Summary
Sarcopenia is a common geriatric syndrome characterized by progressive decrease of muscle mass and function leading to an increased risk of physical disability, poor quality of life, and mortality. Increasing evidence shows that sarcopenia is related with fragility fractures. This systematic review aimed to summarize the following: (1) the prevalence of sarcopenia in patients with fragility fracture and (2) the associated risk factors for fragility fracture in patients with sarcopenia. Literature search was conducted in PubMed and Cochrane databases. Studies with the prevalence of sarcopenia in elderly patients with fragility fracture and associated risk factors in patients with sarcopenia were included. A total of 15 papers were included, with 10 reporting sarcopenia prevalence, and 5 on fracture risk in patients with sarcopenia. The prevalence of sarcopenia after fracture ranged from 12.4 to 95% in males and 18.3 to 64% in females. The prevalence of sarcopenia in elderly patients with fragility fracture was high, especially in men. Two studies showed that sarcopenia was a risk factor for fragility fracture when associated with low bone mineral density (BMD) but only in men. Caution should be taken for male patients with sarcopenia and low BMD, which is related to significantly increased risk of fractures. There is a pressing need for further research on sarcopenia and its risk on fragility fracture to better understand the relationship, pathophysiology, and mechanisms, which may shed light on potential interventions to improve clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia is a common geriatric syndrome. In normal aging, muscle mass usually decreases at a rate of 1% annually after the age of 40 years [1]. The disease is characterized by progressive loss of lean body mass and function leading to an increased risk of physical disability, poor quality of life, and mortality [2]. The prevalence of sarcopenia among community-dwelling people ranges from 1 to 29% [3]. The disease is also one of the indicators of frailty in the elderly [4].
Several definitions of sarcopenia have emerged in recent years. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) developed a clinical definition of sarcopenia [2]. The EWGSOP recommends using the presence of both low muscle mass and function (strength or performance) for the diagnosis of sarcopenia [2]. In 2011, the International Working Group on Sarcopenia (IWGS) emphasized assessment of physical function including the ability to rise from chair or gait speed to be included in targeting sarcopenia [5]. In 2014, the Asian Working Group for Sarcopenia (AWGS) and the Foundation for the National Institutes of Health (FNIH) of the USA also established the consensus for sarcopenia diagnosis for their respective regions [6, 7]. Table 1 summarizes the definition of sarcopenia recommended by the above working groups. However, due to several definitions, the same population adopting different definitions recommended by the EWGSOP, IWGS, AWGS, and FNIH leads to the prevalence of sarcopenia ranging from 2.6%–22.1% in men to 1.3%–18.25% in women as shown in a study conducted in Hong Kong [8].
Recent evidence showed that sarcopenia was associated with low bone mineral density (BMD) and osteoporosis in elderly men [9, 10]. In postmenopausal women, low lean body mass was associated negatively with femoral neck BMD [11] and structural parameters of bone [12].m
Elderly people with sarcopenia are three times more likely to fall [13]. Increasing evidence showed that sarcopenia is closely related to fragility fracture [14,15,16]. Most importantly, high prevalence of sarcopenia in patients with fragility fractures has been reported recently, which is alarming to clinicians. However, there is currently still few clinical data demonstrating a causal relationship between osteoporosis and sarcopenia. There is a pressing need to understand more on this relationship. The objective of this systematic review and meta-analysis is to summarize the prevalence of sarcopenia in patients with fragility fracture in different countries and risk factors of fragility fracture with sarcopenia.
Methods
Search strategy
Literature search was carried out on PubMed and Cochrane databases. The keywords, “sarcopeni*” AND “fracture*,” were used to search in all fields. Last access to both databases was on Nov. 2, 2018. PRISMA guidelines was used.
Search criteria
Inclusion criteria were as follows: (1) clinical studies that investigate the relationship (prevalence or risk) between sarcopenia and fragility fracture; (2) full-text literature published in English. Exclusion criteria were as follows: (1) non-English papers; (2) not fragility fracture-related; (3) young subjects included; (4) review papers or conference abstracts.
Selection of studies
Two reviewers conducted study selection independently. Duplicates were removed. Irrelevant papers were screened out through the title and abstract by inclusion and exclusion criteria. Full text of potential relevant papers was then retrieved and further assessed for the eligibility. Disagreements were settled by discussion and consensus.
Data extraction
For the studies investigating sarcopenia prevalence, the following data were extracted and presented in tables: (1) study design and sample size; (2) mean age and gender; (3) fracture site; (4) definition of sarcopenia; (5) interval between time of fracture and measurement; (6) prevalence of sarcopenia.
For the studies that investigated risk of fracture in people with sarcopenia, the following data were extracted: (1) sample size; (2) mean age and gender; (3) definition of sarcopenia; (4) follow-up period; (5) fracture risk in term of hazard or odd ratio.
Assessment of quality of selected studies
Two authors independently performed quality assessment of the included studies. Disagreements were resolved by discussion. The methodological quality was assessed using the Newcastle-Ottawa Scale, which has been proven to be valid for nonrandomized studies [32]. The form assigns a maximum of 4 points for selection, 2 points for comparability, and 3 points for exposure or outcome.
Data analysis
All the studies included in this review were clinical studies but there was variability in terms of methodology. Qualitative analysis was performed for studies on prevalence of sarcopenia after fracture, due to the heterogeneity. Subgroup meta-analysis on sarcopenia in patients with history of fragility fracture was performed by computing hazard ratios (HRs) using fixed-effects model. Quantitative analyses were performed on time-to-event basis and were confined to data derived from the period of follow-up. HRs and 95% confidence intervals of fracture were calculated. CMA software version 3.3 was used for all analyses and production of plots. The inverse variance method was used to weight the study effect size.
Results
Results of the search
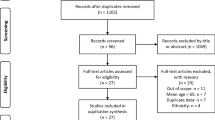
In the search, 354 and 33 papers were identified in PubMed and Cochrane databases respectively. Three hundred sixty-six papers were excluded based on the selection criteria after screening the titles and abstracts. The full texts of the 21 papers selected were retrieved for further assessment of eligibility. After screening the full text in detail, 2 duplicated papers and 4 papers with duplicated population were excluded. Therefore, 15 papers were finally recruited for analysis in this review. Figure 1 shows the selection process of the included papers.
Quality of selected studies
Tables 2, 3, and 4 summarize the quality of the 15 studies using the Newcastle-Ottawa Quality Scale. All studies were of high quality and suitable for qualitative or quantitative analysis.
Prevalence of sarcopenia
Fracture sites
A total of 10 included papers reported the prevalence of sarcopenia in patients with fragility fracture. The prevalence of sarcopenia after fracture ranged from 12.4%–95% in males to 18.3%–67.7% in females. Eight of the 10 papers estimated the prevalence of sarcopenia after hip fracture (17.1%–95%) [17, 19, 20, 22,23,24,25,26]. The remaining 2 papers estimated the prevalence of sarcopenia after distal radius fracture (30%) [18] and vertebral fracture (42.3%) [21]. Table 5 summarizes the details and the prevalence in the 10 studies.
Definitions of sarcopenia
With the new consensus on the definition of sarcopenia, an increasing number of papers have used the criteria recommended by the working groups to define sarcopenia. Most of the 10 included papers related to sarcopenia prevalence used recommended definitions of sarcopenia except 1 in 2009 [26], 2 in 2012, and 2 in 2013 [24, 25].
In hip fracture studies (8 out of 10 studies), 5 using the recommended definition of sarcopenia had prevalence ranging from 12.4%–73.6% in males to 18.3%–67.7% in females after the fracture [17, 19, 20, 22, 23]. The remaining 3 papers did not use the recommended definitions but instead used Skeletal Muscle Index (SMI) or Appendicular Lean Mass (ALM) [24,25,26]. Two had a prevalence of 81.8%–95% in males and 44.7%–64% in females, after hip fracture [24, 25], while the remaining 1 paper had an overall prevalence of 58% for both sexes [26].
Out of the 5 papers using recommended definitions for hip fracture, 2 used the EWGSOP criteria to define sarcopenia in hip fracture patients. The prevalence of sarcopenia was 58.0% and 12.4% in males; 34.9% and 18.3% in females for these 2 papers [19, 20]. Meanwhile, 2 used AWGS criteria to define sarcopenia in hip fracture patients. The prevalence of sarcopenia in hip fracture patients was 73.6% and 68.2% in males; 67.7% and 44.3% in females [22, 23]. Only 1 study used FNIH recommendation, which reported the prevalence of sarcopenia at 72% in males and 28% in females [17].
Time of measurement
For hip fracture studies, the prevalence of sarcopenia was higher if the measurement was taken post-operatively. Using the EWGSOP criteria, the study by Steihaug et al. [19] with measurements after hip surgery had a higher prevalence of sarcopenia (females 34.9%; males 58.0%) compared to the study by González-Montalvo et al. [20] (females 18.3%; males 12.4%) with measurements before the operation. The results were similar using the AWGS criteria. The study by Ho et al. [22] with measurements after hip surgery had a higher prevalence of sarcopenia (females 67.7%; males 73.6%) compared to the study by Yoo et al. [23] (females 44.3%; males 68.2%) with measurements before the operation.
Gender difference
Of the 10 included papers, 8 papers provided the prevalence of sarcopenia separately for both sexes. Seven of the 8 papers showed that the prevalence of sarcopenia after fracture was higher in males [17,18,19, 22,23,24,25]. In contrast, González-Montalvo et al. [20] showed that the prevalence of sarcopenia was higher in females (18.3%) compared to males (12.4%) after hip fracture.
Patient vs. control
Of the 10 included papers, 4 papers used case-control study design [18, 21, 23, 24]. All of them showed that the prevalence of sarcopenia was higher in the fracture group (30% to 50.1%) compared to the control group (10.8% to 33.5%).
Fracture risk of sarcopenia
Definition of sarcopenia
Five prospective cohort studies evaluated sarcopenia status of subjects at baseline and analyzed fracture risks, with a follow-up period ranging from 3 to 15.9 years [27,28,29,30,31]. All of the papers used the definitions of sarcopenia recommended by the working groups, including EWGSOP and AWGS, except the study by Harris et al. [27]. Table 6 summarizes the details and fracture risk of sarcopenic patients in each study.
Sarcopenia alone
Only 2 of the 5 papers concluded that sarcopenia was associated with increased fracture risk. Yu et al. [31] found that multivariate-adjusted fracture risk for those with sarcopenia alone in community-dwelling men aged 65 years or older was 2.33 times more (95% CI, 1.48–3.67) when compared to those with normal BMD and without sarcopenia. Hars et al. [29] also found a higher fracture risk in healthy 63- to 67-year-old community-dwelling elderly with an odds ratio of 2.31 (95% CI, 1.04–5.18) after multi-variable adjustment. However, Chalhoub et al. [30], Harris et al. [27], and Schaap et al. [28] were unable to show a higher fracture risk in sarcopenic patients.
Sarcopenia and low bone mineral density (BMD)
Two studies showed that men had a much higher fracture risk if they were sarcopenic and osteoporotic. Yu et al. [31] found that men with osteoporosis and sarcopenia had a significantly increased risk of fracture (HR, 3.49, 95% CI, 1.76–6.90) than those with sarcopenia alone (HR = 2.33, 95% CI, 1.48–3.67) or low BMD alone (HR = 2.4, 95% CI, 1.63–3.54). Chalhoub et al. [30] also found similar results of significantly higher risk of fracture in sarcopenic and osteoporotic men (HR = 4.08, 95% CI, 2.79–5.96) than those with sarcopenia alone (HR = 1.20, 95% CI, 0.64–2.28) or low BMD alone (HR = 1.82, 95% CI, 1.55–2.13). Meanwhile, the fracture risk in women was higher with low BMD, but the addition of sarcopenia did not further increase the fracture risk [27, 30].
Subgroup analysis on different nationalities and sarcopenia assessment
Asian sarcopenia prevalence, in general, is higher (30–69.5%) compared to Caucasians (17.1–64%). As for the assessments of sarcopenia with different criteria for diagnosis, EWGSOP had a range of 17.1–37%, AWGS 30–69.5%, and SMI 42.3–58% for prevalence.
Subgroup meta-analysis of sarcopenia in patients with fragility fracture
In the fixed-effects model, a 1-SD advantage in intelligence was associated with the sarcopenia patients who had fracture history. There was a low degree of heterogeneity indicated by the effect size (effect size = 2.986 (2.402, 3.711), Q = 7.43, I2 = 32.7%, p value = 0.19) (Fig. 2).
Discussion
Most fragility fractures in elderly people are caused by falls from standing height. Reduced skeletal mass and strength were associated with impaired balance [12, 33] and frailty [34] in elderly people. A systematic review and meta-analysis [35] concluded that muscle weakness, especially lower limb weakness, was a significant risk factor for recurrent falls. This explains how the 4 case-control studies [18, 21, 23, 24] showed the prevalence of sarcopenia to be higher in patients with fracture than control subjects. Sarcopenia is part of the frailty that leads to fracture. Furthermore, it has been shown in studies that approximately 50% of all rehabilitation patients have sarcopenia [36].
Sarcopenia was more prevalent in older men than women after a fragility fracture [17,18,19, 22,23,24,25]. There are a number of risk factors for sarcopenia, and the decline in sex hormone may be a possible reason for the sex difference. In men, the levels of testosterone and bioavailable testosterone, respectively, decreases by 0.8% and 2% per year from age of 40 [37]. In women, there is a marked reduction in estrogen levels after menopause. Testosterone increase synthesis of muscle protein and higher levels of testosterone are associated with less loss of lean body mass [38, 39]. Evidence also shows that estrogen levels are correlated positively with lean body mass [40]. However, the role of estrogen on the onset of sarcopenia is controversial and further research is still required [41]. The loss of muscle strength in men was nearly two-fold compared to women over 3 years of follow-up in a study [42].
Sarcopenia was more prevalent in patients with hip fracture as our review showed that sarcopenia prevalence was up to 95% [17, 19, 20, 22,23,24,25,26], while those with distal radius and vertebral fracture were 30% [18] and 42.3% [21], respectively. A recent study also showed that sarcopenia was more prevalent in the order of hip fracture (41.5%), vertebral fracture (35%), and distal radius fracture (29.6%) [43]. As lean body mass and strength decrease during aging, it is more common to have sarcopenia in hip fracture patients, as the age of these patients is generally higher than distal radius fracture patients. As physical function is highly related to sarcopenia [44], patients with hip fractures would understandably be less mobile.
The prevalence of sarcopenia after fracture should be analyzed with caution if the time of measurement is much delayed after the fracture. Our review showed that the measurement of sarcopenia before and after surgery differed remarkably, from 12.4–18.3% (pre-surgery) to 34.9–58% (post-surgery) using the ESWSOP criteria [19, 20] and from 44.3–68.2% (pre-surgery) to 67.7–73.6% (post-surgery) using AWGS [22, 23]. It is well known that increased bed rest after lower limb fracture can lead to muscle disuse atrophy. The lean body mass remains stable in the first 10 days, but significantly decreases from 10 days to 2 months after a hip fracture [45]. It is recommended to measure the body lean mass within 10 days after hip fracture to minimize bedrest-induced muscle loss and to avoid overestimating sarcopenia prevalence.
In this review, only 2 studies showed that fracture risk was higher in elderly people with sarcopenia alone [29, 31]. The number of falls was not adjusted for the fracture risk in these 2 papers. On the other hand, hazard ratios were adjusted with the number of falls reported in the studies by Chalhoub et al. [30] and Harris et al. [27], where no increased fracture risk was found. As the majority of fragility fractures are caused by fall incidences, adjusting the number of falls may underestimate the observed fracture risk. The hazard ratio may also be underpowered due to relatively small sample size in the study by Schaap et al. [28]. These are the possible reasons that no significant association between sarcopenia and fragility fracture was found.
It is important to note that men had a much higher fracture risk with both sarcopenia and low BMD. In the study by Chalhoub et al. [30], the fracture risk in men with sarcopenia or osteoporosis alone was 1.20 and 1.82 times, respectively, but the risk was significantly increased to 4.08 times in men with both sarcopenia and osteoporosis. The combined effect of sarcopenia and osteoporosis on fracture risk was greater than the sum of the individual risk. This may be the consequence of the synergistic interaction between low bone mass and low lean body mass on bone quality. Muscles and bones are postulated to closely interact with each other in two ways. In the mechanostat theory, muscle contraction is required to impose mechanical force to the bone structure, thus stimulating the osteogenic effects [46]. In the crosstalk theory, there is a feed-forward loop between bone and skeletal muscle by secreting factors that act on each other and influence metabolism [46, 47]. Studies found that low lean body mass and strength were associated with impaired bone quality in terms of bone geometry and microarchitecture [48,49,50]. Good bone strength should be comprised of good bone density as well as bone quality [51]. Weak muscle and bone affect each other negatively, and hence contribute to the additive effect of fracture risk. However, despite the theories, there is currently very few clinical data demonstrating a causal relationship between osteoporosis and sarcopenia.
The addition of sarcopenia did not further increase the fracture risk in osteoporotic women [27, 30]. A possible explanation was that muscle loss in women was slower and the severity of sarcopenia in women was not as high as in men. Our current findings support this as there is close relationship observed mainly in males. This is of clinical importance as clinicians should pay extra attention in the screening of sarcopenia in these patients. This also suggests that fractures in women were mainly associated with low BMD. As the number of papers included in this review is limited, further studies are required to confirm the above phenomenon. There has been increasing concern about the combined effect of sarcopenia and osteoporosis on fracture risk. The terms “sarco-osteopenia” and “sarco-osteoporosis” were first coined by Binkley and Buehring [52] to emphasize the weak bones and weak muscles that may contribute to falls and fractures in elderly adults. Recent studies indicated that elder people with sarco-osteoporosis were frailer with higher burden of comorbidities [53, 54]. However, there is still a knowledge gap in this area and a lack of clinical data. Further research is required for better understanding in the future.
It is important to note that most studies only measure the lean body mass by dual energy X-ray absorptiometry (DXA) scan, which does not have a functional component. Although stating that an established sarcopenia definition was used according to the AWGS, EWGSOP, IWGS, and FNIH, readings of hand grip or gait are often missed. The description as to why these additional assessments was not stated. Following guidelines strictly for the correct diagnosis of sarcopenia is important. It can be assumed that elderlies having fractures would not be able to do gait tests and may decline additional assessments due to pain. Missing values were also identified in the study by Steihaug et al., which was accounted by patients being too ill, refused specific examinations, or discharged before data collection was complete. The study by Schaap et al. had 9 missing falls data but there were no details regarding the cause, but there was complete data on sarcopenic parameters. Furthermore, studies using BIA or triceps skinfold are not the gold standard in diagnosing sarcopenia, which is used by 3 studies. Other confounding factors include the fact that sarcopenia may just be an indicator of frailty in the elderly. Other limitation in this systematic review is that the definitions of sarcopenia still varied in different papers, although more consensus have been reached by several working groups in recent years. This leads to difficult comparison of sarcopenia prevalence among different papers. Secondly, only two databases were used for the electronic search and non-English papers were excluded, which may cause some missing relevant articles. Also, only qualitative review was conducted in this review due to the different nature of papers and data heterogeneity.
Based on current studies, the prevalence of sarcopenia in elderly people with fragility fracture was much higher than non-fracture elderly, especially in men. This is an alarming signal to orthopedic experts, as sarcopenia is under-attention. The combined effect of sarcopenia and low BMD on bone quality in males should be further studied. There is also a pressing need for further research on the fragility fracture associated with sarco-osteoporosis to understand their relationship and mechanism. These can provide more evidence to develop potential interventions to improve clinical outcomes.
References
Janssen I, Heymsfield SB, Wang ZM, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol 89:81–88
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T (2014) Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43:748–759
Frisoli A, Chaves PH, Ingham SJM, Fried LP (2011) Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women’s Health and Aging Study (WHAS) II. Bone 48:952–957
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, de Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 12:249–256
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JSW, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101
Studenski SA, Peters KW, Alley DE et al (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. Journals of Gerontology Series a-Biological Sciences and Medical Sciences 69:547–558
Woo J, Leung J, Morley JE (2015) Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 16:247–252
Blain H, Jaussent A, Thomas E, Micallef JP, Dupuy AM, Bernard PL, Mariano-Goulart D, Cristol JP, Sultan C, Rossi M (2010) Appendicular skeletal muscle mass is the strongest independent factor associated with femoral neck bone mineral density in adult and older men. Exp Gerontol 45:679–684
Verschueren S, Gielen E, O’Neill TW et al (2013) Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int 24:87–98
Orsatti FL, Nahas EAP, Nahas-Neto J, Orsatti CL, Marocolo M, Barbosa-Neto O, da Mota GR (2011) Low appendicular muscle mass is correlated with femoral neck bone mineral density loss in postmenopausal women. BMC Musculoskelet Disord 12
Szulc P, Beck TJ, Marchand F, Delmas PD (2005) Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men - the MINOS study. J Bone Miner Res 20:721–729
Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G (2012) Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 31:652–658
Cheung CL, Tan KCB, Bow CH, Soong CSS, Loong CHN, Kung AWC (2012) Low handgrip strength is a predictor of osteoporotic fractures: cross-sectional and prospective evidence from the Hong Kong Osteoporosis Study. Age 34:1239–1248
Hong W, Cheng Q, Zhu XY, Zhu H, Li H, Zhang X, Zheng S, du Y, Tang W, Xue S, Ye Z (2015) Prevalence of sarcopenia and its relationship with sites of fragility fractures in elderly Chinese men and women. PLoS One 10
Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB, Study HA (2010) Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and Body Composition Study. J Bone Miner Res 25:513–519
Landi F, Calvani R, Ortolani E, Salini S, Martone AM, Santoro L, Santoliquido A, Sisto A, Picca A, Marzetti E (2017) The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int 28:1569–1576
Roh YH, Do Koh Y, Noh JH, Gong HS, Baek GH (2017) Evaluation of sarcopenia in patients with distal radius fractures. Arch Osteoporos 12
Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Ranhoff AH (2017) Sarcopenia in patients with hip fracture: a multicenter cross-sectional study. PLoS One 12
Gonzalez-Montalvo JI, Alarcon T, Gotor P, Queipo R, Velasco R, Hoyos R, Pardo A, Otero A (2016) Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr Gerontol Int 16:1021–1027
Hida T, Shimokata H, Sakai Y, Ito S, Matsui Y, Takemura M, Kasai T, Ishiguro N, Harada A (2016) Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J 25:3424–3431
Ho AWH, Lee MML, Chan EWC, Ng HMY, Lee CW, Ng WS, Wong SH (2016) Prevalence of pre-sarcopenia and sarcopenia in Hong Kong Chinese geriatric patients with hip fracture and its correlation with different factors. Hong Kong Med J 22:23–29
Yoo JI, Ha YC, Kwon HB, Lee YK, Koo KH, Yoo MJ (2016) High prevalence of sarcopenia in Korean patients after hip fracture: a case-control study. J Korean Med Sci 31:1479–1484
Hida T, Ishiguro N, Shimokata H, Sakai Y, Matsui Y, Takemura M, Terabe Y, Harada A (2013) High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int 13:413–420
Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R (2012) Sarcopenia is more prevalent in men than in women after hip fracture: a cross-sectional study of 591 inpatients. Arch Gerontol Geriatr 55:E48–E52
Singh MAF, Singh NA, Hansen RD et al (2009) Methodology and baseline characteristics for the sarcopenia and hip fracture study: a 5-year prospective study. Journals of Gerontology Series a-biological Sciences and Medical Sciences 64:568–574
Harris R, Chang Y, Beavers K, Laddu-Patel D, Bea J, Johnson K, LeBoff M, Womack C, Wallace R, Li W (2017) Risk of fracture in women with sarcopenia, low bone mass, or both. J Am Geriatr Soc 65:2673–2678
Schaap LA, van Schoor NM, Lips P, Visser M (2017) Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures; the Longitudinal Aging Study Amsterdam. J Gerontol Ser A:glx245
Hars M, Biver E, Chevalley T, Herrmann F, Rizzoli R, Ferrari S, Trombetti A (2016) Low lean mass predicts incident fractures independently from FRAX: a prospective cohort study of recent retirees. J Bone Miner Res 31:2048–2056
Chalhoub D, Cawthon PM, Ensrud KE, Stefanick ML, Kado DM, Boudreau R, Greenspan S, Newman AB, Zmuda J, Orwoll ES (2015) Risk of nonspine fractures in older adults with sarcopenia, low bone mass, or both. J Am Geriatr Soc 63:1733–1740
Yu R, Leung J, Woo J (2014) Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc 15:551–558
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Carter ND, Khan KM, Mallinson A, Janssen PA, Heinonen A, Petit MA, McKay HA, Grp F-FBR (2002) Knee extension strength is a significant determinant of static and dynamic balance as well as quality of life in older community-dwelling women with osteoporosis. Gerontology 48:360–368
Yoshimura N, Muraki S, Oka H, Iidaka T, Kodama R, Horii C, Kawaguchi H, Nakamura K, Akune T, Tanaka S (2018) Do sarcopenia and/or osteoporosis increase the risk of frailty? A 4-year observation of the second and third ROAD study surveys. Osteoporos Int 29:2181–2190
Moreland JD, Richardson JA, Goldsmith CH, Clase CM (2004) Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc 52:1121–1129
Churilov I, Churilov L, MacIsaac RJ, Ekinci EI (2018) Systematic review and meta-analysis of prevalence of sarcopenia in post acute inpatient rehabilitation. Osteoporos Int 29:805–812
Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB (2002) Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 87:589–598
LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, Hoffman AR, Laughlin GA, Marshall LM, Orwoll ES (2011) Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab 96:3855–3863
Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A (1995) Testosterone administration to elderly men increases skeletal-muscle strength and protein-synthesis. Am J Physiol Endocrinol Metab 269:E820–E826
van Geel TA, Geusens PP, Winkens B, Sels J-PJ, Dinant G-J (2009) Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: a cross-sectional study. Eur J Endocrinol 160:681–687
Messier V, Rabasa-Lhoret R, Barbat-Artigas S, Elisha B, Karelis AD, Aubertin-Leheudre M (2011) Menopause and sarcopenia: a potential role for sex hormones. Maturitas 68:331–336
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064
Yoon BH, Lee JK, Choi DS, Han SH (2018) Prevalence and associated risk factors of sarcopenia in female patients with osteoporotic fracture. J Bone Metab 25:59–62
Westbury LD, Dodds RM, Syddall HE, Baczynska AM, Shaw SC, Dennison EM, Roberts HC, Sayer AA, Cooper C, Patel HP (2018) Associations between objectively measured physical activity, body composition and sarcopenia: findings from the Hertfordshire Sarcopenia Study (HSS). Calcif Tissue Int 103:237–245
D’Adamo CR, Hawkes WG, Miller RR, Jones M, Hochberg M, Yu-Yahiro J, Hebel JR, Magaziner J (2014) Short-term changes in body composition after surgical repair of hip fracture. Age Ageing 43:275–280
Girgis CM (2015) Integrated therapies for osteoporosis and sarcopenia: from signaling pathways to clinical trials. Calcif Tissue Int 96:243–255
Karsenty G, Mera P (2017) Molecular bases of the crosstalk between bone and muscle. Bone
Edwards MH, Ward KA, Ntani G, Parsons C, Thompson J, Sayer AA, Dennison EM, Cooper C (2015) Lean mass and fat mass have differing associations with bone microarchitecture assessed by high resolution peripheral quantitative computed tomography in men and women from the Hertfordshire Cohort Study. Bone 81:145–151
LeBrasseur NK, Achenbach SJ, Melton LJ, Amin S, Khosla S (2012) Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J Bone Miner Res 27:2159–2169
Szulc P, Blaizot S, Boutroy S, Vilayphiou N, Boonen S, Chapurlat R (2013) Impaired bone microarchitecture at the distal radius in older men with low muscle mass and grip strength: the STRAMBO Study. J Bone Miner Res 28:169–178
Carbonare LD, Giannini S (2004) Bone microarchitecture as an important determinant of bone strength. J Endocrinol Investig 27:99–105
Binkley N, Buehring B (2009) Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitom 12:413–416
Wang YJ, Wang Y, Zhan JK, Tang ZY, He JY, Tan P, Deng HQ, Huang W, Liu YS (2015) Sarco-osteoporosis: prevalence and association with frailty in Chinese community-dwelling older adults. Int J Endocrinol 2015:482940
Patel HP, Dawson A, Westbury LD, Hasnaoui G, Syddall HE, Shaw S, Sayer AA, Cooper C, Dennison EM (2018) Muscle mass, muscle morphology and bone health among community-dwelling older men: findings from the Hertfordshire sarcopenia Study (HSS). Calcif Tissue Int 103:35–43
Funding
This study was partially supported by General Research Fund (Reference: 14113018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1208 kb)
Rights and permissions
About this article
Cite this article
Wong, R.M.Y., Wong, H., Zhang, N. et al. The relationship between sarcopenia and fragility fracture—a systematic review. Osteoporos Int 30, 541–553 (2019). https://doi.org/10.1007/s00198-018-04828-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-04828-0