Abstract
The improvement of spinach growth is proved to relate to N2 fixation by nano-anatase TiO2 in this study. The results show that all spinach leaves kept green by nano-anatase TiO2 treatment and all old leaves of control turned yellow white under culture with N-deficient solution. And the fresh weight, dry weight, and contents of total nitrogen, \( {\text{NH}}^{ + }_{4} \), chlorophyll, and protein of spinach by nano-anatase TiO2 treatment presented obvious enhancement compared with control. Whereas the improvements of yield of spinach were not as good as nano-anatase TiO2 treatment under N-deficient condition, confirming that nano-anatase TiO2 on exposure to sunlight could chemisorb N2 directly or reduce N2 to NH3 in the spinach leaves, transforming into organic nitrogen and improving the growth of spinach. Bulk TiO2 effect, however, was not as significant as nano-anatase TiO2. A possible metabolism of the function of nano-anatase TiO2 reducing N2 to NH3 was discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a photocatalyst, nano-TiO2 under light could cause an oxidation-reduction reaction [1]. Therefore, we have firstly studied the relation between nano-TiO2 and photosynthesis. Our previous results showed that nano-TiO2 treatment could markedly promote aged seeds’ vigor and chlorophyll biosynthesis of spinach, particularly, the ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) activity and the photosynthesis efficiency. The nano-TiO2 treatment also has obvious effects on the improvement of growth and development in spinach. However, bulk TiO2 treatment shows little effect [2]. The researches on improving photosynthesis of spinach suggested that nano-TiO2 could increase light absorbance, accelerate transport and transformation of light energy, protect chloroplasts from aging, and prolong photosynthetic time of chloroplasts [3–5]. Our recent study proved that the complex of Rubsico and Rubisco activase could be induced in nano-TiO2-treated spinach, which promoted Rubsico carboxylation and increased the rate of photosynthetic carbon reaction [6].
Nitrogen is an important composition of chlorophyll, amino acid, protein, etc. The synthesis of those will be inhibited under nitrogen-deficient in vivo. Having an improvement of spinach growth and obvious increase of the chlorophyll and protein contents by nano-TiO2 treatment [2–5], the effects of nano-TiO2 on nitrogen metabolism of spinach were studied in our previous work [7]. It demonstrated that the activities of nitrate reductase, glutamate dehydrogenase, glutamine synthase, and glutamic-pyruvic transaminase were obviously increased by nano-TiO2 treatment. And nano-TiO2 treatment could promote spinach to absorb nitrate, accelerate inorganic nitrogen (such as \( {\text{NO}}^{ - }_{3} - {\text{N}} \) and \( {\text{NH}}^{ + }_{4} - {\text{N}} \)) to be transformed into organic nitrogen (such as protein and chlorophyll), and enhance spinach yield [7].
We hypothesize that the improvement of spinach growth and the increase of N-compound contents might be related to the generation of N2 fixation by nano-TiO2. As known to all, biological N2 fixation deoxidizes N2 to NH3 by nitrogenase [8–11]. Schrauzer et al. [12] demonstrated that bulk TiO2 (rutile) could reduce N2 to NH3 or N2H4 and C2H2 to C2H4 under ultraviolet radiation in the 1970s. Then they found that rutile in desert surface could reduce N2 from the air to NH3 and traces of N2H4 on exposure to sunlight [13]. This finding had an important theory and ecology significance. The total amount of N2 fixed by rutile was previously estimated to be 10 × 106 tons per year, corresponding to about a third of the N2 that is normally oxidized by lightning discharges and to about 10% of N2 reduced biologically. However, they did not investigate N2 fixation of rutile on plants under sunlight. According to their results, we hypothesize that nano-TiO2 in spinach leaves might reduce N2 to NH3 on exposure to sunlight, and accelerate it to be translated into organic nitrogen, thus promote spinach growth. But all of these still need to be proved by experiments. The reaction equations of rutile catalyzing N2 fixation advanced by Schrauzer are defined in Eqs. a, b and c [12, 13].



It is well-known that the quantum effect and the photocatalysis activity of nano-TiO2 are greater than bulk TiO2, i.e., nano-TiO2 could induce an oxidation-reduction reaction and release an energetic electron under light, which might reduce N2 to NH3 directly. Therefore, we believe that the effect of catalyzing N2 fixation of nano-TiO2 might be more significant than that of bulk TiO2. The N2 fixation of nano-TiO2 in spinach leaves was studied in the paper. The results showed that all leaves of nano-TiO2 treatment kept green, one part of the old leaf surface turned green, and the other was yellowish white by bulk TiO2 treatment, whereas all old leaf surface of control displayed yellowish white under nitrogen-deficient condition, demonstrating that nano-TiO2 had N2-fixation effect in spinach leaves.
Materials and Methods
Material Treatment and Culture
Experimental material was Spinacia oleracea. The seeds were purchased from a local seed company. Nano-anatase TiO2 was prepared via controlled hydrolysis of titanium tetrabutoxide. The details of the synthesis are as follow [14]: colloidal titanium dioxide was prepared via controlled hydrolysis of titanium tetrabutoxide. In a typical experiment, 1 ml of Ti (OC4H9)4 dissolved in 20 ml of anhydrous isopropanol was added dropwise to 50 ml of double-distilled water adjusted to pH 1.5 with nitric acid under vigorous stirring at room temperature. Then, the temperature was raised to 60°C and kept 6 h for better crystallization of nano-TiO2 particles. The resulting translucent colloidal suspension was evaporated using a rotary evaporator yielding a nanocrystalline powder. The obtained powder was washed three times with isopropanol and dried at 50°C until complete evaporation of the solvent. The average grain size calculated from broadening of the (101) XRD peak of anatase (Fig. 1) using Scherrer’s equation was ca 5 nm. Spinach seeds were soaked with 0.25% nano-anatase TiO2 and bulk TiO2 suspension for 48 h at 10°C under light (1,200 μmol m-2 s-1 of light intensity), respectively, and with deionized water for control. And the seeds were whole surrounded with the suspension. Bulk TiO2 (rutile) was purchased from Shanghai Chem. Co.; the average grain size was 10–15 μm. Then, the seeds were carefully selected and planted in a perlite-containing pot and placed in porcelain dishes, which were respectively added with 500 ml of the following culture solutions: 1) Hoagland nutrient solution; 2) nitrogen-deficient Hoagland nutrient solution. Hoagland nutrient solution and nitrogen-deficient Hoagland nutrient solution were prepared as described in [9]. These were placed in a glasshouse under sunlight in spring (1,200 μmol m-2 s-1 of light intensity) for 35 days. Spinach seedlings in the stage of two leaves and three leaves were sprayed with 0.25% nano-anatase TiO2 and bulk TiO2 suspension, respectively, and deionized water for control.
Assay of Physiological and Biochemical Indexes
The fresh weight and dry weight of spinach were weighted at the 35th day. The contents of total nitrogen were measured by Kjeldahl’s method [15]. The chlorophyll contents were determined by Arnon’s method [16]. The protein contents were set out using the Lowry method [17]. The contents of \( {\text{NH}}^{ + }_{4} - {\text{N}} \) were measured as in earlier reports [18, 19]. The oxygen evolution of spinach leaves was measured with an Oxygraph oxygen electrode (Hansatech instruments, UK). The assay medium contained 0.5 M sorbitol, 10 mM KCl, 0.5 mM MgCl2, 0.05% (w/v) bovine serum albumin (BSA), 10 mM NaHCO3, and HEPES-KOH (pH 7.6).
Results and Discussion
Growth of Spinach Under N-Deficient Condition
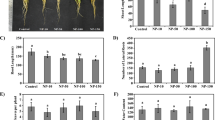
As shown in Fig. 2a, nano-anatase TiO2 could greatly improve spinach growth. The leaf area of spinach treated by nano-anatase TiO2 was larger than those of bulk TiO2-treated groups and the control under culture with Hoagland solution, respectively. The single fresh and dry weights of the nano-anatase TiO2-treated-groups were enhanced by 86.14% and 88.32% as compared with the bulk TiO2-treated groups, and by 91% and 99% as compared with control, respectively (Figs. 3 and 4).
From Fig. 2b, all leaves of nano-anatase TiO2 treatment kept green. After bulk TiO2 treatment, one part of the old leaf surface turned green, and the other was yellowish white. Whereas all old leaf surface of control displayed yellow white under culture with N-deficient Hoagland solution. We can see from Fig. 2b that the growth of spinach was inhibited under N-deficient condition, e.g., the single fresh weight and dry weight of control, bulk TiO2, and nano-anatase TiO2 treatment in N-deficient Hoagland solution were much lower than those of control in Hoagland solution (Figs. 3 and 4). Whereas the single fresh weight and dry weight of nano-anatase TiO2-treated spinach were as 3.7 and 2.0, 4.1 and 2.2 times those of control and bulk TiO2 treatment under N-deficient condition, respectively (Figs. 3 and 4), indicating that nano-anatase TiO2 treatment could decrease the inhibition of N-deficient on spinach growth.
As known to all, nitrogen is the main composition of chlorophyll, which cannot form without it. And it is known as chlorosis that the leaves of plants turn yellowish white. Nitrogen element in the old leaves can be transported to the young leaves under N-deficient condition. Therefore, it is the cardinal symptom of plants under N-deficient condition that the blade area is small, the old leaves turn yellowish white, the young leaves are green, and the plant is short [9]. All leaves were still green and obvious improvement of spinach growth by nano-anatase TiO2 treatment in N-deficient Hoagland solution, implying that nano-anatase TiO2 had a N2 fixation effect in spinach leaves. But bulk TiO2 effect was not as significant as nano-anatase TiO2. We believe that spinach yield fell greatly and the old leaves displayed chlorosis under N-deficient condition that is related to the inhibition of synthesis of organic nitrogen compounds (such as chlorophyll, protein) and the damage of photosynthesis closely.
The Contents of Total Nitrogen of Spinach Under N-Deficient Condition
To demonstrate N2 fixation of nano-anatase TiO2 in spinach leaves under sunlight, the experiments assayed the contents of total nitrogen of spinach. The results, in Fig. 5, showed that the contents of total nitrogen of spinach with nano-anatase TiO2 and bulk TiO2 treatment were 23.35% and 10.38% higher than that of control, separately, under culture with Hoagland solution. On the other hand, the contents of total nitrogen of spinach with nano-anatase TiO2 and bulk TiO2 treatment were 2.03 and 1.33 times that of control, respectively, under culture with N-deficient Hoagland solution. In Hoagland solution, the enhancement of total nitrogen contents of spinach with nano-anatase TiO2 and bulk TiO2 treatment was caused by the increase of absorption of inorganic nitrogen (which was proved by our previous studies). And it was also related to the generation of N2 fixation. However, the significant enhancement of total nitrogen contents of spinach with nano-anatase TiO2 and bulk TiO2 treatment were caused by N2 fixation under N-deficient condition, e.g., there was an obvious N2 fixation in spinach treated by nano-anatase TiO2. Although the treatments of spinach were different, the contents of total nitrogen of seeds were same.
The Contents of \( {\text{NH}}^{ + }_{4} - {\text{N}} \) and O2-Evolving Rate of Spinach Under N-Deficient Condition
\( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) of most plants was reduced from \( {\text{NO}}^{{\text{ - }}}_{{\text{3}}} - {\text{N}} \). Leguminous plants could form \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) by N2 fixation. It is shown in Fig. 6 that \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) contents of nano-anatase TiO2-treated spinach were decreased by 9.93% and 2.39% compared with those of bulk TiO2 and control in Hoagland solution. However, \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) contents of nano-anatase TiO2-treated spinach were increased by 9.48% and 17.51% compared with those of bulk TiO2 and control under culture with N-deficient Hoagland solution, respectively.
\( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) contents of nano-anatase TiO2-treated spinach were lower than those of the control under Hoagland solution condition, which is because it was related to the enhancement of activity of key enzymes in ammonia assimilation and make \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) transform to organic nitrogen (such as proteins and chlorophyll) quickly, which was proved by our previous work [7].
There was no nitrogen nutrition (such as \( {\text{NO}}^{{\text{ - }}}_{{\text{3}}} - {\text{N}} \)) in N-deficient Hoagland solution. Thus, on the one hand, \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) in spinach came from \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) accumulation and the reduction of \( {\text{NO}}^{{\text{ - }}}_{{\text{3}}} - {\text{N}} \) in seeds. On the other hand, it also might come from N2 fixed in spinach leaves by nano-anatase TiO2 and bulk TiO2.
It is well-known that photosynthesis is composed of photocatalyzed chemical reaction and enzyme-catalyzed chemical reaction. Oxygen evolution is an important part of photosynthesis, and the photosynthesis is inhibited under N-deficient environment. Figure 7 displayed that nano-anatase TiO2 and bulk TiO2 treatment could significantly increase the oxygen-evolving rate of spinach chloroplast in Hoagland solution, which was enhanced by 43.41%, 16.95% compared with control. However, the oxygen-evolving of spinach was seriously inhibited under culture with N-deficient Hoagland solution, e.g., the oxygen-evolving rates of control, bulk TiO2 treatment and nano-anatase TiO2 treatment were 54%, 65.14%, and 85.61% of that of control under culture with Hoagland solution. But it can be seen that the oxygen-evolving rate of nano-anatase TiO2-treated spinach was higher than that of control and bulk TiO2 treatment under N-deficient condition, demonstrating that nano-anatase TiO2 treatment could significantly decrease the inhibition of N-deficient on the oxygen evolution of spinach.
Our previous work confirmed that the enhancement of oxygen evolution rate from nano-anatase TiO2-treated spinach was closely related to the enhancement of light absorption and photochemical reaction activity, which was consistent with improvement of chlorophyll synthesis and sensitized nano-anatase TiO2 by chlorophyll in chloroplast [2–6, 20]. Nano-anatase TiO2 and bulk TiO2 can promote the pigments of chloroplast absorbing and transferring light energy, and excite energy-enriched electrons, which cause water photolysis [3–5].
Considering \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} - {\text{N}} \) formation and the enhancement of oxygen-evolving rate of spinach by nano-anatase TiO2 and bulk TiO2 treatment, it can be explained by the reaction equation according to Schrauzer’s researches defined as in Eq. d [12, 13].

The reaction equation is linked to the ability of nano-anatase TiO2 and bulk TiO2 to chemisorb both H2O and N2 and closely related to the photolysis of H2O in spinach leaves. The photolysis of H2O on nano-TiO2 and bulk TiO2 has been described in line with current concepts of the electronic of semiconducting solid [1, 21, 22]. Upon illumination with near-UV light electrons from the valence band are excited into the lowest conduction band; the band gap of rutile is in the order of 2.9–3.2 eV (390–420 nm) [23], corresponding to between 70 and 80 kcal, thus sufficient for reaction to occur as defined in Eq. e [12, 13].

The positive holes generated in the valence band provide the sites for oxygen production or other oxidation reactions in spinach chloroplast. The electrons of nano-anatase TiO2 and bulk TiO2 in the conduction band can be utilized for the reduction of substrates such as H+ and N2 in spinach chloroplast. It is also possible, however, to describe the electrons in the conduction band as excited titanium atoms in lower oxidation states at which the reduction of the substrates occurs. N2 inhibits the H2 evolution and thus may be assumed to be reduced at the same site or sites in spinach chloroplast. As H2 is formed in reactions involving the transfer of two electrons and the production of C2H4 from C2H2 is inhibited by N2, Schrauzer assumed that chemisorbed N2 is reduced, at least in part, via chemisorbed diimide [12, 13].
As mentioned above, the oxygen-evolving rate of spinach with nano-anatase TiO2 and bulk TiO2 treatment was higher than that of control either in Hoagland solution or in N-deficient Hoagland solution. And the NH3 formation and the oxygen evolution of nano-anatase TiO2-treated spinach were more significant than that of bulk TiO2-treated spinach, because the average grain size of nano-anatase TiO2 (5 nm) is much smaller than that of bulk TiO2, which entered spinach cell more easily.
Considering the improvement of spinach growth and oxygen evolution by nano-anatase TiO2 treatment, we believed that nano-anatase TiO2 entered spinach cell and bound to thylakoid membrane of chloroplast, especially photosystem II (PSII) or chlorophyll, and sensitized nano-anatase TiO2 by chlorophyll in chloroplast. Li et al. showed that the sensitized nano-TiO2 (rutile or anatase) by chlorophyll in vitro could strongly absorb light not only in ultraviolet region, but also in visible region from 400 to 800 nm [20]. PSII is capable of water splitting and oxygen evolution, which is a key site of electron transport. Therefore, N2 fixation and oxygen evolution of nano-anatase TiO2 in spinach chloroplast are schemed as follows (Scheme 1): two membrane complexes—PSII and Cytb6f—are shown. The transfer of electrons from nano-TiO2 by Nano-TiO2 entered spinach cell and bound to photosystem II (PSII) or chlorophyll directly, and sensitized nano-anatase TiO2 by chlorophyll in chloroplast. Nano-TiO2 chemisorbed N2 and H2O, N2 is reduced to NH3 and H2O is photolyzed to O2 upon exposure to sunlight. Parts of electrons from nano-TiO2 by oxidation-reduction reaction are transferred to PSII reaction center and then to other electron carriers, such as PQ (plastoquinone), Cytb6f (cytochrome b6f complex), and PC (plastocyanin). The reaction center chlorophyll of PSII, P680, undergoes light-induced oxidation to produce the strong oxidant, P680+, which can oxidize water, releasing O2. The electrons from P680 are transferred to PQ, Cytb6f, PC, etc. Accompanying these electron transfers, a proton gradient is established across the membrane. This electrochemical gradient is ultimately utilized for the synthesis of ATP by the ATP synthase. Oxidation-reduction reaction is illustrated with yellow lines; the transfer of electrons produced from water is illustrated with red lines; the translocation of proton is illustrated with blue lines.
The Protein and Chlorophyll Contents of Spinach Under N-Deficient Condition
To further prove inorganic nitrogen transformation, the protein and chlorophyll contents of spinach were assayed. The results in Fig. 8 indicated that the chlorophyll contents of nano-anatase TiO2-treated spinach and bulk TiO2-treated spinach were 37.48% and 13.34% higher than those of control in Hoagland solution. However, the chlorophyll contents of spinach were inhibited seriously under N-deficient condition, e.g., the chlorophyll contents of control; bulk TiO2- and nano-anatase TiO2-treated spinach were as much as 94.51%, 64.01%, and 31.81% less than those of the control under Hoagland solution, respectively. On the other hand, the chlorophyll contents of nano-anatase TiO2-treated spinach under N-deficient condition were higher than those of control and bulk TiO2-treated spinach, which lead to keeping green of all the leaves of nano-anatase TiO2-treated spinach. It was obvious that the chlorophyll formation of spinach by nano-anatase TiO2 treatment under N-deficient condition was caused by the N2 fixation that occurred in spinach. There was little chlorophyll in control leaves under N-deficient condition, which was formed by parts of nitrogen in seeds, and led to chlorosis of spinach leaves. The increase of chlorophyll contents in spinach leaves with bulk TiO2 treatment also came from N2 fixation under culture with N-deficient Hoagland solution.
The increase of the protein contents of nano-anatase TiO2- and bulk TiO2-treated spinach under Hoagland solution is observed in Fig. 9, which enhanced by 17.55% and 6.33%, respectively, suggesting that nano-anatase TiO2- and bulk TiO2-treatment could promote the synthesis of protein. It can also be seen from Fig. 9, the synthesis of protein in spinach was also inhibited seriously under N-deficient Hoagland solution, whereas the reduction of the protein contents of nano-anatase TiO2-treated spinach was lower than that of control and bulk TiO2-treated spinach, i.e., the protein contents of control, bulk TiO2- and nano-anatase TiO2-treated spinach under N-deficient condition had a reduction of 77.03%, 61.61%, and 31.77% compared with those of control under Hoagland solution, respectively.
The results mentioned above obviously suggested that nano-anatase TiO2 treatment supplied more nitrogen to the synthesis of chlorophyll and protein of spinach than control and bulk TiO2 treatment, which led to notable promotion of spinach growth.
Conclusions
The study of the experiments showed that all spinach leaves kept green by nano-anatase TiO2 treatment and all old leaves of spinach of control turned yellowish white under N-deficient condition. Nano-anatase TiO2 treatment resulted in obvious increase in the fresh weight, dry weight, and contents of total nitrogen, \( {\text{NH}}^{ + }_{4} \), oxygen, chlorophyll, and protein of spinach. After bulk TiO2 treatment, one part of the old leaf surface of spinach turned green, and the other was yellowish white. The improvements of yield and N compounds, oxygen contents of spinach were not as good as nano-anatase TiO2 treatment under N-deficient condition. It proved that nano-anatase TiO2 on exposure to sunlight could chemisorb N2 directly or reduce N2 to NH3 in spinach, which was transformed into organic nitrogen (such as chlorophyll, protein) and improve the growth of spinach. However, bulk TiO2 effect was not as significant as nano-anatase TiO2. According to Schrauzer’s reaction equation, a possible metabolism of the function of nano-anatase TiO2 catalyzing N2 to NH3 was advanced in Eq. f.

This is a pathbreaking study on the N2 fixation by nano-anatase TiO2 in spinach, which is an important theoretical foundation and technical approach for the future of agricultural applications of nano-TiO2.
References
Crabtree RH (1998) A new type of hydrogen bond. Science 282:2000–2001
Zheng L, Hong FS, Lu SP, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:82–93
Hong FS, Yang P, Gao FQ, Liu C, Zheng L, Yang F, Zhou J (2005) Effect of nano-anatase TiO2 on spectral characterization of photosystem particles from spinach. Chem Res Chin Univ 21:196–200
Hong FS, Yang F, Liu C, Gao FQ, Wang ZG, Gu FG, Ma ZN, Zhou J, Yang P (2005) Influences of nano-TiO2 on the chloroplast ageing of spinach under light. Biol Trace Element Res 104:249–260
Hong FS, Zhou J, Liu C, Yang F, Wang ZG, Gu FG, Wu C (2005) Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res 105:269–280
Gao FQ, Hong FS, Liu C et al (2006) Mechanism of Nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach: inducing complex of rubisco-rubisco activase. Biol Trace Element Res 111:239–253
Yang F, Hong FS, You WJ et al (2006) Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Element Res 110:179–190
Buchanan BB, Gruissem W, Johones RL (2002) Biochemistry and molecular biology of plants. Science Press, Beijing, pp 786–824
Wu WH (2003) Plant physiology. Science Press, Beijing, pp 128–165, 105–108, 89, (in Chinese)
Ji LN, Huang JJ, Mo TH (2001) Bioinorganic chemistry introduction. Zhongshan University Press, Guangzhou, pp 119–149, (in Chinese)
Yang P, Gao F (2002) Bioinorganic chemistry theory. Science Press, Beijing, pp 186–189, (in Chinese)
Schrauzer GN, Gut TD (1977) Photolysis of water and photoreduction of nitrogen on titanium dioxide. J Am Chem Soc 99:7189–7193
Schrauzer GN, Strampach N et al (1983) Nitrogen photoreduction on desert sans under sterile conditions. Proc Natl Acad Sci USA 80:3873–3876
Yang P, Lu C, Hua N, Du Y (2002) Titanim dioxide nanoparticles co-doped with Fe3+ and Eu3+ ions for photocatalysis. Mater Lett 57:794–801
Shanghai Plant Physiology Society (eds) (1999) Experimental guide of modern plant physiology. Science Press, Beijing, pp 133–134, 138–139, 86–87, (in Chinese)
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Limani AM, Rouillon C, Glevarec G, Gallais A, Hirel B (2002) Genetic and physiological analysis of germination efficiency in maize in relation to nitrogen metabolism reveals the importance of cytosolic glutamine synthetase. Plant Physiol 130:1860–1870
Lam HM, Coschigano KT, Oliverira IC, Melo-Oliveira R, Coruzzi GM (1996) The molecular genetics of nitrogen assimilation into amino acids in higher plants. Annu Rev Plant Physiol Plant Mol Biol 47:569–593
Li M, Wang ZL, Shi HZ, Zeng Y (2003) Surface morphology, spectra and photocatalytic bactericidal effect of chlorophyll-sensitizing TiO2 crystalline phases. J Inorg Mater 18:1261–1266, (in Chinese)
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature (London) 238:8–37
Frank SN, Bard AJ (1975) Semiconductor electrodes. II. Electrochemistry at n-type titanium dioxide electrodes in acetonitrile solutions. J Am Chem Soc 97:7427–7433
Wrighton MS, Ginley DS, Wolczanski PT, Ellis AB, Morse DL, Linz A (1975) Photoassisted electrolysis of water by irradiation of a titanium dioxide electrode. Proc Natl Acad Sci USA 72:1518–1522
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant No.20671067) and by the Jiangsu Province Universities Natural Science Foundation (grant no. 03KJB180122, 06KJB180094).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, F., Liu, C., Gao, F. et al. The Improvement of Spinach Growth by Nano-anatase TiO2 Treatment Is Related to Nitrogen Photoreduction. Biol Trace Elem Res 119, 77–88 (2007). https://doi.org/10.1007/s12011-007-0046-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-007-0046-4