Abstract
Lead (Pb) is among the most abundant toxic trace elements which causes direct and indirect negative effects on humans, animals, and plants. Thus, there is a need to alleviate the Pb toxicity in plants for good quality food production especially from marginal soils. In this study, the effects of silicon nanoparticles (Si NPs) were investigated on coriander (Coriandrum sativum L.) biomass, vitamin C, flavonoid, antioxidant enzyme activities (i.e., catalase (CAT), peroxidase (POD), and super oxide dismutase (SOD)), malondialdehyde (MDA), and Pb concentration in plants subjected to different Pb concentrations. Treatments included four levels of Pb (0, 500, 1000, and 1500 mg/kg of soil), and two levels of Si NPs (0 and 1.5 mM) in all combinations. The Pb treatments alone decreased the plant biomass and vitamin C while increased the flavonoid, MDA, antioxidant enzyme activities, and Pb concentration in tissues depending upon the Pb treatments. The foliar-applied 1.5 mM Si NPs alleviated the adverse impacts of Pb on coriander plants which were due to the minimization of Pb concentration in plants and improvements in the plant defense system. Si NPs minimized accumulation of MDA in plant tissues and adjusted the activities of POD, CAT, and SOD in plants under Pb stress. Overall, Si NP foliar application might be a suitable approach in reducing the Pb concentrations in plants. However, field studies with various plant species and environmental conditions are required to highlight the role of Si NPs on the plant under toxic trace element stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) destructiveness in living organisms has been able to be a real concern, which is basically due to the use of Pb-contaminated soil everywhere throughout the world (Norton et al. 2014; Xie et al. 2017). Extreme Pb in soil has harmful impacts on soil microbial action and development of plants (Iqbal et al. 2015; Kushwaha et al. 2018). Pb impaired the plant growth of plants at morphological, physiological, and molecular levels (Kaur et al. 2015; Singh et al. 2016). Pb, a non-redox active element, may alter the redox homeostasis in plants via several indirect mechanisms such as replacement of essential elements from cellular biomolecules and variation in metal-related enzyme activities, which increased the production of reactive oxygen species (ROS) in plants (Chen et al. 2017; Shahid et al. 2014; Shu et al. 2012). Therefore, any disruption of redox homeostasis in plants may be the cause of oxidative burst in plants (Adrees et al. 2015a; Kaur et al. 2015). Thus, persistence of oxidative stress environment in plants may be due to physiological disorders in plants which ultimaltey the cause of cell death (Keunen et al. 2011; Adrees et al. 2015b). Pb may accumulate in soil mainly via anthropogenic activities such as use of sewage sludge, and waste water and subsequently enters to food crops mainly vegetables which is due to the cultivation of vegetables near highways and industrial regions. The higher Pb concentrations have been reported in the leafy vegetables than the other vegetables (Feleafel and Mirdad 2013; Rizwan et al. 2018).
Nanoparticles (NPs) have been generally used to improve the human life in various fields (Karimi and Mohsenzadeh 2016; Rizwan et al. 2017a). The application of nanotechnology has been gaining greater attention in recent decades for remediation of contaminated agricultural soils and might be considered one of the important approaches aiming to use for the treatment of metal polluted soils (Ali et al. 2019; Hussain et al. 2019). Nanoparticles have unique characteristics such as high sorption capacity and large surface area which make these NPs different from their bulk materials. The NPs can pass through the plant cell walls and may enter into the plants or NPs may dissociate at the surface of plants and enter into the plants depending upon the conditions and NP types and sizes along with other factors (Rameshaiah et al. 2015; Rizwan et al. 2019). Various published studies demonstrated the potential efficiency of NPs in plants especially in cereals when they were simultaneously exposed to NPs and toxic trace elements (Hussain et al. 2018; Rizwan et al. 2016). Among the different NPs, the nanoparticles of silicon (Si NPs) can improve the photosynthesis and plant biomass under abiotic-stressed conditions (Hussain et al. 2019; Siddiqui et al. 2020). Although it was depicted Si can minimize the metal toxicity in plants, the easily bioavailable sources of Si are not explored at large scale (Adrees et al. 2015b). Thus, there is still a need to explore the bioavailable Si sources which can be easily used in agriculture (Keller et al. 2015; Rizwan et al. 2017b). Silicon can be used in NP form for agricultural purposes which can be considered a novel source of this element (Hussain et al. 2019). Due to wide attention in nanotechnology in recent years, Si NPs can increase biomass quantity and quality and decrease evaporation and transpiration in plants; also, it stimulates production of some antioxidant enzymes and reduces the susceptibility of crops to some fungal diseases, most projects respecting the use of Si NPs in soil conditions (Tripathi et al. 2015; Wang et al. 2015). Moreover, most of the studies have highlighted the effect of Si NPs on element uptake by rice (Cui et al. 2017; Wan et al. 2016), and wheat (Hussain et al. 2019), but less information is available in literature concerning the impacts of Si NPs on other important food crops such as coriander (Coriandrum sativum L.) plants. In addition, short-term studies were mainly conducted to highlight the impact of Si NPs in plants under stressful environments (Cui et al. 2017).
Coriander is one of the annual aromatic plants which belongs to Apiaceae family, known as herb and medicinal plants with special roles in human life. Coriander leaves are a good source of chemicals including vitamin C (160 mg per 100 g fresh weight), vitamin A (beta-carotene 12 mg per 100 g fresh weight), vitamin B12 (60 mg per 100 g), polyphenols, and essential oils (Prakash 1990). Coriander is used in the food industry, and as an essential oil in the pharmaceutical, cosmetic, and sanitary industries, and as fruit oil in the food and pharmaceutical industries (Sefidkon 1999). Considering that coriander is one of the most widely used vegetables and a demanded medicinal plant, its extensive cultivation in unfavorable conditions and the use of unconventional water for irrigation are unavoidable. Such situations have faced the plant to be exposed to the dangers of heavy elements, especially Pb. Since that, antioxidants are one of the important mechanisms of plant against toxic trace element stress; therefore, the present study was performed to explore the effects of Si NPs on enzymatic and non-enzymatic antioxidant and development of coriander under a soil condition with high Pb contents.
Materials and methods
Plant cultivation
In this experiment, air-dried soil (38° 25′ N, 48° 30′ E and 1500 m above the sea level) was spiked with a solution of either one of four amounts (0, 500, 1000, and 1500 mg/kg) of PbCl2 and the soil was incubated under stable conditions (darkness, 40 °C, for 6 months in dry/wet cycle). The soil was sandy loam having pH of 7.3, 1.45% organic carbon, and EC of 2.0 dSm-1. Then, 30 healthy and uniform seeds of selected coriander variety were planted and cultivated in each plastic box with 10 kg soil (40 cm × 25 cm × 20 cm). The seed was prepared from native cultivar from Nahavand, Iran, because the city is considered the biggest producers of coriander in the country. The pots were placed in a greenhouse and regularly irrigated, when necessary. The temperature in the greenhouse during the experiment was 25–32 °C. The Si NPs were of Sunny company, Iran, with the purity of 99%, particle size of 20–35 nm, and active level of 461 g/m2. The weighed amount Si NPs was added in small quantity of deionized water and these NPs were dispersed with the help of ultra-sonication for about 30 min, and final volume was made for further use. During the cultivation period for 12 weeks, plants were foliar-applied with a solution containing either without or with 1.5 mM of Si NPs for three times (both side of leaves) at an interval of 2 weeks. After harvesting (4 months after seeds sowing), the plant samples were washed with the help of distilled water, and then, these samples were divided into two parts. One part of the samples was frozen by using liquid N2 prior to biochemical and enzyme activity measurements while the subsample was dried at 80 °C in an oven for dry weight measurement. The samples were dried for about 48 h until constant dry weight was achieved. The samples were weighted and ground with a Retsch mixer mill and used for Pb measurements.
Vitamin C contents in shoots
For the measurement of vitamin C, coriander leaves were used according to the procedure of Omaye et al. (1979). For this purpose, 1 g of powdered samples was digested in 1 ml of trichloroacetic acid 10%, and for 20 min at 2100 rpm. Ten microliters were taken from the illuminating solution, and 1 ml of dihydrofenylhydrazine-copper sulfate-thiora 6-mM reagent was added and placed in a thermometer at 31 °C for 3 h. Then, 110 μl of 41% sulfuric acid were added to the samples and the absorption of samples and standard was taken at 120 nm wavelength by a 6705-genius spectrophotometer. Vitamin C content was calculated based on the standard curve of milligrams of ascorbic acid per 100 g of weight.
Flavonoid content
Flavonoid contents were measured by the method of Beketov et al. (2005). In brief, 0.2 ml of extract was mixed with 4.5 ml ethanol 90%, 0.2 ml of aluminum chloride (2%), and 0.1 ml acetic acid (33%), and then, the mixture was placed in dark for 30 min. After this, the absorbance was measured at 414 nm with a spectrophotometer (Model: 6850, Jenway, UK).
Activities of antioxidant enzymes and MDA level
Activity of glutathione peroxidase (POD) was assessed according to the protocol of Polle et al. (1994). In brief, 3 ml of the sample includes 100 mM buffer solution of potassium phosphate (pH = 7.0), 20 mM guaiacol, 10 mM H2O2, and 50 ml of an enzyme extract. The absorbance was recorded by guaiacol oxidation at 470 nm for 3 min. Finally, the POD activities were assessed according to coefficient of 2.8 mM-1 cm-1 and denoted as μMol/g FW min.
Measurement of SOD activity was performed in a 1.5-ml mixture comprised of 50 mM phosphate buffer (pH = 7.0), 0.1 mM EDTA, 13 mM methionine, nitrotetrazolium (NBT), riboflavin, and 10 μL sample. 560-nm wavelength was used for the measurement of absorbance by a spectrophotometer (Giannopolitis and Ries 1977).
The catalase (CAT) activity was assessed according to the procedure of Chance and Maehly (1955) with little modifications. The reaction mixture for CAT (3 ml) was comprised of enzyme extract (0.1 ml), phosphate buffer (50 mM at pH 7.0), and 15 Mm H2O2. The reaction was started by using enzyme extract. The variation in the absorbance of the reaction mixture was recorded at 240 nm after every 20 s. One unit of the CAT activity was comprised of the change in absorbance of 0.01 unit min-1. This enzyme activity was measured by measuring the oxidation of ascorbate by a spectrophotometer at 290 nm for 1 min. The reaction solution contained a 25 mM phosphate buffer, 0.1 EDTA, 1.0 mM H2O2, 0.25 mM ascorbate peroxidase, and enzyme extract.
The determination of malondialdehyde (MDA) level was performed by the protocol of Heath and Packer (1968). In brief, 0.2 g of fresh samples were weighed and homogenated in water with 0.5 ml of 0.1% trichloroacetic acid (TCA), followed by centrifugation at 5,000 rpm for 5 min. The reaction mixture was consisted of 1 ml of supernatant, 4 ml of 20% trichloroacetic acid solution comprised of 0.5% thiobarbituric acid (TBA). The resultant solution was heated at 95 °C for 30 min. Then, it was immediately put in ice and centrifuged at 5,000 rpm for 10 min. The intensity of light absorption was noted at 523 nm by a spectrophotometer. The amount of enzyme activity was calculated in 1 min/ml of protein. An enzyme activity unit was a small amount of NBT from the enzyme, which caused 50% reduction in inhibition at 560 nm.
Concentration of Pb
To determine Pb concentrations in roots and shoots of coriander plants, samples were kept in 80 °C oven and then were ground with pestle and mortar. In this step, the sample was digested and heat. Pb was analyzed using an atomic absorption spectrophotometer (GTALLO, model Varian, Model Spectra AA 200, USA).
Statistical analysis
All data obtained were analyzed with a two-way ANOVA with a statistical program (SAS 9.4, SAS institute, USA). Means were separated by the Duncan test and p values less than 0.05 were considered statistically significant.
Results
According to our results, the effect of Pb and Si and interaction effect of Si NPs and Pb have significant effect on fresh and dry biomass, photosynthesis pigment, and antioxidant enzyme, proline, carbohydrate, MAD, and Pb concentration.
Effect of NPs on biomass
The results showed that Pb concentration and Si NP treatments had a significant effect on the biomass of coriander. Plants grown in contaminated soil, especially in high concentrations of Pb (1500 mg/kg), had dramatically lower biomass, almost 60% compared with the control plant. Si NPs have positive effect on biomass especially under Pb stress. Foliar application of Si NPs has beneficial effect on fresh biomass, and the highest value was 70% more than the control, and it obtained in the treatment of 0 mg/kg Pb in the soil combined with 1.5 mM Si foliar application (0.70 g) (Fig. 1). The lowest plant biomass was found in the plant treated by 1500 mg/kg Pb in the soil without any Si NPs application, and it was 56% lower than the control.
Interaction of Si NPs and Pb biomass in coriander plants. Treatments included Pb (0, 500, 1000, and 1500 mg/kg) and Si-NPs (– Si (white) and + Si (black)) represent foliar application with 0 or 1.5 mM Si NPs. Bars are means of four plants per replicates. Bars with different letters differ significantly from each other at p < 0.05 as determined by LSD test
Flavonoid, vitamin C, and anthocyanin content
The data related to flavonoid contents were reported in Fig. 2. The results revealed that the Pb stress increased this trait compared with the control plants, almost 33% in plant treated 1500 mg/kg. The Si NPs increased the flavonoid content by 13, 10, 8, and 2% in 0, 500, 1000, and 1500 mg/kg treatment compared with these Pb treatments without NPs, respectively. Vitamin C in coriander plant was also affected by Pb concentrations, and it was observed by 15 % destruction in 1500 mg/kg compared with control plant. Although Si NPs increased this treated, but it was not significant in most of the treatments (Fig. 2b). The results regarding anthocyanin content in coriander were reported in Fig. 2c. The Pb caused a significant increase in anthocyanin until 500 mg/kg which was almost 93% compared with control, and after that, 1000 and 1500 mg/kg treatment showed a decreasing trend compared with 500 mg/kg. The Si NPs have not influenced on anthocyanin content in coriander plants under Pb stress.
Interaction of Si NPs and Pb biomass on a flavonoid, b vitamin C in coriander plants. Treatments included Pb (0, 500, 1000, and 1500 mg/kg) and Si-NPs (– Si (white) and + Si (black)) represent foliar application with 0 or 1.5 mM Si NPs. Bars are means of four plants per replicates. Bars with different letters differ significantly from each other at p < 0.05 as determined by LSD test
Activities of antioxidant enzymes
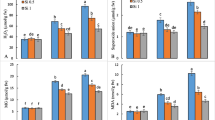
In this study, antioxidant enzyme, POD, CAT, and SOD activities were also measured. The results indicated that Pb stress affected all enzyme activities either alone or under Si NPs application. POD activity significantly increased by Pb level until 1500 mg/kg (Fig. 3a). Si NPs statically diminished POD activity up to 9, 26, 5, and 47% under 0, 500, 1000, and 1500 mg/kg Pb stress conditions, respectively. Nevertheless, Pb and Si NPs interaction in 500 and 1000 mg/kg did not show a significant effect.
Interaction of Si NPs and Pb biomass on a POD, b CAT, c SOD, and d MDA in coriander plants. Treatments included Pb (0, 500, 1000, and 1500 mg/kg) and Si-NPs (– Si (white) and + Si (black)) represent foliar application with 0 or 1.5 mM Si NPs. Bars are means of four plants per replicates. Bars with different letters differ significantly from each other at p < 0.05 as determined by LSD test
CAT activity increased until 500 mg/kg of Pb, while decreased in higher Pb concentration (Fig. 3b). Si NPs decreased the CAT activity under 0, 500, and 1000 mg/kg Pb by 24, 37, and 75%; however, Si NP exposure increased enzyme activity almost 124% at Pb concentration of 1500 mg/kg By increasing in Pb concentration, SOD activity increased significantly but not dramatically, particularly when Pb was present, and Si NPs applied enzyme activity almost was 237, 57, and 15% at 0, 500, and 1000 mg/kg Pb respectively.
Malondialdehyde content
Malondialdehyde content dramatically increased by Pb concentration, as it increased until 1000 mg/kg of Pb, but after that decreased. The interaction effect between Si NPs and Pb for this parameter was significant. Foliar application of Si NPs decreased the MDA content (Fig. 3c), but at the highest concentration of Pb, there is no significant effect between with or without Si application.
Pb concentration
Figure 4 shows that the Pb concentration was higher in root than shoot. Si NPs effectively decreased the Pb concentration in roots by 22, 11, and 10%, respectively compared with related control. Also, Pb concentration decreased in shoots by 2, 5, and 21%, respectively in 500, 1000, and 1500 mg/kg Pb.
Interaction of Si NPs and Pb biomass on a Pb concentration in root, b Pb concentration in shoot in coriander plants. Treatments included Pb (0, 500, 1000, and 1500 mg/kg) and Si-NPs (– Si (white) and + Si (black)) represent foliar application with 0 or 1.5 mM Si NPs. Bars are means of four plants per replicates. Bars with different letters differ significantly from each other at p < 0.05 as determined by LSD test
Discussion
The Pb is one of the non-essential elements for crops but is accumulating in the soils due to human activities which is serious thread to agriculture (Parys et al. 2014; Silva et al. 2017; Rizwan et al. 2018). In general, Pb exists in various forms in the soil including free metal ions or complexes with organic and inorganic materials. Pb toxicity is related to many factors including concentration, species chemical form (Kroukamp et al. 2016). Our results indicated that Pb had adverse effects on plant biomass dramatically (Fig. 1). It is clear that both biomass and photosynthesis are related to each other while heavy metal stress decreased the photosynthesis and cell division (Dallas and Ho 2005; Keller et al. 2015) by altering the performance of Calvin cycle enzymes and the electron transport chain and also damaged the stomata cells (Souza et al. 2005) and thus greatly reduce the biomass. Additionally, declining in biomass due to Pb stress has been revealed in Brassica napus (Shakoor et al. 2014), and water hyacinths (Malar et al. 2016) and coriander (Fatemi et al. 2020a, b). In this study, Si NPs helped the plants to alleviate the negative effect of Pb (Fig. 1). A similar improvement in plant biomass was reported in many studies under the application of Si NPs and metal stress (Asgari et al. 2018; Tripathi et al. 2015; Hussain et al. 2019). Plant biomass seems to be affected positively by Si NPs which might be due to its placement in the leaf bundle which would affect the structure of chloroplasts and leaf yield at the unit level and thus increase the plant’s ability to use light. Some researchers also noted this increase in biomass as the effect of Si on improving the efficiency of photosystems II (Torabi et al. 2013; Adrees er al. 2015b). Some studies demonstrated the recovering of plant biomass by using Si NPs under stressed conditions (Asgari et al. 2018; Wang et al. 2015; Rizwan et al. 2019). Si NPs may enhance the growth of metal-stressed plants by improving the nutritional status, photosynthesis, and morphology and physiology of crops (Hussain et al. 2019; Keller et al. 2015).
The MDA content increased in the studied Pb-stressed plants, which is sign of peroxidation of lipids via Pb toxicity for coriander plants, and our results further suggest that Si NPs alleviated the oxidative stress (Fig. 3). Si NPs have beneficial effect in plants via improving enzymatic and non-enzymatic antioxidant and decreasing MDA. Similar results have been shown in cucumber plant under Mn stress (Mishra et al. 2006), cotton plant under Pb stress (Bharwana et al. 2013) and also spinach, barley, and tomato (Zhang and Selim 2008). Reddy et al. (2005) conducted that one of the important damaging of Pb is the ROS production in plants. ROS destroys plasma membrane via different mechanisms such as composition alteration of lipids in membrane, combination with thiols in membrane, and destruction of transporter in membrane. Previous studies indicated that Pb stress increased the oxidative stress in numerous plant species under various experimental conditions (Hattab et al. 2016; Rizwan et al. 2018). The results demonstrated that Si NPs enhanced the antioxidant system which may protect the plants under Pb stress. Similarly, Si supply minimized the H2O2 and MDA contents in different plant species (spinach, rice, and wheat) under various metal toxicities (Chalmardi et al. 2014; Gunes et al. 2007; Song et al. 2011; Tripathi et al. 2013).
Antioxidants are one of the important and critical mechanisms in plants under abiotic stress; there are two kinds of important antioxidants such as enzymatic and non-enzymatic (Gill et al. 2015; Suzuki et al. 2012). Changing of the antioxidants during Pb-stressed condition along with Si NPs has been rarely reported especially in leafy plants like coriander. According to our result, vitamin C was greatly affected by Pb stress in coriander plants (Fig. 2) and Pb stress decreased vitamin C in plants dramatically (Leiva-Brondo et al. 2012; Rizwan et al. 2018). Vitamin C mainly functions in plants as an antioxidant and cofactor in many redox reactions. In addition, vitamin C can control cell differentiation along with other functions (Fenech et al. 2019). The vitamin C is strongly altered by stressful conditions. For human health, vitamin C is involved in many biochemical processes, and thus, the absorption of this vitamin is required for better growth (Wohlrab et al. 2017), but for this case probably, Pb deactivate enzyme involved in vitamin C production. Our findings have shown that flavonoids, one of the important phenolic compounds, increased by increasing the Pb levels (Fig. 2). Phenolic compound is important antioxidant under stressful condition that this compound is produced by shikimic acid or phenyl propanoid pathways (Ren and Sun 2014). Phenolic compounds may act as antioxidants as the hydroxyl groups can donate hydrogen and can react with ROS in the termination reaction, which ultimately breaks the cycle of producing new radicals. The exogenous exposure of Si NPs further improved the production of flavonoid in Pb-stressed coriander plants which may enhance the tolerance against metal stress.
According to our results, antioxidant enzyme activities varied under Pb stress or Pb + Si NPs (Fig. 3). The alteration in enzyme activities due to the Pb stress is the typical response of the plants under stressed conditions (Rizwan et al. 2018). It plays a crucial role in preventing damage to the protein and membrane of the cell during the early stages of stress. By persistence of the stress and increase of the peroxide accumulation, cells would be damaged, and death may occur (Ibrahim and Bafeel 2011). Some studies highlighted that the high activities of enzymes are required for tolerance of plants to heavy metal exposure such as Pb (Li et al. 2012; Semane et al. 2007). Wang et al. (2010) stated that reducing germination of the seeds and decreasing seedling growth in Pb-contaminated soils was due to increased cell peroxidation and increased activity of enzymes such as SOD and POD, to reduce the harmful effects of hydrogen peroxide. Si NP foliar application decreased POD and SOD activities under all Pb treatments, and also, CAT activity decreased. This might be due to the removal of ROS in a non-enzymatic way by enhancing the activities of non-enzymatic antioxidants (Adrees et al. 2015a) or due to the enhancement of other antioxidant enzyme activities (Liu et al. 2013). Increasing antioxidant enzyme activities was also confirmed in other studies for many plants like the following: cotton, soybean, and ramie (Farooq et al. 2013; Miao et al. 2010; Tang et al. 2015). Plants suffer from oxidative stress when they are grown under stressful environmental conditions (Adrees et al. 2015b; Ali et al. 2015; Habibi 2015; Noman et al. 2015). It is indicated that Si supply may overcome the oxidative stress in plants by improving antioxidants that scavenge ROS in plants. This Si NP-mediated reduced ROS may reduce the injuries to cell membranes (Alzahrani et al. 2018)
Similarly, in our result, the growth of coriander was decreased under Pb toxicity, and this reduction was concentration-dependent. Pb is mainly entered the path of apoplastic or calcium ion channel to the root. Higher accumulation of Pb in root compared than shoot is also confirmed by other studies (Hou et al. 2014; Malar et al. 2016). Indeed, it is a defense mechanism by plant that accumulate Pb in root (Soares et al. 2016; Rizwan et al. 2018) and it depends on genotypic and environmental variations (Lal 2010). The accumulation of Pb in seedlings depends on its concentration in the culture medium. Also, the Pb accumulated in root part was more than leaves in coriender plants (Fig. 4); this is in agreement with other studies (Malar et al. 2016; Rizwan et al. 2018). Our results showed a decreasing in Pb absorption by plants with 1.5 mM Si NP foliar application in both root and leaf. The studies conducted that the Si coprecipitation with heavy metals in apoplast may decline the absorption of these metals, and then, adverse effects would be ameliorated (Iwasaki et al. 2002; Keller et al. 2015; Shi et al. 2018). Declining metal accumulation by plants via Si NPs was also confirmed by other studies (Ali et al. 2019; Fatemi et al. 2020a). This reduction in Pb uptake by coriander under Si NPs might be due to several mechanisms. For example, Si can trigger root exudates those can chelate with metals and decrease their uptake by plants (Kidd et al. 2001; Adrees et al. 2015b). Furthermore, Si may decrease free metals ions in plants (Iwasaki et al. 2002; Rogalla and Römheld 2002), and Si may reduce cell wall porosity (da Cunha and do Nascimento 2009). Overall, Si NP foliar spray might be helpful to reduce the adverse effects of Pb in coriander and probably in other plants.
Conclusions
Our results indicated that Si NPs have beneficial effect on plants especially under Pb stress. The obtained results suggested that the effect of Pb stress on the biomass and enzymatic parameters of the plant was varied with the levels of Pb applied. The alteration in activities of POD and CAT enzymes of leaves showed the effects of Pb toxicity and the production of free oxygen radicals. Si NPs minimized these toxic effects of Pb in plants by altering the physiological and metabolic processes of coriander plants. It seems enzymatic antioxidant affected more than non-enzymatic by Pb stress and Si NP applicant. It can be concluded that spraying with Si NPs could be used as a ration to reduce the harmful effects of Pb stress on coriander plants. Si NPs can adjust antioxidant enzyme activities and minimize the oxidative stress in plants. The most important mechanism of Si NPs is the reduction of the Pb concentration in plants and its transfer from roots to aerial parts of the plants.
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Rehman MZ, Irshad MK, Bharwana SA (2015a) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162
Adrees M, Ali S, Rizwan M, Rehman MZ, Ibrahim M, Abbas F, Farid M, Qayyum MF, Irshad MK (2015b) Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol Environ Saf 119:186–197
Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD (2015) Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ Sci Pollut Res 22:10601–10609
Ali S, Rizwan M, Hussain A, ur Rehman MZ, Ali B, Yousaf B, Wijaya L, Alyemeni MN, Ahmad P (2019) Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol Biochem 140:1–8
Alzahrani Y, Kuşvuran A, Alharby HF, Kuşvuran S, Rady MM (2018) The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol Environ Saf 154:187–196
Asgari F, Majd A, Jonoubi P, Najafi F (2018) Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiol Biochem 127:152–160
Beketov E, Pakhomov V, Nesterova O (2005) Improved method of flavonoid extraction from bird cherry fruits. Pharm Chem J 39:316–318
Bharwana S, Ali S, Farooq M, Iqbal N, Abbas F, Ahmad M (2013) Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J Bioremed Biodeg 4:1–12
Chalmardi ZK, Abdolzadeh A, Sadeghipour HR (2014) Silicon nutrition potentiates the antioxidant metabolism of rice plants under iron toxicity. Acta Physiol Plant 36:493–502
Chance B, Maehly A (1955) Assay of catalases and peroxidases. 764-775
Chen Q, Zhang X, Liu Y, Wei J, Shen W, Shen Z, Cui J (2017) Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul 81:253–264
Cui J, Liu T, Li F, Yi J, Liu C, Yu H (2017) Silica nanoparticles alleviate cadmium toxicity in rice cells: mechanisms and size effects. Environ Pollut 228:363–369
da Cunha KPV, do Nascimento CWA (2009) Silicon effects on metal tolerance and structural changes in maize (Zea mays L.) grown on a cadmium and zinc enriched soil. Water Air Soil Pollut 197:323
Dallas S, Ho G (2005) Subsurface flow reedbeds using alternative media for the treatment of domestic greywater in Monteverde, Costa Rica, Central America. Water Sci Technol 51:119–128
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf 96:242–249
Fatemi H, Pour BE, Rizwan M (2020a) Isolation and characterization of lead (Pb) resistant microbes and their combined use with silicon nanoparticles improved the growth, photosynthesis and antioxidant capacity of coriander (Coriandrum sativum L.) under Pb stress. Environ Pollut:114982
Fatemi H, Esmaielpour B, Sefidkon F, Soltani AA, Nematollahzadeh A (2020b) How mycorrhiza symbiosis help coriander (Coriandrum sativum L.) plants grow better under contaminated soil? J Plant Nutr:1–14
Feleafel MN, Mirdad ZM (2013) Hazard and effects of pollution by lead on vegetable crops. J Agri Environ 26(3):547–567
Fenech M, Amaya I, Valpuesta V, Botella MA (2019) Vitamin C content in fruits: biosynthesis and regulation. Front Plant Sci 9:2006
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill RA et al (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Gunes A, Inal A, Bagci E, Coban S, Pilbeam D (2007) Silicon mediates changes to some physiological and enzymatic parameters symptomatic for oxidative stress in spinach (Spinacia oleracea L.) grown under B toxicity. Sci Hortic 113:113–119
Habibi G (2015) Effects of soil-and foliar-applied silicon on the resistance of grapevine plants to freezing stress. Acta Biol Szegediensis 59:109–117
Hattab S, Hattab S, Flores-Casseres ML, Boussetta H, Doumas P, Hernandez LE, Banni M (2016) Characterisation of lead-induced stress molecular biomarkers in Medicago sativa plants. Environ Exp Bot 123:1–12
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hou X, Liu A, Call L, Zhou C, Wu P, Zou X, Ma X (2014) Effects of Pb stress on growth and Pb accumulation of Paspalum notatum. J Agro-Environ Sci 4
Hussain A, Ali S, Rizwan M, Rehman MZ, Javed MR, Imran M, Chatha SA, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut 242:1518–1526
Hussain A, Rizwan M, Ali Q, Ali S (2019) Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ Sci Pollut Res 26:7579–7588
Ibrahim MM, Bafeel SO (2011) Molecular and physiological aspects for Lepidium sativum tolerance in response to lead toxicity. Fresenius Environ Bull 20:1871–1879
Iqbal MM, Murtaza G, Saqib ZA, Rashid A (2015) Growth and physiological responses of two rice varieties to applied lead in normal and salt-affected soils. Int J Agric Biol 17:1–10
Iwasaki K, Maier P, Fecht M, Horst WJ (2002) Leaf apoplastic silicon enhances manganese tolerance of cowpea (Vigna unguiculata). J Plant Physiol 159:167–173
Karimi J, Mohsenzadeh S (2016) Effects of silicon oxide nanoparticles on growth and physiology of wheat seedlings. Russ J Plant Physiol 63:119–123
Kaur G, Singh HP, Batish DR, Kohli RK (2015) Adaptations to oxidative stress in Zea mays roots under short-term Pb2+ exposure. Biol 70:190–197
Keller C, Rizwan M, Davidian JC, Pokrovsky O, Bovet N, Chaurand P, Meunier J-D (2015) Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 μM Cu. Planta 241:847–860
Keunen E, Remans T, Bohler S, Vangronsveld J, Cuypers A (2011) Metal-induced oxidative stress and plant mitochondria. Int J Mol Sci 12:6894–6918
Kidd P, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52:1339–1352
Kroukamp E, Wondimu T, Forbes PB (2016) Metal and metalloid speciation in plants: overview, instrumentation, approaches and commonly assessed elements. TrAC Trends Anal Chem 77:87–99
Kushwaha A, Hans N, Kumar S, Rani R (2018) A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol Environ Saf 147:1035–1045
Lal N (2010) Molecular mechanisms and genetic basis of heavy metal toxicity and tolerance in plants. In: Plant adaptation and phytoremediation. Springer, pp 35–58
Leiva-Brondo M, Valcárcel M, Cortés-Olmos C, Roselló S, Cebolla-Cornejo J, Nuez F (2012) Exploring alternative germplasm for the development of stable high vitamin C content in tomato varieties. Sci Hortic 133:84–88
Li L, Zheng C, Fu Y, Wu D, Yang X, Shen H (2012) Silicate-mediated alleviation of Pb toxicity in banana grown in Pb-contaminated soil. Biol Trace Elem Res 145:101–108
Liu M, Huang B, Bi X, Ren Z, Sheng G, Fu J (2013) Heavy metals and organic compounds contamination in soil from an e-waste region in South China. Environ Sci Process Impacts 15:919–929
Malar S, Vikram SS, Favas PJ, Perumal V (2016) Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot Stud 55:1–11
Miao B-H, Han X-G, Zhang W-H (2010) The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann Bot 105:967–973
Mishra S, Srivastava S, Tripathi R, Govindarajan R, Kuriakose S, Prasad M (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Noman A, Ali S, Naheed F, Ali Q, Farid M, Rizwan M, Irshad MK (2015) Foliar application of ascorbate enhances the physiological and biochemical attributes of maize (Zea mays L.) cultivars under drought stress. Arch Agron Soil Sci 61:1659–1672
Norton GJ et al (2014) Lead in rice: analysis of baseline lead levels in market and field collected rice grains. Sci Total Environ 485:428–434
Omaye ST, Turnbull JD, Sauberlich HE (1979) Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. In: Methods in enzymology, vol 62. Elsevier, pp 3–11
Parys E, Wasilewska W, Siedlecka M, Zienkiewicz M, Drożak A, Romanowska E (2014) Metabolic responses to lead of metallicolous and nonmetallicolous populations of Armeria maritima. Arch Environ Contam Toxicol 67:565–577
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol 106:53–60
Prakash V (1990) Leafy spices. CRC Press, Inc.
Rameshaiah G, Pallavi J, Shabnam S (2015) Nano fertilizers and nano sensors–an attempt for developing smart agriculture. Int J Engin Res General Sci 3:314–320
Reddy AM, Kumar SG, Jyothsnakumari G, Thimmanaik S, Sudhakar C (2005) Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 60:97–104
Ren S-C, Sun J-T (2014) Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. J Funct Foods 7:298–304
Rizwan M, Ali S, Adrees M, Rizvi H, Rehman MZ, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Res 23:17859–17879
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Rehman MZ, Farid M, Abbas F (2017a) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater 322:2–16
Rizwan M, Ali S, Adrees M, Ibrahim M, Tsang DC, Rehman MZ, Zahir ZA, Rinklebe J, Tack FM, Ok YS (2017b) A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 182:90–105
Rizwan M, Ali S, Rehman MZ, Javed MR, Bashir A (2018) Lead toxicity in cereals and its management strategies: a critical review. Water Air Soil Pollut 229:1–16
Rizwan M, Ali S, Rehman MZ, Malik S, Adrees M, Qayyum MF, Alamri SA, Alyemeni MN, Ahmad P (2019) Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol Plant 41:1–9
Rogalla H, Römheld V (2002) Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ 25:549–555
Sefidkon F (1999) Effect of essential oil in shoot and fruit of coriander. Iran J Med Aromat Plant 13:32–38
Semane B, Cuypers A, Smeets K, Van Belleghem F, Horemans N, Schat H, Vangronsveld J (2007) Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol Plant 129:519–528
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E (2014) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. In: Reviews of Environmental Contamination and Toxicology, vol 232. Springer, pp 1–44
Shakoor MB et al (2014) Citric acid improves lead (Pb) phytoextraction in Brassica napus L. by mitigating Pb-induced morphological and biochemical damages. Ecotoxicol Environ Saf 109:38–47
Shi Z, Yang S, Han D, Zhou Z, Li X, Liu Y, Zhang B (2018) Silicon alleviates cadmium toxicity in wheat seedlings (Triticum aestivum L.) by reducing cadmium ion uptake and enhancing antioxidative capacity. Environ Sci Pollut Res 25:7638–7646
Shu X, Yin L, Zhang Q, Wang W (2012) Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environ Sci Pollut Res 19:893–902
Siddiqui H, Ahmed KBM, Sami F, Hayat S (2020) Silicon nanoparticles and plants: current knowledge and future perspectives. In: Sustainable Agriculture Reviews 41. Springer, pp 129–142
Silva S, Pinto G, Santos C (2017) Low doses of Pb affected Lactuca sativa photosynthetic performance. Photosynthetica 55:50–57
Singh A, Parihar P, Singh R, Prasad SM (2016) An assessment to show toxic nature of beneficial trace metals: too much of good thing can be bad. Int J Curr Multidiscip Stud 2:141–144
Soares C, de Sousa A, Pinto A, Azenha M, Teixeira J, Azevedo RA, Fidalgo F (2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bot 122:115–125
Song A, Li P, Li Z, Fan F, Nikolic M, Liang Y (2011) The alleviation of zinc toxicity by silicon is related to zinc transport and antioxidative reactions in rice. Plant Soil 344:319–333
Souza JF, Dolder H, Cortelzaao A (2005) Influence of Mn toxicity on photosynthesis in Vigna umbellate seedlings. Phptosynthetica 38:449–453
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Tang H et al (2015) Effects of selenium and silicon on enhancing antioxidative capacity in ramie (Boehmeria nivea (L.) Gaud.) under cadmium stress. Environ Sci Pollut Res 22:9999–10008
Torabi F, Majd A, Enteshary SH, Ayrian S (2013) Study of effect of silicon on some anatomical and physiological characteristics of borage (Borago officinalis L.) in hydroponic conditions. J Cell Tissue 4:275–285
Tripathi P et al (2013) Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol Eng 52:96–103
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK, Rai AK (2015) Silicon-mediated alleviation of Cr (VI) toxicity in wheat seedlings as evidenced by chlorophyll florescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicol Environ Saf 113:133–144
Wan J et al (2016) Synthesis and evaluation of a new class of stabilized nano-chlorapatite for Pb immobilization in sediment. J Hazard Mater 320:278–288
Wang J, Li W, Zhang C, Ke S (2010) Physiological responses and detoxific mechanisms to Pb, Zn, Cu and Cd in young seedlings of Paulownia fortunei. J Environ Sci 22:1916–1922
Wang S, Wang F, Gao S (2015) Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ Sci Pollut Res 22:2837–2845
Wohlrab C, Phillips E, Dachs GU (2017) Vitamin C transporters in cancer: current understanding and gaps in knowledge. Front Oncol 7:74
Xie L et al (2017) The cadmium and lead content of the grain produced by leading Chinese rice cultivars. Food Chem 217:217–224
Zhang H, Selim H (2008) Reaction and transport of arsenic in soils: equilibrium and kinetic modeling. Adv Agron 98:45–115
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatemi, H., Esmaiel Pour, B. & Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid, and antioxidant enzyme activities of coriander (Coriandrum sativum L.) plants grown in lead (Pb)-spiked soil. Environ Sci Pollut Res 28, 1417–1425 (2021). https://doi.org/10.1007/s11356-020-10549-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10549-x