Abstract
A study based on geochemical and environmental isotope data was performed in two low-temperature hydrothermal systems in NW Portugal (Caldelas and Gerês hydrothermal systems). This study aims to demonstrate the role of integrated hydrogeological tools for developing conceptual models of groundwater circulation. The studied hydrothermal systems are ascribed to groundwater circulation in fractured calc-alkaline/alkaline granitic contexts, responsible for different groundwater geochemical types. Caldelas hydrothermal system is dominated by Ca/Na-HCO3 waters, while at Gerês, the hydrothermal system is characterized by Na-HCO3-type waters. The isotopic signatures indicate that the preferential recharge areas are located at very different altitudes (Caldelas around 171 m a.s.l. and Gerês between 912 and 1118 m a.s.l.). The thermomineral waters issue with a mean temperature of 27 °C (Caldelas) and 43 °C (Gerês). Several geothermometers were used to estimate the reservoir temperature and the corresponding depth reached by the hydrothermal systems. At Caldelas, the mean estimated reservoir temperature was 42 ± 6 °C, using only the chalcedony and K2/Mg geothermometers, which suggests depths around 0.93 km. In the Gerês thermal area, the chalcedony, K2/Mg, and Na/K/Ca (β = 4/3) geothermometers gave a mean estimated reservoir temperature of 96 ± 5 °C, suggesting depths close to 2.8 km. In both case studies, conceptual circulation models are proposed, based on: geological heterogeneities, geochemical and isotopic signatures, mean preferential recharge altitudes, groundwater circulation paths, and mean residence time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
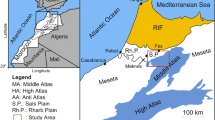
One of the main objectives in hydrogeological research is to establish the most likely conceptual models to explain observations in groundwater systems (e.g., Moore 2002; Baalousha 2008; Kresik and Mikszewski 2013). With this goal, integrated and comparative studies using hydrogeochemical and isotopic determinations were performed at Caldelas and Gerês hydrothermal systems, located in NW Portugal (Fig. 1). Using interdisciplinary approaches in both aquifer systems will allow the development of strong conceptual hydrogeological models that can lead to practical applications like the estimation of groundwater’s mean residence time and preferential recharge altitudes for each system. This type of knowledge provides basis for future studies on the vulnerability of the hydrothermal systems, to anthropogenic effects. The thermomineral waters from both hydrothermal systems are used in local Spas, playing an important role in the regional socio-economic development.

(adapted from Pedrosa 1999)
Regional geology and hydrogeological setting of Caldelas and Gerês hydrothermal systems.
Among the techniques most frequently applied in hydrogeological studies to characterize groundwater circulation and to establish conceptual circulation models, environmental isotopes, particularly 2H, 13C, 18O, 3H, and 14C, usually provide valuable insights to understand the aquifer systems dynamics (e.g., preferential recharge altitude, groundwater flow paths, and mean residence time). In fact, environmental isotopes coupled with hydrogeochemical signatures often play an important role in the identification of mixing processes between different aquifer units (e.g., Sidle 1998; Diamond and Harris 2000; Marques et al. 2006; Ayenew et al. 2008). Frequently, in the case of recharge altitude estimation, the environmental signals obtained through the 18O and 2H content represent important tools in the evaluation of water vapor masses moving through the continents (Gonfiantini et al. 2001; Carreira et al. 2009, 2014; Liota et al. 2013; Giustini et al. 2016). The distribution of δ18O and δ2H mimics the topography of the continents; waters of mountain chains are denoted by more depleted δ values, known as “the altitude effect” (Dansgaard 1964; Rozanski et al. 1982, 1992, 1993). The lowering of temperature with increasing elevation in mountain regions usually leads to enhanced condensation and therefore to a progressive depletion of heavy isotopes in precipitation with altitude. This altitude effect has been used in numerous hydrological studies to identify the preferential recharge areas and to investigate the origin and interconnection of water bodies (Darling et al. 2003; Marques et al. 2003; Galego Fernandes and Carreira 2008; Carreira et al. 2011, 2014).
Another tool, which is very often used in the characterization of such type of hydrothermal systems, is tritium (3H) content. According to Gonfiantini et al. (1990), 3H in precipitation at most coastal stations located in the northern hemisphere has returned to the presumed pre-bomb values, and this trend has also been observed at all the stations in the southern hemisphere. Nevertheless, the use of 3H in the characterization of groundwater dynamics and identification of mixing between different water units can be useful. For example, knowing that the seasonal variations of tritium in the atmosphere (spring leak) can be followed in precipitation, one can link for example this feature to the relative shallow groundwater systems; besides, the presence of 3H can be used to recognize an active aquifer recharge.
This work is focused on a comparative study of the thermomineral waters of Caldelas and Gerês. The main goal of the present work is to develop a hydrogeological conceptual model of the hydrothermal systems, using the geological, morphotectonical, hydrogeochemical, and isotopic data available for each area. Furthermore, the identification of possible mixing processes between the hydrothermal systems and the local aquifers (from unconfined and semi-confined aquifer systems) was investigated, since, in both regions, the intense land use for agriculture should be considered as a pollution source and risk to the hydrothermal systems. Furthermore, attention was put on the estimation of reservoir temperature and maximum groundwater flow depth reached by Caldelas and Gerês hydrothermal systems, based on the chemical composition of the discharging fluids.
The collected information allowed a better understanding of the thermomineral aquifers and could potentially support the selection of strategic sites for future drilling, as well as delimitation of the preferential protection areas, always bearing in mind that these Spas are expanding their activities in response to increased demand. Furthermore, a conceptual model for each studied system will be presented using a cross-section displaying the (1) tectonics and subsurface geology, (2) preferential distribution of recharge, (3) main flow path directions, and (4) discharge areas.
Geological setting
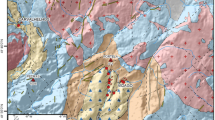
At Caldelas and Gerês, the thermomineral water circulation occurs mainly in calc-alkaline/alkaline granitic contexts, respectively, with different mineralogical compositions (e.g., Neiva 1993; Mendes and Dias 1996, 2004; Jaques et al. 2016). The study sites are located in the geotectonic framework of the Galicia—Trás-os-Montes Zone (Ribeiro et al. 2007). In Northern Portugal, the following types of granitic rocks can be identified based on their geometric relationships and internal deformation (Ferreira et al. 1987): (1) syn-tectonic granites with minute flakes of muscovite and biotite, and metasomatic assemblage, and strongly correlated with migmatites (Ribeiro and Moreira 1986); (2) late-tectonic granites (at Caldelas region), frequently associated with granodiorites, with abundant biotite, and muscovite being a secondary mineral (Moreira and Simões 1988); (3) post-tectonic granites (at Gerês region) characterized by the presence of megacrystals of potassium feldspar and biotite. The lack of metamorphic minerals in these granites point to an age younger than the last Variscan deformation phase (Moreira and Simões 1988).
Fluvial sandstones and conglomerates of quaternary age (sometimes with clay layers) constitute the most recent formations in the region, particularly at the Gerês and Homem valley bottoms (Coudé-Gaussen 1981; Moreira and Simões 1988; Vidal-Romaní et al. 1990).
The main fracture systems in the region are represented by tectonic lineaments (some of them related with strike-slip faults), trending NNE–SSW, NNW–SSE, ENE–WSW, and WNW–ESE of late Variscan age, and still active during the Meso-Cenozoic (e.g., Cunha et al. 2019; Azañón and Cabral 2020). The morphology of the Gerês region shows that the regional fracture system of NNE–SSW direction is responsible for the impressive landscape of the Gerês valley. In the region, the intersection of the NNE–SSW with ENE–WSW fracture systems is the major control of the thermal springs occurrence (e.g., Medeiros et al. 1975; Carvalho et al. 2005; Lima and Oliveira 2007).
According to Lima (2011), in the study region, the ENE–WSW fault system is the most important in the springs occurrence, since this fracture system has a wide spreading along hundreds of kilometers, defining also the main surface drainage catchments, i.e., underling the network drainage axes.
The studied sites have similar structural, tectonic, and hydrogeological features, namely: (1) the local and regional high-altitude lands, associated with highly fractured granitic rocks, play an important role in conducting the infiltrated meteoric waters toward the deep aquifer systems; (2) the discharge zones are related to the intersection of the major regional deep fault structures (Ribeiro et al. 2007).
Methodology: sampling and analytical methods
Two fieldwork campaigns were performed in Caldelas and Gerês region, the first in 2002 and the second in 2003 (March of each year). Groundwater samples from the hydrothermal systems and from the local shallow aquifers were collected for chemical (major cations and anions) and isotopic (δ2H, δ18O, and 3H) determinations. Temperature (°C), pH, and electrical conductivity (EC: in μS/cm) measurements were performed in situ. All water samples were specifically treated by ultrafiltration for chemical analyses determinations. Total alkalinity was determined a few hours after collection. The thermomineral water samples were collected in boreholes and in springs in the case of Caldelas system. The water samples from Gerês hydrothermal system, as well as the water samples from the local shallow aquifers were sampled only in springs.
The water chemical analyses were performed at the Laboratório de Mineralogia e Petrologia of Instituto Superior Técnico (LAMPIST), Portugal, by the following methods: atomic absorption spectrometry for Ca2+ and Mg2+; emission spectrometry for Na+ and K+; colorimetric methods for SiO2; ion chromatography for SO42−, NO3−, nd Cl−; potentiometry for alkalinity, here referred to as HCO3−.
The δ2H and δ2O were determined three times for each sample, and isotopic composition for each sample stands for the mean value of these measurements. The measurements were conducted on a mass spectrometer (SIRA 10, VG-Isogas) using the methods proposed by Friedman (1953) and Epstein and Mayeda (1953) for 2H and 18O, respectively, with a precision of ± 1‰ for δ2H and ± 0.1‰ for δ18O. The tritium (3H) content was determined using the electrolytic enrichment and liquid scintillation counting method (IAEA 1976; Lucas and Unterweger 2000) using a Packard Tri-Carb 2000 CA/LL. The error associated with the 3H measurements [usually around 0.6 tritium units (TU)] varies with the 3H concentration in the sample. All isotopic determinations were performed at Instituto Tecnológico e Nuclear (ITN), presently Centro de Ciências e Tecnologias Nucleares (C2TN/IST), Portugal.
Results and discussion
Hydrogeochemical approach: Caldelas and Gerês hydrothermal systems
Geologic and geochemical investigations, carried out in granitic regions, have demonstrated that the hydrolysis of rock-forming silicate minerals is an important process that controls the chemical composition of natural waters (Bowser and Jones 2002; Sung et al. 2012; Cho et al. 2015).
The studied hydrothermal aquifer systems are located within calc-alkaline/alkaline granitic environments responsible for the occurrence of two groundwater chemical types. A predominance of Ca/Na-HCO3-type waters is found at Caldelas hydrothermal system, while Na-HCO3-type waters characterize the Gerês hydrothermal system (Table 1).
The local shallow aquifers (Table 1) present low total dissolved solids (TDS—mean value of 36.09 ± 6.99 mg/L). The low mineralization and temperatures observed in this group of groundwater samples seem to indicate shallow and short flow paths, and slight water–rock interaction processes responsible for the typical Na–Cl facies.
The hydrolysis of plagioclases should be considered the main water–rock interaction process responsible for the occurrence of Ca/Na-HCO3- and Na-HCO3-type waters at Caldelas and Gerês hydrothermal systems, respectively. In fact, as referred by Medeiros et al. (1975), in the granitic rocks from Caldelas region, the dominant feldspar is plagioclase (oligoclase–andesine) whose composition varies around An25–34. More recently, Dias et al. (2010) refer that in the granitic rocks from Caldelas region, the plagioclase ranges from An14–36, while the previous studies presented by Medeiros et al. (1975) indicated the presence of Na-rich plagioclase (An8) in the granitic rocks of Gerês region. Mendes (2001) reported the presence of Na-rich plagioclases from Ab70–98 to Ab81–99 in the Gerês granitic rocks.
Plotting the chemical composition of the thermomineral and of the local shallow groundwaters in a Piper Diagram (Fig. 2), three groups can be recognized, namely:
-
(1)
the cold dilute groundwaters, with greater hydrogeochemical variability, which could be related to anthropogenic inputs; these samples were collected in different springs, frequently in areas of intensive agricultural activities;
-
(2)
the cluster of Gerês hydrothermal system, presenting the greatest hydrogeochemical homogeneity;
-
(3)
Caldelas hydrothermal cluster, showing a greater heterogeneity, particularly in major cation content, higher than the Gerês thermomineral waters.
Two hypothesis can be formulated to explain the two hydromineral clusters, which should be ascribed to the mineralogical heterogeneities (mainly the plagioclases) of the percolated calc-alkaline/alkaline granitic rocks. In fact, the heterogeneity observed in the cations content of Caldelas thermomineral waters may be related to Ca2+ content of the feldspars of local igneous rocks (e.g., Mendes and Dias 2004; Jaques et al. 2016). According to Jaques et al. (2016), the albitization and quartz dissolution of Gerês granitic rocks occurred at depths shallower than 5 km induced by the fluids flow along the geological structures.
The different geochemical signatures, found between Caldelas and Gerês thermomineral waters, indicate underground flow paths with water–rock interaction processes where calcium and sodium dissolution is controlled by the Ca- and Na-plagioclases’ hydrolysis present in the rock matrix, respectively (Fig. 2). These hydrogeochemical signatures are well observed when the Na+ + K+ or Ca2+ + Mg2+ contents are plotted as a function of the bicarbonate concentration (Fig. 3). Dissimilarities between Caldelas and Gerês hydrothermal systems are also found in the alkalinity.
From the observation of Fig. 3, mixing between hydrothermal fluids and local shallow groundwater, either at Caldelas or Gerês areas, appears to be unlikely. Nevertheless, the shift within Caldelas hydrothermal system (AC4 borehole when compared with AC6 borehole or even with Bica de Fora spring waters) is noticed in temperature and silica content (see Table 1). However, this deviation cannot be assumed to be the result of mixing with shallow groundwaters, since the silica content of the cold groundwaters does not support the hypothesis of a mixing pattern (Fig. 4). A possible explanation could be ascribed to different exploitation rates, or to an analytical error.
Following the results presented previously (Figs. 2, 3, 4), the three groups of groundwaters can also be identified (Table 1), based on electrical conductivity (EC), dry residuum (DR), temperature, and pH. The water EC determined in the thermomineral waters of Caldelas and Gerês is correlated to the DR. The DR mean values range from 105.9 ± 6.2 (at Caldelas) to 246.3 ± 5.6 mg/L (at Gerês). The local shallow groundwaters in the region display a mean DR value of 37.9 ± 10.1 mg/L. Although mixing between the mineral waters and the local shallow aquifers, in overall terms, seems not to exist in both areas, the deviation observed in some physico-chemical signatures of Caldelas AC4 waters could be explained by mixing process with local shallow aquifers. This hypothesis can explain the temperature decrease and the increase in the nitrate content. An alternative hypothesis is that the thermomineral waters from borehole AC4 are related to another hydrothermal system ascribed to a different underground flow path.
According to May (2005), an indicator of anthropogenic contamination is the relation Ca-NO3, parameter usually associated with agriculture. However, in Caldelas region, calcium dissolution seems to be controlled by the hydrolysis of Ca-feldspars present in the rock-matrix silicate minerals and is not associated with an anthropogenic source. Furthermore, according to May (2005), the correlation between Ca and SO4 can also be a fingerprint of pollution. However, no “positive correlation” is found between the Ca and the SO4 content [rCa-SO4 = − 0.76 (n = 4)], corroborating the formulated hypothesis of no mixing. In fact, during the 2003 campaign, no NO3 was detected in Caldelas thermomineral waters (AC6 borehole and Bica de Fora spring).
In the study regions, Na+ dominates in the local shallow groundwaters, when compared with Ca2+ and Mg2+. In addition, Caldelas NS4 and NS5 springs and, possibly, AC4 borehole, as well as the Gerês NS6 spring present signatures of probable anthropogenic contamination (e.g., NO3− concentration), maybe due to their location, downhill from agricultural areas.
The different chemical facies (Ca/Na-HCO3 and Na-HCO3) found at Caldelas and Gerês hydrothermal systems, and the different emergence temperatures (20–31 °C and 40–45 °C, respectively), are essentially dependent (not only from the plagioclase hydrolysis and the local geological heterogeneities), but also from:
-
(1)
the initial CO2 content (pH values of 6.64–7.54 for Caldelas waters and 8.18–8.47 for Gerês waters), indicating higher CO2 pressure in the Caldelas system;
-
(2)
different degrees of the water–rock (granite) reaction progress and of reservoir temperature (much lower concentrations of calcium and magnesium and higher concentrations of sodium and bicarbonate for Gerês waters indicate a major degree of water–granite interaction processes).
The dissolution process is a function of water pH and temperature, where the water–rock interaction processes will be faster in acidic than in alkaline waters, where the role of pH is strongly influenced by dissolved CO2 in the aqueous system (Custódio and Llamas 1983). Na, K, Ca, and Mg ions have relatively weak connections within the silicate structure, easily passing to the water. In granitic regions, the Cl and SO4 contents in the groundwater samples are usually low, except when in the presence of pyrite crystals whose presence in oxidizing media may lead to sulfates. With respect to Cl, the presence of this ion is usually attributed to the presence of accessory minerals such as apatite.
In Caldelas and Gerês thermomineral waters, the values obtained in the [rCl − r(Na + K)]/rCl ratio (Table 2) are always negative (except in AC6—2002). These negative values are characteristics of granitic environments, particularly visible in Gerês mineral waters (− 7.73 to − 5.90), where the hydrolysis of feldspar will result in the supply of more alkaline ions (Na and K) to the solution than Cl (Custódio and Llamas 1983). A clear difference between the thermomineral waters and the local shallow groundwaters is evident in Gerês hydromineral system. However, this difference is not well marked in Caldelas thermomineral waters, probably due to the shorter circulation of the mineral waters when compared with Gerês thermomineral aquifer.
In the rMg/rCa ratio, positive values close to zero (0.01–0.14) were obtained for both thermomineral aquifers, indicating greater mobility of Ca in relation to Mg. In the local shallow groundwaters, this ratio increases to around 0.7, although still minor than 1 (Table 2). Additionally, in Caldelas mineral waters, the ratio r (Ca + Mg)/rCl is much higher in Gerês hydrothermal system, probably due to the above-mentioned lithological heterogeneities in the geological setup, with higher percentage of minerals rich in the alkaline earth elements. Nevertheless, the rK/rNa ratio in both thermomineral systems is quite similar and comparable to those from the local shallow aquifers.
The origin of Cl in groundwaters may be associated with rock leaching. However, some authors point out that Cl may be often associated with a deep magmatic origin, similar to that proposed for other groundwaters in N of Portugal connected with or near deep faults (Marques et al. 2006, 2010a, b; Carreira et al. 2008, 2010, 2014). The [r (Na + K)/rCl] ratios obtained for Caldelas and Gerês hydrothermal systems are rather unlike (see Table 2). Several authors (Schoeller and Schoeller 1979; Chae et al 2006; Li and Zeng 2017) draw attention to the fact that when the chloro-alkaline imbalance index values, expressed by the ratio [r (Na + K)/rCl], are < 4.0, the Cl diluted in the waters has a characteristic magmatic origin, not being the result of water–rock interaction processes. Regarding the thermomineral waters under study, they have distinct values: in Caldelas, the Cl content seems to be mainly associated with a deep origin and in Gerês mainly from the rocks leaching, where Cl concentration could increase through deeper and longer circulation paths.
Reservoir temperature: chemical geothermometers
Chemical geothermometer is an important tool used in the exploration of hydrothermal resources, using the data acquired from surface manifestations (e.g., thermal spring and borehole waters). During the last decades, several chemical geothermometers have been widely used to estimate deep reservoir temperatures in hydrothermal systems (e.g., D'Amore et al. 1987; Gokgoz and Tarcan 2006; Arnórsson et al. 2000). The various chemical geothermometers, when applied to the same fluids, often yield a wide range of values for reservoir temperatures. Therefore, care must be taken in interpreting the estimated temperatures, requiring expert knowledge of the chemical geothermometers to distinguish between reliable and ambiguous results (e.g., D'Amore et al. 1987; Arnórsson et al. 2000; Gokgoz and Tarcan 2006). It is a good practice to compare temperatures indicated by different geothermometers.
Table 3 lists the geothermometers that were used to estimate reservoir temperature and circulation in depth at Caldelas and Gerês hydrothermal systems. To obtain a clear visualization of the results of chemical geothermometer, the mean reservoir temperatures and the mean maximum reservoir depths for Caldelas and Gerês hydrothermal systems were plotted (Fig. 5). From the diagram of Fig. 5, it is possible to observe similar results of the chalcedony and K2/Mg chemical geothermometers, as opposed to the dispersion of values resulting from the application of the other chemical geothermometers.
From the six different chemical geothermometers used to estimate the mean reservoir temperature, only chalcedony and K2/Mg will be discussed; the other four geothermometers were dismissed based on:
-
(1)
in the case of Na–K–Ca geothermometer, the discharge temperature at Caldelas boreholes is higher (≈ 30 °C) than the obtained reservoir temperature;
-
(2)
concerning quartz, Na/K, and Na/Li geothermometers, they were not considered for discussion since, the reservoir depths obtained are not compatible with the geological features in both areas, and unreliable considering the tritium content determined in the hydrothermal systems.
For the Caldelas thermomineral waters, the mean reservoir temperature using the chalcedony and K2/Mg geothermometers is close to 44 ± 5 °C, suggesting chemical equilibrium reactions with chalcedony, chlorite, muscovite, and K-feldspars (Bowers et al. 1984; Giggenbach et al. 1983), at this temperature, in the hydrothermal reservoir. Caldelas hydrothermal reservoir should present higher CO2 pressure values than the Gerês thermomineral reservoir, considering the initial CO2 content (pH values of 6.64–7.54 for Caldelas thermomineral waters and 8.18–8.47 for Gerês thermomineral waters).
Knowing that (1) the mean geothermal gradient (gg) in the region is 30 °C/km (IGM 1998), and (2) the mean regional annual atmospheric temperature (Ta) is 14 °C at Caldelas, and 13 °C at Gerês, using the chemical geothermometers (Table 3), the maximum circulation depth reached by the thermomineral waters was calculated through the following equation:
where Tr stands for the mean reservoir temperature.
The mean maximum circulation depth for Caldelas hydrothermal system using chalcedony and K2/Mg geothermometers varies between 0.90 ± 0.05 and 0.95 ± 0.26 km, respectively.
Considering the geological and structural features (Almeida Soares 2019) of Caldelas area and the issue temperatures of the thermomineral waters, a maximum circulation depth for Caldelas waters in the range of 0.93 ± 0.16 km seems to be more realistic. Concerning the Gerês thermomineral waters, we can see that the temperatures estimated using the Chalcedony, K–Mg, and Na–K–Ca (β = 4/3) geothermometers (Table 3) are relatively close (ranging from 89 to 108 °C, with a mean value of 96 °C ± 5 °C), except for the sample NM2. These estimated temperature values suggest similar circulation depths close to 2.8 km. For both hydrothermal systems, the temperature values estimated using the Na–K (Michard 1990) and Na–Li (Fouillac and Michard 1981) are too high, giving reservoir depths unfitted with the tritium content determined in the hydrothermal systems.
Isotope hydrology
Oxygen-18 and deuterium signatures
The deviations observed in the stable isotopic composition of rainfall in a given place will depend on many factors like seasonality, moisture source, rainfall amount, and meteorological conditions during evaporation, condensation, and precipitation, occurring throughout the year (Rozanski et al. 1982, 1992, 1993; Celle-Jeanton et al. 2001; Gourcy et al. 2005; Lambs et al. 2013; González-Trinidad et al. 2017). However, besides this variation, the distribution of δ18O and δ2H mimics the topography of the continents, and, therefore, the precipitation in mountain chains is marked by more depleted δ values, the so-called “altitude effect” (Dansgaard 1964; Rozanski et al. 1982, 1992, 1993). The lowering of air temperature with increasing altitude leads to a progressive depletion in the heavy isotopes (18O and 2H) in precipitation isotopic composition. The “altitude effect” has been used in numerous hydrogeological studies to identify the preferential recharge altitude and to investigate the origin and interconnection of groundwater bodies (e.g., Marques et al. 2006, 2010a, 2017; Carreira et al. 2011, 2014). The vertical isotope gradient established with the δ18O and δ2H precipitation values varies between − 0.15 and − 0.50‰/100 m for oxygen-18 and about − 1 to − 4‰/100 m for deuterium (e.g., Yurtsever and Gat 1981; Araguás-Araguás et al. 2000; Gonfiantini et al. 2001).
The δ2H and δ18O average values of groundwater samples from hydrothermal systems generally match the mean isotope composition of rainfall in the region (Table 4). This, combined with the altitude effect, makes it possible to infer the altitude of recharge of Caldelas and Gerês hydrothermal systems from their isotopic compositions (Fig. 6). The scatter of values should be ascribed to the existence of different recharge altitudes. Similar dispersion in the isotopic data in the regional precipitation has been reported in other works (e.g., Diamond and Harris 2000; Glok Galli et al. 2017).
In this study, the evaluation of the preferential altitudes of the recharge areas (Caldelas and Gerês hydrothermal systems) was performed using the regional equation proposed by Lima (2011): alt. (m a.s.l.) = − 588 δ18O to 2734. This equation was obtained by measuring the isotopic composition of shallow spring waters in the region. Based on the δ18O values of these shallow groundwater samples, the author estimated the local isotopic gradient with the altitude (Fig. 7). Within the research region, the isotopic gradient obtained was 18O = − 0.17 ‰ per 100 m of altitude, using the shallow groundwater isotopic composition and the issuing springs altitudes.
The depleted isotopic composition of Gerês thermomineral waters points to a higher altitude of the preferential recharge area, when compared with Caldelas thermomineral waters. Using the equation proposed by Lima (2011) the mean recharge altitude at Caldelas is around 170 m a.s.l. (value estimated without AC4), while in Gerês, the preferential recharge altitudes are varying between 900 and 1100 m a.s.l. (Fig. 7).
Tritium signatures
Groundwater samples for 3H determinations were collected in all sampling sites (Table 4). The tritium content found in the local shallow aquifers and in Caldelas hydrothermal system is indicating an active recharge. On the other hand, very low (or zero) 3H content was determined at Gerês thermomineral waters, which are indicating a mean residence time greater than 60 years.
No 3H was found in the thermomineral waters from Gerês (with the exception of sample NM4). Gerês thermomineral water samples besides of the absence of 3H present the highest mineralization (represented in Fig. 8c by the SiO2 values), suggesting a longer and deeper circulation path promoting more extensive water–rock interaction processes (Fig. 8d, e) corroborating the results from chemical geothermometers. The 3H and 18O isotopic signatures of the Caldelas thermomineral water samples (Fig. 8b) reflect, in a first approach, the preferential recharge at low-altitude sites (18O data), when compared with Gerês thermomineral waters, and relatively shallow and short underground flow paths (3H data).
Analyzing Table 4 and Fig. 8, some important issues can be identified, namely:
(1) In the local shallow groundwater systems, the 3H content ranges from 3.5 ± 0.5 to 2.1 ± 0.5 TU, values similar to those found in the precipitation samples from the Portuguese Network “Isotopes in Precipitation” (Carreira et al. 2005). However, the AC6 borehole waters (Caldelas hydrothermal system) present higher 3H content (4.1 TU in both campaigns). In the GNIP (Global Network Isotopes in Precipitation) website (http://www-naweb.iaea.org/napc/ih/IHS_resources_isohis.html), the tritium variation in the precipitation over Portuguese mainland can be found, and a decrease of 3H content is observed along the years (Portuguese GNIP stations initiated in 1988) until the present time. The variation of the precipitation can be used to explain the higher tritium content measured in the mineral waters (AC4 and AC6—Caldelas) when compared with the local shallow aquifers.
(2) Tritium values found in regional precipitation are in the order of 5 TU, in two meteorological stations, in the N of Portugal, from the Portuguese Network Isotopes in Precipitation (weight arithmetical annual mean at Porto = 4.5 TU and Vila Real = 6.4 TU: in Carreira et al. (2005). Therefore, based on the tritium input from regional precipitation data and considering the 3H half-life of 12.32 years (Lucas and Unterweger 2000), one can classify these thermomineral waters as modern waters with active recharge.
Most of the Gerês thermomineral water samples do not present tritium. However, during the 2003 campaign, a 3H content of 1.2 ± 0.5 TU was determined at NM4 thermomineral spring water. The easiest explanation is possible mixing with local shallow groundwaters. However, this hypothesis does not seem reliable since the results from the 2003 field work campaign showed that the Gerês thermal spring waters NM3 and NM4 presented almost similar chemical signatures (see Table 1), being the NM3 spring waters characterized by very low 3H content (see Table 4). Another possible explanation is in situ 3H production, at depth. Moser et al. (1989) described this source of 3H associated with the rock matrix at Stripa (Sweden). These authors proposed a tritium source associated with underground production in the granitic rocks. However, those authors also mentioned the importance of constant tritium content in the groundwater, should be observed, during several years in the groundwater samples. In Gerês hydrothermal system, this isotopic homogeneity in the tritium content is not evident (see Table 4), indicating a negligible tritium production at depth to justify the tritium measured in Gerês Bica NM4, another hypothesis that can be formulated is this 1.2 ± 0.5 TU is due to laboratory errors.
Conceptual flow models of Caldelas and Gerês hydrothermal systems
The usual definition of a groundwater conceptual model is frequently a qualitative and often a graphic description of the groundwater system, including an explanation of the hydrogeologic units, the system boundaries, inputs/outputs, and details of soils and rocks (Moore 2002). Preferably, after the conceptualization of groundwater systems, which should be grounded on Earth-based models, the application of mathematical models should be faced to outline scenarios using diverse integrated approaches. Useful models must be robust, calibrated, and supported on a permanent back-analysis scale based on a logical understanding of the real hydrological functioning framework, i.e., an evaluation with in situ measurements, of some parameters of the rock mass behavior (Chaminé et al. 2015). In the same way, a conceptual flow model is a simplified representation of a given aquifer system within a geological environment (Albu et al. 1997; Kresik and Mikszewski 2013). These are normally developed based on important data sets collected in the scope of regional investigations.
In this study, a special emphasis has been put on the contribution of a multidisciplinary approach (geology, morphotectonics, hydrogeology, geochemistry, and isotope hydrology) to the development of the conceptual flow models of Caldelas and Gerês hydrothermal systems. The models presented reflect the recharge and the discharge areas and identify the local/regional underground flow paths of both shallow and deep groundwaters, highlighting similarities and differences between both systems.
Concerning Caldelas hydrothermal system (Fig. 9a), the preferential recharge areas are located at low-altitude sites, around 170 m a.s.l., as indicated by the isotopic composition of the thermomineral waters (δ18O ≈ − 4.92‰ vs. V-SMOW). The local meteoric waters (recharge waters) infiltrate at low-altitude sites along rock discontinuities (diaclases, fractures, and faults), percolate at considerable depths (about 0.93 km), interacting with the calc-alkaline and alkaline granitic rocks (mean reservoir temperature of 44 ± 5 °C, using the chalcedony and K2/Mg chemical geothermometers), with relatively high CO2 pressures, promoting the development of Ca/Na-HCO3-type thermomineral waters. The deep circulating waters emerge along pathways linked to major NNE–SSW-trending faults, in a lower altitude site, with issue temperatures around 30 °C (mean issuing temperature 27 °C). Local shallow groundwater systems are ascribed to short underground flow paths as revealed by the related Na–Cl-type waters (similar to rain waters) presenting low mineralization (≈ 37 mg/L).
Gerês hydrothermal system (Fig. 9b), is characterized by preferential recharge areas located at high-altitude sites, between 900 and 1100 m a.s.l., evidencing the so-called “altitude effect” in the isotopic composition of the thermomineral waters (δ18O ≈ − 6.42‰ vs. V-SMOW). In this case, the local meteoric waters infiltrates at high-altitude sites, also along rock discontinuities (diaclases, fractures, and faults), percolate at greater depths (about 2.80 km) interacting with the granitic rocks (mean reservoir temperature of 96 ± 15 °C, using the chalcedony, K2/Mg and Na/K/Ca—β = 4/3 chemical geothermometers) promoting the development of Na-HCO3-type thermomineral waters. The deep circulating waters emerge in places where the major ENE–WSW and NNE–SSW faults intersect, in a lower altitude site, with issue temperatures between 40 and 46 °C. In this case, local shallow aquifers seem to be ascribed to longer underground flow paths (when compared to Caldelas local shallow groundwaters) as revealed by the evolution to Na-HCO3-type waters with rather high SiO2 values (hydrolysis of the plagioclases). Although, these waters are presenting low mineralization (≈ 38 mg/L) due to weak water–granite interaction dominated by the hydrolysis of Na-plagioclase.
The effect of the distance to the Atlantic coast “continental effect” in this particular case study should not be very relevant, since the two areas of research are approximately 50 km apart with the same water vapor masses origin.
Concluding remarks
The main goal of this study, conducted in two low-temperature hydrothermal systems in the north of Portugal (Caldelas and Gerês hydrothermal systems), was to demonstrate the applicability of combined geological, morphotectonical, geochemical, and environmental isotope data as important hydrogeological tools to improve the conceptual circulation models. In both case studies, the proposed conceptual circulation models were developed taking into consideration the fact that local/regional geology (dominated by calc-alkaline/alkaline fractured granitic rocks) can be complex, heterogeneous, and that local/regional hydrogeological anisotropy would favor the development of irregular distribution of groundwater pathways. The main differences between these two thermomineral water systems are ascribed to the different recharge altitudes. From the geochemical point of view, the presence of calc-alkaline/alkaline granitic rocks is responsible for the evolution to Ca/Na-HCO3-type thermomineral waters (at Caldelas) and to Na-HCO3-type thermomineral waters at Gerês. However, the hydrolysis mechanism is influenced by the different hydrothermal systems temperatures along the groundwater flow (emergence temperatures 21–31 °C in Caldelas and 41–46 °C in Gerês), and from the initial CO2 content (pH values of 6.64–7.54 and 8.18–8.47 for Caldelas and Gerês thermomineral waters, respectively). In the case of Gerês thermomineral waters, the much lower concentrations of calcium and magnesium and higher concentrations of sodium and bicarbonate indicate a major degree of the granite–water reaction progress at higher reservoir temperatures. If the concentrations of chloride and sulfate are similar for both mineral waters, the r (Na + K)/rCl ratio obtained in Caldelas and Gerês thermomineral waters is rather different (see Table 2). The distinct values in this ratio indicate that the Cl have different origins: in Caldelas thermomineral waters, the Cl content seems to be mainly associated with a deep origin (magmatic), while in Gerês thermomineral waters, the Cl is mainly due to water–rock processes, where Cl concentration could increase through a deeper and longer circulation paths.
A circulation depth for Caldelas waters in the range of 0.93 km seems to be realistic, considering the geological and structural features of Caldelas area and the issue temperatures of the thermomineral waters. The mean reservoir depths obtained from temperatures estimated using the chalcedony and K2/Mg chemical geothermometers are 42 ± 6 °C. Concerning the Gerês thermomineral waters, the chalcedony, K2/Mg, and Na/K/Ca (β = 4/3) chemical geothermometers indicate similar estimated temperatures (mean value 96 °C ± 5 °C), suggesting chemical equilibrium reactions with chalcedony, chlorite–muscovite–K-feldspar, calcite, and plagioclases at this temperature in an hydrothermal reservoir, located at depths close to 2.8 km. In addition, the maximum depth reached by the two systems will determine the reservoir temperatures at Caldelas and at Gerês, and the extension of water–rock interaction mechanisms occurring during groundwater flow.
The “altitude effect” represents the main factor responsible for the obtained isotopic differences (δ18O and δ2H values) in the thermomineral waters of these two case studies.
The different groundwater circuits are also responsible for the different 3H content, ascribed with lower (at Caldelas) and higher (at Gerês) mean residence time of the thermomineral waters.
This study demonstrates that interdisciplinary approaches allow the development of strong conceptual hydrogeological models that can lead to practical applications like the mean residence time of groundwaters and preferential recharge areas, providing a basis for future studies on the vulnerability of the hydrothermal systems, to anthropogenic effects. Some of the potential applications of conceptual models in hydrogeological studies are the identification of preferential recharge altitudes and areas for protection of water resources for a sustainable management. Besides, after the development of conceptual models, the application of numerical models can be used as testing sensitivity of the system to anthropogenic effects. The visualization of the aquifer system dynamics is extremely useful for reporting the scientific information, sometimes not very accessible, to Portuguese Directorate-General for Energy and Geology (DGEG) water authorities that is responsible for the promotion of proper management and protection of the hydromineral and geothermal resources. In fact, such models promote an easier to visualization and better understanding of the difficulties.
References
Albu M, Banks D, Nash H (1997) Mineral and thermal groundwater resources. Chapman and Hall, London
Almeida Soares MAM (2019) Estudo gravimétrico em contexto granítico no Concelho de Amares. MSc thesis, Faculdade de Ciências da Universidade do Porto
Araguás-Araguás L, Froehlich K, Rozanski K (2000) Deuterium and oxygen-18 isotope composition of precipitation and atmospheric moisture. Hydrol Process 14:1341–1355
Arnorsson S, Gunnlaugsson E, Svavarsson H (1983) The chemistry of geothermal waters in Iceland. III. Chemical geothermometry in geothermal investigations. Geochim Cosmochim Acta 47(3):567–577. https://doi.org/10.1016/0016-7037(83)90278-8
Arnórsson S, D’Amore F, Gerardo J (2000) Isotopic and chemical techniques in geothermal exploration. International Atomic Energy Agency, Vienna
Ayenew T, Kebede S, Alemyahu T (2008) Environmental isotopes and hydrochemical study applied to surface water and groundwater interaction in the Awash River basin. Hydrol Process 22:1548–1563. https://doi.org/10.1002/hyp.6716
Azañón JM, Cabral J (2020) Active processes in Iberia: an introduction. In: Quesada C, Oliveira JT (eds) The geology of Iberia: a geodynamic approach, vol 5. Springer Nature, Cham, pp 1–3. https://doi.org/10.1007/978-3-030-10931-8_1
Baalousha H (2008) Fundamentals of groundwater modelling. In: Konig LF, Weiss JL (eds) Groundwater: modelling, management and contamination. Nova Science Publishers, New York, pp 113–130
Bowers TS, Jackson KJ, Helgeson HC (1984) Equilibrium activity diagrams. Springer, Berlin
Bowser CJ, Jones BF (2002) Mineralogic controls on the composition of natural waters dominated by silicate hydrolysis. Am J Sci 302:582–662
Carreira PM, Valério P, Nunes D, Araújo MF (2005) Temporal and seasonal variation of stable isotopes and tritium in precipitation over Portugal. In: Proceedings of the International Conference Isotopes in Environmental Studies, Aquatic Forum 2004. IAEA, Vienna, 370–373
Carreira PM, Marques JM, Graça R, Aires-Barros L (2008) Radiocarbon application in dating “complex” hot and cold CO2—rich mineral water systems: a review of case studies ascribed to the Northern Portugal. Appl Geochem 23:2817–2828. https://doi.org/10.1016/j.apgeochem.2008.04.004
Carreira PM, Nunes D, Valerio P, Araujo MF (2009) A 15-year record of seasonal variation in the isotopic composition of precipitation water over continental Portugal. J Radioanal Nucl Chem 281:153–156. https://doi.org/10.1007/s10967-009-0064-0
Carreira PM, Marques JM, Carvalho MR, Capasso G, Grassa F (2010) Mantle-derived carbon in hercynian granites. Stable isotope signatures and C/He associations in the thermomineral waters, N-Portugal. J Volcanol Geotherm Res 189:49–56. https://doi.org/10.1016/j.jvolgeores.2009.10.008
Carreira PM, Marques JM, Espinha Marques J, Chaminé HI, Fonseca PE, Monteiro Santos F, Moura RM, Carvalho JM (2011) Defining the dynamics of groundwater in Serra da Estrela Mountain area, central Portugal: an isotopic and hydrogeochemical approach. Hydrogeol J 19(1):117–131. https://doi.org/10.1007/s10040-010-0675-0
Carreira PM, Marques JM, Carvalho MR, Nunes D, Antunes da Silva M (2014) Carbon isotopes and geochemical processes in CO2-rich cold mineral water, N-Portugal. Environ Earth Sci 71:2941–2953. https://doi.org/10.1007/s12665-013-2671-x
Carvalho JM, Chaminé HI, Afonso MJ, Espinha Marques J, Medeiros A, Garcia S, Gomes A, Teixeira J, Fonseca PE (2005) Productivity and water costs in fissured-aquifers from the Iberian crystalline basement (Portugal): hydrogeological constraints. In: López-Geta JA, Pulido-Bosch A, Baquero-Úbeda JC (eds) Water, mining and environment. Book Homage to Professor Rafael Fernández Rubio. Instituto Geológico y Minero de España, Madrid, pp 193–207
Celle-Jeanton H, Travi Y, Blavoux B (2001) Isotopic typology of the precipitation in the Western Mediterranean region at three different time scales. Geophys Res Lett 28(7):1215–1218. https://doi.org/10.1029/2000GL012407
Chae G-T, Yun S-T, Kim K, Mayer B (2006) Hydrogeochemistry of sodium bicarbonate type bedrock groundwater in the Pocheon spa area, South Korea: water–rock interaction and hydrologic mixing. J Hydrol 321:326–343. https://doi.org/10.1016/j.hydrol.2005.08.006
Chaminé HI, Carvalho JM, Teixeira J, Freitas L (2015) Role of hydrogeological mapping in groundwater practice: back to basics. Eur Geol J 40:34–42
Cho B-W, Choo CO, Kim MS, Hwang J, Yun U, Lee S (2015) Spatial relationships between radon and topographical, geological, and geochemical factors and their relevance in all of South Korea. Environ Earth Sci 74:5155–5168. https://doi.org/10.1007/s12665-015-4526-0
Coudé-Gaussen G (1981) Les Serra da Peneda et do Gerês: étude géomorphologique. Memór Centro Estudos Geogr Lisboa 5:1–254
Craig H (1961) Standard for reporting concentrations of deuterium and oxygen-18 in natural waters. Science 133:1833–1834
Cunha PP, Vicente G, Martín-González F (2019) Cenozoic sedimentation along the piedmonts of thrust related basement ranges and strike-slip deformation belts of the Iberian Variscan Massif. In: Quesada C, Oliveira JT (eds) The geology of Iberia: a geodynamic approach, vol 4. Springer Nature, Cham, pp 1–3. https://doi.org/10.1007/978-3-030-11190-8_5
Custódio E, Llamas MR (1983) Hidrología subterránea. Ed. Omega, Tomo 1, 2ª edição. Barcelona, Spain (in Spanish)
D’Amore F, Fancelli R, Caboi R (1987) Observations on the application of chemical geothermometers to some hydrothermal systems in Sardinia. Geothermics 16(3):271–282
Dansgaard W (1964) Stable isotopes in precipitation. Tellus XVI 4:436–468
Darling WG, Bath AH, Talbot JC (2003) The O and H stable isotopic composition of fresh waters in the British Isles. 2. Surface waters and groundwater. Hydrol Earth Syst Sci 7:163–181. https://doi.org/10.5194/hess-7-163-2003
Diamond RE, Harris C (2000) Oxygen and hydrogen isotope geochemistry of thermal springs of the Western Cape, South Africa: recharge at high altitude? J Afr Earth Sci 31(3–4):467–481. https://doi.org/10.1016/S0899-5362(00)80002-0
Dias G, Noronha F, Almeida a, Simões PP, Martins HCB, Ferreira N (2010) Geocronologia e petrogénese do plutonismo tardi-Varisco (NW de Portugal): síntese e inferências sobre os processos de acreção e reciclagem crustal na Zona Centro-Ibérica. Ciências Geológicas—Ensino e Investigação e sua História Vol 1 Cap II Petrologia e Geoquímica. pp 143–160 (in Portuguese)
Epstein S, Mayeda T (1953) Variation of 18O content of waters from natural sources. Geochim Cosmochim Acta 4:213–224
Ferreira N, Iglesias M, Noronha F, Pereira E, Ribeiro A, Ribeiro ML (1987) Granitóides da Zona Centro Ibérica e seu enquadramento geodinâmico. In: Bea F, Carnicero A, Gonzalo JC, López-Plaza M, Rodríguez-Alonso MD (eds) Geologia de los Granitoides y Rocas Asociadas del Macizo Hespérico. Ediciones Rueda, Madrid, pp 37–51
Fouillac C, Michard G (1981) Sodium/lithium ratio in water applied to geothermometry of geothermal reservoir. Geothermics 10(1):55–70. https://doi.org/10.1016/0375-6505(81)90025-0
Fournier RO, Truesdell AH (1973) An empirical Na–K–Ca geothermometer for natural waters. Geochim Cosmochim Acta 37(5):1255–1275. https://doi.org/10.1016/0016-7037(73)90060-4
Friedman I (1953) Deuterium content of natural waters and other substances. Geochim Cosmochim Acta 4:89–103
Galego Fernandes P, Carreira PM (2008) Isotopic evidence of aquifer recharge during the last ice age in Portugal. J Hydrol 361:291–308. https://doi.org/10.1016/j.jhydrol.2008.07.046
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na–K–Ca–Mg geoindicators. Geochim Cosmochim Acta 52(12):2749–2765. https://doi.org/10.1016/0016-7037(88)90143-3
Giggenbach W, Gonfiantini R, Jangi BL, Truesdell AH (1983) Isotopic and chemical composition of the Parbati Valley geothermal discharges, North-West Himalaya, India. Geothermics 12:199–222. https://doi.org/10.1016/0375-6505(83)90030-5
Giustini F, Brilli M, Patera A (2016) Mapping oxygen stable isotopes of precipitation in Italy. J Hydrol Reg Stud 8:162–181. https://doi.org/10.1016/j.ejrh.2016.04.001
Glok Galli M, Damons ME, Siwawa S, Bocanegra EM, Nel JM, Mazvimavi D, Martínez DE (2017) Stable isotope hydrology in fractured and detritic aquifers at both sides of the South Atlantic Ocean: Mar del Plata (Argentina) and the Rawsonville and Sandspruit river catchment areas (South Africa). J S Am Earth Sci 73:119–129. https://doi.org/10.1016/j.jsames.2016.12.006
Gokgoz A, Tarcan G (2006) Mineral equilibria and geothermometry of the Dalaman–Koycegiz thermal springs, southern Turkey. Appl Geochem 21(2):253–268. https://doi.org/10.1016/j.apgeochem.2005.08.010
Gonfiantini R, Araguas LA, Rozanski K (1990) Tritium in precipitation: comparison between the years of high tritium (1963–1967) and recent years. Bull Radiat Prot 13(1):1
Gonfiantini R, Roche MA, Olivry JC, Fontes J-C, Zuppi GM (2001) The altitude effect on the isotopic composition of tropical rains. Chem Geol 181:147–167
González-Trinidad J, Pacheco-Guerrero A, Júnez-Ferreira H, Bautista-Capetillo C, Hernández-Antonio A (2017) Identifying groundwater recharge sites through environmental stable isotopes in an alluvial aquifer. Water 9(8):569. https://doi.org/10.3390/w9080569
Gourcy LL, Groening M, Aggarwal PK (2005) Stable oxygen and hydrogen isotopes. In: Aggarwal PK, Gat JR, Froehlich KFO (eds) Isotopes in the water cycle. Past, present and future of a developing science. Springer, Amsterdam, pp 39–51
IAEA (1976) Procedure and technique critique for tritium enrichment by electrolysis at IAEA laboratory. In: Technical Procedure no. 19. International Atomic Energy Agency. Vienna
IGM (1998) Recursos Geotérmicos em Portugal Continental: Baixa Entalpia. http://www.lneg.pt/CienciaParaTodos/edicoes_online/diversos/rec_geotermicos/texto
Jaques L, Noronha F, Liewig N, Bobos N (2016) Paleofluids circulation associated with the Gerês late-orogenic granitic massif, northern Portugal. Chem Erde 76(4):659–676
Kresik N, Mikszewski A (2013) Hydrogeological conceptual site models: data analysis and visualization. CRC Press, Boca Raton
Lambs L, Moussa I, Brunet F (2013) Air masses origin and isotopic tracers: a study case of the oceanic and Mediterranean rainfall southwest of France. Water 5:617–628. https://doi.org/10.3390/w5020617
Li F, Zeng J (2017) Characterization of origin and evolution of formation water in buried hill of Jizhong depression, China, using multivariate statistical analysis of geochemical data. Geofluids. https://doi.org/10.1155/2017/5290686
Lima AS (2011) Modelo conceptual da ocorrência hidromineral do Gerês: fundamentos sobre a delimitação da área de recarga do sistema CIG-R Livros de Actas, pp 169–182 (in Portuguese)
Lima A, Oliveira ACV (2007) Conceptualização de modelos hidrogeológicos em águas sulfúreas. In: Chaminé HI, Carvalho JM (eds) O valor acrescentado das ciências da terra no termalismo e no engarrafamento da água (II Fórum Ibérico de Águas Engarrafadas e Termalismo). Instituto Superior de Engenharia do Porto, Porto, pp 141–160 ((in Portuguese))
Liotta M, Grassa F, D’Alessandro W, Favara R, Gagliano Candela E, Pisciotta A, Scaletta C (2013) Isotopic composition of precipitation and groundwater in Sicily, Italy. Appl Geochem 34:199–206. https://doi.org/10.1016/j.apgeochem.2013.03.012
Lucas LL, Unterweger MP (2000) Comprehensive review and critical evaluation of the half-life of tritium. J Res Natl Inst Technol 105:541–549
Marques JM, Espinha Marques J, Carreira PM, Graça RC, Aires-Barros L, Carvalho JM, Chaminé HI, Borges FS (2003) Geothermal fluids circulation at Caldas do Moledo area, Northern Portugal: geochemical and isotopic signatures. Geofluids 3(3):189–201. https://doi.org/10.1046/j.1468-8123.2003.00059.x
Marques JM, Andrade M, Carreira PM, Eggenkamp HGM, Graça RC, Aires-Barros L, Antunes da Silva M (2006) Chemical and isotopic signatures of HCO3/Na/CO2-rich geofluids, North Portugal. Geofluids 6:273–287
Marques JM, Carreira PM, Espinha Marques J, Chaminé HI, Fonseca PE, Monteiro Santos FA, Eggenkamp HGM, Teixeira J (2010a) The role of geosciences in the assessment of low-temperature geothermal resources (N-Portugal): a review. Geosci J 14:329–446. https://doi.org/10.1007/s12303-010-0034-0
Marques JM, Matias MJ, Basto MJ, Carreira PM, Aires-Barros LA, Goff FE (2010b) Hydrothermal alteration of Hercynian granites, its significance to the evolution of geothermal systems in granitic rocks. Geothermics 39:152–160. https://doi.org/10.1016/j.geothermics.2010.03.002
Marques JM, Matos C, Carreira PM, Neves MO (2017) Isotopes and geochemistry to assess shallow/thermal groundwater interaction in a karst/fissured-porous environment (Portugal): a review and reinterpretation. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-017-0207-3
May F (2005) Alteration of wall rocks by CO2-rich water ascending in fault zones: natural analogues for reactions induced by CO2 migrating along faults in siliciclastic reservoir and cap rocks. Oil Gas Sci Technol 60(1):19–32
Medeiros AC, Teixeira C, Lopes JT (1975) Carta Geológica de Portugal na escala 1:50000. Notícia Explicativa da Folha 5-B (Ponte da Barca). Direcção—Geral de Minas e Serviços Geológicos, Lisboa (in Portuguese)
Mendes AC (2001) Geocronologia e petrogénese do maciço granítico pós-tectónico de Peneda-Gerês (ZCI, Norte de Portugal e Galiza). PhD dissertation, Universidade do Minho, Braga (in Portuguese)
Mendes A, Dias G (1996) Petrology and geochemistry of late-Hercynian subalkaline plutonism in the Central Iberian Zone: the Peneda-Gerês granitic massif. C R Acad Sci Paris 323(IIa):665–672
Mendes AC, Dias G (2004) Mantle-like Sr–Nd isotope composition of Fe–K subalkaline granites: the Peneda-Gerês Variscan massif (NW Iberian Peninsula). Terra Nova 16:109–115
Michard G (1990) Behaviour of major elements and some trace elements (Li, Rb, Cs, Sr, Fe, Mn, W, F) in deep hot waters from granitic areas. Chem Geol 89:117–134
Moore JE (2002) Field hydrogeology: a guide for site investigations and report preparation. Lewis Publishers, New York
Moreira A, Simões M (1988) Carta Geológica de Portugal na escala 1:50,000. Notícia Explicativa da Folha 1-D (Arcos de Valdevez). Serviços Geológicos de Portugal (in Portuguese)
Moser H, Wolf M, Fritz P, Fontes J-C, Florkowski T, Payne B (1989) Deuterium, oxygen-18 and tritium in Stripa groundwater. Geochim Cosmochim Acta 5:1757–1763
Neiva AMR (1993) Geochemistry of granites and their minerals from Gerez mountain, northern Portugal. Chem Erde 53:227–258
Pedrosa MY (1999) Notícia Explicativa da Carta Hidrogeológica de Portugal, escala 1:200000, Folha 1. Instituto Geológico e Mineiro, Lisboa ((in Portuguese))
Ribeiro ML, Moreira A (1986) Carta Geológica de Portugal na escala 1:50 000. Notícia Explicativa da Folha 1-B (Monção). Serviços Geológicos de Portugal (in Portuguese)
Ribeiro A, Munhá J, Dias R, Mateus A, Pereira E, Ribeiro L, Fonseca PE, Araújo A, Oliveira JT, Romão J, Chaminé HI, Coke C, Pedro J (2007) Geodynamic evolution of the SW Europe Variscides. Tectonics. https://doi.org/10.1029/2006TC002058
Rozanski K, Sonntag C, Munnich KO (1982) Factors controlling stable isotope composition of European precipitation. Tellus 34:142–150
Rozanski K, Araguás-Araguás L, Gonfiantini R (1992) Relation between long-term trends of oxygen-18 isotope composition of precipitation and climate. Science 258:981–985
Rozanski K, Araguás-Araguás L, Gonfiantini R (1993) Isotopic patterns in modern global precipitation. Climate change in continental isotopic records. Wiley, New York, pp 1–36
Schoeller H, Schoeller M (1979) Une etude des eaux thermominerales du Massif central francais. Bull Bureau Recherches Geol Mineres Sect III 2:121–156
Sidle WC (1998) Environmental isotopes for resolution of hydrology problems. Environ Monit Assess 52:389–410
Sung K-Y, Yun S-T, Park M-E, Koh Y-K, Choi B-Y, Hutcheon I, Kim K-H (2012) Reaction path modeling of hydrogeochemical evolution of groundwater in granitic bedrocks, South Korea. J Geochem Explor 118:90–97. https://doi.org/10.1016/j.gexplo.2012.05.004
Truesdell AH (1975) Summary of Section III. Geochemical techniques in exploration. In: Proceedings of the second United Nations symposium on the development and use of geothermal resources San Francisco, California: Lawrence Berkley Laboratory, University of California, pp 53–79
Vidal-Romaní JR, Brum A, Zêzere J, Rodrigues L, Monge C (1990) Evolución cuaternaria del relieve granítico en la Serra de Gêrez-Xurés, (Minho, Portugal—Ourense, Galicia). Cuaternario Geomorfol 4:3–12
Yurtsever Y, Gat JR (1981) Atmospheric waters. In: Gat JR, Gonfiantini R (eds) Stable isotope hydrology. Deuterium and oxygen-18 in the water cycle. Technical Reports Series No. 210. IAEA, Vienna STI/DOC/10/210. 103-142
Acknowledgements
C2TN|IST authors acknowledge the FCT (Portuguese Science and Technology Foundation) support through the FCT-UIDB/04349/2020 project; CERENA|IST recognize the FCT support through the strategic project FCT-UIDB/04028/2020. JEM is thankful to the funding provided by the Institute of Earth Sciences (ICT), under FCT contracts UID/GEO/04683/2019 and COMPETE POCI-01-0145-FEDER-007690. HIC was supported partially under the framework of the labcarga|ISEP re-equipment program (IPP-ISEP|PAD’2007/08) and Centre GeoBioTec|UA (UID/GEO/04035/2019). An early draft of this manuscript was critically read by two anonymous reviewers and the authors gratefully acknowledge their contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a part of the Topical Collection in Environmental Earth Sciences on “Mineral and Thermal Waters” guest edited by Drs. Adam Porowski, Nina Rman and Istvan Forizs, with James LaMoreaux as the Editor-in-Chief.
Rights and permissions
About this article
Cite this article
Carreira, P.M., Marques, J.M., Guerra, A. et al. Caldelas and Gerês hydrothermal systems (NW Portugal): a comparative study based on geochemical and isotopic signatures. Environ Earth Sci 80, 100 (2021). https://doi.org/10.1007/s12665-021-09389-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09389-w