Abstract
Several common age-related mechanisms and factors influence muscle and bone, affecting functionality of both tissues. Sarcopenia is closely linked with osteoporosis, and their combined effect may exacerbate negative health outcomes. Fall-related fractures are some of the most serious consequences of these two systemic pathologies, with hip fracture being a major complication affecting osteoporotic and sarcopenic elderly. This work aims to review the literature on the current state of knowledge about the relations between sarcopenia and osteoporosis and to present the association between sarcopenia and osteoporosis and the risk of hip fracture. A literature search was performed in PubMed and Scopus databases for articles with the predefined terms “sarcopenia,” “muscular atrophy,” “femoral fractures,” “hip fractures,” “osteoporosis,” and “bone density.” There is a growing and significant interest being directed to sarcopenia and associated risk for osteoporotic hip fracture, but there still is a notorious heterogeneity in the methodology and cohort size of the available studies. Collectively, most of the studies herein analyzed indicate that sarcopenia could be a predictor of risk for hip fracture. The simultaneous evaluation of sarcopenia and osteoporosis may be of importance in identifying those patients in higher risk of suffering an osteoporotic hip fracture and who could benefit from preventive or therapeutic interventions, or both.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia is a term that was first used 20 years ago [1] and comes from the Greek “sarx” (meaning flesh) and “penia” (meaning deficiency). Originally, the term sarcopenia was strictly used to describe the decrease of muscle mass with ageing. More recently, however, it has been defined as the deficiency in muscle mass along with impaired muscle strength and a decrease in physical performance [2]. In other words, it is necessary to document not only low skeletal muscle mass but also either low muscle strength or low physical performance to diagnose this syndrome. This is important mainly because muscle mass is not the only factor influencing muscle strength and because there is no linear relationship between mass and strength.
There are a number of different techniques which are useful for the diagnosis of sarcopenia, namely because they allow the measurement either of muscle mass, strength, or function. Some of these techniques are of interest only for research, but others also have clinical application [3, 2].
Muscle mass can be assessed in routine clinical practice with dual-energy X-ray absorptiometry (DXA), bioelectrical impedance analysis (BIA), or by anthropometry. DXA and other body image techniques (e.g., CT and MRI) can be used to assess muscle mass. DXA is less expensive and can be almost as precise as those other two body image techniques in separating lean tissue from fat and bone keeping the exposure to radiation to a minimum. BIA is a technique that has been frequently used to measure muscle mass, and because it only requires a portable equipment, it constitutes a real alternative to DXA [4]. Anthropometry measures skinfold thickness and member circumferences in order to estimate body fat. However, it is not recommended for routine diagnosis of sarcopenia due to its vulnerability to errors in obese and elderly individuals and to its large variability.
There are numerous techniques for measuring muscle strength, like knee flexion and extension, peak expiratory flow, or handgrip strength. This last one has been the preferred alternative because of its simplicity and mainly because it correlates well with lower extremity muscle.
Finally, there are several methods to assess physical performance, but the two used more frequently are the Short Physical Performance Battery (SPPB) and the gait speed. The SPPB is a composite measure of strength, endurance, balance, and gait of an individual when performing some well-defined tests [5]. The usual gait speed (over a 6-m course) is one of these tests in SPPB but is also a measurement method for physical performance assessment by itself.
Sarcopenic individuals can also be identified in clinical practice with the help of an algorithm developed by the European Working Group on Sarcopenia in Older People (EWGSOP) [2]. Following these recommendations, the gait speed should be measured and compared to the cut-off point of 0.8 m/s. If the gait speed is higher than 0.8 m/s, then the grip strength should be measured. A normal value for grip strength excludes sarcopenia, but a low value for grip strength implies that muscle mass should be measured. On the other hand, if the firstly measured gait speed was inferior or equal to 0.8 m/s, muscle mass should also be measured. A low muscle mass indicates sarcopenia, whereas a normal value for muscle mass excludes this diagnosis.
Sarcopenia can be classified as primary when caused by ageing itself or secondary when other causes are involved. The above mentioned authors (EWGSOP) have also proposed three stages for classifying sarcopenia. A low muscle mass alone characterizes the first of those stages named pre-sarcopenia. The second stage, identified as sarcopenia, is defined as the presence of not only low muscle mass but also decreased muscle strength or decreased muscular physical performance. Finally, the third stage, or severe sarcopenia, is characterized as the presence of low muscle mass and also decreased muscle strength and decreased muscular physical performance.
The onset of sarcopenia and its progression have been related to several factors and mechanisms, namely endocrine (corticosteroids, GH, IGF-1, abnormal thyroid function, and insulin resistance), primarily related to age (sex hormones, apoptosis, and mitochondrial dysfunction), neurodegenerative diseases (motor neuron loss), cachexia, inadequate nutrition or gastrointestinal malabsorption, and disuse (immobility, physical activity, and zero gravity). In a sarcopenic patient, one or more of these mechanisms may be implicated, and even relative contributions of each of these mechanisms may vary over time [6, 2].
Most of those factors and age-related mechanisms influence not only the muscle but also the bone, affecting the functionality of both tissues. Moreover, sarcopenia is closely linked with osteoporosis, and the combined effect of sarcopenia and osteoporosis could exacerbate negative health outcomes and healthcare costs (e.g., death, disability, falls, or increased risk of fractures) [7, 8].
Osteoporosis is a skeletal pathology characterized by a reduced bone density and an impairment of bone micro-architecture, thus making the bone more fragile and susceptible to fracture. Osteoporosis has been defined based on the World Health Organization (WHO) [9] criteria as a bone mineral density (BMD) 2.5 standard deviations (SD) or more below the average BMD for a young healthy adult (i.e., with a T-score ≤−2.5 SD). Similarly, osteopenia has been defined as a BMD lying between −2.5 and −1.0 SD below the average BMD for a young healthy adult (i.e., with a T-score greater than −2.5 SD but lower than −1.0 SD). BMD can be measured either by X-ray methods (quantitative computed tomography and DXA) or by quantitative ultrasound methods [10]. Currently, DXA is considered the gold standard for diagnosing osteoporosis and, as mentioned before, is also of great importance in diagnosing sarcopenia.
Fall-related fractures are some of the most serious consequences of these two systemic pathologies, with hip fracture being a major complication affecting osteoporotic and sarcopenic elderly [11]. The incidence of hip fractures steeply rises with age. These fractures not only have high associated mortality rates but also represent a leading cause of permanent disability, morbidity, and hospital admissions for the patients. Consequently, the associated health care costs are already considerable and predicted to escalate. In European Union, the economical burden of the estimated 620.000 of annual hip fractures is expected to double in the next three decades [12].
In this work, the current state of knowledge on the relations between sarcopenia and osteoporosis and the association between sarcopenia and osteoporosis and the risk of hip fracture is reviewed and described.
Methods
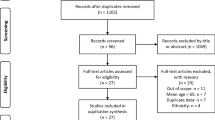
A literature search was performed in PubMed and Scopus databases (October 2014 to November 2014). The search was limited to articles written in English, French, Portuguese, and Spanish. The following MeSH terms were used to identify studies on sarcopenia: (sarcopenia) OR (muscular atrophy). The following MeSH terms were used to identify studies on hip fractures and bone mineral density reduction: (femoral fractures) OR (hip fractures) OR (osteoporosis) OR (bone density). These two searches were then combined by using the Boolean operator “AND.” Animal studies were excluded from the search as well as those studies with unavailable full article access. From the results, and after removing duplicates, we identified 180 journal articles, 17 of which were considered relevant (Fig. 1).
Results
Sarcopenia has been the focus of a recent and growing interest, and there are already several studies that have addressed the role of sarcopenia in the risk of hip fractures in specific cohorts and the putative links between sarcopenia and osteoporosis, these being considered by Crepaldi and Maggi [13] as a hazard duet.
In Italy (Torino), Di Monaco et al. [14] enrolled into a cross-sectional study of 591 patients of both sexes and which had been admitted to a rehabilitation institution due to a previous hip fracture. In all 591 patients, DXA was used to assess appendicular skeletal muscle index (SMI) (muscle mass/height2, kg/m2). Diagnosis of sarcopenia in a patient was made by the authors when that index was lower than the limit of −2 SD of the mean of one of two reference populations from other studies. The results showed that, regardless of which of the two reference populations used, sarcopenia was more prevalent in men than in women (p < 0.001; OR = 10.54, 95 % CI = 3.25–34.16 or OR = 23.64, 95 % CI = 10.8–51.6). It was also notorious within the results, and again regardless of which of the two reference populations used, that sarcopenia was highly prevalent in those 591 patients with a hip fracture, both in men and in women (64.0 or 21.8 % in women and 95.0 or 86.7 % in men).
In a previous work and using similar methodologies [15], those same authors analyzed the association between osteoporosis and sarcopenia in 313 women who suffered recent fractures of the hip. From these 313 women, 58 % were diagnosed with sarcopenia and 74 % were diagnosed with osteoporosis, revealing a high prevalence of these two conditions. The analysis of the results also revealed, at least in this population of women with a hip fracture, that sarcopenia is associated with osteoporosis and that the odds for osteoporosis is 1.8 higher in sarcopenic women (95 % CI = 1.07–3.02). In other two studies [16, 17], these authors observed a clear association between fat mass and indexed appendicular lean mass (LM), (lean mass/height2, kg/m2), but with a stronger association between fat mass and BMD than between fat mass and appendicular LM.
In another cross-sectional study, Hida et al. [18] examined a total of 2868 patients of both sexes admitted to a health institution in Japan. Some of these patients (n = 357) had a hip fracture in the previous days (HF group) with the other 2511 patients being admitted for other causes than hip fracture (NF group). DXA was used to assess BMD and SMI (muscle mass/height2, kg/m2). Analysis by stepwise logistic regression showed a higher prevalence of sarcopenia and a lower appendicular SMI (p < 0.001) in the HF group. On the other hand, there were no differences in the upper appendicular SMI between the two groups (HF and NF) (p > 0.95). Additionally, multivariate analysis revealed that not only older age and lower BMD, but also sarcopenia, were associated with a hip fracture (respectively OR = 1.103, OR = 0.082, and OR = 1.476, p < 0.001).
Using data from a prospective cohort study (a total of 2941 patients with ages between 70 and 79 years, of which 1345 were women and 1286 were men), Lang et al. [19] evaluated the usefulness of some body composition measures as predictors for hip fractures. Some of these body composition measures were assessed by computed tomography (e.g., lower appendicular muscle mass and related intramuscular fat), others by DXA (e.g., BMD) or by using a dynamometer (e.g., muscle strength). The authors validated 63 hip fracture events in the cohort during the observational period. The results showed that, in addition to BMD, coexistent decreased muscular mass, strength and performance, and increased muscular fatty infiltration are associated with a higher risk of suffering a hip fracture. In these circumstances and for these four descriptors, RR (95 % CI) of having a hip fracture varied between 1.21 (1.06–1.39) and 1.83 (1.22–2.72).
Yu et al. [20] conducted a large study in Hong Kong with community-dwelling men aged 65 years or older (n = 2000). The main objective of this work was to assess the value of sarcopenia as a predictor for fractures in elderly osteoporotic men. BMD and appendicular SMI were both measured by DXA, with sarcopenia being assessed by appendicular muscle mass/height2 (kg/m2). During the follow-up period, 11.3 % of the individuals suffered at least one fracture. It was observed that there was a significant association between sarcopenia and the risk of fracture (OR = 1.87, 95 % CI = 1.26–2.79). This association was independent of other clinical risk factors, such as BMD. However, when combining sarcopenia and osteoporosis, a stronger association with fractures was observed (OR = 3.49, 95 % CI = 1.76–6.90), which reflects an increased risk of fracture when comparing sarco-osteoporotic men with non-sarcopenic men or with men with normal BMD.
In a large retrospective study, Capozza et al. [21] also evaluated the association between osteoporosis (using as descriptor the bone mineral content, BMC) and the LM in the whole body, both assessed by DXA. To evaluate that relationship, those authors used an SD score previously developed and named as BMC-LM which allowed the comparison between premenopausal and postmenopausal women (n = 3205) either with (n = 2591) or without (n = 614) recent fractures. The two groups with no fractures (premenopausal women without fractures and postmenopausal women without fractures) were considered the reference groups. Compared to the reference group, premenopausal women that suffered a recent fracture exhibited similar BMC-LM SD scores. Likewise, postmenopausal women that suffered a recent fracture but not in an osteoporotic site (e.g., in the hip) exhibited similar BMC-LM SD scores. On the other hand, BMC-LM SD scores were significantly lower in postmenopausal women who suffered a recent fracture in an osteoporotic site, particularly in women with a hip fracture.
Sixty-two elderly hip-fractured patients were enrolled in a study carried by Calvani et al. in Rome, Italy [22]. The mean age of these patients was 84.6 (±7.6 years) and 84 % were women. Muscle mass was estimated by BIA within 24 h from admission for hip fracture due to accidental fall. Then, skeletal muscle index (SMI) was calculated as absolute muscle mass/height2 (kg/m2). There were positive correlations between the intake of calories (r = 0.384, p = 0.003), protein (r = 0.367, p = 0.005), and leucine (r = 0.311, p = 0.005) and muscle mass, indicating an association between sarcopenia and low intake of calories, proteins, and leucine in hip-fractured elderly patients.
Sjoblom et al. [23] examined a cohort of 590 postmenopausal women (age: mean 67.9 years, range 65–72 years) selected from a Finnish population-based randomized controlled trial (OSTPRE-FPS). The authors assessed both BMD and LM by DXA and then assigned all women into categories. Women were assigned to one of three categories based on their BMD according to the WHO definitions: osteoporotic, osteopenic, and normal. Women were also assigned to one of four categories based on three variables, the relative SMI (appendicular muscle mass/height2, kg/m2), the hand grip strength, and also the walking speed. These four categories were sarcopenic, pre-sarcopenic, non-sarcopenic, and unclassified. Logistic regression analysis showed that osteoporosis was significantly associated with sarcopenia, with odds of having osteoporosis in sarcopenic women being 12.9 higher (p ≤ 0.001, 95 % CI = 3.1–53.5). In comparison to non-sarcopenic women, sarcopenic women also had 2.7 times higher odds of suffering a fracture (p = 0.005; OR = 2.732, 95 % CI = 1.4–5.5) and 2.1 times higher odds of having had at least a fall in the previous 12 months (p = 0.021; OR = 2.1, 95 % CI = 1.1–3.9).
Another study [24] enrolled 169 patients, 62.1 % of which were male, with a mean age of 84 years (age interval 66–96 years). In this study, Chen et al. validated 130 recent fragility fractures, with 115 patients exhibiting vertebral fractures, 12 other exhibiting hip fractures, and 3 patients with pelvic fractures. DXA measurements revealed differences in BMD, with these patients exhibiting fractures having significantly lower T-scores (−2.33 ± 1.10) when compared with patients with no fractures (−1.87 ± 1.23, p = 0.030). It was also observed that low blood levels of albumin and creatinine (respectively 3.5 and 0.8 mg/dL) were associated with recent fractures (OR = 4.6, 95 % CI = 1.1–18.2; p = 0.032 and OR = 10.8, 95 % CI = 1.2–97.3; p = 0.033). The authors suggested that, at least in this population, there is a strong association between fragility fractures and low muscle mass and thus sarcopenia. However, they emphasized that the retrospective design of the study did not allow establishing a definitive causal relation, mainly because sarcopenia may have preceded the occurrence of fractures but could also have been a consequence of fragility fractures.
In a large study within a Canadian hip fracture population of both sexes [25], 36900 fracture events (either non-traumatic or resulting from a minor accidental trauma) were identified by Auais et al. over a 8-year period. These authors observed significant differences between sexes, with women being older (mean = 4.6 years) and having more sarcopenia risk factors than men.
Singh et al. [26] used data from the Sarcopenia and Hip Fracture Study (SHIP), a prospective cohort study on sarcopenia and hip fracture, to assess the baseline characteristics of patients admitted to one of the three Sydney (Australia) hospitals after having suffered a hip fracture. A total of 193 community-dwelling patients, 139 women and 54 men aging between 60 and 97 years, were included. The authors collected numerous of those baseline characteristics, but it is worthwhile highlighting some of the results obtained. This is the case of characteristics related to sarcopenia, namely SMI and LM assessed by BIA. Patients were considered sarcopenic if they presented a SMI lower than 7.0 or 9.5 kg/m2, respectively, for females and males. On admission, 70 % of patients from this population were diagnosed with sarcopenia or sarcopenic obesity, which represents a high level when compared with most of the available data obtained with BIA [27]. In addition, 58 % of all patients also were considered undernourished and 55 % showed vitamin D deficiency. However, the same authors [28] used data from the same prospective cohort study on sarcopenia and hip fracture (SHIP) to investigate risk factors for recurrent falls and hip fractures. They identified 227 falls in the year immediately after a hip fracture and only nine new hip fractures. Some independent predictors for recurrent falls and hip fractures were identified by multivariate analyses, including nutritional status and older age, but not sarcopenia or physical performance.
Lee et al. [29] did a study with a cohort of elderly participants in two Korean national surveys, having selected 1596 males and 1886 females older than 60 years. DXA was used to assess appendicular skeletal muscle mass (SMM) and BMD. Blood levels of 25-hydroxyvitamin D [25(OH)D] were also measured in each individual. The authors defined a ratio between appendicular SMM and body weight (appendicular SMM/Wt × 100) and used it to define sarcopenia. Individuals were considered sarcopenic if they had a ratio lower than the limit of −1 SD of the mean for a young (20–30 years old) reference group from the same surveys. In other words, the limits for that ratio were 32.2 % for men and 25.4 % for women. The results showed that, in both sexes, whole femoral BMD and local BMD in the femoral neck were significantly lower in sarcopenic individuals exhibiting vitamin D insufficiency (<20 ng/mL) in comparison with individuals with adequate vitamin D levels and in comparison with non-sarcopenic individuals regardless of their vitamin D levels. In this population, a significant association between BMD and appendicular SMM and also with other factors such as body mass index (BMI) and a recent event of fracture was also observed. Among others, sarcopenia and BMI were considered independent predictors of low BMD in the femur, both in men and in women.
In a relatively small study, in which 27 postmenopausal obese women were enrolled (age interval 50–75 years), Aubertin-Leheudre et al. [30] examined the impact of sarcopenia on BMD. These authors did not find any influence of sarcopenia on hip BMD, one of the main sites for osteoporosis and fractures. Based on the results, the authors hypothesize that obesity could have preserved BMD and thus reduced the fracture risks associated with sarcopenia in obese women.
Palombaro et al. [31] conducted a study with 54 women in early postmenopause, 31 of which presented low BMD. The objective was to determine if this population had decreased physical performance, differences in gait (balance, strength, or gait speed) and in muscle strength, and an associated increase in fall and fracture rates. In general, there were no significant differences between patients with low or normal BMD, except for step time and stance time variability. Particularly, no differences were found when comparing specific sarcopenia characteristics such as muscle strength and gait speed between women with low and normal BMD. However, the authors admitted that several limitations, including technical ones, could have had an influence on the results.
Finally, Salmaso et al. [32] studied 44 community-dwelling women (mean age = 78.27 ± 6.74 years, range = 67–94 years) in Brazil in order to examine the possible association between nutritional status, sarcopenia, and osteoporosis. BMD and body composition (fat mass and appendicular lean mass) were assessed by DXA. Sarcopenia was defined as appendicular LM/height2 <5.45 kg/m2. The results of this study revealed that 15.9 % of those women were diagnosed with sarcopenia, whereas 34.1 % had osteoporosis, 52.3 % were osteopenic, and 31.8 % have had a recent osteoporotic fracture. The authors did not, however, present any association between the risk of fractures and those conditions.
Discussion
The prevalence of sarcopenia in all the studies herein analyzed varied considerably, from 8.3 to 70.0 % in patients with normal BMD and aged over 60 years [20, 18]. This variation was due mainly to differences in the study sample (namely the age and sex), the definitions, and the methods of assessing sarcopenia and osteoporosis.
The aim of the present work was to describe studies concerning the association between sarcopenia and osteoporotic hip fracture (Table 1). First of all, it should be highlighted that there is still a limited number of studies which specifically focused on this association [14, 15, 18, 19, 21]. Nevertheless, it should also be emphasized that not only these are recent studies that have analyzed large samples but also they have a relative homogeneity on the techniques used and on the methodologies employed. Three other large studies focused on the association between sarcopenia and osteoporotic fracture in general, but not differentiating hip fractures from other fractures [23, 20, 24].
In all those studies (n = 8), the authors found a significant association between sarcopenia and fractures, either in women or men. More specifically, it was clear that sarcopenic patients have an increased risk of suffering a hip fracture. On the other hand, in two other studies [30, 28], the authors did not find any influence of sarcopenia on femur BMD nor on recurrent falls or hip fractures. In one of these studies, the authors attributed the lack of association to the technique used (BIA). In the other study with obese women, the authors attributed the lack of association to a protective effect of obesity, which could have reduced the fracture risk associated with sarcopenia.
There are two aspects of sarcopenia which are fundamental when studying osteoporotic hip fractures in the elderly. Firstly, sarcopenia implies a decrease in the number of type II (fast) muscle fibers and motoneurons with significant effects on muscle mass and strength, on balance, and on enhancing the risk of falls [33]. Secondly, sarcopenia is involved in the genesis of hip fracture because it is closely linked with the loss of bone mass and strength.
Frequently, sarcopenia and osteoporosis have been associated with frailty, a major syndrome in elderly patients, which includes weight loss, low muscle strength, decreased walking speed, and instability and, more importantly, resulting frequently in falls and fractures [34]. Both sarcopenia and osteoporosis are diseases characterized by a state of disequilibrium. In sarcopenia, the rate of degradation of skeletal muscle protein is not in balance with the rate of its synthesis. Similarly, in osteoporosis, the rate of bone resorption is not in balance with the rate of its synthesis, and therefore, there is an impairment of bone micro-architecture and a reduced bone density.
Several pathways have been identified in those imbalances. The RANK-RANKL/osteoprotegerin signaling pathway plays an essential role in osteoclastogenesis, which is promoted by the binding of RANKL to RANK, but inhibited by the binding of osteoprotegerin to RANKL [35]. On the other hand, the osteoblastic activity is controlled mainly by the canonical Wnt signaling pathway.
Those pathways involved in osteoporosis have been much more studied and are now better understood than those involved in sarcopenia, although the cellular interactions observed on both pathologies are similar. Interestingly, the abovementioned Wnt signaling pathway is also one of the signal transduction pathways involved in muscle regeneration [36]. Another process involved in muscle regeneration is the activation of the phosphatidylinositol-3-kinase/Akt (PI3K/Akt) pathway, which promotes the synthesis of muscle proteins by activating the mammalian target of rapamycin (mTOR) and SGK1. In addition, inactivation of the FoxO proteins also inhibits protein degradation and prevents muscle atrophy. In contrast, several processes result in increased muscle protein breakdown. The production of satellite cells (myogenic stem cells) is inhibited by myostatin, while glucocorticoids inhibit PI3K/Akt pathway by direct inhibition of Akt, and inflammatory cytokines (e.g., IL-1, IL-6 and TNF-α) provoke DNA fragmentation and apoptosis in skeletal muscle cells. Furthermore, several hormones and other molecules may influence muscle protein metabolism, being involved in its anabolism or catabolism, such as growth hormone (GH), insulin-like growth factor I (IGF-1), corticosteroids, androgens, estrogens, insulin, and vitamin D [37]. Finally, it is worthwhile highlighting that muscle action is a mechanical stimulus to the bone, inducing a local production of several growth factors such as IGF-1 and FGF-2 and thus stimulating osteogenesis.
In fact, most of the studies herein reviewed and that demonstrated an association between sarcopenia and hip fracture also found an association between sarcopenia and osteoporosis. Some other studies here described [29, 32] also found this association, with sarcopenia being considered a predictor of low BMD, namely in the femur. On the contrary, in one study here examined, no association was found between sarcopenia and osteoporosis [31]. This lack of a significant association was also referred by previous authors [38, 34, 39, 40]. In general, however, these were relatively smaller studies, not purposely designed and with some limitations which could influence the comparisons with other studies.
Finally, other significant associations were demonstrated in some of the studies, namely the associations between sarcopenia, BMD, and fat mass and between sarcopenia, low intake of calories, and proteins.
Taking into account all the described studies, there is evidence that sarcopenia can be a risk factor for a hip fracture and consequently its treatment is an important goal in order to prevent osteoporotic fractures. However, evidence about the efficacy of potential therapies in preventing fragile fractures is still scarce. At the present, there are several therapeutic approaches being researched, namely exercise, nutrition, and pharmacological treatment.
Exercise, more specifically physical resistance exercise practice by active elderly patients, is considered highly effective for sarcopenia. Frequently, however, this therapy is of limited use because most sarcopenic patients have motor and cognitive deficits along with other complications, and thus, performing the exercises could represent an additional risk of fracture [36].
Nutrition is another major field of study on the treatment of sarcopenia, as it is common in elderly people to have a deficient protein and caloric intake. Therapy with nutritional supplements (proteins and amino acids) combined with exercise therapy may have an effect on sarcopenia [6], though the effect on the prevention of fractures is not clear.
Many pharmacological treatments for sarcopenia have been investigated, most of them in recent years. The drugs more thoroughly studied were testosterone, growth hormone (GH), dehydroepiandrosterone (DHEA), and vitamin D [41]. The available data on the effects of testosterone in the elderly show that testosterone not only increases contraction strength but is also associated with severe complications, such as thrombosis and prostate cancer. Similarly, GH has severe secondary effects though it has been shown that its use is associated with an increase in muscle mass and a decrease in fat mass and in bone demineralization rate. DHEA induces an increase in bone density, but has no effects on muscle mass or function and its secondary effects are unknown. In respect to vitamin D, its supplementation improves muscle strength and is associated with a decrease in falls and mortality and with functional improvement.
Conclusions
We conclude that there is already a substantial knowledge on the relations between sarcopenia and osteoporosis, with evidence that there is a significant association between both. Additionally, we also conclude there is now evidence on the association between sarcopenia, osteoporosis, and hip fracture, with most authors considering that sarcopenia is a predictor of fracture risk in the elderly and provides incremental predictive value if integrated with BMD and other factors.
Abbreviations
- BIA:
-
Bioelectrical impedance analysis
- BMC:
-
Bone mineral content
- BMD:
-
Bone mineral density
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DHEA:
-
Dehydroepiandrosterone
- DXA:
-
Dual-energy X-ray absorptiometry
- FGF-2:
-
Fibroblast growth factor 2
- GH:
-
Growth hormone
- IGF-1:
-
Insulin-like growth factor 1
- LM:
-
Lean mass
- MRI:
-
Magnetic resonance imaging
- mTOR:
-
Mammalian target of rapamycin
- OR:
-
Odds ratio
- RANK:
-
Receptor activator of nuclear factor kappa-B
- RANKL:
-
Receptor activator of nuclear factor kappa-B ligand
- RR:
-
Relative risk
- SD:
-
Standard deviation
- SGK1:
-
Serum/glucocorticoid-induced kinase 1
- SMI:
-
Skeletal muscle index
- SMM:
-
Skeletal muscle mass
- SPPB:
-
Short Physical Performance Battery
- TNF-α:
-
Tumor necrosis factor α
- WHO:
-
World Health Organization
- Wt:
-
Body weight
References
Rosenberg I (1989) The epidemiologic and methodologic problems in determining nutritional status of older persons. (Summary comments). Am J Clin Nutr 50:1231–1233
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Rubbieri G, Mossello E, Di Bari M (2014) Techniques for the diagnosis of sarcopenia. Clin Cases Miner Bone Metab 11(3):181–184
Dodds R, Sayer AA (2014) Sarcopenia. Arq Bras Endocrinol Metabol 58:464–469
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49(2):M85–M94
Cederholm T, Cruz-Jentoft AJ, Maggi S (2013) Sarcopenia and fragility fractures. Eur J Phys Rehabil Med 49(1):111–117
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52(1):80–85
Lynch GS (2008) Update on emerging drugs for sarcopenia—age-related muscle wasting. Expert Opin Emerg Drugs 13(4):655–673
Kanis JA (2007) on behalf of the World Health Organization Scientific Group. Assessment of osteoporosis at the primary health care level. Technical Report. University of Sheffield UK: WHO Collaborating Center
Pisani P, Renna MD, Conversano F, Casciaro E, Muratore M, Quarta E, Di Paola M, Casciaro S (2013) Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World J Radiol 5(11):398–410
Rolland Y, Abellan van Kan G, Benetos A, Blain H, Bonnefoy M, Chassagne P, Jeandel C, Laroche M, Nourhashemi F, Orcel P, Piette F, Ribot C, Ritz P, Roux C, Taillandier J, Tremollieres F, Weryha G, Vellas B (2008) Frailty, osteoporosis and hip fracture: causes, consequences and therapeutic perspectives. J Nutr Health Aging 12(5):335–346
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1–2):136
Crepaldi G, Maggi S (2005) Sarcopenia and osteoporosis: a hazardous duet. J Endocrinol Invest 28(10 Suppl):66–68
Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R (2012) Sarcopenia is more prevalent in men than in women after hip fracture: a cross-sectional study of 591 inpatients. Arch Gerontol Geriatr 55(2):E48–E52
Di Monaco M, Vallero F, Di Monaco R, Tappero R (2011) Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr 52(1):71–74
Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A (2007) Skeletal muscle mass, fat mass, and hip bone mineral density in elderly women with hip fracture. J Bone Miner Metab 25(4):237–242
Di Monaco M, Vallero F, Di Monaco R, Tappero R, Cavanna A (2007) Fat mass and skeletal muscle mass in hip-fracture women: a cross-sectional study. Maturitas 56(4):404–410
Hida T, Ishiguro N, Shimokata H, Sakai Y, Matsui Y, Takemura M, Terabe Y, Harada A (2013) High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int 13(2):413–420
Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB (2010) Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 25(3):513–519
Yu R, Leung J, Woo J (2014) Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the osteoporotic fractures in men (MrOs) study. J Am Med Dir Assoc 15(8):551–558
Capozza RF, Cure-Cure C, Cointry GR, Meta M, Cure P, Rittweger J, Ferretti JL (2008) Association between low lean body mass and osteoporotic fractures after menopause. Menopause 15(5):905–913
Calvani R, Martone AM, Marzetti E, Onder G, Savera G, Lorenzi M, Serafini E, Bernabei R, Landi F (2014) Pre-hospital dietary intake correlates with muscle mass at the time of fracture in older hip fractured patients. Front Aging Neurosci 6(269):1–6
Sjoblom S, Suuronen J, Rikkonen T, Honkanen R, Kroger H, Sirola J (2013) Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 75(2):175–180
Chen LY, Liu CL, Peng LN, Lin MH, Chen LK (2012) Associative factors of existing fragility fractures among elderly medical inpatients: a hospital-based study. J Clin Gerontol Geriatr 3(3):94–96
Auais M, Morin S, Nadeau L, Finch L, Mayo N (2013) Changes in frailty-related characteristics of the hip fracture population and their implications for healthcare services: evidence from Quebec, Canada. Osteoporos Int 24(10):2713–2724
Singh MAF, Singh NA, Hansen RD, Finnegan TP, Allen BJ, Diamond TH, Diwan AD, Lloyd BD, Williamson DA, Smith EUR, Grady JN, Stavrinos TM, Thompson MW (2009) Methodology and baseline characteristics for the sarcopenia and hip fracture study: a 5-year prospective study. J Gerontol A Biol Sci Med Sci 64(5):568–574
Pagotto V, Silveira EA (2014) Methods, diagnostic criteria, cutoff points, and prevalence of sarcopenia among older people. Sci World J 2014:1–11
Lloyd BD, Williamson DA, Singh NA, Hansen RD, Diamond TH, Finnegan TP, Allen BJ, Grady JN, Stavrinos TM, Smith EUR, Diwan AD, Fiatarone Singh MA (2009) Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the sarcopenia and hip fracture study. J Gerontol A Biol Sci Med Sci 64(5):599–609
Lee SG, Lee Y, Kim KJ, Lee W, Kwon OH, Kim JH (2013) Additive association of vitamin D insufficiency and sarcopenia with low femoral bone mineral density in noninstitutionalized elderly population: the Korea National Health and Nutrition Examination Surveys 2009–2010. Osteoporos Int 24(11):2789–2799
Aubertin-Leheudre M, Lord C, Labonte M, Khalil A, Dionne IJ (2008) Relationship between sarcopenia and fracture risks in obese postmenopausal women. J Women Aging 20(3–4):297–308
Palombaro KM, Hack LM, Mangione KK, Barr AE, Newton RA, Magri F, Speziale T (2009) Gait variability detects women in early postmenopause with low bone mineral density. Phys Ther 89(12):1315–1326
Salmaso FV, Vigário PS, de Mendonça LMC, Madeira M, Netto LV, Guimarães MRM, de Farias MLF (2014) Analysis of elderly outpatients in relation to nutritional status, sarcopenia, renal function, and bone density. Arq Bras Endocrinol Metabol 58(3):226–231
Cederholm T (2012) Sarcopenia, osteoporosis and fractures. In: Sarcopenia. pp 168–180
Frisoli A Jr, Chaves PH, McNeill Ingham SJ, Fried LP (2011) Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women’s Health and Aging Study (WHAS) II. Bone 48(4):952–957
Cooper C, Dere W, Evans W, Kanis JA, Rizzoli R, Sayer AA, Sieber CC, Kaufman JM, van Kan GA, Boonen S, Adachi J, Mitlak B, Tsouderos Y, Rolland Y, Reginster JYL (2012) Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int 23(7):1839–1848
Hida T, Harada A, Imagama S, Ishiguro N (2014) Managing sarcopenia and its related-fractures to improve quality of life in geriatric populations. Aging Dis 5(4):226–237
Kim TN, Choi KM (2015) Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab 20(1):1–10
Coin A, Perissinotto E, Enzi G, Zamboni M, Inelmen EM, Frigo AC, Manzato E, Busetto L, Buja A, Sergi G (2008) Predictors of low bone mineral density in the elderly: the role of dietary intake, nutritional status and sarcopenia. Eur J Clin Nutr 62(6):802–809
Gillette-Guyonnet S, Nourhashemi F, Lauque S, Grandjean H, Vellas B (2000) Body composition and osteoporosis in elderly women. Gerontology 46(4):189–193
Walsh MC, Hunter GR, Livingstone MB (2006) Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int 17(1):61–67
Malafarina V, Úriz-Otano F, Iniesta R, Gil-Guerrero L (2012) Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas 71(2):109–114
Acknowledgments
The authors would like to thank Catarina Gomes for the advice on the text.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Oliveira, A., Vaz, C. The role of sarcopenia in the risk of osteoporotic hip fracture. Clin Rheumatol 34, 1673–1680 (2015). https://doi.org/10.1007/s10067-015-2943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-2943-9