Abstract
Background
Sarcopenia and osteopenia/osteoporosis show a high prevalence in old age and incur a high risk for falls, fractures, and further functional decline. Physical performance and bone metabolism in patients suffering from the so-called osteosarcopenia—the combination of sarcopenia and osteopenia—are currently still unknown.
Aims
This study investigates physical performance and bone metabolism in osteosarcopenic, prefrail, community-dwelling older adults.
Methods
68 prefrail adults between 65 and 94 years were assigned to four groups according to mean DXA results: osteosarcopenic [low T-score and low appendicular lean mass (aLM)], sarcopenic (low aLM), osteopenic (low T-score), and controls. Multiple linear regression analysis, adjusted for age, gender, physical activity, and 25-OH-vitamin D3 serum level, was used to identify the influence of being osteosarcopenic, sarcopenic, or osteopenic on physical performance (hand grip, chair rise test, sit-to-stand power, gait speed, SPPB) and serum markers for increased bone turnover [osteocalcin, β-crosslaps and procollagen type 1 amino-terminal propeptide (P1NP)].
Results
Only osteosarcopenic participants showed significantly reduced hand grip strength, increased chair rising time, and STS power time as well as significantly increased bone turnover markers.
Discussion
Due to low physical performance and high bone turnover, older adults with osteosarcopenia have to be regarded as the most at-risk population for fractures and further functional decline.
Conclusions
Up-to-date osteoporosis and post-fracture management of older persons should aim at both, bone and muscle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical manifestation of osteopenia/osteoporosis (OP), by WHO definition, the loss of bone mineral density in comparison to the mean level for a young–adult reference population [1], is the fragility fracture, which is associated with significant morbidity and mortality. It also causes a high risk of disability. Fragility fractures may occur spontaneously but most frequently they are the consequence of falls. Sarcopenia (SP), the age-related loss of muscle mass and physical performance, is becoming an increasing medical and financial concern in ageing societies. Sarcopenia is evident in around 20 % of over 70-year-olds; the figure rises to 50 % for those over the age of 80 [2]. Those affected by this syndrome exhibit impaired mobility, a higher disability rate, and also a higher risk for falls and fractures [3]. The pathophysiology of OP and SP reveals overlapping features. There is an intensive and complex interaction, both mechanically (mechanostat hypothesis) [4] and biochemically (e.g., estrogen, testosterone, GH, IGF-1, IL-6, osteocalcin) [5, 6]. Mechanical capacity may be tested by standardized methods, such as hand grip, chair rise test or sit-to-stand power. Bone turnover markers, such as osteocalcin, β-crosslaps, and procollagen type 1 amino-terminal propeptide (P1NP), increase with age and menopause and serum levels may predict bone loss and fracture risk [7]. Due to these interactions between muscle and bone, individuals suffering from both, sarcopenia and osteopenia, were classified as osteosarcopenic (OS) or sarcoosteopenic [8, 9]. Fracture risk is increased 3.5-fold in male OS patients and herewith significantly higher than in SP and OP alone [10]. Although it is just shown in male, it gives a first insight in a potential additive risk. About one-third of older patients (mean age 79) with a history of falls were osteosarcopenic in a large (N = 679) cross-sectional study [11], reflecting 47 %/63 % of the osteopenic/osteoporotic population, respectively. As the OS patients were significantly older, the authors hypothesized that OS results from progression of SP or OP. However, OS, SP, and OP may be three distinct, although partially, overlapping phenomena with different pathogeneses. As most of the current therapeutic interventions (nutrition, exercise, vitamin D) should be started early in the development of the “hazardous duet,” we examined a prefrail population with rather mild loss of bone and muscle. This study investigated whether the OS phenotype could be distinguished from the SP and the OP phenotype in this prefrail population with respect to clinically relevant measures of physical performance and bone turnover.
Methods
Participants
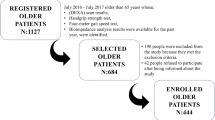
Participants were drawn from a randomized, controlled training study in prefrail, community-dwelling older adults [12]. Briefly, prefrail participants aged between 65 and 94 years were recruited and supplemented with vitamin D3 up to 2000 IU per day for 2 months. Therefore, just one participant has shown a 25-OH-vitamin D level below 50 nmol/L. Analyses in this study refer to the period after supplementation and right before the start of the training intervention. The data on 68 prefrail older individuals were included. Prefrailty was defined according to Fried and colleagues [13]. Exclusion criteria were depression (GDS >5), dementia (MMSE <25), BMI >35 kg/m2, intake of immunosuppressive drugs, history of kidney stones, sarcoidosis, multiple myeloma, chronic obstructive pulmonary disease, inflammatory bowel disease, angina pectoris, history of cancer, and current attendance of muscle training. The study was approved by the Medical Ethics Committee of the University of Erlangen-Nürnberg. Informed consent was obtained from all individual participants included in the study.
Measurements
Clinical chemistry
Blood was drawn in the morning (9 a.m.–12 p.m.) in a fasting state. 25-OH-vitamin D3 serum concentration was measured in HPLC technique with a kit from Chromsystems (München, Germany) according to the instructions of the manufacturer on an Agilent 1100 ChemStation (Waldbronn, Germany). Osteocalcin, β-crosslaps, and P1NP (Roche Diagnostics, Mannheim, Germany) were measured on the cobas e601twin immunoassay system (Roche Diagnostics).
Physical performance
Physical performance was measured using the score from the short physical performance battery (SPPB) established by Guralnik [14]. SPPB is a well-established, reliable, and valid measure of lower extremity performance that is predictive for adverse health outcomes like morbidity, institutionalization, and mortality. The score comprises assessment of standing balance, timed 4-m walk, and timed test of five chair-rise repetitions. The global score ranges from zero (low performance) to 12 points (high performance). Muscular power of the lower limbs was tested by the sit-to-stand transfer test (STS Power) described by Lindemann et al. [15]. Participants were seated on the front part of a chair (height: 49 cm) with arms crossed over chest, eyes fixed straight ahead, and both feet on a force plate (Zebris Medical, Germany). They were then asked to rise as fast as possible into a standing position and to stand as motionlessly as possible until the end of data recording. The test was performed three times with a 1 min rest between the trials. During all trials, the participants were verbally encouraged by the investigator to move explosively. STS Power is defined as P = F·s/t and was calculated by using the changes of vertical ground reaction force (collected at 558 Hz) during the rising phase (time in seconds between peak force and end of the rising phase: t), vertical ground reaction force during quiet standing (F = m·g, m: weight of subject in kg, g: 9.81 m/s2), and the difference between body height standing and body height sitting in meters (s). The trial that showed the highest power was used for analysis. Isometric hand grip strength was measured with a hand-held dynamometer (Jamar, USA).
DXA measurements
Body composition and whole bone mineral density (BMD) were assessed using a Dual-Energy X-ray absorptiometry (DXA) scanner (Lunar Prodigy, GE Healthcare Technologies, USA). Appendicular lean mass (aLM) was calculated as the sum of the lean mass of both arms and legs, and was divided by squared body height. For aLM, Cronbachs alpha for repeated measures on a sub sample of 20 participants was 0.998.
Statistical analysis
The relation between BMD (T-score) and aLM was used for classification of osteosarcopenia (OS: low T-score and low aLM), sarcopenia (SP: low aLM and high T-Score), osteopenia (OP: low T-score and high aLM), and controls (CO: high T-score and high aLM). The 50th percentile of each sex was used as cut-off to dichotomize T-score and aLM into low versus high values. Values of bone turnover markers from one SP patient were removed due to hyperparathyroidism. Participant characteristics were expressed as the mean and standard deviation of values assessed (Table 1). Multiple linear regression analysis was used to identify the influence of being osteosarcopenic, sarcopenic, or osteopenic compared to controls on physical performance and markers of bone turnover (Table 1). Models were adjusted for age, gender, physical activity, and 25-OH-vitamin D3 serum level. The level of significance was set at 5 %. PASW 23.0 (IBM-SPSS Inc., Chicago, Il, USA) was used for statistical analysis.
Results
After adjustment for age, gender, 25-OH-vitamin D3 level, and physical activity, only the group of OS individuals showed significantly lower hand grip strength, longer chair rise time, lower STS Power, and higher levels of osteocalcin, β-crosslaps, and P1NP compared to the control group (Table 1).
Figure 1 shows the distribution of bone mineral density (T-score) and appendicular lean mass (kg/m2) of the investigated cohort and the cut-offs that were set to separate OS from OP and SP phenotype [50th percentile: aLM (female): 6.398 kg/m2; aLM (male): 7.367 kg/m2; BMD (male): −0.6; BMD (male): −0.9]. Horizontal and vertical lines in Fig. 1 show classification exemplarily for female.
Two-thirds were female (Table 1). The mean age was highest in the OP group (81 years) and lowest in the control group (74 years) with SP and OS being rather early events (76 and 78 years, respectively). 13 participants fulfilled the criteria of EUGMS definition of sarcopenia (six males, seven females). Seven of them are at least osteopenic (three males, four females). Regarding the 25-OH-vitamin D level, the four groups do not differ significantly (p = 0.206, one factorial ANOVA).
Discussion
In our study, only osteosarcopenic, prefrail, community-dwelling older adults showed a significant reduction in physical performance and a significantly increased bone turnover, suggesting that osteosarcopenic individuals are at a higher risk for further functional decline and fractures compared to sarcopenic and osteopenic/osteoporotic individuals.
We examined a cohort of prefrail individuals, in which falls, fractures, and/or further decline in physical performance may rapidly lead to care dependency, significant morbidity, and increased mortality. However, it should be appreciated that prefrail individuals are in the majority of cases fit enough to get specific treatment in terms of physical training [16], nutritional interventions, and/or specific pharmacological compounds. We chose this prefrail cohort to learn more about the relevance of osteosarcopenia in patient care. This study not only contributes to a deeper understanding of the development of osteosarcopenia, but also sets the basis for planning future interventional studies. Yet, the limited generalizability of the results may be considered as an important limitation of the study.
In contrast to the results of the first, and so far only study that tried to describe the clinical phenotype of osteosarcopenia [11], osteosarcopenic individuals in our study were not significantly older than OP and SP patients. We hypothesize that the group of OS patients, classified according to strict criteria, not only consists of end-stage osteoporosis patients with severe muscle loss, but also of end-stage sarcopenia patients with severe bone loss and of patients that develop the combination of both early. The latter form may be caused by a separate pathophysiology. Indeed, gait speed and SPPB were slightly reduced in OS in comparison to OP and SP and more so in comparison to controls, although statistical significance was not met. However, we found a statistically significant decline in hand grip strength, chair rise time, and STS power in OS, but not in OP and SP in comparison to controls. This means that the combination of mild bone loss and mild muscle loss acts negatively on physical performance, suggesting a higher risk for falls and fractures. A study with 198 male patients above 60 years of age showed a 9-fold risk of abnormal BMD in individuals diagnosed as sarcopenic, but also a 8-fold increase in those that were diagnosed as pre-sarcopenic [17]. This observation refers to the interaction of muscle and bone in older individuals during early stages of disease. Interestingly, only those parameters of physical performance that mainly reflect muscle strength and muscle power (hand grip strength, chair rise time, and STS power) were strongly associated with OS, while parameters more focused on coordinative capabilities (gait speed and balance, both included in SPPB) were not. Similarly, our findings show a close relationship between muscle and bone and that weak muscle in OS patients, with reduced strength and power, is not able to stimulate bone mechanically to promote adequate osteogenesis (mechanostat hypothesis). Therefore, recommendations on high protein intake (1.0–1.2 g/kg BW/d) for sarcopenic patients should be considered for osteosarcopenic patients also [18]. This is mainly due to the fact that the sarcopenic muscle is less able to use available protein for synthesis (anabolic resistance). Regarding the distribution of protein intake over the day, 20–25 g protein with a large proportion of leucine per meal is suggested to exceed the anabolic threshold. Leucine is demonstrated to increase rates of postprandial muscle protein synthesis in the elderly [19].
Additionally, in the OS group, but not in the OP or the SP group, bone turnover—assessed by osteocalcin, β-crosslaps, and P1NP—was significantly increased. We therefore assume a faster BMD loss in the OS population as compared to those individuals that were affected by osteopenia or sarcopenia only. However, our observation needs to be confirmed in larger prospective trials. The relationship between markers of high bone turnover and muscle is yet unclear and somewhat contradictory. For example, osteocalcin has—besides its inhibiting impact on bone mineralisation—positive, hormone-like effects on insulin sensitivity of muscle cells and also on testosterone production [20]. Furthermore, it was shown that osteocalcin-deficient mice exhibited a decrease in muscle mass and function, suggesting that osteocalcin might exert muscle anabolic effects [6]. Therefore, more information on bone metabolism in OS individuals is needed and has to be assessed prospectively.
Conclusions
The findings of the study underline the close interaction between bone and muscle in the pathogenesis of osteopenia/osteoporosis and sarcopenia. In addition, our results highlight the associated negative functional outcome that has to be expected when both tissues are affected simultaneously. For the first time, we demonstrate the negative synergistic effects of osteosarcopenia on physical performance and bone turnover. Our analysis may provide the basis for future prospective observational and interventional studies in this field.
References
Kanis JA, on behalf of the World Health Organization Scientific Group. Assessment of osteoporosis at the primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK. 2007: Printed by the University of Sheffield.World Health Organization: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Technical Report Series 843, Geneva WHO 1994
Thomas DR (2010) Sarcopenia. Clin Geriatr Med 26:331–346
Landi F, Liperoti R, Russo A et al (2012) Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 31:652–658
Isaacson J, Brotto M (2014) Physiology of mechanotransduction: how do muscle and bone “talk” to one another? Clin Rev Bone Miner Metab 12:77–85
Edwards MH, Dennison EM, Aihie Sayer A et al (2015) Osteoporosis and sarcopenia in older age. Bone 80:126–130
Kawao N, Kaji H (2015) Interactions between muscle tissues and bone metabolism. J Cell Biochem 116:687–695
Garnero P, Sornay-Rendu E, Duboeuf F et al (1999) Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res 14:1614–1621
Binkley N, Buehring B (2009) Beyond FRAX??: it’s time to consider “sarco-osteopenia”. J Clin Densitom 12:413–416
Kull M, Kallikorm R, Lember M (2012) Impact of a new sarco-osteopenia definition on health-related quality of life in a population-based cohort in northern Europe. J Clin Densitom 15:32–38
Yu R, Leung J, Woo J (2014) Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc 15:551–558
Huo YR, Suriyaarachchi P, Gomez F et al (2015) Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc 16:290–295
Drey M, Pfeifer K, Sieber CC et al (2010) The fried frailty criteria as inclusion criteria for a randomized controlled trial: personal experience and literature review. Gerontology 57:11–18
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Guralnik JM, Simonsick EM, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49:M85–M94
Lindemann U, Claus H, Stuber M et al (2003) Measuring power during the sit-to-stand transfer. Eur J Appl Physiol 89:466–470
Liu CK, Fielding RA (2011) Exercise as an intervention for frailty. Clin Geriatr Med 27:101–110
Pereira FB, Leite AF, De Paula AP (2015) Relationship between pre-sarcopenia, sarcopenia and bone mineral density in elderly men. Arch Endocrinol Metab 59:59–65
Cederholm T, Cruz-Jentoft AJ, Maggi S (2013) Sarcopenia and fragility fractures. Eur J Phys Rehabil Med 49:111–117
Rizzoli R, Stevenson JC, Bauer JM et al (2014) The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 79:122–132
Zoch ML, Clemens TL, Riddle RC (2015) New insights into the biology of osteocalcin. Bone 8:229–236
Acknowledgement
The authors want to thank the volunteer participants and John Hoppe for providing language help.
The members of the FiAT intervention group: Astrid Zech, Department of Exercise Physiology, Institute of Sports Science, Friedrich Schiller University of Jena, Jena, Germany; Ellen Freiberger, Institute for Biomedicine of Aging, University of Erlangen-Nürnberg, Nürnberg, Germany; Klaus Pfeifer, University of Erlangen-Nürnberg, Institute of Sport Science and Sport, Erlangen, Germany.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
Michael Drey and Jürgen M. Bauer were supported by a Forschungskolleg Geriatrie Grant from the Robert Bosch Foundation, Stuttgart, Germany. The reagents for bone marker measurements were kindly provided from Roche Diagnostics.
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
The members of the FiAT intervention group are mentioned in the Acknowledgement.
Rights and permissions
About this article
Cite this article
Drey, M., Sieber, C.C., Bertsch, T. et al. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res 28, 895–899 (2016). https://doi.org/10.1007/s40520-015-0494-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-015-0494-1