Abstract
As a distinctive fingerprint of the groundwater sources and water–rock interaction within a karst/fissured-porous environment, isotopic (e.g. δ13C, δ18O, δ34S, 3H, and 14C) and geochemical data have been used to establish flow paths (diffuse flow) of thermal waters used in Caldas da Rainha Spa (Portuguese mainland). These thermal waters (T ≈ 32 °C) discharge from springs and boreholes located close to an N–S-oriented oblique fault (60°E). This hydrothermal system is dominated by deep karst/fissured-porous Lower Jurassic carbonate formations containing slow flowing groundwater. 14C determinations in the total dissolved inorganic carbon (TDIC) indicate a mean “age” of about 1600 years BP for the thermal waters. The HCO3−, Ca2+, and Mg2+ signatures are related to water/calcite–dolomite interaction, whereas Na+, Cl−, and SO42− concentrations are mainly associated with halite and gypsum dissolution. δ18O values indicate that the hydrothermal aquifer system is depleted in heavy isotopes comparing to the shallow aquifer systems, signifying that the main recharge must be related to the Lower Jurassic carbonate formations of the Candeeiros Mountain. The δ13C values measured in the TDIC are typical of carbonate dissolution enhanced by CO2 soil air dissolution. The δ34Ssulphate and δ18Osulphate values of the thermal waters indicate that the sulphate is clearly the result of water–rock interaction with evaporitic layers at depth. Considering a mean geothermal gradient in the region of about 30 °C/km, the silica and K2/Mg geothermometry seems to indicate more reliable circulation depths (1–2 km) for the thermal waters than the SO42−–H2O isotope geothermometer (3–5 km depth). The lack of mixing evidences between the thermal and the local shallow cold groundwaters indicates that both water sources are distinct. Furthermore, increasing knowledge on the local/regional hydrogeology is extremely important to achieve the sustainable use of such “invisible” georesources, since most thermal and mineral waters from karst aquifers worldwide are used both as a source of bottled water and a recreational resource (spa facilities, tourism, etc.).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater represents about 96% of unfrozen freshwater resources and about 35% of the world’s drinking water (UNESCO-WWAP 2006). Furthermore, groundwater is less vulnerable to surface-induced pollution than surface waters. Among groundwaters, thermal waters are the “noblest relative”, since these waters originate from atmospheric precipitation which infiltrates underground via deep crustal discontinuities, retaining particular physical and chemical characteristics according to the mineralogical composition of the geological formations to which they interact (Freeze and Cherry 1979; IAEA 1981; Albu et al. 1997). In addition, their emergence temperature is a function of the depth at which these waters have been circulating. Since thermal waters come into contact with rock at higher temperatures, their total mineralization is greater than in other so-called “normal” groundwaters of the region where they issue (Albu et al. 1997).
Karst aquifers represent, in many parts of the world, a very important source of thermal waters resources (e.g., Albu et al. 1997; Goldscheider and Drew 2007). In most of karst hydrothermal systems, this is due to an efficient drainage network where the influence of karst flow control is very high (e.g., Abusaada and Sauter 2013; Anaya et al. 2014; Delbart et al. 2014).
Groundwaters (thermomineral or not) associated with karst aquifer systems are more vulnerable to mixing processes than other types of aquifers, thus requiring particular attention (e.g., Goldscheider and Drew 2007). Ravbar and Goldscheider (2009) applied different methods, which consider the specific nature of karst aquifers such as heterogeneity and duality of infiltration processes, of groundwater vulnerability mapping in a karst catchment (Slovenia) which were validated by tracer tests. Thermal and mineral waters from karst aquifers supply spas all over the world such as the famous bath in Budapest (Hungary) and geothermal installations use such type of resources for electricity production, district heating, or other purposes (e.g., Goldscheider et al. 2010). According to these authors, regional fault and fracture zones are often the most productive zones, but are sometimes difficult to locate, resulting in a relatively high exploration uncertainty. Dotsika et al. (2010) used O, H, B, Sr, and S isotopes for tracing the origin of dissolved boron in groundwaters ascribed to the Katsika–Petralona karst system, in Central Macedonia, Greece, consisting of extensively fissured and karstified Jurassic limestone under thin sandstone layers. The results obtained indicated that the B dissolved in groundwater is geogenic, i.e., it does not derive from an anthropogenic contaminant source. Erõss et al. (2012) used radionuclides as natural tracers to identify mixing of fluids in the Buda Thermal karst system (Hungary) and to infer the temperature and chemical composition of the end members. Pu et al. (2013) analysed oxygen isotopes and major ions in river and spring waters in Lijiang basin-Yulong Mountain region, SW China to identify the geochemical evolution affecting water quality and groundwater recharge sources. Those authors concluded that water–rock interaction was the significant contributor to water geochemistry, while there was a little anthropogenic influence as a whole in the region.
There are about 50 Spas in the Portuguese mainland with waters whose temperatures range from 20 to 76 °C. They are located mostly in the northern part of the country due to the greater variety of geological formations and tectonic features founded there. The surface temperature of most of these waters lends itself to a great variety of uses: balneotherapy (its classic function), urban heating, fish-farming, and as a low-enthalpy geothermal resources, with great potential for the future (Marques 2007).
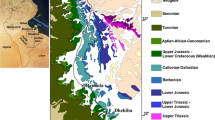
The Caldas da Rainha carbonate hydrothermal system (Fig. 1) is dominated by deep fissured-porous Jurassic limestone formations containing mainly slow flowing groundwater in the rock fissures and joints. As further discussed, in the case of Caldas da Rainha carbonate hydrothermal system, the recharge takes place only where the karstified formations outcrop.

Adapted from Zbyszewski and Moitinho de Almeida (1960)
Geological sketch map of the study region. (E) stands for sandy-silty sediments; (D) stands for loamy and dentritic–sandstone–rocks; (C) and (B) stand for carbonate rocks; (A) stands for marls (and evaporitic deposits). In the place indicated as (7) are located the Caldas da Rainha thermal springs and the thermal waters exploitation boreholes. (1) stands for an unexploited thermal spring located outside the Caldas da Rainha spa area, (2) stands for a stream water, (3, 4, 5, and 10–16) stands for the sampling sites of shallow cold dilute groundwaters (well and borehole waters) mentioned throughout the text.
To establish effective global protection measures for thermal water bodies, it is highly advisable to specifically consider the risk of contamination, which should be the conceptual basis of the methodology for defining safeguard zones in thermal water bodies intended for Spas supplies. Unfortunately, sometimes, these vital and rather limited georesources are polluted due to anthropogenic activities as: agricultural runoff loaded with chemical fertilizers, pesticides and animal wastes, septic tanks, landfill, municipal wastewater, and industrial wastes (e.g., Ryan and Meiman 1996).
The aim of this study is to increase knowledge on the existence of possible mixing processes between Caldas da Rainha deep thermal waters (T ≈ 32 °C), used in local Spas, and local shallow cold groundwaters. For this purpose, a structural geology, hydrogeomorphology, hydrogeochemistry, and isotope hydrology interdisciplinary geosciences approach was used. The main goal is to demonstrate the usefulness of coupled isotopic (δ18O) and geochemical (major ions) approach to assess shallow/thermal water interactions in a karst aquifer system, providing a clear picture on the potential application to other case studies (see Verbovšek and Kanduč 2016).
Field sampling and laboratory methods
Fieldwork campaigns were carried out in the study region (Fig. 1) to collect groundwater samples from boreholes and springs related to the thermal, local surface (e.g. stream) and shallow cold dilute groundwater systems, for isotopic (δ2H, δ18O, δ13C, 3H, and 14C) and chemical analyses.
Temperature (°C), electric conductivity (μS/cm), and pH were measured in the field. The chemical parameters of the water samples were analysed at the Instituto Superior Técnico, Laboratório de Mineralogia e Petrologia (Portugal), using the following methods: total alkalinity (here referred to HCO3−) was measured a few hours after collection by titration; atomic absorption spectrometry for Ca2+ and Mg2+; emission spectrometry for Na+ and K+; spectrophotometry UV/V for SiO2; ion chromatography for SO42−, NO3−, and Cl−. Sulphide was precipitated in situ as CdS and at the laboratory titrated (by potentiometry) with sodium thiophulfate. Dry residuum (D.R.) was calculated following the US Geological Survey procedure, which recommends drying a filtrate sample at 180 °C (Hem 1970).
Environmental isotopes (δ2H, δ18O and 3H) were measured at the Centro de Ciências e Tecnologias Nucleares (C2TN/IST—Bobadela, Portugal). The δ18O and δ2H determinations were carried out using a SIRA 10 VG-ISOGAS mass spectrometer, following analytical methods described by Epstein and Mayeda (1953) and by Friedman (1953) and Tanweer (1990), respectively. The results are reported in δ notation in o/oo vs. V-SMOW. The accuracy of the measurements is 1o/oo for δ2H and 0.1o/oo for δ18O. Tritium content in the water samples was determined (also at C2TN/IST), using electrolytic enrichment followed by liquid scintillation counting measurements (PACKARD TRI-CARB 2000 CA/LL) with a detection limit of 0.5 TU. The associated error to the measurements (≈ 0.6 TU) varies with the tritium concentration in the water samples. This analytical method is described in IAEA (1976). The δ13C and carbon-14 determinations were performed on the total inorganic dissolved carbon (TIDC), at the University of Georgia, Centre for Applied Isotope Studies, USA, by AMS. The radiocarbon content is given in pmC (percentage of modern Carbon) and the δ13C values are reported in ‰ vs. V-PDB standard, with an accuracy of ± 0.1‰. The physico-chemical parameters and isotopic composition of representative waters from Caldas da Rainha region are presented in Table 1.
Geological setting
Geological and morphotectonic data are essential when studying thermal waters vs. karst hydrogeology. It is very problematic to understand a thermal karst aquifer system without taking into account the lithological heterogeneity, geostructures, rock fracturing and weathering degree, as well as the topography, morphostructure, and landscape history (Albu et al. 1997; Goldscheider and Drew 2007).
In the study region, favorable climatic conditions, lithological characteristics, and tectonics generate promising thermal aquifer systems (IGM 1998). Due to topographic gradients, stratum dip, fault behavior, and geological contacts, thermal springs (≈ 32 °C) emerge at Caldas da Rainha city (Fig. 1).
The study region is dominated by a huge syncline structure responsible for the regional flow paths of the thermal waters, discharging (at the W part of the syncline) from springs and boreholes located close to a locally N–S-oriented oblique fault (60°E) (see Fig. 1). This fault puts in contact loamy and dentritic rocks from the Upper Jurassic and marls (and evaporitic deposits) from the Hetangian–Retian (Zbyszewski and Moitinho de Almeida 1960; Marques et al. 2007, 2013).
The thermal aquifer system is ascribed to Middle and Lower Jurassic carbonate series, being covered by Upper Jurassic formations (Fig. 1). Regional diapiric structures were responsible for the uplift and consequent folding of the Jurassic formations (Zbyszewski and Moitinho de Almeida 1960; Marques et al. 2009). These salty domes had been eroded and the Hetangian–Retian becomes exposed at the surface. The most recent formations are Plio-Plistocene sandy–silty sediments (Zbyszewski and Moitinho de Almeida 1960), which contain superficial unconfined aquifers in the porous dentritic formations (mainly sands and argillaceous sandstones).
Recharge into carbonate aquifers may both originate from the carbonate area itself or from adjacent non-carbonate areas. In the present study, the thermal waters infiltrate into shallow holes or dolines (at Candeeiros Mountain; Fig. 1E part of the syncline—on the karstified Middle and Lower Jurassic rocks) rather than diffusely through the overlying loamy and dentritic rocks.
Results and discussion
Hydrogeochemical and isotopic signatures
Caldas da Rainha thermal waters belong to the Cl–Na sulphurous-type. The presence of reduced species of sulphur in the thermal waters could be related to bacterial sulphate reduction (Krouse and Mayer 2001). Considering the present knowledge of the lithology of the study region, the clear correlation between Na+ and Cl− in the thermal waters (Fig. 2; dashed line) indicates different degrees of water–rock interaction mainly ascribed to different underground flow paths within the same geological environment, including dissolution of halite founded along the regional syncline (Zbyszewski and Moitinho de Almeida 1960), since the rCl/rNa ratio in the thermal waters is close to 1 (≈ 0.98). Figure 3 shows that higher Ca2+ and SO42− concentrations found in the thermal waters are strongly pointing to dissolution of gypsum lenses, located in the deepest parts of the syncline. Nevertheless, the contribution of the Jurassic carbonate series to the Ca2+ signatures of the thermal waters (as well as for some of the cold dilute groundwaters) should not be overlooked. The dashed line in Fig. 3 has a similar explanation as in Fig. 2. The cold dilute groundwaters percolating the Middle and Lower Jurassic carbonate formations belong to the Ca-HCO3-type waters likewise the groundwaters associated with the Upper Jurassic sandstones. The cold dilute groundwaters percolating through Upper Jurassic rocks present higher Cl−, SO42−, and NO3− concentrations (see Table 1) which could be related to the fact that the shallow cold dilute groundwaters (most from dug wells) were sampled from sites with intense agriculture practices (this approach will be developed in item 4.2).
Diagram Ca2+ vs. SO42− for groundwaters ascribed to different geological formations. Symbols as in Fig. 2
The 18O and 2H content is widely used to support the results achieved by hydrogeochemical approaches (see Verbovšek and Kanduč 2016). Nevertheless, they can provide independent assessment of the origin and pathways of groundwaters. Water oxygen and hydrogen isotopes can be viewed as environmental tracers of water flow paths, since waters flowing along a particular flow path may have distinctive O and/or H isotopic fingerprints (e.g., Geyh 2000; Zhao et al. 2012). Marques et al. (2009, 2013) reported that Caldas da Rainha thermal waters are meteoric waters rather depleted in heavy isotopes, when compared to the shallow cold dilute groundwaters sampled in the Upper Jurassic loamy and dentritic formations (see Fig. 4), indicating the presence of distinct aquifer systems with different recharge altitudes. As stated by those authors, stable isotope (δ18O; δ2H) data seem to indicate that the recharge of the thermal aquifer is rather diffuse, being mainly ascribed to precipitation that infiltrates on the Middle and Lower Jurassic formations on the W block of the Candeeiros Mountain (see Fig. 1).
Karst groundwater systems are dynamic and in the case of karstified/fissured-porous aquifer systems (as in the present case study) groundwater is in “slow motion” from recharge (where huge karstified areas are present) to discharge areas. In these cases, there are significant differences in groundwater flow regimes and residence times, which can be recognized by the use of radioactive (3H and 14C) environmental isotopes. The use of 14C measurements in karst groundwater systems must be treated with great caution, since the tendency of dissolved inorganic carbon (DIC) from the atmosphere and soil (during recharge) to mixture with the carbonate matrix is very high. Therefore, the observed 14C contents in the total dissolved inorganic carbon (TDIC) of groundwater are best viewed as an approximate measure of the extent of isotopic exchange with the host rock (e.g., Horvatincic et al. 1996; Aggarwal et al. 2014). As reported by Criss et al. (2007), groundwater in regional karst hydrogeological systems can have more than 1000 years old. Gonfiantini and Zuppi (2003) combined the radioactive decay equation with the stable isotope exchange model and concluded that the radioactive decay rate of 14C is three times faster than the isotope exchange rate between carbonate water and rock. They suggested that in karst systems, only a small/simple “apparent age” correction is required.
The 14C content obtained in the Caldas da Rainha thermal waters ranges between 37.58 ± 0.18 and 44.39 ± 0.20 pmC. The 14C “apparent age” determinations (with δ13CTDIC correction) indicate a considerable “apparent age” for the Caldas da Rainha thermal waters (Marques et al. 2012), between 1650 and 1500 years BP (Before Present). Nevertheless, the 14C “apparent age” calculations can be seen as reliable taking into consideration the extension of the regional syncline (≈ 15 km) and the geochemical signatures (Na+, Cl−, SO42−, and Ca2+ concentrations), indicating that the thermal waters should flow deep through the subsurface rocks where the evaporitic deposits are mainly found (Zbyszewski and Moitinho de Almeida 1960). The thermal spring waters from Piscina da Rainha (see Table 1) with no 3H (below detection limits) and low 14C values (37.58 ± 0.18 pmC) seem to corroborate the above-mentioned hypothesis (Marques et al. 2012). The 3H contents (2.5 TU) detected in Caldas da Rainha AC2 thermal borehole waters is not followed by a major change in the chemical composition (i.e., no mixing with shallow groundwaters). This trend seems to indicate a shallower and shorter underground flow path, mostly ascribed to most karstified parts of the system. In this case, the thermal spring waters (with no 3H) should be considered as most representative of the deepest thermal water flow system, where the carbonate rocks are not so intensively karstified, being the flow path mainly associated with a fissured-porous environment and with lower flow velocity.
In Caldas da Rainha thermal waters, δ13C values range from − 9.9 to − 7.8 o/oo vs. V-PDB (Marques et al. 2012). These values are typical for carbonate dissolution enhanced by dissolved soil CO2 during rainy seasons (at the recharge area) and at the end of the vegetation cycle, when the CO2, formed by the decay of organic matter and by the plants roots respiration, is leached into the water from the soil and the vadose zone.
Interaction between shallow and deep (thermal) groundwaters
The problem facing scientists involved in the protection of thermal water resources is focused on the identification of areas and mechanisms by which pollutants can enter the thermal flow system (Albu et al. 1997; Carpenter et al. 1998). This is a required basis for minimizing the impacts of existing or proposed industrial, agricultural, or municipal activities on thermal waters quality (Albu et al. 1997; Sharma 1997). An important issue that should be always present is whether, in the next decades, thermal water development could be done in a way that will be economically and environmentally sustainable.
In this paper, much of the emphasis in thermal water investigations is shifted from the subject of quantity (not a major problem in the study region) to that of quality. The problem of thermal waters quality degradation is vital for the sustainable development of the Spas where the thermal water is used for diversified treatments. In general, solutions to this issue have been found by the implementation of the concept of boreholes and aquifer protection areas (e.g., Field 1993, 2006; Schmidt and Balke 1980, 1985; Zhang et al. 2001). This issue is extremely important, since Caldas da Rainha thermal waters are ascribed to a carbonate aquifer system. Thus, our focus was put on an origin/pathway-target hydrogeological conceptual model, where the groundwater contamination risk is evaluated based on the potential sources of contamination and aquifer vulnerability (see Rezaei et al. 2013).
Tracing groundwaters by means of environmental stable isotopes such as oxygen-18 (18O) offers unique and additional information on the origin and movement of groundwater and its dissolved constituents, allowing a quantitative evaluation of mixing processes between different aquifer systems (Kendall and McDonnell 1998).
Since the isotopic composition of meteoric groundwater generally matches the mean composition of precipitation over the recharge area, it is usual to use locally derived shallow groundwaters (springs and shallow wells for agricultural uses) instead of resorting to precipitation sampling (IAEA 1981). In the study region, the stable isotopic composition of cold shallow dilute waters from springs and dug wells also lies on or is close to the Global Meteoric Water Line (see Fig. 4) and the more depleted shallow groundwaters (from the Upper, Middle, and Lower Jurassic formations) are those related to sampling sites located at higher altitudes (e.g., Marques et al. 2007, 2009, 2012, 2013).
The potential environmental impact on the Caldas da Rainha thermal waters, derived from mixing of thermal waters with shallow contaminated groundwaters, will be evaluated based on the use of δ18O values, as environmental mixing tracer. The methodology established in this section consisted of developing several orthogonal diagrams that relate the isotopic signatures (δ18O values) of groundwaters (thermal and shallow systems), representing the different aquifer features of the study region, with its geochemical signatures.
The graphics SO42− vs. δ18O and Ca2+ vs. δ18O (Fig. 5a, b) show similar trends. The group of thermal waters shows SO42− and Ca2+ concentrations exceeding 600 and 250 mg/L, respectively. The high SO42− and Ca2+ content measured in the thermal waters is mainly due to the intense water–rock interaction with evaporite deposits in the study region (e.g. dissolution of gypsum—CaSO4·2H2O). Through the application of SOLVEQ code (Reed and Spycher 1984) allowed us to calculate the saturation indexes Ω (defined as the ratio between the ionic activity product and the solubility product) for a set of minerals including gypsum. The results obtained (see Table 1) show that Caldas da Rainha thermal waters are considerably undersaturated with respect to gypsum (log Ω < − 0.4). The presence of calcium is also linked to the calcium carbonate (CaCO3) dissolution from the limestone formations, aquifer matrix. The relative distribution of the different thermal water samples in the diagrams of Fig. 5a, b highlights that within the thermal waters group, the waters from AC2 borehole exhibit the most enriched δ18O value (most probably ascribed to a lower recharge altitude) and the lowest SO42− and Ca2+ concentrations, probably as the result of less intense water–rock interaction due to a shorter and shallow underground flow path, through the most karstified parts of the system. In fact, thermal waters from AC2 borehole present a 3H concentration that supports this hypothesis (see Table 1). The second group, the shallow cold groundwaters (from the Upper, Middle, and Lower Jurassic formations), has SO42− and Ca2+ concentrations below 130 and 134 mg/L, respectively. In this group, the water sample from Casal do Guerra (sampling location 12—Fig. 1, Lower Jurassic) stands out due to its depleted δ18O values. This isotopic depletion ought to be related to a higher recharge altitude, probably in the area of Candeeiros Mountain, and a water–rock interaction mainly related with limestone rocks. Comparing the diagrams of Fig. 5a, b, the Ca2+ (between 69 and 129 mg/L) and SO42− (between 45 and 129 mg/L) concentration in water samples associated with the Upper Jurassic formations is higher than the concentration of these ions in groundwater samples from the Middle Jurassic geological formations indicating slight water–rock interaction processes. The electrical conductivity values of the groundwater samples from the Upper Jurassic formations (between 605 and 1104 µS/cm) also contrasts with the low conductivity values of groundwater samples from the Middle Jurassic formations (between 143 and 503 µS/cm). This trend could suggest contamination of the shallow groundwaters circulating in the Upper Jurassic formations by anthropogenic practices, particularly agriculture. In the group of waters associated with the Upper Jurassic geological formations, the waters from Mina da Mata and Mina do Hospital (sampling locations 10 and 11, respectively—Fig. 1) stand out for their highest sulphate content, suggesting diffuse contamination by fertilizers used in the green areas involving the Caldas da Rainha Thermal Hospital. However, it should be noted that none of the samples exceed the Portuguese value established for Human consumption, which is 250 mg/L for the SO42− (DL 306/2007).
a SO42− vs. δ18O and b Ca2+ vs. δ18O for groundwaters sampled in different geological formations. Symbols as in Fig. 2
a Na+ vs. δ18O and b Cl− vs. δ18O for groundwaters sampled in different geological formations. Symbols as in Fig. 2
From the observation of the diagrams Na+ vs. δ18O and Cl− vs. δ18O from Fig. 6a, b, we can consider that the higher concentrations of Na+ and Cl− in the thermal waters [900 and 600 mg/L, respectively (see Table 1)] are due to the intense water–rock interaction with halite (NaCl) present in the evaporite deposits. The group of the shallow cold groundwaters has Na+ and Cl− concentrations less than 80 and 120 mg/L, respectively. In this group, the shallow groundwater samples from Mina da Mata and Mina do Hospital (No. 10 and 11—in Fig. 1, Upper Jurassic) have higher Na+ and Cl− as compared with the other water samples from the Upper Jurassic formations. This fact also suggests the existence of diffuse pollution in the sampling area, possibly ascribed to septic tanks (or sanitation) located in the area of the Caldas da Rainha Thermal Hospital garden, where these springs are located.
Shallow groundwater samples ascribed to the Middle Jurassic geological formations display very low Na+ and Cl− concentrations. No shallow cold groundwaters samples exceed the parameter values set for human consumption, which are of 200 mg/L for Na+ and 250 mg/L for Cl− (DL 306/2007). As in Fig. 5a, b, the water sample from Lower Jurassic (Fig. 1, No. 12—Casal do Guerra) stands out due to its depleted δ18O values, and present very low Na+ and Cl− concentrations, corroborating that water–rock interaction is mainly ascribed with limestone rocks.
Concerning the NO3− concentration in the studied waters, it is shown that in the thermal waters, this species was not detected (Fig. 7). With regard to the shallow cold groundwaters, the Casal da Azenha, the sampling point (14) has the highest NO3− concentration (60 mg/L). This water sample is representative of the waters from the upper most vulnerable unconfined aquifer (Upper Jurassic), so that the high concentration of nitrate can result from fertilizers used in agriculture activities in the surrounding farms of the sampling site where the water sample was collected. In groundwater samples associated with the Upper Jurassic geological formations, some water sampling sites (No. 2, 13 and 14—in Fig. 1) present NO3− concentration greater than 50 mg/L (parametric value set for drinking water).
a NO3− vs. δ18O for groundwaters sampled in different geological formations. Symbols as in Fig. 2
SO4 origin [δ34S(SO4); δ18O(SO4)] and reservoir temperature (SO4–H2O isotope geothermometry)
Since the chemical analysis of waters can only provide the sulphate concentration, but not its source, isotopic techniques [δ34S(SO4) and δ18O(SO4)] have been used to reach the origin of sulphate in the thermal waters and in the shallow cold dilute groundwaters from the Upper Jurassic (D) geological formations (Marques et al. 2012, 2013), where diffuse pollution sources are mainly agricultural activities. The results obtained clearly indicate two groundwater groups with different δ34Ssulphate and δ18Osulphate values: (1) the Caldas da Rainha thermal waters having δ34Ssulphate and δ18Osulphate values between + 14.9 and + 19.1‰ and from + 11.1 to + 16.2‰, respectively; and (2) the shallow groundwater samples collected from boreholes (~ 150 m depth) and dug wells in the Upper Jurassic formations displaying δ34Ssulphate and δ18Osulphate values between + 1.5 and + 4.1‰ and + 8.6 and + 9.3‰, respectively. In the case of Caldas da Rainha thermal waters, the isotopic signatures are clearly the result of water–rock interaction with evaporitic formations at depth (see Mayer and Krouse 2004). In the shallow groundwaters, where sulphate concentrations range between 40 and 60 mg/L, sulphate concentrations may be from atmospheric deposition that has cycled through the soil zone and infiltrated shallow cold dilute groundwaters (see Mayer and Krouse 2004). However, the control of fertilizers used in agriculture activities should also not be ruled out.
The usefulness of the SO42−–H2O isotope geothermometer in the case of water-dominated hydrothermal systems is well proven (e.g., Dowgiallo et al. 2005; Martin et al. 2012). The fractionation factor between sulphate and water has been proposed by Lloyd (1968) and by Mizutani and Rafter (1969). The proposed equations are valid for the temperature range of 0–300 °C. Lloyd (1968) stated that the isotopic exchange rate between sulphate and water is slow. Assuming a pH of 7, the time required to reach 99.9% equilibrium varies with the system temperature at depth and with the period of time water–rock matrix (i.e. 2 years at 300 °C, 18 years at 200 °C and up to as much as 500 years at 100 °C). Based on the apparent carbon-14 ages, the residence time of Caldas da Rainha thermal waters seems to be sufficient to ensure isotopic equilibrium. In the case of Caldas da Rainha thermal water system, a reservoir temperature between 100 and 150 °C was obtained by the SO42−–H2O isotope geothermometer (Fig. 8). Therefore, one question arises: are these reservoir temperatures more suitable than those previously presented by Marques et al. (2012, 2013), using the silica and K2/Mg chemical geothermometers (40 < T°C < 60)? Assuming a mean geothermal gradient in the region of about 30 °C/km (Correia and Ramalho 2010), the reservoir temperatures estimated by isotope geothermometry implies a circulation depth of 3–5 km, which seems to be unreasonable given the shape of the regional syncline ascribed to the thermal water system (Zbyszewski and Moitinho de Almeida 1960). In fact, a 5 km depth thermal water circulation is fairly uncommon in such a geological environment, as the lithostatic pressure would tend to seal the rock joints and fractures. On the other hand, at 40–60 °C (the previous estimate), circulation depths are closer to 1–2 km, which seems more accurate.
Oxygen isotope geothermometry based on the SO4–H2O pair, applied to Caldas da Rainha thermal waters. The iso-temperature lines are based on Lloyd (1968)
Hydrogeological conceptual model: an integrated approach
Most karst aquifer systems present considerable differences such as the distribution of conduits and fissures, permeability, flow paths, and residence time (related with groundwater amount and storage). Thus, in conceptualizing karst flow systems, a wide variety of data sources has to be addressed (e.g., White 1969, 2012; Lewis 2012; Goldscheider and Drew 2007).
In the present case study, environmental isotope data (δ18O values) indicate that the main recharge area of the Caldas da Rainha thermal aquifer system seems to be the Middle and Lower Jurassic geological formations located on the eastern side of Candeeiros Mountain (see Fig. 9). In this case, a spatial assessment of vulnerability is rather difficult as the most sensitive parts of the recharge area, such as dolines, shallow holes, bare karst, and sinking streams, are intensely disseminated. Therefore, concerning groundwater resource protection, the studied karst recharge area, of latent vulnerability, should be protected as a whole. The local meteoric waters enter the system at the surface, at the most karstified areas of Candeeiros Mountain and flows through the joint and fracture network with matrix interactions (i.e., a fissured-porous aquifer system). The discharge zone is situated at the western border of the regional syncline, close to the locally oriented N–S oblique fault (60°E). The geochemical signatures point out that the infiltrated meteoric waters percolate along the syncline (ca. 15 km length) promoting water–carbonate–evaporitic rock interaction. Coupled isotopic and geochemical data indicate that no evidences of mixing seem to occur between the shallow groundwaters and the thermal aquifer. The data obtained support the hypothesis that the thermal aquifer system seems to be “isolated” from anthropogenic contamination, due to the existence of impermeable layers composed of a series of loamy and dentritic rocks of the Upper Jurassic. The conceptual circulation model “build up” for Caldas da Rainha thermal waters acknowledges the existence of independent underground flow paths, as revealed by the 3H data (Marques et al. 2012, 2013). The results obtained through the calculation of the saturation indexes Ω with respect to calcite, dolomite, halite, and gypsum (see Table 1) seem to confirm the above-mentioned hypothesis, where AC2 and Piscina da Rainha thermal waters should be faced as surface manifestations of the same aquifer system ascribed to different underground flow paths.

Adapted from Zbyszewski (1959)
Schematic cross section of the research region. Geological formations: (A) Hetangian–Retian; (B) Lower and (C) Middle Jurassic (thermal aquifer formations); (D) Upper Jurassic; (E) Plio-Plistocene. Sampling sites: (1) Caldas da Rainha diapir; (2) Candeeiros Mountain; (3) Fonte da Bica diapir. (i) thermal spring at Caldas da Rainha Spa; (ii) exploitation borehole of thermal water. δ18O values in ‰ vs. V-SMOW.
The hydrogeochemical characteristics (HCO3−/Ca2+/Na+/Mg2+–Cl−/SO42−facies), issue temperature ≈ 32 °C, and 14C signatures—mean “apparent age” of about 1600 years BP—of Caldas da Rainha thermal waters reflect a depth circulation of about 1–2 km through the subsurface limestone rocks. The results of the SO42−–H2O isotope geothermometer, indicating a reservoir temperature between 100 and 150 °C, seem to be unreliable, not corroborating the above-mentioned thermal water circulation depth.
In this case, geological, hydrogeological, hydrogeochemical, and environmental isotopic techniques provided important information on recharge areas, underground flowpaths, water–rock interaction, non-existence of contamination processes, and transit times in the system, which will be extremely useful for the sustainable management of Caldas da Rainha thermal water system.
Concluding remarks and outlook
This paper illustrates the wide applicability of geochemical and environmental isotopic methods in thermal waters assessment and management in karst regions. Groundwater is a “hidden resource” for which exploration is much more difficult than for surface waters due to its inaccessibility. The conceptual model described above incorporates geochemical and isotopic techniques to gain a more holistic understanding of this type of carbonate rock aquifers. This approach allowed us to: (1) update and strength the conceptual model for one of Portugal’s most important Spa ascribed to a karst environment; (2) improve knowledge on flow paths, water–rock interaction, and possible mixing processes; (3) contribute to enhance environmental issues, which are vital for a sustainable development of Spas, in karst regions; and (4) stimulate local authorities for the importance of thermal water’s sustainable management.
In the future, the procedures, strategies, and guidelines to be adopted by the Caldas da Rainha Spa owner must be focused on the control of the storage, handling, use, and disposal of potential contaminants within the source zone, and of activities that may adversely affect groundwater quantity and quality. Such issues should be achieved through a combination of activities, programs for best practices, and rules and protocols by local/regional governmental institutions.
References
Abusaada M, Sauter M (2013) Studying the flow dynamics of a karst aquifer system with an equivalent porous medium model. Groundwater 51(4):641–650
Aggarwal PK, Araguas-Araguas L, Choudhry M, van Duren M, Froehlich K (2014) Lower groundwater 14C age by atmospheric CO2 uptake during sampling and analysis. Groundwater 52(1):20–24
Albu M, Banks D, Nash N (1997) Mineral and thermal groundwater resources. Chapman and Hall, London
Anaya AA, Padilla I, Macchiavelli R, Vesper DJ, Meeker JD, Alshawabkeh AN (2014) Estimating preferential flow in karstic aquifers using statistical mixed models. Groundwater 52(4):584–596
Carpenter SR, Caraco NF, Correl DL, Howarh RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorous and nitrogen. Ecol Appl 8:559–568
Correia A, Ramalho EC (2010) Update heat flow density map for Portugal. Proceedings world geothermal congress 2010:7
Criss R, Davisson L, Surbeck H, Winston W (2007) Isotopic methods. In: Goldscheider N, Drew D (eds) Methods in karst hydrogeology. Taylor & Francis, London, pp 123–145
Delbart C, Barbecot F, Valdes D, Tognelli A, Fourre E, Purtschert R, Couchoux L, Jean-Baptiste P (2014) Investigation of young water inflow in karst aquifers using SF6–CFC–3H/He–85Kr–39Ar and stable isotope components. Appl Geochem 50:164–176
DL 306/2007 (2007) Portuguese legislation on water quality (Decreto Lei 306/2007). Diário da República, 1ª série—N.º 164–27 de Agosto de 2007 (in Portuguese)
Dotsika E, Poutoukis D, Kloppmann W, Guerrot C, Voutsa D, Kouimtzis TH (2010) The use of O, H, B, Sr and S isotopes for tracing the origin of dissolved boron in groundwater in Central Macedonia, Greece. Appl Geochem 25:1783–1796
Dowgiallo J, Halas S, Porowski A (2005) Isotope temperature indicators of thermal waters in South-Western Poland. Proceedings world geothermal congress 2005:8
Epstein S, Mayeda T (1953) Variations of 18O content of waters from natural sources. Geochim Cosmochim Acta 4:213–224
Erőss A, Mádl-Szőnyia J, Surbeckb H, Horváthc Á, Goldscheider N, Csomae AÉ (2012) Radionuclides as natural tracers for the characterization of fluids in regional discharge areas, Buda Thermal Karst, Hungary. J Hydrol 426–427:124–137
Field MS (1993) Karst hydrology and chemical contamination. United States Environmental Protection Agency, Washington, [EPA/600/J-93/510 (NTIS PB94135134)]
Field MS (2006) Tracer-test design for losing stream–aquifer systems. Int J Speleol 35:25–36
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, Englewood Cliffs
Friedman I (1953) Deuterium content of natural waters and other substances. Geochim Cosmochim Acta 4:89–103
Geyh MA (2000) Environmental isotopes in the hydrological cycle. Technical documents in hydrolology 39 (IV)—groundwater. UNESCO, Paris
Goldscheider N, Drew D (2007) Methods in karst hydrogeology. Taylor & Francis Group, London
Goldscheider N, Mádl-Szőnyi J, Erőss A, Schill E (2010) Review: thermal water resources in carbonate rock aquifers. Hydrogeol J 18(6):1303–1318
Gonfiantini R, Zuppi GM (2003) Carbon isotope exchange rate of DIC in karst groundwater. Chem Geol 197:319–336
Hem JD (1970) Study and interpretation of the chemical characteristics of natural water. In: Geological survey water, 2nd edn. United States Department of the Interior. Washington, USA
Horvatincic N, Srdoc D, Krajcar Bronic I, Pezdic J, Kapelj S, Sliepcevic A (1996) A study of geothermal waters in northwest Croatia and east Slovenia. Isotopes Water Res Manag IAEA 2:470–474
IAEA [International Atomic Energy Agency] (1976) Procedure and technique critique for tritium enrichment by electrolysis at IAEA laboratory. Technical procedure 19, International Atomic Energy Agency, Vienna
IAEA [International Atomic Energy Agency] (1981) Stable isotope hydrology. Deuterium and Oxygen-18 in the water cycle. IAEA, Vienna, Austria, Technical reports series 210
IGM [Instituto Geológico e Mineiro] (1998) Recursos geotérmicos em Portugal Continental. Baixa entalpia. Direcção de Serviços de Gestão e Recursos Geológicos, Divisão de Recursos Hidrogeológicos e Geotérmicos, Lisboa
Kendall C, McDonnell JJ (1998) Isotope tracers in catchment hydrology. Elsevier, Amsterdam
Krouse HR, Mayer B (2001) Sulphur and oxygen isotopes in sulphate. In: Cook P, Herczeg AL (eds) Environmental tracers in subsurface hydrogeology, 2nd edn. Kluwer Academic Publishers, Boston, pp 195–231
Lewis J (2012) The application of ecohydrological groundwater indicators to hydrogeological conceptual models. Groundwater 50(5):679–689
Lloyd RM (1968) Oxygen isotope behavior in the sulphate-water system. J Geophys Res 73:6099–6110
Marques JM (2007) Portugal: um dos países mais ricos da Europa em termalismo. Veiga da Cunha Serra L, Vieira da Costa A, Ribeiro J, Proença L de Oliveira R (eds) Reflexos de Água, Livro Comemorativo dos 30 anos da Associação Portuguesa dos Recursos Hídricos (APRH), Lisboa, pp 80–81 (in Portuguese)
Marques JM, Graça H, Carreira PM, Matias MJ, Graça RC, Nunes D (2007) Updating Caldas da Rainha thermomineral waters conceptual model (Central Portugal): a preliminary isotopic (18O, 2H and 3H) approach. In: Marques JM, Chambel A, Ribeiro L (eds) Proceedings of the symposium on thermal and mineral waters in hard rock terrains, pp 131–144
Marques JM, Graça H, Eggenkamp HGM, Carreira PM, Matias MJ, Mayer B, Nunes D, Trancoso VN (2009) Karst groundwater quality based on geochemical and isotopic tracers: Caldas da Rainha thermomineral water system (Central Portugal). Proceedings of the 2nd international multidisciplinary conference on hydrology and ecology “HydroEco 2009”, Vienna, Austria, pp 75–78
Marques JM, Graça H, Eggenkamp HGM, Carreira PM, Mayer B, Nunes D (2012) Contribuição de traçadores geoquímicos e isotópicos para a avaliação das águas termais das Caldas da Rainha. Comun Geol 99(2):43–51
Marques JM, Graça H, Eggenkamp HGM, Neves O, Carreira PM, Matias MJ, Mayer B, Nunes D, Trancoso VN (2013) Isotopic and hydrochemical data as indicators of recharge areas, flow paths and water-rock interaction in the Caldas da Rainha—Quinta das Janelas thermomineral carbonate rock aquifer (Central Portugal). J Hydrol 476:302–313
Martin JB, Kastner M, Henry P, Le Pichon X, Lallement S (2012) Chemical and isotopic evidence for sources of fluids in a mud volcano field seaward of the Barbados accretionary wedge. J Geophys Res. doi:10.1029/96JB00140
Mayer B, Krouse HR (2004) Procedures for sulphur isotope abundance studies. In: de Groot P (ed) In: Handbook of stable isotope analytical techniques. Elsevier, Amsterdam, pp 538–596
Mizutani Y, Rafter TA (1969) Oxygen isotopic composition of sulphates, part 3: Oxygen isotopic fractionation in the bisulphate ion-water system. NZ J Sci 12:54–59
Pu T, Hea Y, Zhanga T, Wua J, Zhuc G, Changa L (2013) Isotopic and geochemical evolution of ground and river waters in a karst dominated geological setting: a case study from Lijiang basin, South-Asia monsoon region. Appl Geochem 33:199–212
Ravbar N, Goldscheider N (2009) Comparative application of four methods of groundwater vulnerability mapping in a Slovene karst catchment. Hydrogeol J 17(3):725–733
Reed MH, Spycher NF (1984) Calculation of pH and mineral equilibria in hydrothermal waters with application to geothermometry and studies of boiling and dilution. Geochim Cosmochim Acta 48:1479–1492
Rezaei A, Zare M, Raeisi E, Ghanbari RN (2013) Interaction of a fresh water lake and a karstic spring via a syncline fold. Groundwater 51(2):305–312
Ryan M, Meiman J (1996) An examination of short-term variations in water quality at a karst spring in Kentucky. Groundwater 34(1):23–30
Schmidt H, Balke KD (1980) Possibilities for artificial groundwater recharge and storage in Germany. Z Dtsch Geol Ges 131:93–109
Schmidt H, Balke KD (1985) Standards and laws of artificial groundwater recharge in Germany. Environmental Office, UBA-FB, Berlin, pp 80–179 (German)
Sharma BR (1997) Environmental and pollution awareness. Technical India Publications, New Delhi
Tanweer A (1990) Importance of clean metallic zinc for hydrogen isotope analysis. Anal Chem 62:2158–2160
UNESCO-WWAP (2006) Water: a shared responsibility. The United Nations world water development report 2, UNESCO, Paris
Verbovšek T, Kanduč T (2016) Isotope Geochemistry of groundwater from fractured dolomite aquifers in Central Slovenia. Aquat Geochem 22(2):131–151
White WB (1969) Conceptual models for carbonate aquifers. Groundwater 7(3):15–21
White WB (2012) Conceptual models for carbonate aquifers. Groundwater 50(2):180–186
Zbyszewski G (1959) Étude structurale de la vallée typhonique de Caldas da Rainha (Portugal). Memórias dos Serviços. Geológicos de Portugal 3:1–184
Zbyszewski G, Moitinho de Almeida F (1960) Carta Geológica de Portugal, escala 1/50,000. Notícia Explicativa da Folha 26-D—Caldas da Rainha
Zhang K, Wen Z, Xhang X (2001) China’s water environment at the beginning of the 21st century: challenges and countermeasures. Water Sci Technol 46(11–12):245–251
Zhao L, Xiao H, Dong Z, Xiao S, Zhou M, Cheng G, Yin L, Yin Z (2012) Origins of groundwater inferred from isotopic patterns of the Badain Jaran Desert, Northwestern China. Groundwater 50(5):715–725
Acknowledgements
This study was proposed and funded by the Ministry of Health/Centro Hospitalar das Caldas da Rainha/Portugal, under the Research Contract HIDROCALDAS No. 1577. CERENA/IST thankfully acknowledges the FCT support through the UID/ECI/04028/2013 Project, and C2TN/IST gratefully acknowledges the FCT support through the UID/Multi/04349/2013. The authors would like to acknowledge Dr. Henrique Graça, Technical Director of Caldas da Rainha Spa, for all the support during field work campaigns and for helping the drawing of the geological sketch map of the study region. Some of the figures were improved by José Teixeira and Helder Chaminé and we thank them both.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special issue on Mineral and Thermal Waters.
Rights and permissions
About this article
Cite this article
Marques, J.M., Matos, C., Carreira, P.M. et al. Isotopes and geochemistry to assess shallow/thermal groundwater interaction in a karst/fissured-porous environment (Portugal): a review and reinterpretation. Sustain. Water Resour. Manag. 5, 1525–1536 (2019). https://doi.org/10.1007/s40899-017-0207-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-017-0207-3