Abstract
Background
Age-related loss of muscle and bone (sarcopenia and osteoporosis), increases the risk of falls and fractures and consequently leads to a substantial economic burden for the society. The combined condition, osteosarcopenia, may identify patients at a higher risk of those outcomes and could be relevant for assessment and treatment in clinical practice.
Aim
To evaluate the current knowledge of the prevalence of osteosarcopenia and the fracture risk in older people.
Method
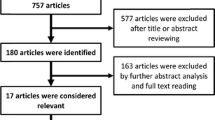
A systematic literature review was conducted until 10th March 2018. A total of 1105 papers were detected, whereof 1049 and 29 were excluded by title/abstracts and full-text assessment, respectively. Twenty-seven original papers were included in the systematic review, whereof 17 were suitable for meta-analysis.
Results
The prevalence of osteosarcopenia varied (5–37%) depending on the classification of sarcopenia and whether participants were classified initially according to sarcopenia or osteoporosis. In patients with low-energy osteoporotic fractures, sarcopenia was present in 7.8–58% and 1.3–96.3% of the cases, women and men, respectively. The meta-analysis of prevalence of sarcopenia in patients with low-energy fracture (n = 9) was 46% (95% CI 44, 48; p < 0.001). The relative risk of fracture (sarcopenic versus non-sarcopenic) in meta-analysis of four studies was 1.37 (95% CI 1.18, 1.59; p < 0.001). Mean bone mineral density (n = 5) and T-score (n = 3) of femoral neck was significantly lower in sarcopenic participants [− 0.07 g/cm2 (95% CI 0.08, 0.06) and − 0.34 (95% CI − 0.46, − 0.23), respectively].
Conclusion
Osteosarcopenia is frequent and the relative risk of fracture is higher among sarcopenic patients. A standard and strict classification of sarcopenia is needed to assess its true relationship and consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The age-related loss of skeletal muscle and bone, i.e., sarcopenia and osteoporosis, both represent a substantial and growing public health issue as both conditions individually contribute to frailty, poor balance, fall and fractures [1,2,3,4]. Back in 2009, Binkley et al. [5], classified individuals with both conditions as “Sarco-osteopenic” and suggested that the combination of these two conditions could be expected to identify individuals at a higher fracture risk than currently thought.
Definitions
Sarcopenia was first named by Rosenberg in 1989 and referred to the putative age-dependent decline in muscle mass [6]. In 1998, Baumgartner et al. [7] defined the first cut-off values for sarcopenia based on whole body dual-energy X-ray absorptiometry (DXA) of relative appendicular lean mass (RaLM) as 2 standard deviations (SD) below the mean of a young reference group (women: 5.45 kg/m2; men: 7.26 kg/m2). Although the cut-off values are still widely used, the current recommendations suggest parameters of muscle strength and physical performance to be incorporated in the definition of sarcopenia [7,8,9,10,11]. Consequently, The European Working Group on Sarcopenia in Older People (EWGSOP) suggested a conceptual staging of sarcopenia as pre-sarcopenia (low muscle mass only), sarcopenia (low muscle mass plus either low muscle strength or low physical performance) or severe sarcopenia (low muscle mass, strength and physical performance) [8]. In parallel, The International Working Group on Sarcopenia (IWG/IWGS) have suggested a definition based on appendicular muscle mass (adjusted for squared height) and gait speed (GS), whereas The Foundation of the National Institute of Health and Sarcopenia Project (FNIH) defines sarcopenia based on appendicular muscle mass (adjusted for BMI) and hand grip strength (HGS) [9, 10]. Despite an overall agreement that sarcopenia should be defined from a combination of low muscle mass, strength and/or function, the introduction of a wide number of different cut-off values makes it difficult to compare results from different populations and studies as the prevalence varies significantly depending on the cut-off values used [12, 13].
In contrast, there has been consensus regarding the definition of osteoporosis since 1994, where WHO defined a T-score (i.e., the SD of bone mineral density (BMD) in reference to the mean of healthy young adults of the same gender) between − 1.0 and − 2.5 and a T-score ≤ − 2.5 as osteopenia and asymptomatic osteoporosis, respectively. Low-energy fracture of the hip or spine defined symptomatic osteoporosis even with a normal BMD T-score [14].
As a more recent approach, Binkley et al. [15] introduced an even more comprehensive syndrome, the “Dysmobility Syndrome” to reflect a geriatric condition, identifying patients at high risk of adverse musculoskeletal conditions, in line with the metabolic syndrome in patients with increased risk of cardiovascular diseases. The syndrome includes osteoporosis, low muscle mass, history of fall, slow GS, low HGS and high fat mass—all components included in an arbitrary equally weighted scale—and has been demonstrated to better identify individuals with a previous fall or fracture than the sarcopenia definitions alone [15]. This finding is supported in a new review by Hill et al. [16], who found the dysmobility syndrome to be associated with a functional decline, increased number of falls and fractures, as well as increased mortality.
Prevalence
Both sarcopenia and osteoporosis are common conditions in the older population. The International Sarcopenia Initiative, based on EWGSOP criteria, found sarcopenia to be present in 1–29% of the home-dwelling populations, in 14–33% of the long-term care population and in 10% of acute hospitalized geriatric patients [17]. In a more recent paper, though, the prevalence of sarcopenia in patients hospitalized at a geriatric ward was 6.6 and 18.7% sarcopenia and severe sarcopenia, respectively [18]. In line, the “EU27-report” from 2013 stated that the prevalence of osteoporosis in Europe in 2010 was approximately 22.0 million (22.1%) and 5.5 million (6.6%) in 50- to 84-year-old women and men, respectively [19]. Furthermore, the annual fracture incidence related to osteoporosis is approximately 8.9 million worldwide, with a higher incidence in Scandinavia [20].
The linkage of bones and muscle disorder
The genesis of both osteoporosis and sarcopenia are multifactorial, and it is believed that some of the known factors causing osteoporosis also contribute to the development of sarcopenia [21, 22]. Notably, bone and muscle tissue mass are tightly correlated throughout life and share environmental, endocrine and paracrine influences [23]. Moreover, parallel changes in muscle and bone mass are known to be brought about by exercise, disuse and aging [21]. “The mechanostat theory”, e.g., states that a decline in mechanical loading reduces the bone formation leading to a fragile bone status [24,25,26,27]. Thus, muscle has long been recognized as the primary source of anabolic mechanical stimuli for bone tissue [23]. However, the precise mechanisms responsible for orchestrating bone and skeletal mass are still not well-characterized and little is known about potential mechanisms other than loading [10]. Secreted factors such as myostatin, activins and pro-inflammatory cytokines represent potential underlying common mechanisms linking bone and muscle, yet little is known regarding how these factors work and affect muscle and bone mass. However, emerging data supports the concept that muscle secretes factors that target other tissues and are involved in glucose metabolism while simultaneously similar data has been emerging for bone [28]. An integrated physiology is therefore hypothesized to exist between muscle and bone tissue.
Another key player in the muscle bone cross-talk is thought to be the Wnt/β-catenin signaling pathway, a major regulator of bone mass and of muscle development and growth. The regulation of this pathway by osteocytes may play a central role in regulating bone mass [22]. In particular, sclerostin (encoded by the SOST gene), is highly express in osteocytes and inhibits Wnt signaling [29].
The consequences of sarcopenia and osteoporosis
Falls and/or fractures leads to immobilization, hospitalization, impaired level of function and a markedly increased risk of mortality. Consequently, sarcopenia [30,31,32,33,34] and osteoporosis [19, 35,36,37] creates significant economic, societal, and social burden for individuals. This burden is expected to increase with the increasing life expectancy.
The future therefore brings challenges: the increasing aging population and their increased demands for healthy old age. In this context, clinicians, prevention, early detection and better treatment of both sarcopenia and osteoporosis may be necessary to meet these demands.
Aim and research question
The aim of the present systematic review was to provide an overview of the current knowledge of sarcopenia and osteoporosis in older Caucasians (aged 65 years or older) as well as a meta-analysis of studies comparing sarcopenic with non-sarcopenic patients with regard to BMD/T-score and risk of low-energy fractures.
Current knowledge of the following topics is sought:
-
1.
The prevalence of sarcopenia among older people suffering from osteopenia, osteoporosis and/or low-energy fractures.
-
2.
Fracture risk assessment among older people suffering from sarcopenia and symptomatic osteoporosis.
-
3.
Markers of bone turnover as assessed in osteosarcopenic older people compared to non-sarcopenic osteoporotic or osteopenic.
Method
Search strategy and study eligibility
The following search queries in PubMed until the 10th of March 2018 were used with the filters “abstract and full text available” added.
Search strings: “Osteoporosis OR Osteopenia AND Sarcopenia AND older”; “Fracture AND Sarcopenia AND Older”; “Osteoporosis OR Osteopenia AND Sarcopenia AND Bone Markers”; “Fracture AND Sarcopenia AND Bone Markers”; and “BMD AND Sarcopenia AND Bone Markers”. The results were exported to EndNote X8 (Clarivate Analytics 2017; Philadelphia; PA 19130; USA) to remove duplicates. Title and abstracts were screened for eligibility with regard to the following inclusion criteria: “original papers only”; “human studies only”; “mean age ≥ 65 years”; “Caucasian ancestry only”; and as well as “primary focus on osteosarcopenia”. Full-text papers were screened according to the same inclusion criteria. Afterwards, additional records were screened and identified through other sources as described above.
Data extraction for meta-analysis
Eligible studies were screened to provide data comparing sarcopenic vs non-sarcopenic patients with regard to osteopenia/osteoporosis from baseline BMD or T-score as well as with regard to fractures. Studies on fractures were required to provide numbers of fracture events in both groups. Studies not providing such data were excluded.
All demographic data and prevalence of sarcopenia were extracted from each study. In fracture studies wherein all patients had fractures, the number (prevalence) of sarcopenic participants were calculated as well. Percentages were recalculated back to absolute numbers and medians with ranges were recalculated to their means with standard deviations wherever required. In case of missing information the author of the study was contacted. The first author (BRN) conducted the primary search and data extraction, which were subsequently confirmed by an experienced author (JA). Disagreements were solved by consensus.
Statistics
Weighted mean difference method was used to compare the baseline BMD and T-score in sarcopenic vs non-sarcopenic participants, by pooling their means and standard deviations. The relative risk (RR) ratio with 95% confidence interval (CI) of fractures was estimated by pooling the number of events and total populations together in both groups. The prevalence of sarcopenia in the included studies was pooled as proportions. Heterogeneity among studies was tested using the Chi squared method and the I2 statistic. We used fixed effect model in all analyses to restore the real sizes of the influential studies. All analyses were performed with 95% CI and all p values < 0.05 were considered statistically significant. All analyses and plots were performed using the meta-analysis package of the statistic software program STATA version 15 (STATA Corporation, Lakeway Drive, College Station, TX, USA).
Results
Twenty-seven original papers were included after assessment of eligibility. Of those, 17 were included in the meta-analysis. The results of the search strategy are presented in the PRISMA flow chart [38] in Fig. 1 and selected data from the papers included are presented in Table 1.
Overall, study settings were very diverse due to heterogeneous populations, designs and methodology. The populations comprised healthy volunteers; volunteers with increased risk of fall; participants referred to outpatient clinics treating osteoporosis and preventing fall; as well as patients with verified low-energy fractures. The designs were mostly cross-sectional; however, few prospective observational designs were present (Table 1). Furthermore, the methods and classification used to determine sarcopenia were diverse, even though a greater consensus appeared after the introduction of the EWGSOP recommendations [8]. The main difference though was whether sarcopenia was classified with regard to muscle mass only or whether strength and/or physical performance were included (Table 1).
The associations of sarcopenia with low bone mass and fracture in older people
Prevalence
The overall prevalence of osteosarcopenia (from T-score and diverse sarcopenic classifications) in the cohorts including both gender varied between 5.0 and 37.0% [39,40,41,42,43]. In studies including women only, osteosarcopenia varied between 0.5 and 45.0% [2, 44, 45]. However, the prevalence of osteosarcopenia seemed to vary depending on whether participants were classified primarily with sarcopenia or osteopenia/osteoporosis. Notably, there was a tendency, that in subjects classified as sarcopenic, osteopenia was more frequent than osteoporosis. In a population of 171 women and 118 men, it was observed that sarcopenia was more frequently associated with osteopenia than osteoporosis, 81.3 vs 30.2% [39]. This finding was supported by Kirchengast et al. [46] who found an increased prevalence of osteopenia (women = 58.8%; men = 50.0%) compared to osteoporosis (women = 25.5%; men = 16.7%), in sarcopenic women and men. On the other hand, in participants suffering from osteoporosis Locquet et al. [39] and Genaro et al. [47] found 36.1 and 21.4%, respectively, to be sarcopenic. In line with these results, Huo et al. [41] found sarcopenia to be more frequent in osteoporotic (62.7%) vs osteopenic (47.7%) participants, as well as Gillette-Guyonnett et al. [48] who found sarcopenia to be present in 32.2% of the osteoporotic participants vs 26.2% in non-osteoporotic participants.
In general, the relative percentage of participants with osteosarcopenia is greater in women (25.5–82.6%) compared to men (16.4–32.0%) [40, 41, 46].
Another general finding is a marked variance in the prevalence of sarcopenia in low-energy fracture participants depending on the studied population. In participants with low-energy osteoporotic fractures, sarcopenia was present in 7.8–58% and 1.3–96.3% of the cases, women and men, respectively [2, 32, 49,50,51,52,53]. Noteworthy, the very high prevalence in men were from small-size studies based on the initial definition of sarcopenia [52]. When both genders were included, sarcopenia was present in 17.1–58% [39, 54,55,56,57] of participants with fractures. Interestingly, it has been demonstrated that participants with dysmobility syndrome, i.e., the suggested geriatric syndrome, are more likely to have incurred a prior facture (66.2%), whereas participants with sarcopenia (FNIH classification) and severe sarcopenia (EWGSOP classification) had suffered a prior fracture in only 7.8 and 10.4% of the cases, respectively (Table 1) [49]. This finding may point towards the dysmobility syndrome as a more sensitive marker of frailty.
Meta-analysis of the pooled mean prevalence of sarcopenia in patient populations with fractures is 46% (95% CI 44, 48; p < 0.001) (Fig. 2), although, the heterogeneity of the included studies was significant.
The included studies demonstrated a substantial difference in observed fracture incidences among the old patients and the really old patients. Patients with a mean age of 67.2 years had a history of fractures in 7.8–10.4% of sarcopenic cases [49], whereas sarcopenic participants with mean age between 80 and 85 years had a fracture incidence that ranged between 17 and 58% [2, 54, 55]. This difference may solely be ascribed to the aging bone, though.
Risk of low bone mineral density/T-score and fracture with regard to sarcopenia
Of the 27 original studies included in this review, 17 studies addressed BMD/T-score (8 eligible for meta-analysis) and 16 studies addressed low-energy fractures (4 were eligible for meta-analysis) (Table 1).
In our meta-analysis, we found statistically significant lower weighted mean differences of BMD (0.07 g/cm2; p < 0.001) and T-score (0.34; p < 0.001) in sarcopenic compared to non-sarcopenic individuals (Fig. 3).
Moreover, there was an increased risk of osteoporotic fractures in sarcopenic compared to non-sarcopenic participants with a relative risk of 1.37 (95% CI 1.18, 1.59; p < 0.001) (Fig. 4). However, the heterogeneity between studies was significant (p < 0.001).
Studies on fracture, not eligible for meta-analysis, estimated the odds ratio (OR) of osteoporotic fractures in sarcopenic vs non-sarcopenic at 2.43 (95% CI 1.12, 5.27; p < 0.05) and 2.7 (95% CI 1.4, 5.5; p = 0.005), respectively [58, 59]. Considering osteosarcopenic vs normal BMD/normal muscle mass—participants, the OR of fracture was estimated by Hars et al. [58] and Huo et al. [41] at 3.39 (95% CI 1.54, 7.45; p = 0.002) and 2.71 (95% CI 1.7, 4.4; p < 0.001), respectively. One study, however, estimated the hazard ratio of fracture in sarcopenic and non-sarcopenic participants and did not find a significant difference (Hazard ratio 1.53, 95% CI 0.70, 3.31) [60].
Osteosarcopenia and markers of bone turnover
Markers of bone turnover was assessed in two of the included 27 studies within the given search criteria. In the work by Gonelli et al. [61] markers of bone turnover were included in the regression models to predict BMD at different skeletal sites from different body composition measures, however, it was not explored whether markers of bone turnover were altered in osteosarcopenic compared to osteopenic or sarcopenic participants. In the study by Drey et al. [40] osteosarcopenic participants had increased markers of bone turnover compared to controls, in contrast to osteopenic and sarcopenic participants. Consequently, it was hypothesized, that bone loss was faster in osteosarcopenic compared to participants with only one condition (sarcopenia or osteopenia).
Discussion
In summary, the presented systematic review and meta-analysis demonstrated a high prevalence of osteosarcopenia in various geriatric populations. Somewhat surprisingly, the prevalence of sarcopenia combined with osteopenia was in some studies more frequent, compared to sarcopenia with osteoporosis [39, 46], whereas others has found sarcopenia to more frequent in osteoporotic participants, as expected [41]. This finding could partly be explained by the different classifications of sarcopenia used. Despite the various definitions of sarcopenia, the present meta-analysis showed a high prevalence of sarcopenia in patients with osteoporotic fractures [2, 49,50,51,52, 54,55,56,57]. Importantly, the risk of incurring a low-energy fracture when being sarcopenic was much higher compared to non-sarcopenic controls [32, 39, 41, 58]. Moreover, BMD and T-scores were lower in sarcopenic subjects [32, 39, 41, 47, 58, 62, 63], although there was a high heterogeneity among the included studies.
Future studies on the prevalence of osteopenia and osteoporosis with sarcopenia within different study populations (healthy volunteers, outpatient clinics and admitted patients) are, however, needed to understand the true relationship.
In a clinical setting, the association of markers of bone turnover and importance in muscle metabolism is somewhat unclear and the hypothesis of an association may be contradictory. To our knowledge, only one study in humans have reported increased plasma bone turnover markers in osteosarcopenic participants as compared with either sarcopenic or osteopenic participants, indicating that markers of bone turnover might supplement other clinical and para-clinical biomarkers in identifying participants at high risk of bone fractures [40]. This observation is supported by an in vitro study investigating cultured osteoblasts with serum from participants classified as normal, obese, obese osteopenic, obese sarcopenic and obese osteopenic sarcopenic [64]. In this study the authors found that the RunX2 (an essential transcription factor in osteoblast maturation) level was decreased in all pathological groups compared to healthy controls. However, the osteoblast marker osteocalcin was only altered in obese participants and not in the obese osteopenic sarcopenic participants indicating that different amounts of adipose tissue and muscle mass may alter the bone biology [64]. This could be of interest in future studies on markers of bone turnover, osteosarcopenia and whole body composition with increased age.
In general, it was difficult to compare the evaluated studies in this review due to the lack of consensus regarding the definition of sarcopenia. Since 2010 though, more and more studies rely on the sarcopenia classification including muscle mass, strength and/or physical performance, as suggested by the EWGSOP [8]. However, even by this simple classification a lot of different tests and cut-offs are suggested making the conclusions tricky, as discussed by Bijlsma et al. [65]. In a new review by Reijnierse et al. [12], including patients from a geriatric outpatient clinic, three widely used definitions of sarcopenia did not capture the same patients. With regard to fractures, this was supported by Cawthon et al. [32] who found that participants classified from the Baumgartner and Newman criteria did not have an increased risk of osteoporotic fractures, but participants classified from EWGSOP, IWG and NHIH criteria were more exposed. As a consequence of the lack of a well-defined definition of sarcopenia, the bone field is far more advanced than the muscle field in clinical terms. In contrast to the diagnostics and treatment of sarcopenia, the bone field has been successful in developing therapeutics for prevention and treatment of osteoporosis on the basis of well-defined clear parameters, while the definition of sarcopenia still remains somewhat unsettled [9, 66].
Strengths and limitations
In the present systematic review, both cross-sectional data and prospective studies were included, well aware of the higher evidence level (and the opportunity to consider causality) from the latter design which in this context expresses the relevant outcome “fracture” due to osteosarcopenia. However, regarding participants of interest, most studies were population surveys including home-dwelling older individuals with a high functional level, but also more frail patients groups (patients referred to outpatient clinics and hip-fracture patients) were represented. A great strength of the present study was that we were able to perform 3 meta-analyses, which enabled a comparison.
What could be of interest in future studies?
Studies to gain consensus on the classification of sarcopenia in relevant clinical settings is highly warranted. To gain more knowledge about the underlying mechanisms leading to sarcopenia and osteosarcopenia, biomarkers of bone and muscle should be included in future studies.
Of special interest in the clinical setting may be the dysmobility syndrome, which has been shown to be associated with a functional decline, increased number of falls and fractures, as well as increased mortality [16]. This syndrome may pinpoint participants at high risk of bone fracture and functional disability better than participants with osteosarcopenia, sarcopenia or osteopenia alone. No intervention studies, however, are currently present, as well as prospective studies are limited.
The high prevalence of osteosarcopenia and its consequences may very well alter future assessment of osteoporotic patient to include assessment of sarcopenia to focus on sarcopenic prevention strategies beside the well-known medical treatment of osteoporosis.
Conclusion
The concept “osteosarcopenia” was very frequent in older people and might be a better predictor of physical decline, falls and fractures than osteopenia or sarcopenia alone. From meta-analysis, the relative risk of fracture was higher in sarcopenic subjects making this field interesting in future studies. However, the lack of consensus regarding the classification of sarcopenia is a large barrier for clarification and future treatment targets.
References
Frisoli A Jr, Chaves PH, Ingham SJ, Fried LP (2011) Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women’s Health and Aging Study (WHAS) II. Bone 48(4):952–957
Di Monaco M, Vallero F, Di Monaco R, Tappero R (2011) Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr 52(1):71–74
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res Off J Am Soc Bone Miner Res 22(3):465–475
Marks R (2010) Hip fracture epidemiological trends, outcomes, and risk factors, 1970–2009. Int J Gen Med 3:1–17
Binkley N, Buehring B (2009) Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitometry Off J Int Soc Clin Densitometry 12(4):413–416
Rosenberg I (1989) Summary comments: epidemiological and methodological problems in determining nutritional status in older persons. Am J Clin Nutr 50:1231–1233
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM et al (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on sarcopenia in older people. Age Ageing 39(4):412–423
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12(4):249–256
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM et al (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol Ser A Biol Sci Med Sci 69(5):547–558
Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2003) Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 51(11):1602–1609
Reijnierse EM, Trappenburg MC, Blauw GJ, Verlaan S, de van der Schueren MA, Meskers CG, Maier AB (2016) Common ground? The concordance of sarcopenia and frailty definitions. J Am Med Dir Assoc 17(4):371.e377
Masanes F, Rojano ILX, Salva A, Serra-Rexach JA, Artaza I, Formiga F, Cuesta F, Lopez Soto A, Ruiz D, Cruz-Jentoft AJ (2017) Cut-off points for muscle mass—not grip strength or gait speed—determine variations in sarcopenia prevalence. J Nutr Health Aging 21(7):825–829
WHO (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 843:1–129
Binkley N, Krueger D, Buehring B (2013) What’s in a name revisited: should osteoporosis and sarcopenia be considered components of “dysmobility syndrome?”. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 24(12):2955–2959
Hill KD, Farrier K, Russell M, Burton E (2017) Dysmobility syndrome: current perspectives. Clin Interv Aging 12:145–152
Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP et al (2014) Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43(6):748–759
Smoliner C, Sieber CC, Wirth R (2014) Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc 15(4):267–272
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1–2):136
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 17(12):1726–1733
Hamrick M (2010) JMNI special issue: basic science and mechanisms of muscle–bone interactions. J Musculoskelet Neuronal Interact 10(1):1–2
Chien KR, Karsenty G (2005) Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell 120(4):533–544
Sharir A, Stern T, Rot C, Shahar R, Zelzer E (2011) Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development 138(15):3247–3259
Kaji H (2014) Interaction between muscle and bone. J Bone Metab 21(1):29–40
Kaji H (2013) Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care 16(3):272–277
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism. J Cell Biochem 116(5):687–695
Edwards MH, Dennison EM, Sayer AA, Fielding R, Cooper C (2015) Osteoporosis and sarcopenia in older age. Bone 80:126–130
Schafer MJ, Atkinson EJ, Vanderboom PM, Kotajarvi B, White TA, Moore MM, Bruce CJ, Greason KL, Suri RM, Khosla S et al (2016) Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab 23(6):1207–1215
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res Off J Am Soc Bone Miner Res 26(2):229–238
Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G (2013) Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 42(2):203–209
Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, Tosato M, Bernabei R, Onder G (2012) Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 13(2):121–126
Cawthon PM, Blackwell TL, Cauley J, Kado DM, Barrett-Connor E, Lee CG, Hoffman AR, Nevitt M, Stefanick ML, Lane NE et al (2015) Evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the observational osteoporotic fractures in men cohort study. J Am Geriatr Soc 63(11):2247–2259
Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G (2012) Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr (Edinburgh, Scotland) 31(5):652–658
Scott D, Hayes A, Sanders KM, Aitken D, Ebeling PR, Jones G (2014) Operational definitions of sarcopenia and their associations with 5-year changes in falls risk in community-dwelling middle-aged and older adults. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 25(1):187–193
Johnell O (1997) The socioeconomic burden of fractures: today and in the 21st century. Am J Med 103(2A):20S–25S (discussion 25S-26S)
Kannus P, Niemi S, Parkkari J, Palvanen M, Vuori I, Jarvinen M (1999) Hip fractures in Finland between 1970 and 1997 and predictions for the future. Lancet 353(9155):802–805
van Staa TP, Dennison EM, Leufkens HG, Cooper C (2001) Epidemiology of fractures in England and Wales. Bone 29(6):517–522
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Locquet M, Beaudart C, Bruyere O, Kanis JA, Delandsheere L, Reginster JY (2018) Bone health assessment in older people with or without muscle health impairment. Osteoporos Int 29(5):1057–1067. https://doi.org/10.1007/s00198-018-4384-1
Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R (2016) Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res 28(5):895–899
Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Muir SW, Montero-Odasso M, Gunawardene P, Demontiero O, Duque G (2015) Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc 16(4):290–295
Buehring B, Krueger D, Binkley N (2013) Effect of including historical height and radius BMD measurement on sarco-osteoporosis prevalence. J Cachexia Sarcopenia Muscle 4(1):47–54
Waters DL, Hale L, Grant AM, Herbison P, Goulding A (2010) Osteoporosis and gait and balance disturbances in older sarcopenic obese New Zealanders. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 21(2):351–357
Gentil P, Lima RM, de Oliveira RJ, Pereira RW, Reis VM (2007) Association between femoral neck bone mineral density and lower limb fat-free mass in postmenopausal women. J Clin Densitometry Off J Int Soc Clin Densitometry 10(2):174–178
Patil R, Uusi-Rasi K, Pasanen M, Kannus P, Karinkanta S, Sievanen H (2013) Sarcopenia and osteopenia among 70-80-year-old home-dwelling Finnish women: prevalence and association with functional performance. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 24(3):787–796
Kirchengast S, Huber J (2012) Sex-specific associations between soft tissue body composition and bone mineral density among older adults. Ann Hum Biol 39(3):206–213
Genaro PS, Pereira GA, Pinheiro MM, Szejnfeld VL, Martini LA (2010) Influence of body composition on bone mass in postmenopausal osteoporotic women. Arch Gerontol Geriatr 51(3):295–298
Gillette-Guyonnet S, Nourhashemi F, Lauque S, Grandjean H, Vellas B (2000) Body composition and osteoporosis in elderly women. Gerontology 46(4):189–193
Iolascon G, Moretti A, Giamattei MT, Migliaccio S, Gimigliano F (2015) Prevalent fragility fractures as risk factor for skeletal muscle function deficit and dysmobility syndrome in post-menopausal women. Aging Clin Exp Res 27(Suppl 1):S11–S16
Di Monaco M, Castiglioni C, De Toma E, Gardin L, Giordano S, Di Monaco R, Tappero R (2015) Presarcopenia and sarcopenia in hip-fracture women: prevalence and association with ability to function in activities of daily living. Aging Clin Exp Res 27(4):465–472
Di Monaco M, Castiglioni C, Di Carlo S (2018) Lean mass and functional recovery in men with hip fracture: a short-term prospective pilot study. Am J Phys Med Rehabil 97(6):401–406. https://doi.org/10.1097/PHM.0000000000000875
Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A (2007) Muscle mass and functional recovery in men with hip fracture. Am J Phys Med Rehabil 86(10):818–825
Clynes MA, Edwards MH, Buehring B, Dennison EM, Binkley N, Cooper C (2015) Definitions of sarcopenia: associations with previous falls and fracture in a population sample. Calcif Tissue Int 97(5):445–452
Gonzalez-Montalvo JI, Alarcon T, Gotor P, Queipo R, Velasco R, Hoyos R, Pardo A, Otero A (2016) Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr Gerontol Int 16(9):1021–1027
Fiatarone Singh MA, Singh NA, Hansen RD, Finnegan TP, Allen BJ, Diamond TH, Diwan AD, Lloyd BD, Williamson DA, Smith EU et al (2009) Methodology and baseline characteristics for the sarcopenia and hip fracture study: a 5-year prospective study. J Gerontol Ser A Biol Sci Med Sci 64(5):568–574
Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Ranhoff AH (2017) Sarcopenia in patients with hip fracture: a multicenter cross-sectional study. PLoS One 12(9):e0184780
Landi F, Calvani R, Ortolani E, Salini S, Martone AM, Santoro L, Santoliquido A, Sisto A, Picca A, Marzetti E (2017) The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 28(5):1569–1576
Hars M, Biver E, Chevalley T, Herrmann F, Rizzoli R, Ferrari S, Trombetti A (2016) Low lean mass predicts incident fractures independently from FRAX: a prospective cohort study of recent retirees. J Bone Miner Res Off J Am Soc Bone Miner Res 31(11):2048–2056
Sjoblom S, Suuronen J, Rikkonen T, Honkanen R, Kroger H, Sirola J (2013) Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 75(2):175–180
Schaap LA, van Schoor NM, Lips P, Visser M (2017) Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures; the Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/glx245
Gonnelli S, Caffarelli C, Cappelli S, Rossi S, Giordano N, Nuti R (2014) Gender-specific associations of appendicular muscle mass with BMD in elderly Italian subjects. Calcif Tissue Int 95(4):340–348
Lima RM, Bezerra LM, Rabelo HT, Silva MA, Silva AJ, Bottaro M, de Oliveira RJ (2009) Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. J Clin Densitometry Off J Int Soc Clin Densitometry 12(1):35–41
Pereira FB, Leite AF, de Paula AP (2015) Relationship between pre-sarcopenia, sarcopenia and bone mineral density in elderly men. Arch Endocrinol Metab 59(1):59–65
Wannenes F, Papa V, Greco EA, Fornari R, Marocco C, Baldari C, Di Luigi L, Emerenziani GP, Poggiogalle E, Guidetti L et al (2014) Abdominal fat and sarcopenia in women significantly alter osteoblasts homeostasis in vitro by a WNT/beta-catenin dependent mechanism. Int J Endocrinol 2014:278316
Bijlsma AY, Meskers MC, Molendijk M, Westendorp RG, Sipila S, Stenroth L, Sillanpaa E, McPhee JS, Jones DA, Narici M et al (2013) Diagnostic measures for sarcopenia and bone mineral density. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 24(10):2681–2691
Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ et al (2011) Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 12(6):403–409
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Barbara Rubek Nielsen (BRN), Jawdat Abdulla (JA), Hanne Elkjær Andersen (HEA), Peters Schwarz (PS) and Charlotte Suetta (CS) declare that they have no conflict of interest, that the study was not founded.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. All the authors certify that they comply with the standard ethical guidelines for authorship and publishing.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Nielsen, B.R., Abdulla, J., Andersen, H.E. et al. Sarcopenia and osteoporosis in older people: a systematic review and meta-analysis. Eur Geriatr Med 9, 419–434 (2018). https://doi.org/10.1007/s41999-018-0079-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-018-0079-6