Abstract
To our knowledge, no comprehensive meta-analysis has examined the association between sarcopenia and the risk of fractures. This systematic review and meta-analysis of prospective cohort studies aims to summarize whether sarcopenia is a risk factor for fractures among community-dwelling older adults. We searched four electronic literature databases (Ovid MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, PubMed) for relevant publications from inception to December 2017, using relevant keywords. We conducted a pooled analysis of the association between sarcopenia and the risk of fractures by employing a random-effects model. Subgroup analyses were conducted based on definitions of sarcopenia and gender. In total, nine studies were included in our systematic review and meta-analysis. The prevalence of sarcopenia ranged from 4.3 to 33.1%. The pooled RR of fractures for the sarcopenic versus the nonsarcopenic was 1.34 (95% CI = 1.13–1.58, P = 0.001, I2 = 5.5%, P-heterogeneity = 0.391). Subgroup analyses showed that associations between sarcopenia and fractures were significant when using the AWGS definition (combined effect size = 1.78, 95% CI = 1.25–2.54, P = 0.001), and studies in males (combined effect size = 1.39, 95% CI = 1.13–1.71, P = 0.002). In conclusion, we found that compared to nonsarcopenic, the association between sarcopenia and fractures among community-dwelling older people was significant when using the AWGS definition, and only for males. Future studies are needed to establish a possible association between sarcopenia definitions and risk of fractures of different sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone fractures, especially osteoporotic fractures, represent a great burden on the individual and public health care system [1]. They not only have high associated morbidity, hospitalization, permanent disability but also mortality. However, it is estimated that approximately half of the clinical fractures occur in postmenopausal women without osteoporosis based on bone mineral density (BMD) [2]. Hence, it is necessary to identify other risk factors that may help identify people at high risk of fractures.
Sarcopenia is regarded as one of the four major components of muscle wasting disease [3] and an independent condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code [4]. There are several international published definitions of sarcopenia, including Newman [5], the European Working Group on Sarcopenia in Older People (EWGSOP) [6], Asian Working Group for Sarcopenia (AWGS) [7], International Working Group on sarcopenia (IWGS) [8], and Foundation of the National Institutes of Health (FNIH) [9]. Newman was defined only in terms of low muscle mass; the latter criteria were defined as a loss of muscle mass associated with a loss of muscle function. The cut-off points are the major difference in these definitions, because of the lifestyle, environmental, ethnic differences in body shape or sizes, and cultural backgrounds [7, 10].
In the past several years, sarcopenia has been recognized as an important geriatric condition. There are increasing volumes of articles on the impact of sarcopenia on health outcomes. The decline in muscle mass, function, and strength could contribute to adverse consequences on individual and public health, such as poor quality of life [11, 12], function decline [13], physical disabilities [14], hospitalization [15], and ultimate mortality [16]. In addition, several studies found that sarcopenia was associated with fractures in older adults [17,18,19,20]. However, these consequences were determined from both cross-sectional studies and longitudinal studies, which lead to potential difficulties in attributing causality. Furthermore, the association between sarcopenia and fractures can also differ depending on the definition used for the diagnosis of sarcopenia. Some studies have failed to show that sarcopenia alone significantly associated with fractures [21,22,23,24].
Oliveira and colleagues [25] performed a systematic review describing the association between sarcopenia and osteoporotic hip fracture in 2015. However, almost all studies included in this review were cross-sectional studies, and they did not perform a pooled analysis. In addition, another systematic review [26] that purposed to assess the association sarcopenia and the clinical and socioeconomic consequences (fractures, falls, mortality, functional decline, hospitalization and the length of hospital stay) was published in last year. This review was comprehensive. However, only two studies reported the relationship of sarcopenia and fractures, and the outcomes were simply analyzed and described. Because of the aforementioned reasons, we performed a comprehensive systematic review and meta-analysis of current cohort studies to explore whether sarcopenia is a risk factor for fractures among community-dwelling older adults.
Methods
Search strategy
This study was carried out based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol for reporting systematic reviews and meta-analyses [27]. The following electronic databases were searched: EMBASE (from January 1, 1974, to December 2017), Ovid MEDLINE (from January 1, 1946, to December 2017), Cochrane Central Register of Controlled Trails (from inception to December 2017), and PubMed (from inception to December 2017). Our search terms used for this research include sarcopenia (sarcopenic; muscle mass; muscle strength; muscle atrophy; muscle wasting; myopenia; myopenic; dynapenia; dynapenic) and fracture (broken bone; osteoporotic fractures; fracture*; fractures, bone) and aged (aging; geriatrics; elderly; geriatric*; older adult*; older people). In addition, the reference lists of the eligible articles were also searched to avoid missing any relevant studies.
Inclusion and exclusion criteria
In this systematic search, rigorous inclusion criteria were as follows: (1) participants: older adults (aged 60 years old and older); (2) definition of sarcopenia: studies using the clear definition of sarcopenia; (3) studies reporting hazard ratios (HRs), odds ratios (ORs) or risk ratios(RRs), and their corresponding 95% confidence intervals (CIs) for the relationship between sarcopenia and fractures; (4) study design: cohort studies; and (5) data limitation: from inception to November 2017. Studies excluded were as follows: (1) article type: reviews, conference abstract; (2) language: not English or Chinese.
Study selection and data extraction
The review of potentially eligible studies identified by the searches was completed by two independent reviewers to identify reports for review in full text. Each full-text study was reviewed for eligibility by these reviewers. Data were extracted and summarized independently, according to a standardized data extraction form. The presence or absence of sarcopenia at study baseline was the primary exposure variable. The key outcome variable was the incidence of fracture during follow-up. Any study reported HRs, ORs, or RRs for fracture in individuals with sarcopenia and nonsarcopenia was extracted. Other data from the eligible studies included the following: first author, publication years, sociodemographic (country, type of population, age, the proportion of male), sample size, description of groups, duration of follow-up, frequency of follow-up, methods used for assessing fracture, and statistical adjustment for confounding variables (Table 1). We also extracted sarcopenia criteria, the prevalence of sarcopenia, and the tool and cutoff points of muscle mass, muscle strength, and physical performance (Table 2). If the dataset from the same population had been published in not only one publication, then only the study with longer follow-up period was included in our systematic review and meta-analysis. If studies divided the entire cohorts into male cohort and female cohort separately, then we considered the study as two independent studies. If some studies did not provide required estimates, we calculated those using standard methods. For studies with insufficient information, we contacted the primary authors, when possible, to acquire the data. All extracted data were cross-checked by reviews, and disagreements were resolved by consensus with a third reviewer.
Assessment of risk of bias
Two authors independently assessed the risk of bias using the Newcastle-Ottawa Scale (NOS), designed for nonrandomized studies [28], which consists of three factors: patient selection (four items), comparability of the study groups (one item), and assessment of outcome (three items). The overall score ranges from one to nine, nine points represented the highest methodological quality, and NOS scores of ≥ 5 were considered to be of high-quality publications [29]. Disagreements were also settled down by discussion among the third author.
Statistical analysis
HRs, ORs, RRs, and their 95% CIs were assessed for determining the relationship between sarcopenia and fractures. Although published studies presented several estimates of the association between sarcopenia and bone fractures, HRs and ORs were deemed approximate to the RRs [30]. Cochran’ s Q statistic using chi-square and I square (I2) test were performed to assess the impact of study heterogeneity on the results of the meta-analysis, and I2 value was considered to reflect mild heterogeneity (0–25%), moderate heterogeneity (26–75%), and high heterogeneity (76–100%) [31]. Random effect models were chosen if heterogeneity existed. Otherwise, the fixed effect models were used. We performed subgroup analysis to detect probable sources of heterogeneity. The predefined criteria for subgroup analyses were as follows: gender (male, female or both sexes), follow-up duration (< 10 or ≥ 10 years), methods used to assess bone fractures (self-reported or medical records), sarcopenia criteria (EWGSOP, AWGS, and other), and fracture site (all fractures, hip fracture). Medical records included radiology reports and hospital authority electronic database. Because of the limited number of studies, we just perform subgroup analyses based on gender and sarcopenia criteria. Moreover, sensitivity analyses were done evaluating whether the overall estimate depended on the effect size from a single study. We drew funnel plot for outcomes, and Egger’s and Beggar’s tests were done to plot the log RR against its standard error for assessment of potential publication bias [32]. All analyses were analyzed using the statistical software (STATA, version 11.0, Stata Corp, College Station, TX, USA). We used two-tailed P values and P < 0.05 was considered significant.
Results
Search processes
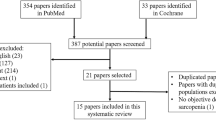
A total of 1468 articles were initially identified through three electronic databases. Among these articles, 675 duplicate articles were removed and 774 articles were excluded by screening the title and abstract. Therefore, only 19 articles remained for full-text review. Of these articles, nine were removed due to being not cohort studies (e.g., cross-sectional study, case-control study), and one [33] was removed due to having no clear definition of sarcopenia. Finally, nine prospective studies [17, 18, 22,23,24, 34,35,36,37] were included in our systematic review. Two studies contained the same population in men (n = 2000), so we choose the outcome which was longer periods of follow-up to avoid double counting data. The flow diagram of article search process is available in Fig. 1.
Included studies
Table 1 presents the characteristic of seven included studies. The overall sample size was 31,513 individuals. All of these studies were quite recent since they were published between 2014 and 2017. Three studies [22, 23, 34] were performed in the USA, two [17, 24] in China, two [35, 37] in Australia, one [18] in Switzerland, and one [36] in Netherlands. All of these studies included community-dwelling older adults. Three studies [17, 22, 35] were conducted on only male subjects, one study [34] was conducted on only female subjects, two [18, 36] on both gender, and three studies [23, 24, 37] divided the entire cohorts into male cohort and female cohort separately. The number of participants ranged from 496 [36] to 10,937 [34], and the follow-up periods varied from 3.4 [18] to 15.9 years [34]. The outcome included incident hip fracture, incident fracture, nonspine fractures, and nonvertebral fractures. Three studies [17, 24, 36] had collected data on fractures using hospital electronic database, four studies [18, 23, 34, 37] used self-reported questionnaires, and two studies [22, 35] used radiology reports. Seven studies directly reported adjusted ORs and HRs and 95% CIs for the association between sarcopenia and fractures. Two studies [35, 37] divided participants into four categories: nonsarcopenic nonobese, nonsarcopenic obese, sarcopenic nonobese, and sarcopenic obese. We merged the first two into nonsarcopenic group, and merged the latter two into the sarcopenic group. Then, we calculated the RR and 95% CI of this study by 2 × 2 contingency tables, which were presented in the tables describing the demographics of study populations. Two studies [23, 34] included more than two groups; we choose the outcome of sarcopenia-alone subjects compared with the nonsarcopenic and normal BMD subjects.
Sarcopenia criteria and prevalence
Five studies [18, 22, 23, 35, 36] adopted European Working Group on Sarcopenia in Older People (EWGSOP), two studies [17, 24] adopted Asian Working Group for Sarcopenia (AWGS), and two studies [34, 37] used the definition of Newman. Table 2 presents the sarcopenia criteria among included studies and shows the different tools and cutoff points of muscle mass, muscle strength, and physical performance. Muscle mass was measured with dual-energy X-ray absorptiometry (DXA) in all of studies. Six studies used both low muscle mass and low muscle function to define sarcopenia, and three studies [18, 34, 37] determined sarcopenia only by low muscle mass. The prevalence of sarcopenia varied from 4.3 [22] to 31.9% [36], 7.3 [24] to 9.4% [17], and 19.9 [34] to 33.1% [37] in EWGSOP, AWGS, and Newman, respectively.
Quality assessment
The quality assessment using NOS is shown in supplemental Table 1. The scores ranged from 6 to 8. Four studies [17, 22, 35, 36] achieved 8 points, three studies [18, 23, 24] achieved 7 points, and two studies [34, 37] achieved 6 points. All included studies had high quality.
The relationship between sarcopenia and risk of fractures
All studies were included in meta-analysis. The association between sarcopenia and risk of fractures is shown in Fig. 2. Sarcopenia was significantly associated with the risk of fractures (combined effect size = 1.34, 95% CI = 1.13–1.58, P = 0.001). Mild heterogeneity was observed in this outcome (I2 = 5.5%, P-heterogeneity = 0.391). We performed subgroup analyses stratified by sarcopenia criteria and sex based on a random effects model. We found a significant positive association when sarcopenia was defined by AWGS (combined effect size = 1.78, 95% CI = 1.25–2.54, P = 0.001). However, the association between sarcopenia and fractures was not significant when EWGSOP (combined effect size = 1.20, 95% CI = 0.96–1.50, P = 0.112) or Newman (combined effect size = 1.25, 95% CI = 0.79–1.98, P = 0.339) was used (Fig. 3). In addition, we found that sarcopenia was significantly associated with the higher incidence of fractures in male (combined effect size = 1.39, 95% CI = 1.13–1.71, P = 0.002), but not in female (combined effect size = 1.09, 95% CI = 0.63–1.87, P = 0.169) and both gender (combined effect size = 1.41, 95% CI = 0.58–3.39, P = 0.447) (Fig. 4).
Forest plot of effect sizes for the association between sarcopenia and fractures across different definitions of sarcopenia by using a random effects model. ES, effect size; EWGSOP, the European Working Group on Sarcopenia in Older People; AWGS, Asian Working Group for Sarcopenia. *The RRs reported in the Scott et al. studies were crude RRs. All other outcomes were adjusted
Sensitivity analysis
We performed a sensitivity analysis by omitting one single study each time and pooling the others to check which study influenced the main effect. The result of sensitivity analysis for all studies is shown in supplemental Fig. 1. The stability of results had no significant changes, which validated the reliability of our analysis.
Publication bias
We used Egger’s and Beggar’s tests to assess the publication bias in this meta-analysis. Finally, asymmetry was observed by visual inspection of funnel plot (supplemental Fig. 2). However, the results did not show any statistically significant publication bias among the studies using Egger’s test (P = 0.876) and Beggar’s test (P = 0.495).
Discussion
We performed this systematic review and meta-analysis to evaluate whether sarcopenia is a predictive factor for fractures in community-dwelling older adults. We found that associations between sarcopenia and fractures were significant when using the AWGS definition and studies in males. However, sarcopenia was not associated with the risk of fractures when EWGSOP or Newman was used and studies in females. No significant heterogeneity was observed across these studies (I2 = 5.5%, P-heterogeneity = 0.391).
One systematic review [25] described the association between sarcopenia and osteoporotic hip fracture in 2015. However, the authors only included one cohort study in their review, which may have reduced the strength of their conclusion. In addition, this review only examined hip fracture risk but did not assess the risk of all fractures. Another meta-analysis [26] that purposed to assess the association sarcopenia and the clinical and socioeconomic consequences was published last year. This review was comprehensive. However, only two studies reported the relationship of sarcopenia and fractures, and the outcomes were simply described. In comparison with this previous meta-analysis, our meta-analysis included nine cohort studies and focused on all fractures. To the best of our knowledge, this is the most comprehensive review to summarize earlier cohort studies on all definitions of sarcopenia and fractures.
Several plausible mechanisms have been proposed for the associations between sarcopenia and the risk of fractures. On the one hand, low muscle mass changes in muscle related proteins, such as myokines, and also is associated with abnormal glucose metabolism, which has a great impact on bone metabolism [38]. Second, sarcopenic individuals are at high risk of falls, which leads to higher incidence of fractures. At last, the decline in muscle function and strength is associated with low mechanical loading, thus also affecting bone mass directly. Ormsbee et al. also researched that loss of muscle commonly combined with the loss of bone, making these individuals at high risk of fractures [39]. Therefore, sarcopenia is considered an effective predictor of fracture risk in older adults.
A positive result was found when sarcopenia was defined by AWGS. Asia is the most populated region in the world with a wide range of society, lifestyles, culture, ethnic, and religious backgrounds. The population size and the rapid population aging, thus the impact of sarcopenia, may be stronger than other regions [7]. In addition, our finding demonstrated the gender differences in the relationship of sarcopenia and fractures. Sarcopenia was associated with fractures in men, but we did not find an increased risk of fractures with sarcopenia in women, which may be partly explained by the fact that muscle strength decline in men is generally two times faster compared to women [40]. Though higher muscle mass was observed at baseline in men, it has been suggested that men could have a rapid age-related decline in muscle mass compared to women [41]. What is more, epidemiological studies have shown that higher testosterone levels have been associated with less loss of muscle mass and strength in older man [42, 43]. Men lose more testosterone than women with age increasing, which leads to the onset and severity of sarcopenia [44]. Therefore, the impact of sarcopenia on fracture risk prediction in men is more prominent.
Prevalence of sarcopenia is difficult to establish. A meta-analysis found a prevalence ranging from 1 to 29% in community-dwelling older adults [45]. In our research, the prevalence of sarcopenia ranged from 4.3 to 31.9%, 7.3 to 9.4%, and 19.9 to 33.1% in EWGSOP, AWGS, and Newman, respectively. This prevalence could differ depending on geographic regions, study population and the definitions of sarcopenia.
There are multiple highlights in our study. The most important is that all the studies included in this meta-analysis were prospective cohort studies, and thus, they do provide a higher level of evidence. The second advantage is that almost all of our results were based on adjusted estimates, which considered some potential confounding factors and provided results that are more accurate. In addition, the setting of the participants only included community-dwelling older adults, and we performed subgroup analyses to evaluate the pooled effect, which can help to reduce and examine the heterogeneity and analyze the results in detail.
Despite these strengths, some limitations in our study should also be taken into account. First, we choose different diagnostic criteria of sarcopenia, and different cutoff points were used in studies, thus may influence the research outcome and quality. The second limitation is that the number of studies included in this analysis was insufficient, especially in terms of a subgroup analysis. Only results of published studies were included in this meta-analysis. There may be numerous cohort studies currently in progress around the world, and a number of these studies could potentially have measured the relevant constructs, but without publishing these results yet. Moreover, some studies reporting negative results are difficult to publish. Therefore, potential publication bias is likely to exist, in spite of no evidence obtained from our statistical tests. Thirdly, fractures were assessed by self-report in some studies and may be subject to recall bias. However, fractures are major life events and inaccuracy of recall is unlikely. At last, some studies included in this meta-analysis reported nonspine fractures or nonvertebral fracture risk, and we treated these fractures as all fractures. Future studies are needed to focus on the association between sarcopenia and fracture of different sites.
Conclusion
We found that compared to nonsarcopenic, the associations between sarcopenia and fractures among community-dwelling older people were significant only when using the AWGS definition, and only for males in our study. Future studies are needed to establish a possible association between sarcopenia definitions and risk of fracture of different sites.
References
Rivas A, Romero A, Mariscal-Arcas M, Monteagudo C, Feriche B, Lorenzo ML, Olea F (2013) Mediterranean diet and bone mineral density in two age groups of women. Int J Food Sci Nutr 64(2):155–161. https://doi.org/10.3109/09637486.2012.718743
Siris ES, Brenneman SK, Barrett-Connor E, Miller PD, Sajjan S, Berger ML, Chen YT (2006) The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50-99: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 17(4):565–574. https://doi.org/10.1007/s00198-005-0027-4
Anker SD, Coats AJ, Morley JE, Rosano G, Bernabei R, von Haehling S, Kalantar-Zadeh K (2014) Muscle wasting disease: a proposal for a new disease classification. J Cachexia Sarcopenia Muscle 5(1):1–3. https://doi.org/10.1007/s13539-014-0135-0
Cao L, Morley JE (2016) Sarcopenia is recognized as an independent condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) code. J Am Med Dir Assoc 17(8):675–677. https://doi.org/10.1016/j.jamda.2016.06.001
Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2003) Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 51(11):1602–1609. https://doi.org/10.1046/j.1532-5415.2003.51534.x
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39(4):412–423. https://doi.org/10.1093/ageing/afq034
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15(2):95–101. https://doi.org/10.1016/j.jamda.2013.11.025
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 12(4):249–256. https://doi.org/10.1016/j.jamda.2011.01.003
Dam TT, Peters KW, Fragala M, Cawthon PM, Harris TB, McLean R, Shardell M, Alley DE, Kenny A, Ferrucci L, Guralnik J, Kiel DP, Kritchevsky S, Vassileva MT, Studenski S (2014) An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci 69(5):584–590. https://doi.org/10.1093/gerona/glu013
Woo J, Arai H, Ng TP, Sayer AA, Wong M, Syddall H, Yamada M, Zeng P, Wu S, Zhang TM (2014) Ethnic and geographic variations in muscle mass, muscle strength and physical performance measures. Eur Geriatr Med 5(3):155–164. https://doi.org/10.1016/j.eurger.2014.04.003
Beaudart C, Reginster JY, Petermans J, Gillain S, Quabron A, Locquet M, Slomian J, Buckinx F, Bruyere O (2015) Quality of life and physical components linked to sarcopenia: the SarcoPhAge study. Exp Gerontol 69:103–110. https://doi.org/10.1016/j.exger.2015.05.003
Manrique-Espinoza B, Salinas-Rodriguez A, Rosas-Carrasco O, Gutierrez-Robledo LM, Avila-Funes JA (2017) Sarcopenia is associated with physical and mental components of health-related quality of life in older adults. J Am Medl Dir Assoc 18(7):636.e631–636.e635. https://doi.org/10.1016/j.jamda.2017.04.005
Kelley GA, Kelley KS (2017) Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp Gerontol 96:100–103. https://doi.org/10.1016/j.exger.2017.06.008
Eguchi Y, Suzuki M, Yamanaka H, Tamai H, Kobayashi T, Orita S, Yamauchi K, Suzuki M, Inage K, Fujimoto K, Kanamoto H, Abe K, Aoki Y, Toyone T, Ozawa T, Takahashi K, Ohtori S (2017) Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord 12:9. https://doi.org/10.1186/s13013-017-0116-0
Bianchi L, Ferrucci L, Cherubini A, Maggio M, Bandinelli S, Savino E, Brombo G, Zuliani G, Guralnik JM, Landi F, Volpato S (2016) The predictive value of the EWGSOP definition of sarcopenia: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 71(2):259–264. https://doi.org/10.1093/gerona/glv129
Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B (2017) Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas 103:16–22. https://doi.org/10.1016/j.maturitas.2017.04.007
Yu R, Leung J, Woo J (2014) Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc 15(8):551–558. https://doi.org/10.1016/j.jamda.2014.02.005
Hars M, Biver E, Chevalley T, Herrmann F, Rizzoli R, Ferrari S, Trombetti A (2016) Low lean mass predicts incident fractures independently from FRAX: a prospective cohort study of recent retirees. J Bone Miner Res 31(11):2048–2056. https://doi.org/10.1002/jbmr.2878
Hong W, Cheng Q, Zhu X, Zhu H, Li H, Zhang X, Zheng S, Du Y, Tang W, Xue S, Ye Z (2015) Prevalence of sarcopenia and its relationship with sites of fragility fractures in elderly Chinese men and women. PLoS One 10(9):e0138102. https://doi.org/10.1371/journal.pone.0138102
Hida T, Shimokata H, Sakai Y, Ito S, Matsui Y, Takemura M, Kasai T, Ishiguro N, Harada A (2016) Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J 25(11):3424–3431. https://doi.org/10.1007/s00586-015-3805-5
Clynes MA, Edwards MH, Buehring B, Dennison EM, Binkley N, Cooper C (2015) Definitions of sarcopenia: associations with previous falls and fracture in a population sample. Calcif Tissue Int 97(5):445–452. https://doi.org/10.1007/s00223-015-0044-z
Cawthon PM, Blackwell TL, Cauley J, Kado DM, Barrett-Connor E, Lee CG, Hoffman AR, Nevitt M, Stefanick ML, Lane NE, Ensrud KE, Cummings SR, Orwoll ES (2015) Evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the Observational Osteoporotic Fractures in Men Cohort Study. J Am Geriatr Soc 63(11):2247–2259. https://doi.org/10.1111/jgs.13788
Chalhoub D, Cawthon PM, Ensrud KE, Stefanick ML, Kado DM, Boudreau R, Greenspan S, Newman AB, Zmuda J, Orwoll ES, Cauley JA (2015) Risk of nonspine fractures in older adults with sarcopenia, low bone mass, or both. J Am Geriatr Soc 63(9):1733–1740. https://doi.org/10.1111/jgs.13605
Yu R, Leung J, Woo J (2014) Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc 15(12):918–923. https://doi.org/10.1016/j.jamda.2014.07.011
Oliveira A, Vaz C (2015) The role of sarcopenia in the risk of osteoporotic hip fracture. Clin Rheumatol 34(10):1673–1680. https://doi.org/10.1007/s10067-015-2943-9
Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O (2017) Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One 12(1):e0169548. https://doi.org/10.1371/journal.pone.0169548
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London, England) 8(5):336–341. https://doi.org/10.1016/j.ijsu.2010.02.007
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Sadeghi O, Saneei P, Nasiri M, Larijani B, Esmaillzadeh A (2017) Abdominal obesity and risk of hip fracture: a systematic review and meta-analysis of prospective studies. Adv Nutr (Bethesda, Md) 8(5):728–738. https://doi.org/10.3945/an.117.015545
Zhang J, Yu KF (1998) What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280(19):1690–1691. https://doi.org/10.1001/jama.280.19.1690
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Chang KV, Hsu TH, Wu WT, Huang KC, Han DS (2017) Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 46(5):738–746. https://doi.org/10.1093/ageing/afx094
Balogun S, Winzenberg T, Wills K, Scott D, Jones G, Aitken D, Callisaya ML (2017) Prospective associations of low muscle mass and function with 10-year falls risk, incident fracture and mortality in community-dwelling older adults. J Nutr Health Aging 21(7):843–848. https://doi.org/10.1007/s12603-016-0843-6
Harris R, Chang Y, Beavers K, Laddu-Patel D (2017) Risk of fracture in women with sarcopenia, low bone mass, or both. https://doi.org/10.1111/jgs.15050
Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, Handelsman DJ, Waite LM, Hirani V (2017) Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: the Concord Health and Ageing in Men Project. J Bone Miner Res 32(3):575–583. https://doi.org/10.1002/jbmr.3016
Schaap LA, van Schoor NM, Lips P, Visser M (2017) Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures; the Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/glx245
Scott D, Chandrasekara SD, Laslett LL, Cicuttini F, Ebeling PR, Jones G (2016) Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5-10 years in community-dwelling older adults. Calcif Tissue Int 99(1):30–42. https://doi.org/10.1007/s00223-016-0123-9
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism. J Cell Biochem 116(5):687–695. https://doi.org/10.1002/jcb.25040
Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A, Panton L (2014) Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle 5(3):183–192. https://doi.org/10.1007/s13539-014-0146-x
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61(10):1059–1064. https://doi.org/10.1093/gerona/61.10.1059
Janssen I, Heymsfield SB, Wang ZM, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (Bethesda, Md : 1985) 89(1):81–88. https://doi.org/10.1152/jappl.2000.89.1.81
LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, Hoffman AR, Laughlin GA, Marshall LM, Orwoll ES (2011) Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab 96(12):3855–3863. https://doi.org/10.1210/jc.2011-0312
Mouser JG, Loprinzi PD, Loenneke JP (2016) The association between physiologic testosterone levels, lean mass, and fat mass in a nationally representative sample of men in the United States. Steroids 115:62–66. https://doi.org/10.1016/j.steroids.2016.08.009
Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB (2002) Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87(2):589–598. https://doi.org/10.1210/jcem.87.2.8201
Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T (2014) Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the international sarcopenia initiative (EWGSOP and IWGS). Age Ageing 43(6):748–759. https://doi.org/10.1093/ageing/afu115
Acknowledgements
The authors thank the staff of the Department of Geriatrics Medicine, West China Hospital, for their guidance and support. We also thank Professor Xiangfa Zeng, from Chengdu Medical College, for helping with language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
ESM 1
(DOC 28 kb)
Supplemental Table 1
(DOCX 14 kb)
Supplemental Figure 1
(DOCX 188 kb)
Supplemental Figure 2
(DOC 125 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Hao, Q., Ge, M. et al. Association of sarcopenia and fractures in community-dwelling older adults: a systematic review and meta-analysis of cohort studies. Osteoporos Int 29, 1253–1262 (2018). https://doi.org/10.1007/s00198-018-4429-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4429-5