does not show similarity to formulate bioinks for bioprinting applications (Rahim et al. 2019).
15.6.6 Stereolithography (SL)
This method is based on photo-polymerization in which photosensitive material/s is cured to produce a 3D object (Kumar and Kim 2020). Firstly, the appropriate bioink is prepared by mixing cells in the solution of photosensitive prepolymer. The SLA device initiates the photopolymer’s chemical reaction by using the digital mirror that induces the crosslinking of the bioink (Melchels et al. 2010). After repetition of the process, the parts of the object are fabricated in a layer-by-layer manner. Finally, curing is the essential step to improve mechanical integrity and to remove the uncrosslinked portion of the bioink. SLA printers transfer the energy into a liquid photopolymerizable resin in the form of UV laser beam. However, certain resins have shown potential carcinogenic properties. This is the way the FDA-approved resin should be used for clinical applications (Wang et al. 2016b; Goole and Amighi 2016). SLA-based technologies are broadly applicable in the biomedical field to build prosthetics, custom implants, bone, or cartilage tissues. Although the SLA is a rapid printing process, it is a low-resolution and time-consuming technique for designing 3D tissue. Therefore, compared to the visible light-based photoinitiation, the bioprinting UV light-based process consumes more time during exposure to light and possesses a low cell survival rate (Ozbolat et al. 2016).
15.7 Applications of 3D Bioprinting
It is an exciting platform for developing medical and engineering technologies and biomimicking living animal cells, tissues, and organs. After modifying conventional technology, the contemporary 3D bioprinting technique forms a base for the evaluation of numerous biomedical applications such as scaffolds, tissue fabrication, artificial organs, development of drugs, and medical devices. Apart from the above-cited applications, it can also be used to create prosthetics, implants, anatomical models, and drug delivery.
15.7.1 Tissues and Organs Printing
3D printing technology provides benefits in the fabrication of cells, cell-laden biomaterials, and tissues to create 3D organs (Ozbolat and Yu 2013). 3D printers have been employed to develop a spinal disk, knee meniscus, heart valve, cartilage and bone, and an artificial ear (Gross et al. 2014).
15.7.2 Customized Implants and Prostheses
Due to advancements in 3D technology, many techniques such as X-ray, MRI, or CT scans are used for making similar geometry of organs that can transfer images into digital 3D files. The strategy has been practiced in spinal, dental, and hip implants (Šljivić et al. 2019).
15.7.3 Anatomical Models
Anatomical models are customized for specific patients and pathology. On the basis of customized models, it helps in educating patients on their exact condition. Neurosurgeons widely use 3D-printed neuroanatomical models to understand the complexity of the human body (Klein et al. 2013).
15.7.4 3D-Printed Dosage Forms and Drug Delivery Devices
Bioprinting has earned worldwide popularity in the last decades, making a significant impact on the pharmaceutical industry. It has shown high reproducibility and capability in manufacturing dosage forms with complex drug release profiles. This technique can also create complex drug manufacturing methods. For the control release rate of the drug, internal structure and shape structure can be adjusted by choosing suitable models, parameters, and materials. The surface area is modified to create a customized shape of the pill by 3D printing (Goyanes et al. 2015). It works on the principle of the concentration gradient to regulate and control the strength and time of the drug release. ZipDose Technology enables the administration of a high drug load, up to 1000 mg in a unit dose. SPRITAM is one example through which the largest quantity of levetiracetam is administered with only a single sip, thereby enhancing patient compliance (Jose and Christoper 2018).
15.7.5 Unique Dosage Forms
3D printing offers the opportunity to produce abundant dosage forms that overcome the limitations of conventional drug fabrication. It has currently been used to manufacture various novel drug formulations, for instance, microcapsules, antibiotic printed micropatterns, nanosuspensions, mesoporous bioactive glass scaffolds, and multilayered drug delivery devices (Ursan et al. 2013). Sustained-release formulation can be developed as single or multilayer printed tablets. Such type of dosage forms are known to reduce the frequency and number of dosage form units taken by the patient on a regular basis. One of the major potentials of 3D printing is to manufacture an individualized dosage form known as polypill. Any polypill is a combination of two or more doses, which includes sustained-release (SR) layer and immediate-release (IR) layer. For example, aspirin and hydrochlorothiazide are placed in the IR layer, while the SR layer helps in the separation of the three drugs, namely, atenolol, pravastatin, and ramipril, to ensure the peculiar release of the individual drug. The polypill helps in treating patients with comorbidities, such as hypertension, chronic renal failure, and diabetes (Khaled et al. 2015).
15.7.6 Drug Testing
3D printed biomimetic model has been employed in drug testing, where it is accelerating the new drug development process and ultimately reduces the usage of animals for preclinical studies. For this, personalized cell-laden pattern and fabricate models such as in vitro liver for drug screening are fabricated. The 3D microenvironment is useful in investigating the toxicological and pharmacological properties of microtissues (Satpathy et al. 2018; Peng et al. 2017).
15.7.7 Other Applications with Some Challenges
Repairing of skin has gained more attention due to its simple structure, but the application of 3D bioprinting on burned skin would be challenging to regenerate scar-free skin. Vascular tissue engineering is complicated as vessels exist throughout the body. Myocardial repair has also attracted massive attention. Currently, there are many cardiac patches used to treat myocardial infarction. Simple tissues (e.g., skin, bone, and cartilage) are fabricated easily, while complex tissues, such as vascular or connective tissues, are hard to fabricate (Aljohani et al. 2018).
15.8 Tissue Engineering and Regenerative Medicine
Tissue engineering is an interdisciplinary field that applies engineering and life sciences principles in developing biological tissue that focuses on creating and fabricating natural substitutes to reproduce functioning tissues or organs. The objective of tissue engineering is to achieve a physiological structure that maintains, restores, or improves damaged tissues or whole organs and to promote health. It constructs alternative structures that mimic the body’s normal tissue for substituting injured or diseased tissue (Wardhana and Valeria 2020).
Regenerative medicine is a multidisciplinary area that involves the body utilizing its systems (directly) or sometimes with the help of foreign biological material (indirectly releasing factors that can induce endogenous tissue healing) to reform damaged cells, tissues, or organs.
The words “tissue engineering” and “regenerative medicine” are often used as interchangeable terms, as the ultimate target is to concentrate on cures rather than treatments for multiple diseases (Tonelli et al. 2016). For example, artificial skin and cartilage have been approved by the FDA as common engineered tissues; however, they have limited use in human patients due to certain limitations.
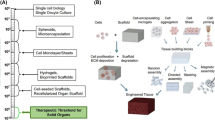
15.8.1 Components of Tissue Engineering
The aim of constructing tissues or regenerating tissues is achieved by tissue engineering. The following three components are required to fabricate artificial tissues.
15.8.1.1 Scaffold
The scaffold is an artificial three-dimensional structure that carries cells and acts as their microenvironment. It mainly contains proteoglycans and glycoproteins that serve as a simulation of the extracellular matrix in a human cell. Scaffold structures should be made of biodegradable materials like collagen and some polyesters. Generally, polylactic acid is used as a scaffold material. To produce an ideal scaffold, it should possess precise regulation of macrostructural (e.g., mechanical strength, spatial form, density, porosity) and microstructural (e.g., pore size, pore interconnectivity, pore distribution) features (Leong et al. 2003). Micropores are necessary for the growth of cells, diffusion of nutrients, and waste. It has been seen that space for blood vessels is imperative and exhibits the limiting factor in scaffold (Chen et al. 2006).
15.8.1.2 Cells
Cells are essential components that fill the empty scaffold and convert it into the target tissue. Currently, the cells available can be classified in several ways. The simplest way is to classify as follows: (1) allogenic cells (cells from the same person), (2) autologous cells (cells from the same individuals), and (3) xenogenic cells (cells from another species) (Hasirci and Hasirci 2018). The other method has classified cells into five types, which are available for tissue engineering (Lanza et al. 2020).
-
(a)
Adult stem cells: These cells are isolated from the same person. It is a good method of production because the donor is the recipient, and rare chances of infection occur. Moreover, immune suppressive therapy is not needed during transplantation.
-
(b)
Mesenchymal stem cells: MSCs exist in the bone marrow and are responsible for the homeostasis of musculoskeletal tissue. They have the potential to differentiate into a variety of cells that are lacking for reparation of tissue by modifying themselves. They are also the right way of replacing adult stem cells.
-
(c)
Embryonic stem cell: ES is capable of differentiating into all somatic cells and proliferating indefinitely. However, they are less reliable due to their tumorigenic properties.
-
(d)
Induced pluripotent stem cells (iPSC): This method is widely accepted. However, these cells are integrated into the host genome and exhibit oncogenic behavior because this type of stem cells rely on the retroviral vector.
-
(e)
Other types of cells: Some of them are from placental tissue, umbilical cord, amniotic fluid, and umbilical cord. Unlike embryonic stem cells, it does not have tumorigenic properties.
15.8.1.3 Growth Factors
It is essential to add bioactive compounds (growth factors) that guide their attachment to the scaffold in tissue regeneration. Growth factors (GFs) are signaling molecules that provide direction for cell growth, helping in stem cell proliferation, migration, and differentiation. Nevertheless, the main limitation of growth factor selection is dosage-related adverse effects (Subbiah and Guldberg 2019).
15.8.2 Limitations
Adequate pore volume and permeability are necessary for the penetration of the cells into the scaffold. Otherwise, it would be confined within the scaffold peripheries leading to cell necrosis. Due to the lack of nutrients, the degradation of scaffold starts, resulting in long-term failure of new tissue grafts (O’Connor et al. 2020). The thickness of artificial tissue is a significant parameter for supplying nutrients to central parts of the tissue by diffusion. This is one of the vital and large areas for developing angiogenesis in tissue engineering (TE) (Shafiee and Atala 2017). The drawback of TE is a deficiency of donor organs for people who are waiting for transplantation. Another drawback is that regular immunosuppressive therapy can harm the health of the recipients. In addition to the cells and scaffolds, temperature (37 °C) and growth and differentiation factors (hormones, chemical, and metabolic factors) are essential for tissue development (Castro et al. 2020).
15.8.3 3D Bioprinting and Tissue Engineering
3D bioprinting is becoming the future of tissue or organ construction. It is difficult to mimic the natural process to establish viable organs due to the slow growth and development of tissues in an individual (Kawasaki et al. 2017; Van Linthout et al. 2014). For these reasons, organ regeneration and complex tissue formation seem to be incompatible through a natural process. Therefore, the biomanufacturing of complex tissue/organ models that imitate specific tissues/organs’ biological functions could help overcome organ failure or rejection (Homan et al. 2016; Pourchet et al. 2017; Neiman et al. 2015; Liu and Wang 2015). The traditional engineering systems are not useful because of their complexity. Each organ has its specific anatomical, histological, and morphological structures that perform various physiological activities (Wang et al. 2010). Thus, the 3D bioprinting technology plays an imperative role in creating external and internal tissue structures. In conventional methods, including cell transplantation, tissue repairing, the anatomy of tissue models, and regulation of vital physiological processes, relies on a self-healing attribute of the recipient cells. Today, 3D bioprinting methods are capable of fabricating tissues in vitro but still face challenges in controlling the process of a complex organ after implantation. The extracorporeal systems can help to regenerate organ function permanently. Thus, bioadditive manufacturing is focusing on engineering extracorporeal organ systems to print, fabricate, and maturation before implantation (Hartupee and Mann 2016).
15.8.4 Tissue Engineering of Different Organs
15.8.4.1 Cardiac
It becomes crucial to design functional myocardium for repairment. Alginate scaffolds can cultivate myocardium models with human cardiomyocyte progenitor cells (hCMPCs) by using 3D bioprinting (Gaetani et al. 2012). Indeed, a cardiac patch of polyesterurethane urea (PEUU) co-cultured with human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (hMSCs) through laser-induced forward transfer (LIFT) cell printing method has been developed. Transplantation of these patches in the infarcted region of the rat heart has shown vascularization and improved physiology of cardiac muscles. Moreover, in 2016, a study has declared that the possibility of 3D bioprinting to recapitulate the whole heart through CAD software (Oklu et al. 2016).
15.8.4.2 Liver
Recently, liver-like microstructures have been fabricated by employing printing technology. For instance, one of the first bioprinting companies, Organovo™ constructed high cell viability and 3D vascularized liver by using bioprinting of high-density hepatocyte cells, hepatoma cells, and endothelial cells, a scaffold which resembles native hepatic lobules. On the other hand, single hepatic cells were replaced by liver spheroids, which can prevent the exerted shear stress on cells throughout the printing process. Consequently, such spheroids demonstrated constant hepatic biomarker secretion of alpha-1 antitrypsin, ceruloplasmin, transferrin, and albumin that helps in the evaluation of hepatotoxic drugs (Nguyen et al. 2015).
15.8.4.3 Cartilage Bone
PCL/nHA/PPF and PCL/nHA scaffolds were set in in the rabbit’s femurs, with or without seeding of rabbit bone marrow mesenchymal stem cells (BMSCs). After 4 and 8 weeks, the evaluation was done by micro-CT, histological test, and mechanical test (Buyuksungur et al. 2017). As evaluated by micro-CT and bone mineral density, scaffolds seeded by BMSC revealed progress in bone tissue regeneration. The test results were significantly better than healthy rabbit femur and showed improvement in regeneration of bone. At present, many researchers are focusing on hybrid biomaterials for the construction of bone scaffolds and their applications. Recently, Oladapo et al. (2019) have fabricated a hybrid bone implant material with the composition of carbohydrate particles (cHA) and polylactic acid (PLA) by using the 3D bioprinting technique (Oladapo et al. 2019). As a result, there is a surge in the multiplicity of components and usage of biomaterials by a combination (Beheshtizadeh et al. 2020).
15.9 Organ-on-Chip
These are the micro-engineered biomimetic systems that combine the ideas of microfluidics, tissue engineering, and diagnosis that can simulate living tissues more closely. It generally consists of transparent 3D polymeric microchannels bound by mammalian cells and replicates three essential aspects of printed organs, which can be employed as remarkable in vitro models for biological activity. Various steps are involved in the printing of organ-on-chip platform (Esch et al. 2015) (Fig. 15.2).
Modification of 3D bioprinting technology with specific cell pattern enables function, tissue regeneration, and high rate of production (organs-on-a-chip) which opens the wide range of research on the organ-on-chip (Park et al. 2018a).
In one such study, living soft tissue was fabricated by using a bioink which combines the shear thinning properties of nanofibrillated cellulose (NFC) and alginate (cross-linker). Moreover, MRI and CT images were used as blueprints to design an atomically shaped human ear and sheep meniscus (cartilage structures) (Markstedt et al. 2015). In another study, Choi et al. (2016) concluded that extracellular muscle matrix (mdECM)-based bioink and bioprinting technology mimicked the functional and structural characteristics of native muscle. This provides new hope for the treatment of muscular injuries. The printed structure is known to enhance myogenic differentiation with high cell viability, contractility, and maturation (Choi et al. 2016). These in vitro tissue models serve as an alternative source in multiple fields, for example, toxicological, drug discovery, and micro-level physiological studies.
15.9.1 Applications of Organ-on-Chip
The main objective of an organ-on a chip is to fabricate a functional unit of the required organ instead of the entire organ that mimics human physiology. This technology can be used for the engineering of tissues that can be used on a large scale for human cell testing and organoids in the integrated fluidic environment (Fetah et al. 2019). It can also be used for the assessment of toxicology parameters in the drug development process where the two-dimensional (2D) cell culture fails to precisely imitate the physiological environment of intra-organ interactions (Wu et al. 2020). So, we can evaluate the impact of drugs upon disease condition in real-time.
15.9.1.1 Liver-on-the-Chip
The liver is the central core of xenobiotic metabolism and is more prone to drug and cosmetic toxicity. During the hepatic injury, liver cirrhosis is encountered in later stages due to the aggregation of collagen-like extracellular matrix proteins from the hepatic stellate cells (HSCs), which are intermediated by numerous pathways within the liver parenchyma. By this technology, human co-culture models are developed to test hepatotoxicity (Leite et al. 2020). The sinusoid is the functional unit of the liver which contains different cells of the liver. It has bile duct cells, hepatic artery, and vein, which perform respective functions such as bile removal, oxygen transport, and toxin removal. Ultimately, several strategies have been printed to mimic the functions of an organ on an organ chip (Radhakrishnan et al. 2020).
15.9.1.2 Tumor-on-the-Chip
Cancers are characterized by cell angiogenesis, proliferation migration, and intravasation of tumor cells. In vitro cell culture fails to replicate the complex nature of the 3D tumor microenvironment. A microfluidic platform has been established to overcome this problem, which mimics the biological tumor microenvironment for appropriate conditions to generate chemical gradients. This makes easier to study the cellular responses of biomolecules and chemicals with changing concentration during cancer metastasis (Yu and Choudhury 2019). Before loading tumor cells or tumor organoids of patients, quiescent perfused 3D microvascular structure (provide nutrients or drugs to the tumor) is printed in an adjacent compartment (Shirure et al. 2018). An easy method to design 3D tumor tissue via employing a microfluidic platform through direct cell writing has been developed. In the first step, an automatic direct cell writing (DCW) technique designs the 3D tissue; secondly, it is placed in a polydimethylsiloxane (PDMS) device engineered with soft lithography (Chang et al. 2008).
15.9.1.3 Lung-on-the-Chip
Horváth et al. (2015) reported the first bioprinted in vitro lung model. This air-blood tissue barrier model is separated by Matrigel membrane, which is made of an epithelial cell layer and endothelial cell layer, printed on porous membrane substrate with a bottom-up approach (Horváth et al. 2015). Decellularized extracellular matrix bioink was obtained from porcine tracheal mucosa (tmdECM), which enclosed fibroblasts and endothelial cells into the polycaprolactone (PCL) frame for printing. From the physiological point of view, this model presents respiratory symptoms such as allergen-induced asthma exacerbation and asthmatic airway inflammation. As 3D bioprinting possesses adaptable nature, it is expected that this will offer a wide platform for preclinical research studies (Park et al. 2018b).
15.10 3D Bioprinting of Medical Devices
3D bioprinting offers many advantages in the area of healthcare and medical devices. The surgeon can use the 3D model of the desired patient anatomy to help MRI or CT scan images for the surgical approach (Aimar et al. 2019). Further enhancement in additive manufacturing technology enables the construction of patient-specific implants from 3D imaging data (Ahangar et al. 2019). The FDA-approved 3D printed titanium device (FastForward Bone Tether Plate) represents a novel approach in treating hallux valgus deformities (FDA 2016).
15.11 3D Bioprinting for Improved Drug Delivery
3D bioprinting has proven to have a disruptive effect on drug delivery systems as this technology has potentially promoted the creativity in fabricating the solid oral dosage form with complex structures, various geometries, torture channels, porosity gradients, and multi-compartment systems, for instance, polypills that consist of multiple active pharmaceutical ingredient in unit dosage form for control release of drug (Samiei 2020). Goyanes et al. (2015) evaluated the drug release property of 3D printed tablets on the basis of geometry and found that printed geometry of acetaminophen tablets resulted in different rate of drug release pattern with high degree of personalization. In this study, acetaminophen-loaded filaments of PVA (polyvinyl alcohol) were produced by taking an aqueous solution of paracetamol (2% w/w) in small pieces of PVA with Varicut (plasticizer). A single-screw filament extruder was printed in various shapes such as a pyramid, cube, cylinder, and sphere. The pyramid-shaped tablet of acetaminophen has the fastest release rate as compared to the cylindrical shaped tablet because the former had the largest surface area-to-volume ratio (Goyanes et al. 2015). Moreover, Spirtam® (levapiracetam) is an immediate-release pyramid-shaped tablet manufactured by powder bed binding method that decreases the lag time for the onset of action because a high amount of the drug is present for absorption through the oral mucosa (Jamróz et al. 2018).
FDM technology was used to enhance the dissolution rate and bioavailability of poorly soluble drug “domperidone.” The conclusion of the study was that the formulation of the 3D tablet could disintegrate slowly and offer a sustained release rate with a floating ability up to 10 h both in vivo and in vitro (Chai et al. 2017). Apart from this, inkjet-based printing systems and nozzle-based deposition systems that rely on natural products have been used for drug delivery systems (Aguilar-de-Leyva et al. 2020). 3D printed implant materials are used for gynecologic and obstetric applications to elute progesterone or estrogen that are entrapped within polycaprolactone (PCL) biodegradable polymer. These implants show extended hormonal release for a period of 1 week (Tappa et al. 2017).
15.12 Disadvantages of 3D Bioprinting
Limitations of raw material: currently, 3D printers can employ approximately 100 different raw materials, which is significantly less than conventional systems. Specifically, the designing of a complex native structure depends on 3D bioprinting, which is still lagging due to the lack of appropriate bioinks with high printability, biocompatibility, and mechanical properties (Noor et al. 2019). Low resolution is caused by inkjet and extrusion-based bioprinting, which is imposed by the physical confinement of the nozzles. Thus, appropriate nozzle size variation is imperative to enhance printing speed, resolution, and compatibility for biomaterials (Xie et al. 2020). Limitations of size: The 3D printing technique is restricted by size limitations. 3D printers are still not capable of printing very large objects. The development of microvasculature plays a pivotal role in restoring the high cell viability of printed organs for long-term survival. However, using 3D bioprinting to fabricate a similar native vascular network is still inadequate because the size of the bioprinted tissues is larger than tens of micrometers (Yu et al. 2020). Cost of printers: There is less probability of purchasing a 3D printer by the average householder. Moreover, printing different types of objects require different types of 3D printers. Even printing of colored objects is costlier than the printing of monochrome objects. Hence, the average population would not be able to afford such expensive treatment. Limited manufacturing jobs: It is an undeniable fact that advancement in technologies leads to a decrease in manufacturing jobs. This drawback can have a heavy impact on the economies of many countries (Ghadage et al. 2019). Unchecked production of dangerous items: One of the major concerns is that the ease of understanding of 3D bioprinting could lead to the undisciplined fabrication of tissues without ethics. Another disadvantage is that it may potentially be used to construct a bioweapon for bioterrorism that endangers people’s lives (Gokhare et al. 2017).
15.13 Conclusion
The chapter encompasses the concept of 3D bioprinting, history, bioinks, type of bioprinting methods, and its varied applications in different research areas. Currently, 3D bioprinting is in the initial stage of development and has generated some notable results by creating a variety of functional tissues. Bioprinting enables the equal distribution of cells throughout a scaffold, but it is still under progress and has to cross multiple obstacles before entering the clinical world, especially for in situ direct use. Various challenges have to be tackled out, which include suitable cell and material selection, tissue maturation, and appropriate vascularization. Major hurdles are biomechanics, sterilized environment, selection of stent material, and scaffolds. 3D bioprinting has shown significant advancement and has garnered the immense interest of the research community. Bioprinting will continue to evolve and develop and will transform the area of organ transplantation.
References
Achilli TM, Meyer J, Morgan JR (2012) Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther 12(10):1347–1360
Agarwal S, Saha S, Balla VK, Pal A, Barui A, Bodhak S (2020) Current developments in 3D bioprinting for tissue and organ regeneration—a review. Front Mech Eng 6:90
Aguilar-de-Leyva Á, Linares V, Casas M, Caraballo I (2020) 3D printed drug delivery systems based on natural products. Pharmaceutics 12(7):620
Ahangar P, Cooke M, Weber M, Rosenzweig D (2019) Current biomedical applications of 3D printing and additive manufacturing. Appl Sci 9(8):1713
Aimar A, Palermo A, Innocenti B (2019) The role of 3D printing in medical applications: a state of the art. J Healthc Eng 2019:1–10
Ali A, Ahmad U, Akhtar J (2020) 3D printing in pharmaceutical sector: an overview. In: Pharmaceutical formulation design-recent practices. IntechOpen, London
Aljohani W, Ullah MW, Zhang X, Yang G (2018) Bioprinting and its applications in tissue engineering and regenerative medicine. Int J Biol Macromol 107:261–275
Anciaux SK, Geiger M, Bowser MT (2016) 3D printed micro free-flow electrophoresis device. Anal Chem 88(15):7675–7682
Arslan-Yildiz A, El Assal R, Chen P, Guven S, Inci F, Demirci U (2016) Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 8(1):014103
Badylak SF, Gilbert TW (2008) Immune response to biologic scaffold materials. In: Seminars in immunology, vol 20. Academic Press, San Diego, CA, pp 109–116
Beheshtizadeh N, Lotfibakhshaiesh N, Pazhouhnia Z, Hoseinpour M, Nafari M (2020) A review of 3D bio-printing for bone and skin tissue engineering: a commercial approach. J Mater Sci 55(9):3729–3749
Buyuksungur S, Tanir TE, Buyuksungur A, Bektas EI, Kose GT, Yucel D, Beyzadeoglu T, Cetinkaya E, Yenigun C, Tönük E, Hasirci V (2017) 3D printed poly (ε-caprolactone) scaffolds modified with hydroxyapatite and poly (propylene fumarate) and their effects on the healing of rabbit femur defects. Biomater Sci 5(10):2144–2158
Campbell T, Williams C, Ivanova O, Garrett B (2011) Could 3D printing change the world. In: Technologies, potential, and implications of additive manufacturing. Atlantic Council, Washington, DC, p 3
Castro N, Ribeiro S, Fernandes MM, Ribeiro C, Cardoso V, Correia V, Minguez R, Lanceros-Mendez S (2020) Physically active bioreactors for tissue engineering applications. Adv Biosyst 4(10):2000125
Chai X, Chai H, Wang X, Yang J, Li J, Zhao Y, Cai W, Tao T, Xiang X (2017) Fused deposition modeling (FDM) 3D printed tablets for intragastric floating delivery of Domperidone. Sci Rep 7(1):2829
Chang R, Nam J, Sun W (2008) Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng Part C Methods 14(2):157–166
Chen QZ, Thompson ID, Boccaccini AR (2006) 45S5 bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 27(11):2414–2425
Choi Y, Kim T, Jeong J, Yi H, Park J, Hwang W, Cho D (2016) 3D cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Adv Healthc Mater 5(20):2636–2645
Cui X, Breitenkamp K, Finn MG, Lotz M, D'Lima DD (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng A 18(11–12):1304–1312
Cui X, Hartanto Y, Zhang H (2017) Advances in multicellular spheroids formation. J R Soc Interface 14(127):20160877
Dababneh AB, Ozbolat IT (2014) Bioprinting technology: a current state-of-the-art review. J Manuf Sci Eng 136(6):061016
Demirci U, Montesano G (2007) Single cell epitaxy by acoustic picolitre droplets. Lab Chip 7(9):1139–1145
Esch E, Bahinski A, Huh D (2015) Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 14(4):248–260
Eshkalak SK, Ghomi ER, Dai Y, Choudhury D, Ramakrishna S (2020) The role of three-dimensional printing in healthcare and medicine. Mater Des 194:108940
FDA clears 3-D printed device for minimally invasive foot surgery. http://www.fiercemedicaldevices.com/story/fda-clears-3-d-printed-device-minimally-invasive-foot-surgery/2015-02-02. Accessed 11 Aug 2016
Fetah K, Tebon P, Goudie M, Eichenbaum J, Ren L, Barros N, Nasiri R, Ahadian S, Ashammakhi N, Dokmeci M, Khademhosseini A (2019) The emergence of 3D bioprinting in organ-on-chip systems. Progr Biomed Eng 1(1):012001
Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, Sluijter JP (2012) Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 33(6):1782–1790
Ghadage S, Aloorkar N, Sudake S (2019) A decisive overview on three dimensional printing in pharmaceuticals. J Drug Deliv Therapeut 9(3):591–598
Gloria A, Russo T, De Santis R, Ambrosio L (2009) 3D fiber deposition technique to make multifunctional and tailor-made scaffolds for tissue engineering applications. J Appl Biomater Biomech 7(3):141–152
Gokhare VG, Raut DN, Shinde DK (2017) A review paper on 3D-printing aspects and various processes used in the 3D-printing. Int J Eng Res Technol 6(6):953–958
Goole J, Amighi K (2016) 3D printing in pharmaceutics: a new tool for designing customized drug delivery systems. Int J Pharm 499(1–2):376–394
Goyanes A, Martinez PR, Buanz A, Basit AW, Gaisford S (2015) Effect of geometry on drug release from 3D printed tablets. Int J Pharm 494(2):657–663
Groll J, Burdick JA, Cho DW, Derby B, Gelinsky M, Heilshorn SC, Juengst T, Malda J, Mironov VA, Nakayama K, Ovsianikov A (2018) A definition of bioinks and their distinction from biomaterial inks. Biofabrication 11(1):013001
Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM (2014) Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem 86(7):3240–3253
Gu Q, Hao J, Lu Y, Wang L, Wallace GG, Zhou Q (2015) Three-dimensional bio-printing. Sci China Life Sci 58(5):411–419
Gu Z, Fu J, Lin H, He Y (2019) Development of 3D bioprinting: from printing methods to biomedical applications. Asian J Pharmaceut Sci 15(5):529–557
Guo SZ, Heuzey MC, Therriault D (2014) Properties of polylactide inks for solvent-cast printing of three-dimensional freeform microstructures. Langmuir 30(4):1142–1150
Hartupee J, Mann DL (2016) Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol 93:143–148
Hasirci V, Hasirci N (2018) Tissue engineering and regenerative medicine. In: Fundamentals of biomaterials. Springer, New York, NY, pp 281–302
Highley CB (2019) 3D bioprinting technologies. In: 3D bioprinting in medicine. Springer, Cham, pp 1–66
Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA (2016) Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6:34845
Horváth L, Umehara Y, Jud C, Blank F, Petri-Fink A, Rothen-Rutishauser B (2015) Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep 5(1):7974
Hospodiuk M, Moncal KK, Dey M, Ozbolat IT (2016) Extrusion-based biofabrication in tissue engineering and regenerative medicine. In: 3D printing and biofabrication, pp 1–27
Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT (2017) The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv 35(2):217–239
Hwang HH, Zhu W, Victorine G, Lawrence N, Chen S (2018) 3D-printing of functional biomedical microdevices via light-and extrusion-based approaches. Small Methods 2(2):1700277
Jamroz W, Szafraniec J, Kurek M, Jachowicz R (2018) 3D printing in pharmaceutical and medical applications—recent achievements and challenges. Pharm Res 35:176. https://doi.org/10.1007/s11095-018-2454-x
Jamróz W, Szafraniec J, Kurek M, Jachowicz R (2018) 3D printing in pharmaceutical and medical applications—recent achievements and challenges. Pharm Res 35(9):176
Ji S, Guvendiren M (2017) Recent advances in bioink design for 3D bioprinting of tissues and organs. Front Bioeng Biotechnol 5:23
Jia J, Richards DJ, Pollard S, Tan Y, Rodriguez J, Visconti RP, Trusk TC, Yost MJ, Yao H, Markwald RR, Mei Y (2014) Engineering alginate as bioink for bioprinting. Acta Biomater 10(10):4323–4331
Jose PA, Christoper PG (2018) 3D printing of pharmaceuticals—a potential technology in developing personalized medicine. Asian J Pharmaceut Res Dev 6(3):46–54
Kawasaki T, Maeno A, Shiroishi T, Sakai N (2017) Development and growth of organs in living whole embryo and larval grafts in zebrafish. Sci Rep 7(1):1–11
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ (2015) 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release 217:308–314
Klein GT, Lu Y, Wang MY (2013) 3D printing and neurosurgery—ready for prime time? World Neurosurg 80(3–4):233–235
Koçak E, Yildiz A, Acartürk F (2020) Three dimensional bioprinting technology: applications in pharmaceutical and biomedical area. Colloids Surf B Biointerfaces 197:111396
Kumar H, Kim K (2020) Stereolithography 3D bioprinting. In: 3D bioprinting. Humana, New York, NY, pp 93–108
Lanza R, Langer R, Vacanti JP, Atala A (2020) Principles of tissue engineering. Academic press, New York
Leite S, Roosens T, El Taghdouini A, Mannaerts I, Smout A, Najimi M, Sokal E, Noor F, Chesne C, van Grunsven L (2020) Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials 78:1–10
Leong KF, Cheah CM, Chua CK (2003) Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 24(13):2363–2378
Liu L, Wang X (2015) Creation of a vascular system for organ manufacturing. Int J Bioprint 1(1):77
Liu W, Heinrich MA, Zhou Y, Akpek A, Hu N, Liu X, Guan X, Zhong Z, Jin X, Khademhosseini A, Zhang YS (2017) Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv Healthc Mater 6:1–11
Liu F, Chen Q, Liu C, Ao Q, Tian X, Fan J, Tong H, Wang X (2018a) Natural polymers for organ 3D bioprinting. Polymers 10(11):1278
Liu W, Zhong Z, Hu N, Zhou Y, Maggio L, Miri AK, Fragasso A, Jin X, Khademhosseini A, Zhang YS (2018b) Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication 10(2):024102
Lorson T, Jaksch S, Lübtow MM, Jüngst T, Groll J, Lühmann T, Luxenhofer R (2017) A thermogelling supramolecular hydrogel with sponge-like morphology as a cytocompatible bioink. Biomacromolecules 18(7):2161–2171
Markstedt K, Mantas A, Tournier I, Martínez Ávila H, Hägg D, Gatenholm P (2015) 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 16(5):1489–1496
Melchels FP, Feijen J, Grijpma DW (2010) A review on stereolithography and its applications in biomedical engineering. Biomaterials 31(24):6121–6130
Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR (2009) Organ printing: tissue spheroids as building blocks. Biomaterials 30(12):2164–2174
Mobaraki M, Ghaffari M, Yazdanpanah A, Luo Y, Mills DK (2020) Bioinks and bioprinting: a focused review. Bioprinting 18:e00080
Moroni L, Boland T, Burdick JA, De Maria C, Derby B, Forgacs G, Groll J, Li Q, Malda J, Mironov VA, Mota C (2018) Biofabrication: a guide to technology and terminology. Trends Biotechnol 36(4):384–402
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785
Neiman JAS, Raman R, Chan V, Rhoads MG, Raredon MSB, Velazquez JJ, Dyer RL, Bashir R, Hammond PT, Griffith LG (2015) Photopatterning of hydrogel scaffolds coupled to filter materials using stereolithography for perfused 3D culture of hepatocytes. Biotechnol Bioeng 112(4):777–787
Ngo TD, Kashani A, Imbalzano G, Nguyen KT, Hui D (2018) Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos Part B 143:172–196
Nguyen D, Robbins J, Crogan-Grundy C, Gorgen V, Bangalore P, Perusse D, Creasey O, King S, Lin S, Khatiwala C, Halberstadt C (2015) Functional characterization of three-dimensional (3D) human liver tissues generated by an automated bioprinting platform. FASEB J 29:LB424
Nguyen D, Hägg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, Kalogeropoulos T, Zaunz S, Concaro S, Brittberg M, Lindahl A (2017) Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep 7(1):1–10
Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T (2019) 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci 6(11):1900344
O’Connor RA, Cahill PA, McGuinness GB (2020) Cardiovascular tissue engineering. In: Biomaterials for organ and tissue regeneration. Woodhead Publishing, London, pp 249–272
Oklu R, Zhang YS, Yue K, Aleman J, Mollazadeh-Moghaddam K, Bakht SM, Yang J, Jia W, Dell'Erba V, Assawes P, Shin SR (2016) 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng 45(1):148–163
Oladapo BI, Zahedi SA, Adeoye AOM (2019) 3D printing of bone scaffolds with hybrid biomaterials. Compos B Eng 158:428–436
Ozbolat I, Gudapati H (2016) A review on design for bioprinting. Bioprinting 3:1–14
Ozbolat IT, Yu Y (2013) Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng 60(3):691–699
Ozbolat IT, Peng W, Ozbolat V (2016) Application areas of 3D bioprinting. Drug Discov Today 21(8):1257–1271
Papaioannou TG, Manolesou D, Dimakakos E, Tsoucalas G, Vavuranakis M, Tousoulis D (2019) 3D bioprinting methods and techniques: applications on artificial blood vessel fabrication. Acta Cardiol Sin 35(3):284
Park JH, Jang J, Lee JS, Cho DW (2017) Three-dimensional printing of tissue/organ analogues containing living cells. Ann Biomed Eng 45(1):180–194
Park J, Jang J, Kang H (2018a) 3D bioprinting and its application to organ-on-a-chip. Microelectron Eng 200:1–11
Park J, Ryu H, Lee B, Ha D, Ahn M, Kim S, Kim J, Jeon N, Cho D (2018b) Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 11(1):015002
Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, Ozbolat IT (2017) 3D bioprinting for drug discovery and development in pharmaceutics. Actabiomaterialia 57:26–46
Peterson GI, Larsen MB, Ganter MA, Storti DW, Boydston AJ (2014) 3D-printed mechanochromic materials. ACS Appl Mater Interfaces 7(1):577–583
Pourchet LJ, Thepot A, Albouy M (2017) Human skin 3D bioprinting using scaffold–free approach. Adv Healthc Mater 6:1601101. https://doi.org/10.1002/adhm.201601101
Radhakrishnan J, Varadaraj S, Dash S, Sharma A, Verma R (2020) Organotypic cancer tissue models for drug screening: 3D constructs, bioprinting and microfluidic chips. Drug Discov Today 25(5):879–890
Rahim TNAT, Abdullah AM, MdAkil H (2019) Recent developments in fused deposition modeling-based 3D printing of polymers and their composites. Polym Rev 59(4):589–624
Rezende RA, Pereira FDAS, Kasyanov V, Kemmoku DT, Maia I, Da Silva JVL, Mironov V (2013) Scalable biofabrication of tissue spheroids for organ printing. Proc CIRP 5:276–281
Salentijn GI, Oomen PE, Grajewski M, Verpoorte E (2017) Fused deposition modeling 3D printing for (bio) analytical device fabrication: procedures, materials, and applications. Anal Chem 89(13):7053–7061
Samiei N (2020) Recent trends on applications of 3D printing technology on the design and manufacture of pharmaceutical oral formulation: a mini review. Beni Suef Univ J Basic Appl Sci 9(1):1–12
Satpathy A, Datta P, Wu Y, Ayan B, Bayram E, Ozbolat IT (2018) Developments with 3D bioprinting for novel drug discovery. Expert Opin Drug Discovery 13(12):1115–1129
Shafiee A, Atala A (2016) Printing technologies for medical applications. Trends Mol Med 22(3):254–265
Shafiee A, Atala A (2017) Tissue engineering: toward a new era of medicine. Annu Rev Med 68:29–40
Shim JH, Lee JS, Kim JY, Cho DW (2012) Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromech Microeng 22(8):085014
Shirure V, Bi Y, Curtis M, Lezia A, Goedegebuure M, Goedegebuure S, Aft R, Fields R, George S (2018) Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip 18(23):3687–3702
Šljivić M, Mirjanić D, Šljivić N, Fragassa C, Pavlović A (2019) 3D printing and 3D bioprinting to use for medical applications. Contemp Mater 10:82
Subbiah R, Guldberg RE (2019) Materials science and design principles of growth factor delivery systems in tissue engineering and regenerative medicine. Adv Healthc Mater 8(1):1801000
Tappa K, Jammalamadaka U, Ballard D, Bruno T, Israel M, Vemula H, Meacham J, Mills D, Woodard P, Weisman J (2017) Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs, a proof of concept study. PLoS One 12(8):e0182929
Tonelli FMP, de Cássia Oliveira Paiva N, de Medeiros RVB, Pinto MCX, Tonelli FCP, Resende RR (2016) Tissue engineering: the use of stem cells in regenerative medicine, pp 315–324
Ursan ID, Chiu L, Pierce A (2013) Three-dimensional drug printing: a structured review. J Am Pharm Assoc 53(2):136–144
Van Linthout S, Miteva K, Tschöpe C (2014) Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res 102(2):258–269
Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJ, Melchels FP, Malda J (2013) Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 5(3):035007
Wang X, Yan Y, Zhang R (2010) Recent trends and challenges in complex organ manufacturing. Tissue Eng Part B Rev 16(2):189–197
Wang X, Ao Q, Tian X et al (2016a) 3D bioprinting technologies for hard tissue and organ engineering. Materials 9:802
Wang J, Goyanes A, Gaisford S, Basit AW (2016b) Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int J Pharm 503(1–2):207–212
Wang Y, Wu S, Kuss MA, Streubel PN, Duan B (2017) Effects of hydroxyapatite and hypoxia on chondrogenesis and hypertrophy in 3D bioprinted ADMSC laden constructs. ACS Biomater Sci Eng 3(5):826–835
Wang Z, Kapadia W, Li C, Lin F, Pereira RF, Granja PL, Sarmento B, Cui W (2020) Tissue-specific engineering: 3D bioprinting in regenerative medicine. J Control Release 329:237–256
Wardhana A, Valeria M (2020) Tissue engineering and regenerative medicine: a review. JurnalPlastikRekonstruksi 7(1):10–17
Wu Q, Liu J, Wang X, Feng L, Wu J, Zhu X, Wen W, Gong X (2020) Organ-on-a-chip: recent breakthroughs and future prospects. Biomed Eng Online 19(1):9
Xie Z, Gao M, Lobo AO, Webster TJ (2020) 3D bioprinting in tissue engineering for medical applications: the classic and the hybrid. Polymers 12(8):1717
Xiong R, Zhang Z, Chai W, Chrisey DB, Huang Y (2017) Study of gelatin as an effective energy absorbing layer for laser bioprinting. Biofabrication 9(2):024103
Xu T, Jin J, Gregory C, Hickman JJ, Boland T (2005) Inkjet printing of viable mammalian cells. Biomaterials 26(1):93–99
Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, Atala A (2012) Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 5(1):015001
Yan Q, Dong H, Su J, Han J, Song B, Wei Q, Shi Y (2018) A review of 3D printing technology for medical applications. Engineering 4(5):729–742
Yeo MG, Kim GH (2017) A cell-printing approach for obtaining hASC-laden scaffolds by using a collagen/polyphenol bioink. Biofabrication 9(2):025004
Yipeng J, Yongde X, Yuanyi W, Jilei S, Jiaxiang G, Jiangping G, Yong Y (2017) Microtissues enhance smooth muscle differentiation and cell viability of hADSCs for three dimensional bioprinting. Front Physiol 8:534
Yu F, Choudhury D (2019) Microfluidic bioprinting for organ-on-a-chip models. Drug Discov Today 24(6):1248–1257
Yu Y, Zhang Y, Martin JA, Ozbolat IT (2013) Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J Biomech Eng 135(9):91011
Yu J, Park SA, Kim WD, Ha T, Xin YZ, Lee J, Lee D (2020) Current advances in 3D bioprinting technology and its applications for tissue engineering. Polymers 12(12):2958
Zhang S, Wang H (2019) Current progress in 3D bioprinting of tissue analogs. SLAS Technol 24(1):70–78
Zhang YS, Duchamp M, Oklu R, Ellisen LW, Langer R, Khademhosseini A (2016) Bioprinting the cancer microenvironment. ACS Biomater Sci Eng 2(10):1710–1721
Zhang B, Luo Y, Ma L, Gao L, Li Y, Xue Q, Yang H, Cui Z (2018) 3D bioprinting: an emerging technology full of opportunities and challenges. BioDesign Manuf 1(1):2–13
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chopra, K., Pawar, S.V., Maurya, M., Gupta, T., Dhaliwal, J. (2022). 3D Bioprinting of Tissues and Organs: A New Paradigm in Regenerative Medicine and Biomedical Engineering. In: Sobti, R., Sobti, A. (eds) Biomedical Translational Research. Springer, Singapore. https://doi.org/10.1007/978-981-16-4345-3_15
Download citation
DOI: https://doi.org/10.1007/978-981-16-4345-3_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4344-6
Online ISBN: 978-981-16-4345-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)