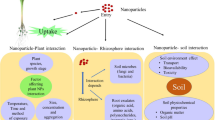
Abstract
Heavy metals (HMs) constitute one of the most detrimental environmental constraints putting at risk diverse life-forms including plants. HMs profoundly hinder plant metabolism, by disrupting the functioning of imperative cellular biomolecules resulting in severely affected crop yields. Among the diverse strategies adopted to alleviate heavy metal (HM) toxicity, application of nanoparticles (NPs) constitutes a comparatively recent, efficient and promising approach as compared to conventional plant growth regulators. The competence of NPs as stress alleviators is endorsed to their ability to decrease the mobility of HMs in soil thereby reducing their availability, improved ability of apoplastic barrier which hinders their translocation in the plant, fortified plant antioxidant system by boosting the activities of the different enzymatic and non-enzymatic antioxidants, mimetic activities of certain NPs as antioxidants and increased production of secondary metabolites particularly phenols. Plant phenolics, in addition to other chemo-ecological roles, serve as potent stress alleviators. The current article encompasses the role of NPs in remediation of HMs from contaminated agricultural soils and aquatic ecosystems. This article also focuses on the role of different types of NPs in alleviating HM toxicity in plants and the possible underlying mechanism.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
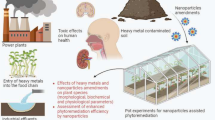
Factors that render ecosystems unfit for survival of life-forms include continuous addition of hazardous contaminants, mainly through anthropogenic activities, and their penetration into diverse aquatic and terrestrial life-forms Kahlon et al. [62, 72]. Amid the sum total of contaminants, HMs, being non-biodegradable and toxic, are remarkably dreadful, inflicting a terrible threat to the existence of different life-forms in view of their chief role in health-related complications [55, 134]. Heavy metal pollution ranks second among the most perilous pollutions and is expected to pull ahead of chief hazardous pollutants notably sulfur dioxide, carbon dioxide and pesticides in near future [22, 72].
HMs are discharged into the ecosystems predominantly through various anthropogenic activities notably smelting, mining of metals, foundries and leaching of metals from diverse sources notably automobiles, landfills, waste dumps, excretion, runoffs, chicken manure and road-works [16, 57, 139, 162]. Natural sources like geological weathering, water and sediment re-suspension, metal corrosion, soil erosion and volcanic eruptions also add to the HM contamination [16, 86, 162]. Besides, agricultural sector, where-in the use of fertilizers, insecticides, pesticides has escalated alarmingly, is considered as secondary source of HM pollution [16, 86, 139]. The concentrations of HMs (Hg, Cd, Pb, Cr, Cu, As) are continuously mounting in the surface water and sediments where-from these find entry into different food chains leaving grave concerns on different life-forms including humans [57, 37, 139]. HMs induce genotoxicity, cytotoxicity, and mutagenicity in humans, animals as well as in plants [24, 114]. Some of the deadly diseases in humans due to HM contaminants include different types of cancers, kidney failure, lung congestion, liver damage and reproductive dysfunction [39]. Prolonged exposure to HMs like As also lead to cardiovascular and neurological disorders, and various types of skin cancers [59, 75, 110]. Millions of people worldwide fall victims to HM contamination chiefly through consumption of contaminated food and drinking water [87, 112].
Plant growth and metabolism are also severely disrupted by HM contamination. Once inside the plant, HMs impede metabolic functions by disrupting protein functioning by forming complexes with their sulfhydryl groups [33], dysfunctioning enzymes cofactors [5, 50], malfunctioning cellular molecules and pigments [50] and severely disrupting the integrity of membranes [5, 33]. These physiological and metabolic disruptions ultimately repress the fundamental primary as well as secondary metabolism pathways in plants including photosynthesis, respiration, nutrient assimilation, etc. [4, 62].
The existing challenges of climate change, diverse environmental constraints, food and energy security, and sustainability compel researchers to explore novel competent technologies to conquer these potential challenges in a proficient manner [131, 146]. Among various such approaches adopted for the purpose, nanotechnology is acknowledged as one of the most imperative, swiftly emerging fields with copious potentialities, contributing to sustainable competitiveness and development in numerous fields including the agricultural field [11, 36, 104, 107, 145, 173]. Application of NPs repairs the perturbed and contaminated ecosystems where traditional agricultural practices have proven unsuccessful [131, 146, 173]. Nanotechnology is proving to be a boon in the agricultural field with copious advantages through the application of nanofertilizers, nanopesticides, and elicitors [19, 78, 151, 155, 173]. Improved performance in plants has been reported due to application of different NPs both under normal as well as perturbed environmental conditions [3, 7, 35, 118, 126, 136, 144]. NPs as elicitors have proven as innovative and efficient resolution to myriads of abiotic environmental pressures including HM toxicity [131, 173]. Application of different NPs (TiO2NPs, SiNPs) alleviates oxidative stress by reducing the content of MDA, H2O2, superoxide radicals by up-regulating the activities of enzymatic and non-enzymatic antioxidants like SOD, CAT, guaiacol and ascorbate peroxidases, GR, GSH [7, 74, 118, 172, 174]. Moreover, NPs also conserve chloroplast structure, improve content of chloroplast pigments, and photosynthetic rate and preserve membrane stability in HM-affected plants [27, 28, 36, 54, 58, 78, 133, 137, 144]. NPs regulate a variety of physiological phenomena in plants notably CO2 fixation, nutrient assimilation, increased activities of imperative primary metabolism enzymes and secondary metabolite production in addition to abiotic stress alleviation [2, 7, 74, 95, 126, 143, 159]. This current review is an attempt to encompass the role of different NPs in regulation of diverse physiological and biochemical phenomena in different plants exposed to HM toxicity.
Impact of nanomaterials on plants
Adequate literature is available on the subject of NPs-mediated impact on plants and studies reveal both positive as well as deleterious effects [26, 102, 144, 145]. For example, exogenously sourced titanium dioxide nanoparticles (TiO2NPs) reduce the oxidative stress imposed by UV-B radiations [74], improve net photosynthesis [46], enrich acquisition of nitrate and assimilation by incorporating inorganic nitrogen into organic molecules in spinach plants [165]. Moreover, TiO2NPs-treated spinach chloroplasts show improved light absorption by chlorophyll a molecules and enhanced electron transfer efficiencies, PSII fluorescence quantum yield and rate of evolution of oxygen [89, 166]. Tomato and spinach plants exposed to TiO2NPs have been reported to improve transfer efficiency and light absorption capacity of PSII [73, 111]. TiO2NPs-triggered enhancement in photosynthesis is attributed to large specific surface area, high photo-catalytic capacity and high thermal conductivity of these NPs [73, 89, 166]. Application of TiO2NPs also serves an essential role in kidney bean in the modulation of enzymatic antioxidant gadgets [56]. Likewise, MnNPs improve activity of PSII by boosting the photolysis of water and evolution of oxygen and also bring an enhancement in the photophosphorylation activity of the electron transport chain in mung-bean [110]. Carbon nanotubes, another important type of NPs, have been observed to potentially enter the seed coat of tomato plants thereby facilitating the water acquisition required during germination [63]. However, no reports of apparent carbon nanotubes induced toxicity in plants are present, except in rice plants in which flowering time has been observed to be delayed by one month [80, 142]. Moreover, carbon nanotubes up-regulate the expression of stress-related genes in tomato plants [66] and increase activity of POX in the seedlings of sainfoin [135]. This feature of carbon nanotubes regulating the expression of stress-related genes in plants can prove handy in the regulation of plant growth and development [71].
Besides the positive effects, deleterious effects of some of the NPs are also documented. Asli and Neumann [10] showed that transpiration is blocked by TiO2NPs and bentonite NPs declining the pace of hydraulic conductivities in maize. Musante and White [97] reported that exogenous application of AgNPs decreased the transpiration rate in Cucurbita pepo. Mukherjee et al. [96] established that ZnONPs application resulted in decreased chlorophyll content in pea. Higher concentration of NPs proves inhibitory to the plants as revealed from the application of CeO2NPs at the concentrations of 1000 and 2000 mg L−1 which recorded a decrease of 60 and 85%, respectively, in the chlorophyll biosynthesis [85]. Likewise, contents of chlorophyll and carotenoids were reported to be significantly decreased by increased concentrations of AgNPs in rice seedlings [100]. TiO2NPs have also been observed to reduce the content of chlorophyll in kidney bean and tobacco [56, 125]. Exogenous supplementation of AlO2NPs reduce the activities of two important enzymes (dehydrogenase and oxido-reductase) in tobacco [108]. ZnONPs application has also been observed to hinder the translocation process in plants like cowpea [153]. Moreover, TiO2NPs have been known to markedly reduce the water absorption and transpiration rate [10]. There are also reports of TiO2NPs-induced cytotoxic impacts in plants and such cytotoxicity in diverse plant cell systems has been endorsed to ROS overproduction [167]. Likewise, ZnONPs are also known to trigger phytotoxic effects in ryegrass which is attributed to membrane lipid peroxidation and ROS production [81]. Improved antioxidant enzymes activities like catalase, SOD have been shown by NiONPs application in tomato [34]. NPs have the ability to impair different growth and developmental aspects in plants as a number of events like flowering, fruiting, timing of senescence, abscission and dormancy are influenced by them [141, 149]. Manufactured NPs can lead to membrane lipid peroxidation by ROS generation [18]. Engineered NPs can significantly impinge on the membrane permeability and fluidity and as a result will affect the acquisition kinetics of the nutrients. So it evolves that the existing literature gives us mixed prospectus as far as the responses of plants to NPs is concerned. Nonetheless, the NMs mediated phytotoxic effects are the focus of most of the prevailing literature, the present review has, therefore, shed-light especially to uncover the NPs-induced remediation of HMs from aquatic ecosystems and HM stress tolerance in plants and the associated underlying mechanisms.
Remediation of HMs from aquatic ecosystems using nanomaterials
Rational water resources’ utilization has emerged as one of the most critical environmental crisis, the resolution to which chiefly lies in the efficient treatment of the contagious wastewater from varied sources. One of the efficient treatment methods is to control the contents of different HMs [9] which are included among the most biologically hazardous and noxious components of the wastewater effluents. Trace elements (metals and metalloids) having atomic density greater than 4 ± 1 g cm−3 are included in the list of HMs and are believed to be the most prevalent toxic soil and water mineral contaminants [17, 92]. Heavy metal accumulation into different soil and water ecosystems signifies a massive threat to the living systems and their bioaccumulation at consecutive trophic positions via biomagnification is adding to its severity [5]. Contrary to the organic pollutants, bulk of the metal/metalloid contaminants are incessantly accumulated in the soil as they are not decomposed and/or degraded chemically or microbially ending in long-term soil eco-toxicity [1]. The invariable enhancement of such toxic contaminants in soil and water ecosystems is a principal global disquiet [106, 129], and the expedition of inventive technological advances has amplified its severity leading to extinction of various living beings and questioning the sustained existence of others dwelling in such contaminated ecosystems by causing noxious ailments [31, 70]. So, it becomes indispensable to find resourceful means of detoxifying/remediating such toxic contaminants. Nevertheless, nanotechnology owns immense potential as environmental cleaner including mitigation of diverse HM toxicities [30]. Several studies of the kind have been spotted in literature dealing with the metal NPs-induced amelioration of heavy metal/metalloid toxicity [152]. A variety of chemical technologies have also been utilized for the remediation purpose, among which, adsorption is incredibly common as well as efficient in view of its cost-efficiency, treatment stability and simplicity [13].
A few techniques are currently accessible to sequester the heavy metals/metalloids from different ecosystems; however, employing nanomaterials is sprouting as a promising option in view of substantial efficiency. Amid the diverse application of NMs in different fields, purification of water so as to trim down the concentration of the toxic contaminants has opened new doors of anticipation toward developing a comparatively feasible environment to thrive in [122]. NPs of different elements are also promisingly effective in remediation of different toxic metal ions from soil ecosystems as well. Fe3O4 NPs impregnated with silica have successfully removed bulk of the toxic contaminants from different ecosystems [161]. Additionally, nanosilica alone has also been applied to eliminate heavy metal ions from the contaminated wastewater (Xin Rong et al. 2001). Zero-valent iron NPs (nZVFe NPs) effectively trims down the concentration of chromium (Cr-VI) and arsenic (As-III) contaminants from waste water [13, 20, 38, 109, 130, 156]. The recent decade has witnessed comprehensive application of ZVFe NPs for the remediating different noxious environmental contaminants [38, 130, 169, 168]. The properties like high surface area and reaction activity permits their employment for rapid decontamination of various aquatic contaminants including toxic metals/metalloids [14]. Furthermore, literature supports that FeONPs scavenge various toxic HMs notably As(III), Cd(II), Cr(VI), Cu(II), Pb(II) and Zn(II) [23, 60, 67, 79, 121, 147, 169]. Moreover ZVFeNPs have been documented to improve plant biomass by decreasing heavy metal contamination of the soil [101, 138]. Environmental engineers, nevertheless, are making relentless efforts to remediate the soil and water ecosystem contaminants, particularly the toxic metal/metalloids through the application of nanomaterials though much more is needed in this regard [41, 84].
Among the most successful NMs widely utilized for the remediation of noxious HMs from the industrial wastewater are single and multi-walled carbon nanotubes, the fullerenes, and graphene oxide [17]. Large surface area and fairly low aggregation capacity of fullerenes makes them ideal to serve as adsorbents for the removal of HMs from industrial wastewater [83, 123]. Significant sorption capacity and competence of oxidized CNTs for [Cd(II), Pb(II)] and [Cr(VI)] ions makes them ideal for removal of these contaminants [17, 76, 119]. In view of their high specific surface area, increased functional groups and active sites on their surface, and reasonably superior chemical stability, the last decade has witnessed a manifold increase in the use of graphene and graphene-based materials for treatment of wastewater [43]. Moreover, graphene is oxidized to add hydrophilic groups for the effective remediation of HMs [14]. Graphene-based materials have a very strong sorption capacity for different metals/metalloids [25, 52, 171]. Heavy metal remediation by ZVFeNPs is predominantly dogged by redox potential of the metal pollutant. Compared to Fe, metal contaminants having more negative or analogous standard redox potential (e.g., Zn and Cd) are eliminated by adsorption capacity with ZVFeNPs; contrarily, metals having positive standard redox potential (e.g., Ni and Pb) are eradicated through reduction as well as adsorption. Cd-spiked soil can be reclaimed using ZVFeNPs (0.01% and Cd accumulation in seeds and leaves of rice grown on such soil gets reduced [158]. Reduced Cd toxicity is endorsed to reduced bioavailability due to the adsorption of the metal contaminant to the nanoparticle surface. Moreover, Liu et al. [82] reported that supplementation of FeNPs under Cd-spiked soils immobilizes Cd leading to reduced bioavailability for the plants. Further support comes from the study of Houben and Sonnet [51] which reveals 45–63% reduction in the amounts of Cd and Zn after the application of powdered FeNPs to the soil.
Substantial attention has been focused on the potential benefits of different nanomaterials in water treatment processes. However, concerns with regard to their looming effects on humans and other ecosystems have arisen. If these concerns are addressed cautiously, NMs can possibly play a cardinal role to ascertain excellent soil and water quality to congregate the escalating demand for clean and safe water and soil for agricultural practices [12, 140].
Nanoparticles-induced heavy metal stress alleviation in plants and the underlying mechanism
Plant resistance against HM stress can be improved through application of NPs which can be applied in the form of aqueous solutions through foliage in addition to application through soil; for example, alleviation of Cd and Pb stress in oryza sativa L. through leaf-applied selenium and silicon NPs [54]. Foliar application of NPs has been reported to be more efficient in HM stress alleviation than their soil application [78]. Although to minimize the detrimental trajectories caused due to HM stress, plants have established varied homeostatic mechanisms that regulate the accumulation and uptake of metals/metalloids, besides, managing their detoxification as well as trafficking; nevertheless, the ability of HM detoxification varies and can be improved. The critical approaches adopted to improve HM resistance in plants comprise; decrease in the quantity of bioavailable metal contaminants, regulation of expression of genes involved in metal/metalloid transport, recuperating the capability of apoplastic barricade to intercept metal contaminants, supplying more nutrients to the plant under stress, fortifying the enzymatic and non-enzymatic antioxidant gadgets and amplified biosynthesis of defensive agents (organic acids, osmolytes, phytochelatins and root exudates) [19, 54, 78, 94, 151, 155]. Apoplastic barrier, though not a complete contaminant blockade, serves vital protective functions in plant roots, controlling the flow ions, oxygen and water [21, 36, 54]. Entry of HMs in plant roots is checked by the apoplastic barriers and their efficiency can be enhanced by the NPs [54, 120]. NPs stick to the HMs in the cell walls forming complexes, thereby making them unavailable. Interaction of NPs with the HMs is crucial while studying the different characteristics of HM stress alleviation. Reduced mobility and, therefore, bioavailability of metal contaminants in the soil has been endorsed to NP application. For example, application of mercapto SiNPs and Fe3O4 NPs increases the stability of Cd, thus, decreasing its mobility [66, 124, 155]. NP-HM complexes, once adsorbed, become immobile, obstructing the mobility of the HMs inside the plants which in-turn reduces their biological activity [28, 154, 173]. Moreover, certain organic acids functioning as metal chelators are bio-concentrated in the cell walls which trim-down the HM-induced damage by chelating the metal contaminants. Biosynthesis of such protective organic acids is known to be improved by NPs as has been reported in case of SiNPs-application reducing the damage caused due to Cd [7, 28, 36, 54, 118, 173]. Moreover, NPs improve soil characteristics; for example, release of phosphate and increased soil pH as a result of hydroxyapatite NPs application in-turn reduces HM toxicity [29, 54]. Furthermore, NPs having high surface to volume ratio are capable of interacting with certain cell biomolecules and elicit different biochemical pathways [30].
ROS production being an indispensable phenomenon of various plant metabolic processes like photosynthesis and respiration, have been reported to act as a defense signal regulating miscellaneous aspects of growth and development. Nevertheless, disproportionate ROS accretion during stressful conditions, damages cell membranes, impairs the structure and functioning of different cellular components as well as proteins [160, 173]. Up-regulation of genes related to various primary physiological phenomena including antioxidative metabolism as well as genes involved in HM stress tolerance requires an optimum concentration of NPs [154, 173]. NP-induced activation of the antioxidative defense gadget of the plant reduces and/or mitigates the HM-induced excessive production of ROS [160, 173].
Among the various types of NPs, role of TiO2NPs has been comprehensively studied in plants. Improved plants’ performance in terms of growth, photosynthesis (net photosynthetic rate, stomatal conductance, rubisco activity), enzyme activities, nutrient status and yield has been worked out in response to the TiO2NPs application both under normal as well as non-biotic pressures [3, 35, 40, 133, 170]. TiO2NPs have been reported to enhance the activities of different antioxidative enzymes (SOD, CAT, APOX, GPOX) in S. olereacea and L. minor (Song et al. 2012); [74]. Moreover, TiO2NPs reduce Cd and free radical accretion and lipid peroxidation by improving enzymatic and non-enzymatic antioxidants and relative water content, conserve chloroplast structure, improve content of chloroplast pigments, and photosynthetic rate and preserve membrane stability in HM affected plants [46, 58, 78, 133] (Table 1). Lei et al. [74], tested the efficacy of TiO2NPs on Spinach and reported the significant decrease in O2•− and H2O2 accumulation, and consequently lipid peroxidation in chloroplasts under oxidative stress. Application of TiO2NPs prevents electrolyte leakage in the chickpea cultivars [74, 91]. Similar results have been validated by Sharma et al. [128] in mustard due to the application of AgNPs (25 and 50 mg L–1). Studies on Spinach have disclosed that TiO2NPs limit oxidative stress by reducing the content of MDA and H2O2, and superoxide radicals and fortify antioxidant enzymatic activities (SOD, APOX, GPOX and CAT) [74, 172]. Application of ZnONPs alleviated Cd-toxicity in Leucaena leucocephala [148]. Likewise, Cd toxicity in mustard by nano-scale hydroxyapatite has been reported [77]. Venkatachalam et al. [148] suggested that ZnONPs-induced mitigation of Cd toxicity is accredited to decreased ROS production which prevents membrane damage as can be confirmed by reduced MDA content thereby increasing plant growth rate, mineral accretion and biomass accumulation. The study also suggested that ZnONPs lead to increased activities of the antioxidative enzymes by enhancing the level of isoenzyme pattern and improved genomic alterations to conquer the heavy metal-induced genotoxicity.
SiNPs alleviate Cr and As toxicity in wheat and maize cultivars [27, 144, 143]. Compared to the organic Si, SiNPs were observed to be more efficient in the study and the authors’ report more efficient protective impact of SiNPs in maize seedlings under AsV stress [27, 137, 144], blockade of As entry leading to reduced AsV accumulation due to blockade in the root endodermis, improved activities of plant antioxidants (SOD, APX, GR, DHAR, GSH) which in-turn re-establishing the redox status and reduces MDA content in As-treated maize [27, 144]. A similar type of protective mechanism has been observed in SiNPs-treated pea seedlings affected with Cr toxicity and the study revealed that SiNPs successfully alleviated the toxic effects induced by Cr [27, 143]. The up-regulated activities of enzymes and the reduced MDA content in the HM-affected plants after their treatment with NPs, is noteworthy [143, 148] (Table 1). SiNPs-induced alleviation of As, Cd, Cr(VI), Pb toxicity has also been reported in different plants [27, 36, 42, 54, 66]. The studies unveil that SiNPs improve the activities of antioxidant enzymes, nitrogen assimilation and maintain the cytosolic homeostasis by optimizing K+/Na+ ratio which is imperative for the stimulation of essential ROS detoxifying enzymes (Alsaeedi et al. 2018; [61, 132, 143]. In a similar study, increased expression of POX, CAT, and Cu/ZnSOD mRNA has been observed in Arabidopsis treated with CuONPs [99]. Likewise, TiO2NPs-induced increase in the activities of CAT and GR on has been reported in an aquatic macrophyte, Hydrilla verticillata, [105]. Similarly, enhancement in the SOD activity has been reported in A. cepa after exposing the plant to AlO2NPs [113].
Reduced oxidative damage in response to CeO2NPs has been validated in rice seedlings by the free radical scavenging ability of the NPs at lower concentrations; however, at higher concentrations, H2O2 concentration has been reported to increase steadily which is endorsed to the SOD mimetic activity of CeO2NPs [44, 47, 116, 117, 163]. Lower CeO2NPs concentrations (200 and 100 mg/L) enhance cellular resistance to metal-induced oxidative stress by reduced oxidative stress suppression of ROS [41]. The ROS scavenging capability of CeO2NPs has been studied in detail compared to other NPs. The surface lattice of CeO2NPs encloses unoccupied oxygen sites which aids them to change their oxidation states [+ 4 (Ce4+) and + 3 (Ce3+], which in-turn facilitates them to trap the membrane damaging toxic free radicals (O2•− and HO•−) [15]. Fascinatingly, the ability of various NPs to mimic the activity of natural antioxidant enzymes has been previously reported (reviewed by [159]. For example, CuONPs and AuNPs mimic POX activity; Fe3O4NPs, Co3O4 NPs and CeO2NPs, imitate CAT and POX activities; CeO2NPs, fullerene and Pt NPs, displays the mimetic activity of SOD [159]. Effect of various NPs on the activities of various antioxidative enzymes is presented in tabulated form (Table 1).
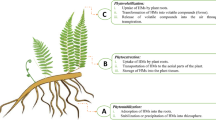
Proteomic study of AgNPs on rice has been an important breakthrough as it has revealed the up-regulation of about twenty-eight responsive proteins including those involved in oxidative stress tolerance, Ca2+ signaling, protection of nucleic acid (DNA/RNA) damage and proteins contributing to the regulation of gene-expression [49, 90]. The enhanced activities have been accredited to the NP-induced de-novo biosynthesis of proteins or the expression of enzyme isoforms, as reveals from different studies. For example, enhanced protein content in Bacopa monnieri and Pisum sativum seedlings due to the application of AgNPs and SiNPs have been reported by Krishnaraj et al. [68] and Tripathi et al. [143], respectively. Venkatachalam et al. [148] have reported that ZnONPs treatment to Cd and Pb-affected plants leads to the over-expression of a POX isoform as revealed from the peroxidase isoenzyme pattern in the study. Over-expression and in-turn over-production of such isoforms are believed to be involved in the alleviation of the heavy metal-induced oxidative stress in crop plants. The molecular study of Venkatachalam et al. [148] clearly indicated that the ROS-induced DNA damage was more pronounced in the Cd and Pb-treated plants, as indicated by the disappearance of several normal bands in the RAPD pattern of the DNA, whereas new DNA amplicons could be located in metal-exposed plants treated with ZnONPs. Moreover, oxidation of proteins is a common HM toxicity symptom as ions of HM directly interact with proteins molecules due to their high binding affinity with carboxyl- thionyl- and histidyl-, groups [48]. Studies have revealed that the NPs within the plant cell systems may interact with these sulfhydryl and carboxyl groups eventually altering the protein activity by acting and reacting similar to the metal ions [48]. As discussed, different NPs up-regulate the expression of different genes in plants speeding-up the biosynthesis of certain secondary metabolism products like essential oils and phenols in addition to the primary metabolic products [95], Ahmad et al. 2019; [126]. A comprehensive survey of the available literature reveals that secondary metabolite accumulation, particularly phenolics, constitutes a crucial adaptive response of plants against HM toxicity [62, 69, 98]. Some additional mechanisms like the NPs-induced biosynthesis of abiotic stress regulators (nitric oxide, methyl jasmonate, salicylic acid) may also be responsible metal stress alleviation; however, scientists are leaving no stone unturned to elucidate such mechanism which may possibly be discovered in future. A pictorial representation of the mechanism of HM stress alleviation induced by NPs is presented in Fig. 1.
showing the nanoparticles-induced mechanism of heavy metal stress alleviation. NPs detoxify the excessive ROS produced due to heavy metal stress by up-regulating the expression of antioxidative enzymes. NPs improve the activity of chelators and help the plant to sequester the toxic metals/metalloids more efficiently. Moreover, improved biosynthesis of phenols due to NP application is accredited to HM stress alleviation
Conclusion and future prospects
Adequate literature is in conformity with the elicitor effect of NPs on different plants. Alleviation of HMs-induced toxicity by the soil and foliage applied NPs has been attributed to the up-regulated activities of imperative primary and secondary metabolic enzymes. The mimetic activities of NPs as antioxidant enzymes, NPs-induced up-regulation of different oxidative metabolism enzymes, osmolytes and chelators, sequestration of HM contaminants into vacuoles, detoxification of HM-induced ROS, increased accretion of secondary metabolites particularly phenols have been endorsed to the elicitor effect of NPs. However, in-depth understanding at the molecular level is the need of the hour to gain insights regarding the absolute mechanism of mitigation of the HM toxicity in plants, and advanced research in this regard is advocated.
The mimetic activities of certain NPs to some antioxidant enzymes as well as their increased expression have opened new doors of anticipation in oxidative stress alleviation. The defense system of the sensitive plants can, therefore, be fortified by exogenous sourcing of NPs and the less tolerant can be reinforced, though further studies are needed in this regard. Further research needs to be conducted at the molecular level on the effect of NPs on phytochelatins and metallothioneins in the plants and the endogenous concentrations of other phytohormones particularly related to stress.
References
Adriano DC, Wenzel WW, Vangronsveld J, Bolan NS (2004) Role of assisted natural remediation in environmental cleanup. Geoderma 122:121–142
Ahmad B, Khan M, Jaleel H, Shabbir A, Sadiq Y, Uddin M (2020) Silicon nanoparticles mediated increase in glandular trichomes and regulation of photosynthetic and quality attributes in Mentha piperita L. J Plant Growth Regul 39(1):346–357
Ahmad B, Shabbir A, Jaleel H, Khan MMA, Sadiq Y (2018) Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Current Plant Biol 113:6–15
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid-A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci 8:1–17
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881
Ali S, Rizwan M, Hussain A, Rehman MZ, Ali B, Yousaf B, Wijaya L, Alyemeni MN, Ahmad P (2019) Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol Biochem 140:1–8
Ali E, El-Shehawi A, Ibrahim O, Abdul-Hafeez E, Moussa M, Hassan F (2021) A Vital Role of Chitosan Nanoparticles in Improvisation the Drought Stress Tolerance in Catharanthus roseus (L.) Through Biochemical and Gene Expression Modulation. Plant Physiol. Biochem. 161:166–175
Alsaeedi AH, El-Ramady H, Alshaal T, El-Garawani M, Elhawat N, Almohsen M (2017) Engineered silica nanoparticles alleviate the detrimental effects of Na+ stress on germination and growth of common bean (Phaseolus vulgaris). Environ Sci Pollut Res 24:21917–21928
Ariffin N, Abdullah MM, Zainol MR, Murshed MF, Faris MA, Bayuaji R (2017) Review on adsorption of heavy metal in wastewater by using geopolymer. In: MATEC web of conferences, vol 97, p 01023. EDP Sciences
Asli S, Neumann PM (2009) Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ 32:577–584
Bandyopadhyay S, Peralta-Videa JR, Gardea-Torresdey JL (2013) Advanced analytical techniques for the measurement of nanomaterials in food and agricultural samples: a review. Environ Eng Sci 3030:118–125
Bhatt I, Tripathi BN (2011) Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 82:308–317
Bhowmick S, Chakraborty S, Mondal P, Van Renterghem W, Van den Berghe S, Roman-Ross G, Chatterjee D, Iglesias M (2014) Montmorillonite-supportednanoscale zero-valen iron removal of arsenic from aqueous solution: kinetics and mechanism. Chem Eng J 243:14–23
Bisht M (2019) Nanomaterials for removal of toxic metals ions from the water. Nanomaterials for healthcare, energy and environment 2019. Springer, Singapore, pp 159–174
Boghossian AA, Sen F, Gibbons BM, Sen S, Faltermeier SM, Giraldo JP, Zhang CT, Zhang J, Heller DA, Strano MS (2013) Application of nanoparticle antioxidants to enable hyperstable chloroplasts for solar energy harvesting. Adv Energy Mater 3:881–893
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9):e04691
Burakov AE, Galunin EV, Burakova IV, Kucherova AE, Agarwal S, Tkachev AG, Gupta VK (2018) Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol Environ Safety 148:702–712
Cabiscol E, Tamarit J, Ros J (2010) Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8
Cao F, Dai H, Hao PF, Wu F (2020) Silicon regulates the expression of vacuolar H(+)-pyrophosphatase 1 and decreases cadmium accumulation in rice (Oryza sativa L.). Chemosphere 240:124907
Chang D, Chen T, Liu H, Xi Y, Qing C, Xie Q, Frost RA (2014) A new approach to prepare ZVI and its application in removal of Cr(VI) from aqueous solution. Chem Eng J 244:264–272
Chao-Dong Y, Xia Z, Guo-Feng L, Jun-Wei Z, Man-Zhu B, Zhi-Xiang Z (2013) Progress on the Structure and Physiological Functions of Apoplastic Barriers in Root. Bull Bot Res 33:114–119
Chen TM, Gokhale J, Shofer S, Kuschner WG (2007) Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci 333:249–256
Chen YH, Li FA (2010) Kinetic study on removal of copper (II) using goethite and hematite nano-photocatalysts. J Colloid Interface Sci 347:277–281
Cirlakova A (2009) Heavy metals in the vascular plants of Tatra mountains. Oecol Montana 18:23–26
Cong HP, Ren XC, Wang P, Yu ShH (2012) Macroscopic multifunctional graphene- based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 6:2693–2703
Cox A, Venkatachalam P, Sahi S, Sharma N (2016) Silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol Biochem 107:147–163
Cui J, Li Y, Jin Q, Li F (2020) Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environ Sci Nano 7(1):162–171
Cui J, Liu T, Li F, Yi J, Liu C, Yu H (2017) Silica nanoparticles alleviate cadmium toxicity in rice cells: mechanisms and size effects. Environ Pollut 228:363–369
Cui H, Shi Y, Zhou J, Chu H, Cang L, Zhou D (2018) Effect of different grain sizes of hydroxyapatite on soil heavy metal bioavailability and microbial community composition. Agric Ecosyst Environ 267:165–173
De La Torre-Roche R, Hawthorne J, Deng Y, Xing B, Cai W, Newman LA, Wang Q, Ma X, Hamdi H, White JC (2013) Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ Sci Technol 47:12539–12547
de Souza TAJ, Souza LRR, Franchi LP (2019) Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol Environ Safety 171:691–700
de Sousa A, Saleh AM, Habeeb TH, Hassan YM, Zrieq R, Wadaan MA, Hozzein WN, Selim S, Matos M, AbdElgawad H (2019) Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci Total Environ 693:133636
Dickinson M, Scott TB (2010) The application of zero-valent iron nanoparticles for the remediation of a uranium-contaminated waste effluent. J Hazard Mater 178:171–179
Dubchak S, Ogar A, Mietelski JW, Turnau K (2010) Influence of silver and titanium nanoparticles on arbuscular mycorrhiza colonization and accumulation of radio caesium in Helianthus annuus. Span J Agric Res 8:103–108
Ebbs SD, Bradfield SJ, Kumar P, White JC, Musante C, Ma X (2016) Accumulation of zinc, copper, or cerium in carrot (Daucus carota) exposed to metal oxide nanoparticles and metal ions. Environ Sci Nano 3:114–126
Emamverdian A, Ding Y, Mokhberdoran F, Xie Y (2015) Heavy metal stress and some mechanisms of plant defense response. Sci World J 2015:1–18
Faisal M, Saquib Q, Alatar AA, Al-Khedhairy AA, Hegazy AK, Musarrat J (2013) Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J Hazard Mat 250:318–332
Faraji J, Sepehri A (2018) Titanium dioxide nanoparticles and sodium nitroprusside alleviate the adverse effects of cadmium stress on germination and seedling growth of wheat (Triticum aestivum L.). Univ Sci 23:61–66
Fatemi H, Pour BE, Rizwan M (2021) Foliar application of silicon nanoparticles affected the growth, Vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ Sci Pollut Res 28:1417–1425
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress and its possible reversal by chelation therapy”. Indian J Med Res 128(4):501–523
Fu F, Ma J, Xie L, Tang B, Han W, Lin S (2013) Chromium removal using resin supported nanoscale zero-valent iron. J Environ Manag 128:822–827
Fu Z, Xi S (2020) The effects of heavy metals on human metabolism. Toxicol Mech Methods 30(3):167–176
Gao F, Hong F, Liu C, Zheng L, Su M, Wu X, Yang F, Wu C, Yang P (2006) Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol Trace Elem Res 111:239–253
Gomez-Garay A, Pintos B, Manzanera JA, Lobo C, Villalobos N, Martín L (2014) Uptake of CeO2 nanoparticles and its effect on growth of Medicago arborea in vitro plantlets. Biol Trace Elem Res 161:143–150
González-Moscoso M, Martínez-Villegas NV, Cadenas-Pliego G, Benavides-Mendoza A, Rivera-Cruz MDC, González-Morales S, Juárez-Maldonado A (2019) Impact of silicon nanoparticles on the antioxidant compounds of tomato fruits stressed by arsenic. Foods 8(12):612
Gopalakrishnan A, Krishnan R, Thangavel S, Venugopal G, Kim SJ (2015) Removal of heavy metal ions from pharma effluents using graphene-oxide nanosorbents and study of their adsorption kinetics. J Ind Eng Chem 30:14–19
Heckert EG, Karakoti AS, Seal S, Self WT (2008) The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 29:2705–2709
Helaly MN, El-Metwally MA, El-Hoseiny H, Omar SA, El-Sheery NI (2014) Effect of nanoparticles on biological contamination of'in vitro'cultures and organogenic regeneration of banana. Australian J Crop Sci 8(4):612–624
Hong FS, Yang P, Gao FQ, Liu C, Zheng L, Zhou J (2005) Effect of nano-anatase TiO2 on spectral characterization of photosystem II particles from spinach. Chem Res Chin Univ 21:196–200
Horie M, Nishio K, Kato H, Fujita K, Endo S, Nakamura A, Miyauchi A, Kinugasa S, Yamamoto K, Niki E, Yoshida Y (2011) Cellular responses induced by cerium oxide nanoparticles: induction of intracellular calcium level and oxidative stress on culture cells. J Biochem 150:461–471
Hossain Z, Mustafa G, Komatsu S (2015) Plant responses to nanoparticle stress. Int J Mol Sci 16:26644–26653
Hossain Z, Mustafa G, Sakata K, Komatsu S (2016) Insights into the proteomic response of soybean towards Al2O3, ZnO, and Ag nanoparticles stress. J Hazard Mater 304:291–305
Hossain MA, Piyatida P, da Silva JA, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation”. J Bot 2012:1–37
Houben D, Sonnet P (2010) Leaching and phytoavailability of zinc and cadmium in a contaminated soil treated with zero-valent iron. In: Proceedings of the 19th World Congress of soil science, soil solutions for a changing World, pp 1–6
Huang ZH, Zheng X, Lv W, Wang M, Yang QH, Kang F (2011) Adsorption of lead (II) ions from aqueous solution on low-temperature exfoliated graphene nanosheets. Langmuir 27:7558–7562
Hussain B, Lin Q, Hamid Y, Sanaullah M, Di L, Khan MB, He Z, Yang X (2020) Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci Total Environ 712:136497
Hussain A, Ali S, Rizwan M, ur Rehman MZ, Javed MR, Imran M, Chatha SAS, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Poll 242:1518–1526
Ishtiyaq S, Kumar H, Varun M, Kumar B, Paul MS (2018) Heavy metal toxicity and antioxidative response in plants: an overview. Plants under metal and metalloid stress. Springer, Singapore, pp 77–106
Jacob DL, Borchardt JD, Navaratnam L, Otte ML, Bezbaruah AN (2013) Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int J Phytoremediat 15:142–153
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some HMs. Interdiscip Toxicol 7(2):60–72
Ji Y, Zhou Y, Ma C, Feng Y, Hao Y, Rui Y, Wu W, Gui X, Han Y, Wang Y, Xing B (2017) Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol Biochem 110:82–93
Jia X, Qiu T, Yao X, Jiang L, Wang N, Wei S, Tao Y, Pei P, Wang Z, Zhang J, Zhu Y (2020) Arsenic induces hepatic insulin resistance via mtROS-NLRP3 inflammasome pathway. J Hazard Mater 399:123034
Jiang W, Pelaez M, Dionysiou DD, Entezari MH, Tsoutsou D, O’Shea K (2013) Chromium(VI) removal by maghemite nanoparticles. Chem Eng J 222:527–533
Kader MA, Lindberg S (2010) Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal Behav 5:233–238
Kahlon SK, Sharma G, Julka JM, Kumar A, Sharma S, Stadler FJ (2018) Impact of heavy metals and nanoparticles on aquatic biota. Environ Chem Lett 16(3):919–946
Kaya C, Ashraf M, Alyemeni MN, Corpas FJ, Ahmad P (2020) Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J Hazard Mater p 123020
Khan ZS, Rizwan M, Hafeez M, Ali S, Javed MR, Adrees M (2020) The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ Sci Pollut Res Int 26:19859–19870
Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3:3221–3227
Khodakovskaya MV, de Silva K, Nedosekin DA, Dervishi E, Biris AS, Shashkov EV, Galanzha EI, Zharov VP (2011) Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc Natl Acad Sci 108:1028–1033
Komarek M, Koretsky CM, Stephen KJ, Alessi DS, Chrastný V (2015) Competitive adsorption of Cd(II), Cr(VI), and Pb(II) onto nanomaghemite: a spectroscopic and modeling approach. Environ Sci Technol 49:12851–12859
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. Plant Growth Metab Proc Biochem 47:651–658
Kısa D, Elmastaş M, Öztürk L, Kayır Ö (2016) Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl Biol Chem 59(6):813–820
Lado LR, Hengl T, Reuter HI (2008) Heavy metals in European soils: a geostatistical analysis of the FOREGS Geochemical database. Geoderma 148:189–199
Lahiani MH, Chen J, Irin F, Puretzky AA, Green MJ, Khodakovskaya MV (2015) Interaction of carbon nanohorns with plants: uptake and biological effects. Carbon 81:607–619
Lajayer AB, Ghorbanpour M, Nikabadi S (2017) HMs in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol Environ Saf 145:377–390
Lei Z, Mingyu S, Chao L, Liang C, Hao H, Xiao W, Xiaoqing L, Fan Y, Fengqing G, Fashui H (2007) Effects of nanoanatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol Trace Elem Res 119:68–76
Lei Z, Mingyu S, Xiao W, Chao L, Chunxiang Q, Liang C, Hao H, Xiaoqing L, Fashui H (2008) Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Elem Res 21:69–79
Li HB, Li J, Zhao D, Li C, Wang XJ, Sun HJ, Juhasz AL, Ma LQ (2017) Arsenic relative bioavailability in rice using a mouse arsenic urinary excretion bioassay and its application to assess human health risk. Environ Sci Technol 51(8):4689–4696
Li YH, Wang S, Wei J, Zhang X, Xu C, Luan Z, Wu D, Wei B (2002) Lead adsorption on carbon nanotubes. Chem Phys Lett 357:263–266
Li Z, Huang J (2014) Effects of nanoparticle hydroxyapatite on growth and antioxidant system in pakchoi (Brassica chinensis L.) from cadmium-contaminated soil. J Nanomater pp 1–7
Lian J, Zhao L, Wu J, Xiong H, Bao Y, Zeb A, Tang J, Liu W (2020) Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 239:124794
Lin S, Lu D, Liu Z (2012) Removal of arsenic contaminants with magnetic c-Fe2O3 nanoparticles. Chem Eng J 211–212:46–52
Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova A, Rao AM, Luo H, Ke PC (2009) Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 5:1128–1132
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585
Liu J, Cai H, Mei C, Wang M (2015) Effects of nano-silicon and common silicon on lead uptake and translocation in two rice cultivars. Front Environ Sci Eng 9:905–911
Lucena R, Simonet BM, Cardenas S, Valcarcel M (2011) Potential of nanoparticles in sample preparation. J Chromatogr A 1218:620–637
Luo JS, Zhang Z (2021) Mechanisms of cadmium phytoremediation and detoxification in plants. Crop J 9(2021):521–529
Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP (2013) Physiological and molecular response of Arabidopsis thaliana (L) to nanoparticle cerium and indium oxide exposure. ACS Sus Chem Eng 1(7):768–778
Masindi V, Muedi KL (2018) Environmental contamination by heavy metals. Heavy Metals 10:115–132
Mazumder DG (2008) Chronic arsenic toxicity and human health. Indian J Med Res 128(4):436–447
Mengting L (2018) Effect of Selenium application on Cadmium accumulation and in Rice (Oryza sativa). Master’s Thesis, Guangxi University, Nanning, China
Mingyu S, Fashui H, Chao L, Xiao W, Xiaoqing L, Liang C, Fengqing G, Fan Y, Zhongrui L (2007) Effects of nano-anatase TiO2 on absorption, distribution of light, and photoreduction activities of chloroplast membrane of spinach. Biol Trace Elem Res 118:120–130
Mirzajani F, Askari H, Hamzelou S, Schober Y, R€ompp, A., Ghassempour, A., Spengler, B., (2014) Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol Environ Saf 108:335–339
Mohammadi R, Maali-Amiri R, Mantri N (2014) Effect of TiO2 nanoparticles on oxidative damage and antioxidant defense systems in chickpea seedlings during cold stress. Russ J Plant Physiol 61:768–775
Mohammed AS, Kapri A, Goel R (2011) Heavy metal pollution: source, impact, and remedies. Biomanagement of metal-contaminated soils. Springer, Dordrecht, pp 1–28
Mohan D, Pittman, Jr.C.U. (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142:1–53
Moharem M, Elkhatib E, Mesalem M (2019) Remediation of chromium and mercury polluted calcareous soils using nanoparticles: Sorption-desorption kinetics, speciation and fractionation. Environ Res 170:366–373
Mukarram M, Khan MMA, Corpas FJ (2021) Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud) Wats) agronomic parameters with a higher essential oil yield. J Hazard Mater 412:125254
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S, Rico CM, Zhao L, Gardea-Torresdey JL (2014) Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138
Musante C, White JC (2012) Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk- size particles. Environ Toxicol 27:510–517
Naikoo MI, Dar MI, Raghib F, Jaleel H, Ahmad B, Raina A, Khan FA, Naushin F (2019) Role and regulation of plants phenolics in abiotic stress tolerance: an overview. Plant Signal Mol. https://doi.org/10.1016/B978-0-12-816451-8.00009-5
Nair PMG, Chung IM (2014) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignification, and molecular level changes. Environ Sci Pollut Res 21:12709–12722
Nair PMG, Chung IM (2014) Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 112:105–113
Nasiri J, Gholami A, Panahpour E (2013) Removal of cadmium from soil resources using stabilized zero-valent iron nanoparticles. J Civil Eng Urban 3:338–341
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicol 17:372–386
Nekrasova GF, Ushakova OS, Ermakov AE, Uimin MA, Byzov IV (2011) Effects of copper (II) ions and copper oxide nanoparticles on Elodea densa Planch. Russian J Ecol 42(6):458–463
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Okupnik A, Pflugmacher S (2016) Oxidative stress response of the aquatic macrophyte Hydrilla verticillata exposed to TiO2 nanoparticles. Environ Toxicol Chem 35:2859–2866
Pandey VC, Pandey DN, Singh N (2015) Sustainable phytoremediation based on naturally colonizing and economically valuable plants. J Clean Prod 86:37–39
Parisi C, Vigani M, Rodríguez-Cerezo E (2015) Agricultural nanotechnologies: what are the current possibilities? Nano Today 10(2):124–127
Poborilova Z, Opatrilova R, Babula P (2013) Toxicity of aluminium oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ Exp Bot 91:1–11
Poguberović SS, Krčmar DM, Maletić SP, Kónya Z, Pilipović DD, Kerkez DV, Rončević SD (2016) Removal of As (III) and Cr (VI) from aqueous solutions using “green” zero-valent iron nanoparticles produced by oak, mulberry and cherry leaf extracts. Ecol Eng 90:42–49
Pradhan S, Patra P, Das S, Chandra S, Mitra S, Dey KK, Akbar S, Palit P, Goswami A (2013) Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata: a detailed molecular, biochemical, and biophysical study. Environ Sci Technol 47:13122–13131
Qi M, Liu Y, Li T (2013) Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol Trace Elem Res 156:323–328
Rahaman MS, Rahman MM, Mise N, Sikder T, Ichihara G, Uddin MK, Kurasaki M, Ichihara S (2021) Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ Pollut p 117940
Rajeshwari A, Kavitha S, Alex SA, Kumar D, Mukherjee A, Chandrasekaran N, Mukherjee A (2015) Cytotoxicity of aluminum oxide nanoparticles on Allium cepa root tip-effects of oxidative stress generation and biouptake. Environ Sci Pollut Res 22:11057–11066
Rascio N, Navari-Izzo F (2011) Heavy metal hyper accumulating plants: how and why do they do it? And what makes them so interesting?”. Plant Sci 180(2):169–181
Rehman AU, Nazir S, Irshad R, Tahir K, ur Rehman K, Islam RU, Wahab Z (2021) Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J Molec Liq 321:114455
Rico CM, Hong J, Morales MI, Zhao L, Barrios AC, Zhang JY, Peralta-Videa JR, Gardea-Torresdey JL (2013) Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ Sci Technol 47:5635–5642
Rico CM, Morales MI, McCreary R, Castillo-Michel H, Barrios AC, Hong J, Tafoya A, Lee WY, Varela-Ramirez A, Peralta-Videa JR, Gardea-Torresdey JL (2013) Cerium oxide nanoparticles modify the antioxidative stress enzyme activities and macromolecule composition in rice seedlings. Environ Sci Technol 47:14110–14118
Rizwan M, Ali S, Rehman MZ, Malik S, Adrees M, Qayyum MF, Alamri SA, Alyemeni MN, Ahmad P (2019) Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol Plant 41(3):1–12
Robati D (2013) Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J Nanostruct Chem 3(1):55
Rossi L, Zhang W, Schwab AP, Ma X (2017) Uptake, uptake, accumulation, and in planta distribution of coexisting cerium oxide nanoparticles and cadmium in Glycine max (L.) Merr. Environ Sci Technol 51:12815–12824
Roy A, Bhattacharya J (2012) Removal of Cu(II), Zn(II) and Pb(II) from water using microwave-assisted synthesized maghemite nanotubes. Chem Eng J 211–212:493–500
Savage N, Diallo MS (2005) Nanomaterials and water purification: Opportunities and challenges. J Nanoparticle Res 7:331–342
Scida K, Stege PW, Haby G, Messina GA, Garcia CD (2011) Recent applications carbon-based nanomaterials in analytical chemistry. Anal Chim Acta 691:6–17
Sebastian A, Nangia A, Prasad MNV (2019) Cadmium and sodium adsorption properties of magnetite nanoparticles synthesized from Hevea brasiliensis Muell. Arg. bark: Relevance in amelioration of metal stress in rice. J Hazard Mater 371:261–272
Servin AD, Morales MI, Castillo-Michel H, Hernandez-Viezcas J, Munoz B, Zhao L, Nunez JE, Peralta-Videa JR, Gardea-Torresdey JL (2013) Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ Sci Technol 47:11592–11598
Shabbir A, Khan MMA, Ahmad B, Sadiq Y, Jaleel H, Uddin M (2019) Efficacy of TiO2 nanoparticles in enhancing the photosynthesis, essential oil and khusimol biosynthesis in Vetiveria zizanioides L. Nash Photosynthetica 57(2):599–606
Shabnam N, Pardha-Saradhi P, Sharmila P (2014) Phenolics impart Au3+–stress tolerance to cowpea by generating nanoparticles. PLoS ONE 9:e85242
Sharma P, Bhattm D, Zaidim MGH, Saradhim PP, Khannam PK, Aroram S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol 167:2225–2233
Sharma P, Pandey S (2014) Status of Phytoremediationin World Scenario. Int J Environ Bioremed Biodegrad 2:178–191
Shi J, Abid AD, Kennedy IM, Hristova KR, Silk WK (2011) To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut 159:1277–1282
Siddiqui MH, Al-Whaibi MH, Firoz M, Al-Khaishany MY (2015) Role of nanoparticles in plants. Nanotechnology and plant sciences. Springer International Publishing, Switzerland, pp 19–35
Siddiqui MN, Mostofa MG, Akter MM, Srivastava AK, Sayed MA, Hasan MS, Tran LSP (2017) Impact of salt-induced toxicity on growth and yield-potential of local wheat cultivars: oxidative stress and ion toxicity are among the major determinants of salt-tolerant capacity. Chemosphere 187:385–394
Singh J, Lee BK (2016) Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J Environ Manage 170:88–96
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6(1143):1–36
Smirnova E, Gusev A, Zaytseva O, Sheina O, Tkachev A, Kuznetsova E, Lazareva E, Onishchenko G, Feofanov A, Kirpichnikov M (2012) Uptake and accumulation of multiwalled carbon nanotubes change the morphometric and biochemical characteristics of Onobrychis arenaria seedlings. Front Chem Sci Eng 6:132–138
Suriyaprabha R, Karunakaran G, Yuvakkumar R, Rajendran V, Kannan N (2012) Silica nanoparticles for increased silica availability in maize (Zea mays L.) seeds under hydroponic conditions. Curr Nanosci 8:902–908
Tafazoli M, Hojjati SM, Biparva P, Kooch Y, Lamersdorf N (2017) Reduction of soil heavy metal bioavailability by nanoparticles and cellulosic wastes improved the biomass of tree seedlings. J Plant Nut Soil Sci 180:683–693
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Mol Clin Environ Toxicol, pp133–164
Theron J, Walker JA, Cloete TE (2008) Nanotechnology and water treatment: applications and emerging opportunities. Crit Rev Microbiol 34:43–69
Thul ST, Sarangi BK (2015) Implications of nanotechnology on plant productivity and its rhizospheric environment. Nanotechnology and plant Sciences. Springer, Cham, pp 37–53
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem 96:189–198
Tripathi DK, Singh S, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2016) Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultivar and hybrid differing in arsenate tolerance. Front Environ Sci 4:46
Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK, Chauhan DK (2017) Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110:70–81
Tuutijärvi T, Vahala R, Sillanpää M, Chen G (2012) Maghemite nanoparticles for As (V) removal: desorption characteristics and adsorbent recovery. Environ Technol 33:1927–1936
Usman M, Farooq M, Wakeel A, Nawaz A, Cheema SA, Rehman H, Ashraf I, Sanaullah M (2020) Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci Total Environ 721:137778
Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma NC, Sahi SV (2017) Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem 110:59–69
Vernay P, Gauthier-Moussard C, Jean L, Bordas F, Faure O, Ledoigt G, Hitmi A (2008) Effect of chromium species on phytochemical and physiological parameters in Datura innoxia. Chemosphere 72(5):763–771
Wang M, Chen L, Chen S, Ma Y (2012) Alleviation of cadmium-induced root growth inhibition in crop seedlings by nanoparticles. Ecotoxicol Environ Saf 79:48–54
Wang Y, Liu Y, Zhan W, Zheng K, Lian M, Zhang C, Ruan X, Li T (2020) Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): a three-year field study. Ecotoxicol Environ Saf 197:110600
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014) Removal of As(III) and As(V) from aqueous solutions using nanoscale zero-valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131
Wang P, Menzies NW, Lombi E, McKenna BA, Johannessen B, Glover CJ, Kappen P, Kopittke PM (2013) Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ Sci Technol 47(23):13822–13830
Wang K, Wang Y, Wan Y, Mi Z, Wang Q, Wang Q, Li H (2021) The fate of arsenic in rice plants (Oryza sativa L.): Influence of different forms of selenium. Chemosphere 264:128417
Wang Z, Yue L, Dhankher OP, Xing B (2020) Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ Int 142:105831
Wang S, Wang F, Gao S (2015) Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ Sci Pollut Res 22:2837–2845
Wang Y, Jiang F, Ma C, Rui Y, Tsang DCW, Xing B (2019) Effect of metal oxide nanoparticles on amino acids in wheat grains (Triticum aestivum) in a life cycle study. J Environ Manag 241:319–327
Watanabe T, Murata Y, Nakamura T, Sakai Y, Osaki M (2009) Effect of zero-valent iron application on cadmium uptake in rice plants grown in cadmium-contaminated soils. J Plant Nut 32:1164–1172
Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42:6060–6093
Wu H, Tito N, Giraldo JP (2017) Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano 11(11):11283–11297
Wu PG, Zhu JH, Xu ZH (2004) Template-assisted synthesis of mesoporous magnetic nano composite particles. Adv Function Mat 14:345–351
Wu B, Wang G, Wu J, Fu Q, Liu C (2014) Sources of heavy metals in surface sediments and an ecological risk assessment from two adjacent plateau reservoirs. PLoS One, 9(7), e102101
Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, Yeh JI, Zink JI, Nel AE (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2:2121–2134
Xin-Rong Z, Ping Y, Meng-Yeu Z (2001) Research on photocatalytic degradation of organophosphorous pesticides using TiO2.SiO2/beads. Indus Water Treat 21(3):13–39
Yang F, Hong FS, You WJ, Liu C, Gao FQ, Wu C, Yang P (2006) Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res 110:179–190
Yang F, Liu C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P (2007) The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res 119:77–88
Yin L, Colman BP, McGill BM, Wright JP, Bernhardt ES (2012) Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 7:47674
Zhang X, Lin S, Lu XQ, Chen Z (2010) Removal of Pb(II) from water using synthe-sized kaolin supported nanoscale zero-valent iron. Chem Eng J 163:243–248
Zhang MY, Wang Y, Zhao DY, Pan G (2010) Immobilization of arsenic in soils by stabilized nanoscale zero-valent iron, iron sulfide (FeS), and magnetite (Fe3O4) particles. Chin Sci Bull 55:365–372
Zhang Y, Yan L, Xu W, Guo X, Cui L, Gao L, Wei Q, Du B (2014) Adsorption of Pb(II) and Hg(II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J Mol Liq 191:177–182
Zhao G, Li J, Ren X, Chen Ch, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion, water pollution. Environ Sci Technol 45(24):10454–10462
Zheng L, Mingyu S, Xiao W, Chao L, Chunxiang Q, Liang C, Hao H, Xiao-qing L, Fashui H (2008) Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Element Res 121:69–79
Zhou P, Adeel M, Shakoor N, Guo M, Hao Y, Azeem I, Li M, Liu M, Rui Y (2021) Application of nanoparticles alleviates heavy metals stress and promotes plant growth: an overview. Nanomaterials 11(1):26
Author information
Authors and Affiliations
Contributions
BA, and AZ conceived the idea. BA, AZ, and FZ wrote the manuscript. FB, and TAD helped in revising the paper. All authors read and approved the MS for submission.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, B., Zaid, A., Zulfiqar, F. et al. Nanotechnology: a novel and sustainable approach towards heavy metal stress alleviation in plants. Nanotechnol. Environ. Eng. 8, 27–40 (2023). https://doi.org/10.1007/s41204-022-00230-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41204-022-00230-8