Abstract
Groundwaters are the primary source of drinking water for half of the world’s population. They represent about 97% of the world’s available freshwater resources, being extensively abstracted and contaminated in many parts of the world. Among groundwaters are the thermal waters, a source of health, well-being, and a major touristic attraction (geoheritage), also providing a link to the natural environment. Therefore, it is strongly important to manage such type of groundwaters in a sustainable way, according to the Sustainable Development Goals (SDGs) of the United Nations 2030 Development Agenda. In this context, geoethics plays an important role to raise in geoscientist’s community their awareness of ethical, social, and cultural responsibilities in conducting scientific Earth Sciences activities, and their possibility to influence decision-makers concerning groundwater sustainable management (hydrogeoethics). This paper aims to highlight the role of environmental isotopes in hydrogeoethics. A brief review of two case studies is presented, showing that nuclear techniques (Isotope Hydrology), combined with other tools of Geosciences (e.g., Geology and Geochemistry), have demonstrated to be essential in the sustainable management of different types of groundwater resources: (i) the Caldas da Rainha thermal waters, where conventional geochemical and isotopic techniques (e.g., δ2H, δ18O, 3H, δ34S(SO4) and δ18O(SO4)), showed that the thermal waters are “geologically protected” from anthropogenic contamination, and (ii) the Santa Margarida Military Camp case study, where δ2H and δ18O data clearly showed no mixing between the Mediterranean temporary pond waters (projectile impact zones associated with real fire training) and the groundwaters of the region, used for agriculture and human consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater is the greatest abstracted raw material on Earth (1000 km3/year), being the main source of drinking water for half of the world’s population. About 70% of groundwater withdrawn worldwide is used for agriculture, and in some areas/regions, groundwater dependence approaches 100% (e.g., Siebert et al. 2010). Concerning both quantity and quality, groundwater is often preferred over surface water (e.g., Howard 2015), based on its relative stability and protection from instantly pollutant inputs. Due to population growth the drinking water demand is increasing (WWAP 2019). Furthermore, it is also needed to meet the necessities of agriculture and industry sectors, being a major concern, since, at the same time, the available drinking water resources are worldwide deteriorating as the result of pollution and overexploitation (e.g., Salameh 2008). Such opposite trends need particular attention in the case of aquifer systems in dry areas that have already suffered the influence of climate change and are ascribed to very low groundwater recharge (e.g., Meixner et al. 2016), enhancing the effects of negligent use of groundwater as the main reservoir of freshwater. Nevertheless, in some terms, it may be ethically accepted in water management strategies to use (some) groundwater well below recharge (if any), namely in dry regions in the tropics, for essential purposes (not for golf courses—even if sources of important revenue, extensive grass lawns in land developments, or for irrigation of water-intensive crops). Groundwater availability is one of the major issues facing mankind today, as the problems associated with it affect the lives of millions of people around the world. We are therefore facing a problem that in recent decades has attracted the attention of many International Agencies (e.g., UN, UNESCO, IAEA, WMO, etc.) and governmental and non-governmental organizations (at regional/international level) related to the “groundwater issue”. In fact, groundwater plays an outstanding role as a base for the achievement of the United Nations Sustainable Development Goals (SDGs), namely the SDG 6 dedicated “to ensure availability and sustainable management of water and sanitation for all’, as the SDGs are defined in the 2030 Agenda as “indivisible and integrated” (United Nations 2015). An effective groundwater management should involve the acquisition of detailed information on groundwater quality and quantity, its capability of being renewed, and the hydrogeological conditions at depth. Socio-economic and ecological aspects must also be taken into due consideration (Vélez-Nicolás et al. 2020). Centered on these data, hydrogeological conceptual models must be established, showing the present-day conditions of a groundwater system (e.g., Moore 2002). Also, mathematical approaches through numerical modeling are of great importance for groundwater management, allowing the study of the evolution of a groundwater system and its possible actions to be taken in case of pollution (e.g., Singh et al. 2019).
Among groundwaters, thermal waters can be considered as the “noblest relative”. In fact, thermal waters originate from meteoric waters which infiltrates through the vadose zone into great depths, in many cases through major crustal discontinuities (e.g., rock fractures, faults, shear zones, etc.). In other cases, “normal” groundwater flows (meaning low gradient) in porous sedimentary formations, being susceptible to reach areas in deep basins where they acquire thermal, geochemical, and isotopic signatures of deep reservoirs that may be connected to the surface by faults or other high gradient discontinuities that allow fast upward groundwater flow that justify the presence of thermal springs. Along such deep and long underground flow paths, thermal waters achieve specific physico-chemical signatures ascribed to the mineralogy of the geological formations which they percolate and interact with (e.g., Marques et al. 2010; Marques and Carreira 2019; Porowski 2019; Carreira et al. 2021). In the case of thermal waters, water-rock interaction processes take place at greater depths and higher temperatures than the normal groundwaters, promoting a higher mineralization. The emergence temperature of the thermal waters is a function of the circulation depth (e.g., Carreira et al. 2021). As stated by Moore (2002), such signatures of the thermal waters should be clearly described in the form of a hydrogeological conceptual model. A conceptual model typically comprises schematic cross-sections displaying the subsurface geology, location of likely/preferential recharge areas, underground flow paths, emergence sites, and main water-rock interactions occurring at depth (e.g., Sharp 1993; Brassington 2007; Marques et al. 2013; Gu et al. 2017; Carreira et al. 2021). The use of thermal waters for balneological proposes at the Thermal Spas plays an important role in the socio-economic development of the areas/regions where this “invisible” georesource occurs. Thus, Thermal Spa stakeholders and local water authorities must be directly involved in the regional development, to promote the success of Thermal Tourism (also called Tourism of Health and Wellness), which encompasses special places and objects that have an important role in increasing our knowledge on the history of the thermal areas/regions, its waters and rocks, and amazing landscapes (see Sadry 2009; Dong et al. 2013).
The geoscientists have specific knowledge and abilities that point to scientific, cultural, social, and ethical responsibilities toward the society and our natural resources (Peppoloni and Di Capua 2015) and should assist decision-makers in their political choices concerning the use of georesources, the prevention/mitigation of natural hazards, in global problems such as climate change, and in the conservation of geodiversity and geotourism attractions (Newsome and Dowling 2018). According to the International Association for Promoting Geoethics (IAPG), geoethics is “the research and reflection on the values which underpin appropriate behaviors and practices, wherever human activities interact with the Earth system” (Di Capua and Peppoloni, 2019; http://www.geoethics.org/definition), putting geoethics as a point of intersection for geosciences, sociology, philosophy, and economy (Peppoloni and Di Capua 2020, 2021). Geoscientists also have a vital role to raise common public consciousness on the theme of groundwater ethics (hydrogeoethics) and its interaction with the societies, and their groundwater practices to protect groundwater and avoid overexploitation and inefficient utilization (e.g., Datta 2005; Abrunhosa et al. 2021). Consequently, isotope hydrology can play an important role on the status and knowledge about groundwater (including thermal water) resources availability (both in terms of quantity and quality) and its use must be systematically assessed and evaluated (e.g., Datta et al. 1996). To protect groundwater (including thermal water) from depletion, withdrawal must be well adjusted by the assessed recharge, which should be studied periodically and reassessed (e.g., Datta et al. 1996). Environmental isotopes can have a key role in such approaches (e.g., Clark and Fritz 1997; Mook 2000). In this view, groundwater ethics, and in a global sense “hydrogeoethics”, can help in promoting ethical, cultural, social, economic, and educational values, starting in schools toward the local water authorities, explaining the significance of the use of environmental isotopes in groundwater/thermal water studies also to the public, including the politicians, administrators, media, among others (e.g., Datta 2013).
The structure, status, and processes of the groundwater system, which can only be acquired through scientific research efforts, are critical aspects of water resource management. In this regard, hydrogeochemical data as well as stable and radioactive isotope data provide essential tools in support of water resource management (Barbieri 2019). The present paper is focused on the role that environmental isotopic techniques can play in sustainable groundwater management practices, providing a brief review of some of the basic theoretical concepts as well as the potential practical applications of Isotope Hydrology, enhancing the importance of expanding Isotope Hydrology methodology throughout the curricula of Universities around the World, as already proposed in 2002 by the UNESCO JIIHP–Joint International Isotopes in Hydrology Program (https://en.unesco.org/themes/water-security/hydrology/programmes/jiihp). In fact, Ferrero and Magagna (2015) reported the importance of extensive education actions from geoscientists who, in the case of isotope hydrology, should constitute pertinent teaching/learning opportunities for teachers and students to identify gaps, inaccuracies, and misconceptions ascribed to the development of hydrogeological conceptual models of a given case study. For this purpose, a brief review of two case studies is made, in which isotopic techniques combined with other tools of Geosciences (e.g., Geology and Geochemistry) have proved to be fundamental in the sustainable management of different types of groundwater resources. In the case study of Caldas da Rainha thermal waters (Central Portugal), the multidisciplinary approach involving conventional geochemical and isotopic techniques (e.g., δ18O, 3H, δ34S(SO4) and δ18O(SO4)) led to the conclusion that the Caldas da Rainha thermal waters are “geologically protected” from anthropogenic contamination ascribed to intensive agriculture practices (Marques et al. 2013, 2019). In the case of the study of the Santa Margarida Military Camp, the use of environmental isotopes (δ2H and δ18O) clearly showed no mixing between the waters from the Mediterranean temporary ponds (projectile impact zones associated with real fire training) and the groundwaters of the region, used as drinking water by the local populations (Matias et al. 2008). These two reviewed case studies are distinct, but at the same time paradigmatic, and contain reference material for groundwater scientists, engineers, policymakers, managers, organizations, and professionals, who may be interested/involved in practical applications of isotope hydrology related to the assessment, development, protection, and sustainable management of groundwater/thermal water resources.
Methodology
Environmental isotopes play a key role in the development of conceptual hydrogeological models. The use of stable isotopes (oxygen-18 (18O) and deuterium (2H)) as groundwater flow tracers from recharge to discharge areas resides in that these isotopes are an integral part of the water molecule, and are conservative after rainwater infiltration (e.g., IAEA 1981). We are thus using water itself to write “the book of its history”, from recharge to discharge.
The use of stable isotopes in hydrogeology takes the form of relative differences compared to a specific standard. Isotopic results are expressed in delta (δ) notation relative to an international standard. The δ value is dimensionless, expressed in permillage (o/oo) and defined by
Rsample represents the 2H/1H, 18O/16O ratio in the sample; Rstandard refers to the same ratio as determined in the standard. The δ18O e δ2H isotopic results determined in water samples are expressed relative to the international standard V-SMOW (Vienna-Standard Mean Ocean Water), which by convention fixes the zero of the δ18O and the δ2H scale. V-SMOW represents a mixture of waters that reflect the average isotopic composition of water from various oceans, which constitute the beginning and end of the Hydrological Cycle (IAEA 1981). In a classical δ2H vs. δ18O diagram, the isotopic composition of atmospheric precipitation and groundwaters of meteoric origin is aligned along the so-called “Global Meteoric Water Line (GMWL)” δ2H = 8 δ18O + 10 defined by Craig (1961), and later improved by Rozanski et al. (1993) and Terzer et al. (2013). Thus, according to Mook (2000), water from atmospheric precipitation, which has not been subject to variation in isotopic composition due to different processes including, among others (i) water-rock interaction at high temperatures at depth, along the underground flow system (usually the case of thermal waters ascribed to high-temperature geothermal systems), and (ii) evaporation effects prior to infiltration (usually the waters from lakes and ponds), will have δ2H and δ18O values that are distributed along and or near GMWL. In fact, a water that has undergone significant evaporation before infiltration (e.g., waters from rivers, dams, lakes, and ponds) will present enrichment in heavy isotopes relative to the initial composition, graphically represented by a straight line with a slope usually ranging between 4 and 6 (see Mook 2000).
Tritium (3H), the radioactive isotope of hydrogen, has a half-life of 12.32 years, being its concentration in natural waters expressed in terms of tritium units (TU) (Lucas and Unterweger 2000). The tritium in precipitation is the result of two distinct processes: (i) the first, of natural origin, is the result of the reaction of neutrons produced by the interaction of cosmic rays, in the upper layers of the atmosphere, with the nuclei of nitrogen atoms; (ii) the second has an anthropogenic origin resulting from thermonuclear explosions carried out in the atmosphere, nuclear industry (nuclear plants, fuel reprocessing plants), and consumer products such as paints, lamps and watch components. In addition, the nuclear industry (e.g., water used for cooling reactors) releases this radioactive isotope into the atmosphere in the form of gaseous and liquid effluents (IAEA 1981; Rozanski et al. 1991). The increase of 3H atmosphere was registered from 1952/3 onwards, with the maximum peak of tritium concentration in precipitation (over 1000 TU) coinciding with the year 1963 (associated with thermonuclear testing during the Cold War). Since then (1963), in the Northern hemisphere, a decrease in tritium concentration in the atmosphere has been observed toward levels (≈ 4–5 TU) similar to those prior to the thermonuclear tests (Rozanski et al. 1991).
It should be noted that both the 3H produced in the atmosphere by natural processes and that resulting from anthropogenic activities are rapidly oxidized, changing to atmospheric water vapor (1H3HO). It thus enters the Hydrological Cycle through precipitation and isotopic exchange between air and oceanic water masses. The use of tritium concentrations present in groundwaters provides qualitative information on the: (i) presence of active recharge in the system; (ii) "age" of groundwater, (iii) extent of underground flow paths, and (iv) possible existence of mixture between recent infiltration and older groundwaters. In determining the “age” of groundwater, it was considered that: a water devoid of tritium has an “age” greater than 70 years; a tritium content of the order of magnitude of a few tritium units indicates that the infiltration will have occurred approximately 30 years after the first thermonuclear tests, or that it is a mixed water; a water with higher tritium values reveals a recent infiltration (e.g., Clark and Fritz 1997).
Dams, lakes, and ponds are the most readily available water resources, being widely used in many parts of the world. Together with other surface water bodies (e.g., wetlands and artificial surface water reservoirs), lakes, and ponds cover millions of km2 of continental areas, constituting an essential component of the hydrological cycle, either regionally or globally (Meybeck 1995). In fact, according to Mook (2000), lakes and ponds interacting with the local environment are complex dynamic systems through surface and ground “inlet” and “outflow” flows, as well as through atmospheric precipitation/evaporation phenomena.
Frequently, it is important to understand the extent of interaction between a lake/pond and the adjacent groundwater systems. The assessment of possible mixing processes between these two end-members is of particular importance for aquifer systems being exploited for water supply, where the vulnerability of the boreholes to pollution originating in the lakes/ponds is a crucial issue. Heavy isotopes (e.g., 2H and 18O) build-up in lakes/ponds can be considerable, depending on climate and system hydrology. Therefore, the lake/pond water entering adjacent groundwater systems can be distinguished from local precipitation. Also, knowing the isotopic composition of the two end-members (lake/pond water and local groundwater), one can estimate the percentage of lake water in the borehole water located downgradient from the lake/pond (e.g., Yehdegho et al. 1997; Stichler et al. 1999). In this paper, we review a case study presented by Matias et al. (2008), where the use of environmental isotope applications has proven to be an important tool to understand the dynamics of pond systems, with an emphasis on assessment of the possible interaction of this type of water system with adjacent groundwater bodies.
Oxygen and sulfur stable isotopes have been widely used to investigate sulfate sources, sulfate oxidation, and reduction zones in the peat, and to assess and compare sulfate sources in numerous wetland systems ascribed to diverse climatic and hydrogeological environments (e.g., Krouse and Mayer 2001; Novak et al. 2005). Sulphate’s isotopic composition has been also used to explain the processes and sources of SO42− released from peatlands to soils, streams, or lakes (e.g., Schiff et al. 2005). In this paper, δ34S(SO4) and δ18O(SO4) values were reviewed as a very important hydrogeological tool (Marques et al. 2013, 2019) for determining sulfate origin (e.g., interaction with evaporites, sulfate from both atmosphere and soil, and sulfate from oxidation of reduced inorganic sulfur species) in shallow cold dilute groundwaters/thermal waters sulfate in a diversified geological environment (limestone rocks, diapir intrusions, and gypsum lenses). It was also useful in assessing shallow cold dilute groundwaters/thermal water interaction and, consequently, in clearness of the vulnerability of the thermal groundwater system to anthropogenic pollution (see Marques et al. 2013, 2019).
Water sampling procedures and storage (for later chemical analyses), type of waters physico-chemical parameters measured in situ, the techniques used for surface waters, shallow cold dilute groundwaters, and thermal waters chemical analysis and isotopic determinations (δ2H, δ18O, δ34S(SO4), δ18O(SO4), and 3H) were those described in detail in Matias et al. (2008) and Marques et al. (2019).
Results and discussion
The case study of Caldas da Rainha thermal waters (a review)
Groundwaters (thermal or not) ascribed to karstified limestone aquifer systems are more vulnerable to mixing processes with shallow groundwaters, when compared to other aquifer types, consequently demanding specific attention (e.g., Goldscheider and Drew 2007). The strategy and setup for sustainable thermal water withdrawal schemes in such hydrogeological environments involve a detailed understanding of the hydrogeological system, including preferential pathways for thermal water flow and the existence (or not) of shallow/thermal waters interaction (e.g., Horvatincic et al. 1996). According to Ravbar and Goldscheider (2009), different methodologies should be applied, considering the nature of karst aquifers such as heterogeneity and duality of infiltration processes. Although groundwaters from fractured and karstified limestones constitutes a main component of drinking water supply all over the world and in particular in many Mediterranean countries (e.g., Custodio et al. 1991; Arfib et al. 2000), thermal waters from karst aquifers supply Spas all over the world, such as the well-known baths in Budapest (Hungary) and geothermal power plants with use such type of georesources for electricity production, district heating, or other purposes (e.g., Goldscheider et al. 2010). Regrettably, as stated by Ryan and Meiman (1996), these types of georesources are sometimes polluted by anthropogenic activities such as: agricultural runoff loaded with chemical fertilizers, effluents from septic tanks, pesticides and animal wastes, municipal wastewater, landfill, and industrial wastes. To create active comprehensive protection procedures for thermal water bodies, it is extremely worthwhile to precisely consider the contamination risk, which should be the conceptual basis for defining safeguard zones in thermal water bodies projected for Spas supplies.
In this section, we review the Caldas da Rainha thermal waters case study (see Marques et al. 2013), one of the most important Spas in Portuguese mainland, with a special emphasis on the usefulness of sulphate’s isotopic composition to explain the sources of SO42− in the thermal waters and the possible existence (or not) of interaction between the local shallow cold dilute groundwaters and the deep circulation thermal waters.
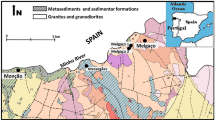
The region where Caldas da Rainha thermal waters emerge (Fig. 1) is dominated by a huge synclinal structure responsible for the regional flow of thermal waters.

Adapted from Marques et al. (2013)
Schematic geological and structural cross-section of the studied area (no scale). (1) Caldas da Rainha diapir; (2) Candeeiros Mountain; (3) Fonte da Bica diapir. (i) Thermal spring at Caldas da Rainha Spa; (ii) exploitation borehole of Caldas da Rainha thermal water.
The Caldas da Rainha thermal waters emerge through springs and are exploited from boreholes close to an N-S oriented oblique fault (60° E), at the geological contact between the Upper Jurassic detrital rocks (e.g., (D)—clayey sandstone) and the Hetangian–Retian Dagorda marls (A) that act as a hydrogeological barrier. The elevation and consequent folding of the Jurassic formations was the result of the regional diapiric intrusions (Zbyszewski and Moitinho de Almeida 1960). At greater depths, there are essentially limestone rocks [(C)—Middle Jurassic and (B)—Lower Jurassic], later covered by Plio-Plistocene sedimentary deposits (E) (see Zbyszewski 1959; Zbyszewski and Moitinho de Almeida 1960).
The Caldas da Rainha thermal waters present an issue temperature around 33 °C. They belong to the group of sulfur waters (presence of H2S, HS−, and S2−) and present a rather complex hydrochemical facies: Cl-SO4-HCO3-Na-Ca. As reported by Marques et al. (2019), noteworthy 3H concentrations (2.8; 2.5 and 1.1 ± 0.6 TU) were detected in Caldas da Rainha thermal waters sampled at AC1-B, AC2, and JK1 boreholes, respectively. On the other hand, the thermal spring waters sampled at the “Piscina da Rainha” spring were characterized by the absence of 3H (and by 3H values close to the detection limit). This type of results is extremely important for the identification of different underground flow paths of thermal waters, basis for a sustainable management of the hydromineral resources, and assessment of their vulnerability to possible contamination of anthropic origin. Traditional springs are usually places of touristic attraction in each Spa. Therefore, the stakeholders have the need to maintain the good quality of spring waters (definition of the protection areas of the thermal springs), which in many cases can be tasted by the visitors, in the local “buvettes”, with the assistance of personnel specialized in thermalism. Concerning the isotopic composition of shallow cold dilute groundwaters and thermal waters sulfate, Marques et al. (2019) reported that these groups of waters presented different δ34S(SO4) and δ18O(SO4) signatures: (i) the shallow cold dilute groundwaters ascribed to the (D) geological formation, present δ34S(SO4) and δ18O(SO4) values between 1.5 and 4.1 ‰ and 8.6 and 9.3 ‰, respectively, and (ii) the thermal waters of Caldas da Rainha present δ34S(SO4) and δ18O(SO4) values between 14.9 and 19.1 ‰ and 11.1 and 16.2 ‰, respectively (Fig. 2).

Adapted from Marques et al. (2013). Unfilled square stands for Caldas da Rainha thermal waters. Filled square stands for shallow cold dilute groundwaters ascribed to the Upper Jurassic (D) geological formations
Variation of δ34S(SO4) and δ18O(SO4) values observed in atmospheric, pedogenic, and geogenic sulfates.
As described by Marques et al. (2013, 2019), the hydrochemical facies of Caldas da Rainha thermal waters (Cl-SO4-HCO3-Na-Ca) is the result of water-rock interaction with limestone (HCO3-Ca), gypsum (SO4-Ca), and diapir (Cl-Na) geological formations along the underground flow paths from recharge (at Candeeiros Mountains) to discharge at the Spa area (at approximately 20 km distance, see Fig. 1).
The Caldas da Rainha thermal spring waters (e.g., “Piscina da Rainha”) should be considered as the most representative of the deep hydrothermal system due to the absence of 3H (Marques et al. 2019).
The results of the isotopic composition of shallow cold dilute groundwaters and thermal waters sulfate are a strong indication that Caldas da Rainha thermal waters are "geologically protected" from any processes of anthropogenic contamination, since the (D)—Upper Jurassic geological formation is a clayey sandstone, typically with low permeability (see Gao et al. 2009), avoiding mixing between shallow cold dilute groundwaters and thermal waters. In fact, as referred by Marques et al. (2019), sulfate concentrations in the local shallow cold dilute groundwaters may be from atmospheric deposition that has traveled through the soil zone (see Mayer and Krouse 2004). Nevertheless, the use of fertilizers in agriculture activities should not be excluded (sulfate concentrations in local shallow cold dilute groundwaters range from 40 to 60 mg/L). Water-rock interaction at depth with diapir formations (see Mayer and Krouse 2004) clearly explain the isotopic composition of Caldas da Rainha thermal waters sulfate, as stated by Marques et al. (2019). The different δ18O values of groundwaters associated with the (D)—Upper Jurassic geological formation and the Caldas da Rainha thermal waters (Fig. 3) reflect different recharge altitudes ascribed to different underground flow systems, corroborating the inexistence of mixing between the local shallow cold dilute groundwaters and the thermal waters, as stated by Marques et al. (2013, 2019).
This study was initially requested by the “Centro Hospitalar das Caldas da Rainha” (CHCR), at that time (2005), the concessionaire of Caldas da Rainha Spa. During the development of this study, particular emphasis was given to the issue of thermal aquifer systems protection from anthropogenic inputs, since the actions to be taken to protect them may arrive too late, when the contamination is already widespread. It should be stated that field work campaigns were always attended by the Technical Director of Caldas da Rainha Spa, providing extensive discussions on the information gathered, as more and more isotopic studies were carried out. As also discussed with the local authorities, in the region under study, diffuse agricultural pollution should be seen as one of the main potential obstacles to achieving an ecologically sustainable exploitation of local thermal water resources. However, in this case study, the isotopic data obtained indicate that Caldas da Rainha thermal waters seem to be “geologically protected” from the human contamination processes detected in the shallow cold dilute groundwaters, given the nature of the geological formations of ground's surface. The results obtained in this study, namely the (i) different δ18O signatures presented by the shallow cold dilute groundwaters [associated with the (D)—Upper Jurassic geological formation] and the Caldas da Rainha thermal waters, and (ii) different isotopic composition of shallow cold dilute groundwaters and thermal waters sulfate, were disseminated to local authorities and the general public, through various Seminars such as the one that took place in December 2008, at the CHCR, were the results obtained were presented and discussed with the Director of the CHCR, the General-Director of Health, Members of the several parties of the Municipal Assembly and the Media of region. It was concluded that such isotopic studies provided the acquisition of fundamental information, so that the due protection can be integral, namely in terms of the delimitation of the thermal aquifer system protection areas, one of the tasks ascribed to the Technical Director of Caldas da Rainha Spa.
The case study of Santa Margarida Military Camp (a review)
This case study is linked to assessment of the environmental impact caused by the Santa Margarida Military Camp (CMSM) after 50 years of use (see Matias et al. 2008). The CMSM covers an area of about 62 km2 and is divided into two sectors with distinct characteristics: (i) an urban area where the headquarters, the residential units, barracks, and support services are located, and (ii) the military training area (the most extensive) including firing ranges, a landing strip, and several areas for tactical military maneuvers of the mechanized divisions (see Matias et al. 2008). The CMSM is the unique Portuguese military training area, comprising firing ranges for tactical military maneuvers of mechanized divisions. Therefore, several negative effects on the environment were predictable ascribed to the military activities, as the Military Camp’s area is classified as a highly vulnerable area to pollution of its multi-layer porous aquifers (Almeida et al. 2000).
The CMSM is in an extensive flat area in the Tertiary Lower Tagus Basin, where the most extensive and important multi-layer aquifer system occurs in Portugal (Rosselló-Graell, 2003), consisting mainly of sandstones alternating with layers of impermeable clays (Almeida et al. 2000), which strongly influence soil porosity and permeability (see Matias et al. 2008) and references therein). The most recent formations are represented by quaternary deposits (see Matias et al. 2008).
In the case study presented by Matias et al. (2008), and reviewed in this paper, a special emphasis was put on the characterization of the surface water/groundwater interaction. In fact, in the CMSM, there is a network of Mediterranean temporary ponds, whose main characteristic is their marked seasonality in the permanence of the water layer, becoming completely dry in the summer months. Superficially, where these Mediterranean temporary ponds occur, there are sands, in some places with a significant percentage of clays (Rosselló-Graell 2003). According to Rosselló-Graell et al. (2000), there were 20 of these ponds present in the CMSM, distributed over an area of 25 km2. They had variable dimensions, with a maximum length between 150 and 300 m, and with an approximate area of 0.1 to 0.9 km2. At the CMSM, some of these Mediterranean temporary ponds are located within, or very close, to the present-day projectile’s impact areas ascribed to real fire training activities, being considered as potentially vulnerable to anthropogenic contamination due to military activities. In fact, as reported by Matias et al. (2008), the waters from such Mediterranean temporary ponds had significant concentrations of Al (0.27 mg/L), Fe (0.12 mg/L), Cr (1.5 μg/L), and Pb (5 μg/L)—example for the Junco (J) Mediterranean temporary pond (see Fig. 4)—constituent elements of ammunition and shells. However, the groundwaters showed no contamination by these elements (Matias et al. 2008). To confirm the existence (or not) of mixing between the surface waters from the Mediterranean temporary ponds and the regional groundwaters, which in some cases are used for human supply (drinking water), the stable isotopic data of the sampled waters were plotted in the diagram of Fig. 5.

Adapted from Matias et al. (2008)
Isotopic composition (δ2H and δ18O values) of surface (from the Mediterranean temporary ponds) and groundwaters of the CMSM.
The observation of Fig. 5 evidences the existence of two water groups. One of the groups (A) is ascribed to local groundwaters (sampled from springs and a borehole). This group of meteoric waters shows no signs of having been subjected to evaporation, being straight infiltrated into the ground, as they are plotted along and close to the Global Meteoric Water Line (δ2H = 8 δ18O + 10). On the other hand, waters from group (B) do not form a cluster, as those from group (A), since they represent the waters from the Mediterranean temporary ponds, where each pond presents signs of its own way of evaporation (in the Mediterranean temporary ponds, the water depth sometimes has no more than 0.5 m). The isotopic signatures of these two groups of waters show no evidence of mixing between them. If there was a mixture between these two groups of waters, one should observe the projection of some water points along a hypothetical mixing line (as suggested in Fig. 5) between the two end-members. These results are extremely important as they demonstrate that, in the CMSM area, military maneuvers using real fire, and having some impact areas in the Mediterranean temporary ponds, do not threaten the sustainable management of groundwater resources (used as tap water and supply for agriculture practices by the local populations). This is one of the major concerns which has been addressed in the context of periodic joint meetings between geoscientists and the CMSM’s military leadership, in the context of the development of the studies reviewed in this paper. To this issue, strongly contributes the fact that where these Mediterranean temporary ponds occur, there are sands with a significant fraction of clays contributing to the natural impermeabilization of the projectile’s impact areas.
Military Training Camps are military facilities that provide training exercises and tactical operations. The greater its size and operational use for military training, the greater the anthropic impacts on the ecosystems. However, the CMSM has already received three awards from the National Défense and Environment, in 1996, 1998, and 2003. The application of an Environmental Management System (EMS), started in 2001, was responsible for the third prize. It is worth mentioning the participation of the authors of this paper, in October 2003, in the CMSM’s Environmental Week, through the presentation of the Lecture “CMSM: Study of the Environmental Impact after half a century of use”. The isotopic studies contributed to the development of the EMS, promoting environmental protection, and integrating the ecological component into military activities. This facility has had an Environmental Certification since 2004, in accordance with the Portuguese Standard ISO 14001, and the results of the studies presented here have contributed to the maintenance of this Environmental Certification, referring to the CMSM as an example of outstanding environmental practices within Portuguese Military Institutions.
Concluding remarks and outlook
This paper highlights the role environmental isotopes in hydrogeoethics, namely (i) in the sustainable management and protection of thermal water systems used in local Spas, and (ii) in identification of possible mixing processes between surface and groundwaters of a given region. Although hydrogeoethics is a relatively new “discipline” in geosciences, it provides important guidelines for human actions by make known to geoethical principles when dealing with the natural water resources of our planet. For this purpose, two paradigmatic case studies were reviewed, providing new insights into hydrogeoethics and its links with a sustainable environment and societal worries. This paper enhances the usefulness of the use of environmental isotopes to assess some of the most important parameters of interest to the groundwater planners and managers, such as groundwater quality and the interaction between surface water and groundwater. The application of environmental isotopic techniques in hydrogeology has demonstrated to be an important tool for improving our understanding on (i) the behavior of groundwater systems, namely thermal water systems, essential for their sustainable management, and (ii) on surface/groundwater interaction ascribed to potential contamination problems. The two case studies reviewed here are well illustrative of the fact that environmental isotopic techniques can provide an independent approach to solving a particular hydrogeological problem, as isotopic groundwater signatures (whether thermal or not) are authentic archives that hold the memory of its evolution. However, we should be aware that these isotopic techniques are just one of the many available tools that can be applied in hydrogeological surveys, and that their use in parallel with other disciplines of geoscience (geology, geochemistry, and geophysics) will certainly be much more successful.
To establish effective global protection measures, it is highly advisable to specifically consider the risk of contamination as the basis of the methodology for defining safeguard zones in thermal water bodies intended for Spas supplies. Unfortunately, sometimes, these vital and rather limited georesources are polluted due to anthropogenic activities such as: agricultural runoff loaded with chemical fertilizers, pesticides and animal wastes, septic tanks, landfill, municipal wastewater, and industrial wastes (which is not the case of Caldas da Rainha area). The future actions, policies, and recommendations to be assumed by the present-day Caldas da Rainha Spa owner (the Caldas da Rainha Municipality) must be focused on the control of the storage, handling, use, and disposal of possible contaminants within the recharge areas, and of actions that may negatively affect groundwater quantity and quality. Such topics should be accomplished through the development of procedures, programs for best practices, and communication protocols by local/regional governmental institutions.
The integration of environmental management systems must be a priority for the government to guarantee the environmental sustainability of the Military Sector. The Armed Forces must agree on the need that the potential environmental impacts of certain activities must be evaluated before a decision is made. To promote the link between the Armed Forces and the environment, research should be increased into methods of developing, measuring, and promoting the integration of environmental practices, at different levels of military organizations, in decision-making, in logistics, and in operational processes.
Though it is a rather new area in geoethics, hydrogeoethics is increasingly assuming an essential role since ethical behavior, while assessing, developing, and managing groundwaters resources have turned into an imperative requirement for the sustainable environment, and societal concerns.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Abrunhosa M, Chambel A, Peppoloni S, Chaminé HI (2021) Advances in geoethics and groundwater management: theory and practice for a sustainable development. In: Proceedings of the 1st Congress on Geoethics and Groundwater Management (GEOETH&GWM’20), Porto, Portugal 2020. Abrunhosa M, Chambel A, Peppoloni S, Chaminé HI (eds) Advances in Science, Technology & Innovation. Springer, Cham, https://doi.org/10.1007/978-3-030-59320-9
Almeida C, Mendonça JJL, Jesus MR, Gomes AJ (2000) Sistemas aquíferos de Portugal Continental. Centro de Geologia da Faculdade de Ciências da Universidade de Lisboa, INAG, Lisboa ([in Portuguese])
Arfib B, de Marsily G, Ganoulis J (2000) Pollution by seawater intrusion into a karst system: new research in the case of the Almyros Source (Heraklio, Crete, Greece). Acta Carsologica/karsoslovni Zbornik 29(1):15–31
Barbieri M (2019) Isotopes in hydrology and hydrogeology. Water 11(2):291. https://doi.org/10.3390/w11020291
Brassington FC (2007) A proposed conceptual model for the genesis of the Derbyshire thermal springs. Q J Eng GeolHydrogeol 40:35–46
Di Capua G, Peppoloni S (2019). Defining geoethics. Website of the IAPG - International Association for Promoting Geoethics, http://www.geoethics.org/definition
Carreira PM, Marques JM, Guerra A, Nunes D, Espinha Marques J, Teixeira J, Chaminé HI (2021) Caldelas and Gerês hydrothermal systems (NW Portugal): a comparative study based on geochemical and isotopic signatures. Environ Earth Sci 80:100. https://doi.org/10.1007/s12665-021-09389-w
Clark I, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1703
Custodio E, Bayó A, Pascual M, Bosch X (1991) Results from studies in several karst formations in southern Catalonia (Spain). In: Gunay G, Johnson AI, Back W (eds) Hydrogeological processes in karst terranes. IAHS-AISH Publication International Association of Hydrological Sciences, Louvain, pp 295–326
Datta PS (2005) Groundwater ethics for its sustainability. Curr Sci 89:1–6
Datta PS, Bhattacharya SK, Tyagi SK (1996) 18O studies on recharge of phreatic aquifers and groundwater flow paths of mixing in Delhi area. J Hydrol 176:25–36
Datta PS (2013) Groundwater vulnerability to changes in land use and society in India. Understanding Freshwater Quality Problems in a Changing World, Proc. of H04, IAHS-IAPSO IASPEI Assembly, Gothenburg, Sweden, (IAHS Publ. 361, 2013), pp 345–352
Dong H, Song Y, Chen T, Zhao J, Yu L (2013) Geoconservation and geotourism in Luochuan Loess National Geopark, China. Quat Int. https://doi.org/10.1016/j.quaint.2013.10.023
Ferrero E, Magagna A (2015) Natural hazards and geological heritage in Earth science education projects. In: Peppoloni S, Di Capua G (ed) Geoethics: the Role and Responsibility of Geoscientists. Geological Society, London, Special Publications 419, pp 149–160
Gao C, Wang Z, Deng J, Zhao J, Yang X (2009) Physical property and origin of lowly permeable sandstone reservoir in Chang 2 division, Zhang-Han oilfield. Ordos Basin Energy Explor Exploit 27(5):367–389
Goldscheider N, Drew D (2007) Methods in karst hydrogeology. Taylor & Francis Group, London
Goldscheider N, Mádl-Szőnyi J, Erőss A, Schill E (2010) Review: thermal water resources in carbonate rock aquifers. Hydrogeol J 18(6):1303–1318
Gu X, Zhang Q, Cui Y, Shao J, Xiao Y, Zhang P, Liu J (2017) Hydrogeochemistry and genesis analysis of thermal and Mineral springs in Arxan, Northeastern China. Water 9:61–77
Horvatincic N, Srdoc D, Krajcar Bronic I, Pezdic J, Kapelj S, Sliepcevic A (1996) A study of geothermal waters in northwest Croatia and east Slovenia. In: IAEA, UNESCO (ed) Symposium on Isotopes in Water Resources Management 2, Vienna, pp 470–474
Howard KWF (2015) Sustainable cities and the groundwater governance challenge. Environ Earth Sci 73(6):2543–2554
IAEA [International Atomic Energy Agency] (1981) Stable Isotope Hydrology. Deuterium and Oxygen-18 in the Water Cycle, Technical Reports Series 210. IAEA, Vienna
Instituto Geográfico do Exército (2008). Carta Militar de Portugal. Série M888/ Escala 1:25 000. Edição 6. Folha 331 (Abrantes) e Folha 343 (Bemposta) [in Portuguese]
Krouse HR, Mayer B (2001) Sulphur and oxygen isotopes in sulphate. In: Cook P, Herczeg AL (eds) Environmental tracers in subsurface hydrogeology, 2nd edn. Kluwer Academic Publishers, Boston, pp 195–231
Lucas LL, Unterweger MP (2000) Comprehensive review and critical evaluation of the half-life of tritium. J Res Nat Inst Stand Technol 105(4):541–549
Marques JM, Carreira PM (2019) Geosciences in the assessment of thermal and mineral groundwater systems in N-Portugal: a review. Sustain Water Resour Manag 5:1511–1523. https://doi.org/10.1007/s40899-017-0190-8
Marques JM, Carreira PM, Espinha Marques J, Chaminé HI, Fonseca PE, Monteiro Santos FA, Eggenkamp HGM, Teixeira J (2010) The role of geosciences in the assessment of low-temperature geothermal resources (N-Portugal): a review. Geosci J 14(4):329–446
Marques JM, Graça H, Eggenkamp HGM, Neves O, Carreira PM, Matias MJ, Mayer B, Nunes D, Trancoso VN (2013) Isotopic and hydrochemical data as indicators of recharge areas, flow paths and water-rock interaction in the Caldas da Rainha-Quinta das Janelas thermomineral carbonate rock aquifer (Central Portugal). J Hydrol 476:302–313
Marques JM, Matos C, Carreira PM, Neves MO (2019) Isotopes and geochemistry to assess shallow/thermal groundwater interaction in a karst/fissured-porous environment (Portugal): a review and reinterpretation. Sustain Water Resour Manag 5(4):1525–1536
Matias MJ, Marques JM, Figueiredo P, Basto MJ, Abreu MM, Carreira PM, Ribeiro C, Flambó A, Feliciano J, Vicente EM (2008) Assessment of pollution risk ascribed to Santa Margarida Military Camp activities (Portugal). Environ Geol 56:1227–1235
Mayer B, Krouse HR (2004) Procedures for sulphur isotope abundance studies. In: de Groot P (ed) Handbook of stable isotope analytical techniques. Elsevier, Amsterdam, pp 538–596
Meixner T, Manning AH, Stonestrom DA, Allen DM, Ajami H, Blasch KW, Brookfield AE, Castro CL, Clark JF, Gochis DJ, Flintj AL, Neff KL, Niraula R, Rodell M, Scanlon BR, Singha K, Walvoord MA (2016) Implications of projected climate change for groundwater recharge in the western United States. J Hydrol 534:124–138
Meybeck M (1995) Les lacs et leur bassin. In: Pourriot R, Meybeck M (eds) Limnologie generale. Masson, Paris, pp 6–59
Mook WG (2000) Environmental isotopes in hydrological cycle. Principles and applications. Technical Documents in Hydrology, 39 (I), IHP-V UNESCO & IAEA, Paris
Moore JE (2002) Field hydrogeology: a guide for site investigations and report preparation. Lewis Publishers, New York
Newsome D, Dowling R (2018) Chapter 17 - Geoheritage and Geotourism. In: Reynard E, Brilha J (eds) Geoheritage: assessment, protection, and management. Elsevier, Amsterdam, pp 305–321
Novak M, Vile MA, Bottrell SH, Stepanova M, Jackova I, Buzek F, Prechova E, Newton RJ (2005) Isotope systematic of sulfate-oxygen and sulfate-sulfur in six European peatlands. Biogeochemistry 76:187–213
Peppoloni S, Di Capua G (2015) Geoethics: the Role and Responsibility of Geoscientists, The Geological Society, Special Publication 419, London
Peppoloni S, Di Capua G (2021) Current Definition and Vision of Geoethics. In: Bohle M, Marone E (ed) Geo-societal Narratives. Palgrave Macmillan, Cham, pp 17–28. https://doi.org/10.1007/978-3-030-79028-8_2
Peppoloni S, Di Capua G (2020) Geoethics as global ethics to face grand challenges for humanity. In: Di Capua G, Bobrowsky PT, Kieffer SW, Palinkas C (eds) Geoethics: status and future perspectives. Geological Society, London, Special Publications, p 508. https://doi.org/10.1144/SP508-2020-146
Porowski A (2019) Mineral and Thermal Waters. In: LaMoreaux J (ed) Environmental Geology. In: Encyclopedia of Sustainability Science and Technology Series, Springer, New York, pp. 149–181
Ravbar N, Goldscheider N (2009) Comparative application of four methods of groundwater vulnerability mapping in a Slovene karst catchment. Hydrogeol J 17(3):725–733
Rosselló-Graell A (2003) Caracterização fito-ecológica das Lagoas temporárias do Campo Militar de Santa Margarida (Ribatejo, Portugal). Portugaliae Acta Biologica 21:245–278 ([in Portuguese])
Rosselló-Graell A, Draper D, Tauleigne Gomes C (2000) Conservation status of Mediterranean temporary ponds in Campo Militar de Santa Margarida (Ribatejo, Portugal). Portugalie Acta Biologica 19:191–199
Rozanski K, Gonfiantini R, Araguás-Araguás L (1991) Tritium in the global atmosphere: Distribution patterns and recent trends. J Phys g Nuclear Part Phys 17:5523–5536
Rozanski K, Araguás-Araguás L, Gonfiantini R (1993) Isotopic patterns in modern global precipitation. Climate change in continental isotopic records. In: Swart PK, Lohmann KC, Mckenzie J, Savin S (eds) Geophysical monograph series 78. AGU, Washington DC, pp 1–36
Ryan M, Meiman J (1996) An examination of short-term variations in water quality at a karst spring in Kentucky. Groundwater 34(1):23–30
Sadry B (2009) Fundamentals of Geotourism with a Special Emphasis on Iran. Samt Organization Publishing, Tehran
Salameh E (2008) Over-exploitation of groundwater resources and their environmental and socio-economic implications: the case of Jordan. Water Int 33(1):55–68. https://doi.org/10.1080/02508060801927663
Schiff SL, Spoelstra J, Semkin RG, Jeffries DS (2005) Drought induced pulses of from a Canadian shield wetland: use of δ34S and δ18O in to determine sources of sulfur. Appl Geochem 20:691–700
Sharp JM Jr (1993) Fractured aquifers/reservoirs: Approaches, problems, and opportunities. In: Banks D, Banks S. (ed) Hydrogeology of Hard Rocks, 24, part 1, Memoires of the 24th Congress, International Association of Hydrogeologists, Oslo, Norway, pp 23–38
Siebert S, Burke J, Faures JM, Frenken K, Hoogeveen J, Doll P, Portmann FT (2010) Groundwater use for irrigation—a global inventory. Hydrol Earth Syst Sci. https://doi.org/10.5194/hess-14-1863-2010
Singh A, Panda SN, Uzokwe VNE, Krause P (2019) An assessment of groundwater recharge estimation techniques for sustainable resource management. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2019.100218
Stichler W, Maloszewski P, Bertleff B, Trapp Ch, Watzel R, Weinsziehr R (1999) Modeling of lake – groundwater interaction based on environmental isotope data. In: Proc. IAEA Int. Symposium on Isotope Techniques in Water Resources Development and Management, C&S Papers Series (CD-ROM) No. 2, IAEA, Vienna
Terzer S, Wassenaar LI, Araguás-Araguás LJ, Aggarwal PK (2013) Global isoscapes for δ18O and δ2H in precipitation: improved prediction using regionalized climatic regression models. Hydrol Earth Syst Sci 17:4713–4728
United Nations (2015) Transforming our World: the 2030 Agenda for Sustainable Development. NY, USA: https://sustainabledevelopment.un.org/post2015/transformingourworld
Vélez-Nicolás M, García-López S, Ruiz-Ortiz V, Sánchez-Bellón A (2020) Towards a sustainable and adaptive groundwater management: lessons from the Benalup aquifer (Southern Spain). Sustainability 12:5215. https://doi.org/10.3390/su12125215
WWAP [UNESCO World Water Assessment Programme] (2019) The United Nations World Water Development Report 2019: Leaving No One Behind. UNESCO, Paris (ISBN 978-92-3-100309-7)
Yehdegho B, Rozanski K, Zojer H, Stichler W (1997) Interaction of dredging lakes with the adjacent groundwater field: an isotope study. J Hydrol 192(1–4):247–270
Zbyszewski G (1959) Étude structurale de la vallée typhonique de Caldas da Rainha (Portugal). Memórias Dos Serviços Geológicos De Portugal 3:1–184
Zbyszewski G, Moitinho de Almeida F (1960) Carta Geológica de Portugal, escala 1/50.000. Notícia Explicativa da Folha 26-D - Caldas da Rainha [in Portuguese]
Acknowledgements
CERENA/|IST author was supported by FCT (Portuguese Foundation for Science and Technology) through the FCT-UIDB/04028/2020 project. C2TN/|IST author was supported by FCT through the strategic project FCT-UIDB/04349/2020. An early draft of this manuscript was critically read by four anonymous reviewers, and we gratefully acknowledge their contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marques, J.M., Carreira, P.M. The use of environmental isotopes in groundwater studies with hydrogeoethics: essential or dispensable?. Sustain. Water Resour. Manag. 8, 74 (2022). https://doi.org/10.1007/s40899-022-00659-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-022-00659-4