Abstract
Hydro-geochemical evaluation is becoming very significant to determine the process involved in the chemical evolution of groundwater. Supply of contaminated drinking water in rural communities is a continuing health issue, leading many resource users to unknowingly consume water with elevated levels of harmful chemicals. The process of pollution risk assessment requires the integration of data that are spatially changeable in nature, making geographic information system (GIS) a perfect tool for such assessments. In this study, GIS was applied to evaluate groundwater quality in Nilakottai block which is one of the endemic fluorosis blocks of Tamil Nadu, South India. The obtained maps may assist water quality personnel in managing and controlling pollution of the study area. The suitability of groundwater quality for drinking and agricultural purposes in the selected area was assessed by measuring physicochemical parameters. For this purpose, 55 water samples were collected from different places of the study area. F− ion leaching depends on groundwater chemistry, including pH and concentrations of HCO3 −, Na+, and Ca2+. HCO3 − and F− were found to have good positive correlation (r = 0.400). Hydro-geochemical classification of ground water samples throws more light on the possible link between the presences of various ions and their contribution to groundwater chemistry. The GIS maps clearly show the presence of Na–Cl, mixed Ca–Mg–Cl and Ca–Cl as the predominant species in specific regions having a direct bearing on water quality. The relative concentrations of the ions occur in the order Na+ > Mg2+ > Ca2+ and Cl− > HCO3 − > SO4 2−. Water quality index (WQI) was calculated to determine the suitability of water for drinking purpose. The WQI values revealed that all groundwater samples were above 100, which is the permissible limit and, therefore, waters above this level cannot be used for human consumption. Calculated values of Percent Na (%Na), Sodium adsorption ratio (SAR) and Magnesium hazard (MH) indicate that most of the groundwater samples are not suitable even for irrigation of most crops. Rainwater harvesting techniques should be promoted in this study area. Supply of drinking water within the permissible limit should be emphasized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Safe drinking water for all is an internationally accepted human right (World Health Organization (WHO) (2004). Water pollution is a serious problem, particularly in developing countries as majority of surface and groundwater resources are contaminated by biological, toxic, organic and inorganic pollutants (Reghunath et al. 2002; Vasanthavigar et al. 2013). Groundwater is an important source of water supply for drinking, irrigation, and industrial purposes. Due to the rising demand for potable and irrigation water and the inadequacy of available surface water and uncertainty in seasonal rainfall, the importance of groundwater is increasing exponentially (Nagarajan et al. 2010; Bozdag and Gocmez 2013). The geochemistry of ground water is complex due to various reasons. Diverse factors such as surface and rainwater infiltration, complicated hydrogeology, human impact on flow systems and sources of pollution decide the chemical content. The rate of reaction with host rocks will be controlled by the residence time of water and the primary mineralogy. Groundwater quality is mostly affected either by natural geochemical characteristics, mainly dissolved ion content, climate, lithology, mineral weathering, nature of geochemical reactions, solubility of salts, dissolution/precipitation reactions, ion exchange, wet and dry deposition of atmospheric salt, and by various anthropogenic activities, such as agriculture, sewage disposal, mining and industrial wastes (Jiang and Yan 2010; Zhang et al. 2012; Bozdag and Gocmez 2013). Hence, to utilize and protect important water sources effectively and to predict the change in groundwater environments, it is necessary to understand the hydrochemical parameters of groundwater, such as pH, electrical conductivity (EC), total dissolved solids (TDS), total hardness (TH), sodium absorption ratio (SAR), major anion (F−, CO3 2−, HCO3 −, Cl−, and SO4 2−) concentrations, major cation (Ca2+, Mg2+, Na+, and K+) concentrations and water quality index (Prasanna et al. 2010; Bozdag and Gocmez 2013). Groundwater contamination problems due to fluoride, arsenic, nitrate, chromium, iron, pesticides, and other industrial chemical/pollutants are emerging with national dimensions. Depletion of groundwater levels and deterioration of water quality require immediate attention.
Dindigul district is a traditional agricultural area located in Tamil Nadu, South India. Nilakottai block of Dindigul district has potential groundwater repositories which could supply water for irrigation to all the 22 constituent villages for almost throughout the year as the area receives maximum rainfall from northeast monsoon. As surface water in this block is scarce, groundwater plays a significant role as a resource for both human consumption and agricultural irrigation.
The chemical imbalance due to decreased rainfall, higher utilization of groundwater and intense water–rock interaction observed has necessitated this study to monitor the water quality issues of this block. The chemical composition of soils and rocks can influence the quality of groundwater through which the water flows. Water quality is dependent upon mineral solubility, ion exchange, oxidation, reduction, etc., in addition to anthropogenic activities (Subba Rao 2002; Naik et al. 2009; Subba Rao et al. 2012). Since every part of the area has its own causative factors of variation in groundwater quality, it is essential to examine the chemistry of groundwater quality of every part of the block for its sustainable development.
Geochemical studies of groundwater provide a better understanding of the possible changes in quality. Many naturally occurring major, minor and trace elements in drinking water can have a significant effect on human health either through deficiency or excessive intake (Frengstad et al. 2001). In India and various parts of the world, numerous studies have been carried out to assess the geochemical characteristics of groundwater (Aghazadeh and Mogaddam 2011; Alexakis 2011; Ahmad and Qadir 2011; Ramesh and Elango 2012) to establish the anthropogenic and geogenic sources affecting groundwater quality as well as the reactions that take place within the aquifer. In the study area, agriculture is the most important economic activity; thus, a hydro-geochemical investigation was carried out to identify groundwater geochemistry and its suitability for irrigation purpose. Salts found in irrigation water can affect both soil structure and crop yield, while the presence of toxic substances may threaten vegetation and reduce the suitability of soil for cultivation (Li et al. 2013).
Assessment of groundwater quality is not easy since contamination depends upon numerous, complex interacting parameters and uncertainty is inherent in all methods of assessing groundwater susceptibility as per the opinion of National Research Council (1993). Hence, reliably and cost effectively modeling groundwater quality remains a major challenge. Mapping the water quality parameters (WQPs) using GIS as a decision support system can provide a useful basis for taking fast and reliable management decisions. GIS is an effective tool widely used for the assessment, monitoring, management, and visual representation of geographic information in several fields; it was widely applied in research fields of natural resources, environment management and their evaluations including disaster management and hydrological fields (Jha et al. 2007; Chenini et al. 2010; Lee et al. 2012).
The focus of the present study is to evaluate the groundwater quality for drinking, domestic and irrigation in Nilakottai block, Dindigul district, Tamil Nadu, India. There has been no attempt to study the statistical nature of the groundwater in this basin. In view of this, an attempt is made on three aspects: the first is on the chemical characteristics of groundwater with respect to the hydro-geochemical signatures and genetic geochemical evolution of groundwater. The second deals with the impacts of groundwater quality on human beings and plant growth. This study paves way to provide baseline information in solving the problems caused by inferior water quality by implementing effective management strategies for better living conditions of the people. The third deals with creation of maps meant for environmental risk communication and to develop preventive and curative measures for future communities of the selected block. This mapping can support the development of highly needed groundwater management. In this block, some bore wells have been abandoned due to bad taste or high salinity and hardness levels. Many of the bore wells containing high levels of fluoride, which are not detectable by taste, color of the water, have been used by the people living in the study area.
Earlier studies in the selected area revealed the existence of endemic fluorosis with excess of fluoride in drinking water. The rate of prevalence of dental fluorosis in the study area is 60–80% and leaching of fluoride ions from fluoride bearing rocks present in these areas was observed as the major cause (Amalraj and Pius 2013). Groundwater F− anomalies could be related to natural origins such as prolonged water–rock interactions and mineral weathering. The F− concentration depends on the groundwater chemistry, including pH and the concentrations of Na+, Ca2+, and HCO3 − (Young et al. 2011; Hu et al. 2013; Brahman et al. 2013). Risk-based prioritization, large-scale risk analyses and proper monitoring of the water quality status in the selected area are very essential.
Materials and methods
Study area
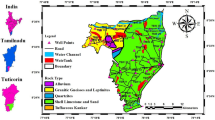
Nilakottai block is located in the southern part of the Dindigul district in Tamil Nadu, India, and covers about 261.12 square km and is distributed in 23 panchayat villages. The selected study area (Fig. 1) geographically lies between 77° 46′ 53.46″E to 10° 3′ 15.92″N and 77° 58′ 6.45″E to 10° 17′43.61″N. The study area is chiefly composed of hornblende biotite gneiss and charnockites. There is distribution of ultramafics, like anorthosites; pyroxene granulites are also found as disseminations in the study area. There is a minor distribution of quartzite and pink migmatites and granites (Ramachandran et al. 2012). Limestone, quartz and feldspar are major sources of minerals present; granite, rough stone, crushed stone (jelly), sand and brick earth are available as minor sources of minerals. Structure hills are the major land form in this block. Red soil, red sandy soil and black cotton soil are the major soil types of this block, but red sandy soils are prevalent. The major part of the block is underlain by Archaean crystalline metamorphic complex. The average annual temperature is from 20 to 36 °C. The normal annual rainfall over the block varies from 700 to 1600 mm. The period from April to June is generally hot and dry. The climate is conducive as the temperature is below 25 °C and with high humidity during the period from November to January. Usually mornings are more humid than afternoons. The relative humidity varies between 65 and 85% in the mornings while in the afternoons it varies between 40 and 70%. Ground water is the main source of drinking, domestic and irrigation purposes in this region. Groundwater occurs under water table condition in weathered and shallow fractures and under semi-confined to confined conditions in deeper fractures. The depth of weather varies from place to place from less than a meter to a maximum of 40 m bgl. The groundwater exploration in deeper aquifer reveals that in about 11% of the wells drilled, the yield was more than 3 lps, whereas in about 15% of the wells, the yield ranged from 1 to 3 lps. A few of the wells have been abandoned due to poor yield. In general, the groundwater in this block is colorless, odorless and slightly alkaline nature (Central Ground Water Board 2008). The principal crops are paddy, maize and coconut. Other crops include jowar (sorghum), ragi (millet), and pearl millet. Cultivation of fruits and vegetables is common in this block. According to Central Ground Water Board report, Nilakottai block comes under the critical category of water quality.
Sampling and analysis
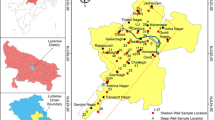
Fifty-five samples from groundwaters that are widely used for drinking, domestic and agricultural purposes were collected in random sampling method into one liter polyethylene bottles. The sampling points are indicated in Fig. 1. The bottles were soaked in water with 10% HNO3 washed and pre-rinsed with Millipore–milliQ distilled water to remove all impurities. All sample bottles were tape to prevent evaporation. Immediately after the sampling the pH, electrical conductivity (EC), resistivity, total dissolved solids (TDS), salinity and dissolved oxygen (DO) were measured in the field using multi-parameter analysis kit (Eutech Instruments PCD 650). The sample locations were identified with the help of global positioning system and were bought to laboratory for determining the chemical constituents such as total hardness (TH), total alkalinity (TA), calcium, magnesium, sodium, potassium, fluoride, chloride, sulfate and nitrate. The analysis of the sample was done on the basis of standard methods suggested by the American Public Health Association (APHA 1995). All aqueous solutions were prepared using Millipore–milliQ distilled water.
Alkalinity of the samples was analyzed by titrating it with 0.1 M HCl. Phenolphthalein alkalinity was completely absent in all the samples, whereas total alkalinity was determined by methyl orange indicator. Total hardness of the samples was analyzed by titration with EDTA using Erichome black-T as an indicator. Calcium (Ca2+) by EDTA titration, Magnesium (Mg2+) by calculation after EDTA titration of calcium and total hardness, sodium (Na+) and potassium (K+) were analyzed by flame photometer (Systronics 128); chloride (Cl−) was analyzed by volumetric titration using AgNO3; Sulfate and nitrate were measured using a double-beam UV–Vis spectrophotometer model Perkin Elmer Lambda 35 by turbidimetric, and colorimetric methods, respectively. Fluoride levels in drinking water samples were measured by fluoride ion selective electrode Orion ion analyzer (Thermo scientific Orion 4 Star pH. ISE Benchtop) by diluting with total ionic strength adjustment buffer (TISAB III) in 10:1 ratio (Orion Star and Star Plus Meter User Guide 2008).
Cation–anion balance
The accuracy of complete chemical analysis of a groundwater sample was checked by computing the cation–anion balance (Eq. 1), where the total cations’ concentrations (TCC) in milliequivalents per liter should be equal to the total anions’ concentrations (TAC) expressed in the same units. The difference between the TCC and TAC should be within the limit of ±5% (Domenico and Schwartz 1990).
Estimation of water quality index
In the case of water quality monitoring, due to the complexity associated with analyzing a large number of measured variables, a method which will reduce the multivariate nature of water quality data is used by employing an index that will mathematically combine all water quality measures and provide a general and readily understood description of water. Water quality index (WQI) is a very useful and efficient method for assessing the quality of water. WQI is also a very useful tool for communicating the information on overall quality of water (Pius et al. 2012). WQI is a single unitless number of 100 point scale that provides a pointer to the quality of water source. To determine the suitability of the groundwater for drinking purposes, the index developed by Tiwari and Mishra (1985) was used. In the present study, ten water quality parameters, namely pH, TDS, TA, TH, Ca2+, Mg2+, F−, Cl−, NO3 −, and SO4 2− were considered for computing WQI, and the unit weight W i of each parameter is obtained depending on its weightage, by adopting the following formula:
where
where q i is the quality rating for the ith water quality parameters (i = 1, 2, 3, … N), V i is the measured value of the ith parameter at a given sampling location and S i is the standard permissible value for the ith parameter. The standard permissible values for various pollutants for drinking water, recommended by WHO (2004) and BIS (2012) are given in Table 1 for the parameters considered for calculating WQI. It is well known that the more harmful a given pollutant is, the smaller is its permissible value. So, the “weights” for various water quality parameters are assumed to be inversely proportional to the recommended standards for the corresponding parameters, i.e., W i = K/S i , where W i is the unit weight for the ith parameter and K is the constant of proportionality and is taken as K = 1. According to this water quality index, the maximum permissible value is 100. Values greater than 100 indicate pollution and are unfit for human consumption.
Irrigational quality parameters
Parameters such as the percent sodium (%Na), sodium adsorption ratio (SAR), Kelly’s index (KI) and magnesium hazard (MH) were estimated to assess the suitability of water from the study area for irrigation purposes.
Percent sodium (%Na)
Sodium content and specific conductivities of the waters are important parameters that control the usage of water in agriculture. Sodium content is usually expressed in terms of percent sodium (%Na) (Raju 2007). It is widely used for evaluating the suitability of water quality for irrigation (Wilcox 1955). %Na is calculated using the formula given below.
Sodium adsorption ratio (SAR)
Salinity indicates leaching of salts into groundwater. This creates a lot of problems, especially in dry climatic regions. The sodium or alkali hazard in the water used for irrigation is determined by the absolute and relative concentration of cations and is expressed as the SAR. SAR has been calculated as follows:
Kelly’s index (KI)
Based on Kelly’s index, water sources are classified for irrigation. Sodium measured against calcium and magnesium was considered by Kelly (1946) and Paliwal (1967) to calculate this parameter. Water with >1.0 Kelly’s index indicates an excess level of sodium and is unsuitable for irrigation. Water with Kelly’s index of <1.0 is only considered suitable for irrigation. Kelly’s index was calculated by means of the following equation:
Magnesium hazard (MH)
The magnesium hazard value is the excess amount of magnesium over calcium. An excess of Mg affects the quality of the soil, resulting in poor agricultural returns. An MH value higher than 50 is considered to be harmful and unsuitable for irrigation (Szabolcs and Darab 1964). MH was calculated by means of the following equation:
where all ionic concentrations are expressed in meq/l.
Results and discussion
The physicochemical parameters of groundwater in the Nilakottai block depicted in Table 2 indicate the minimum, maximum, average, and standard deviation values. The values were compared with the WHO (2004) and BIS (2012) standards (Table 3) to find the suitability of the water samples collected for drinking and irrigation. All natural waters contain water-soluble salts and the concentration and dissolved constituent composition determines the water quality for irrigational use as high concentration in irrigation water affects both plants and soil physically and chemically (Ravikumar et al. 2011; Singh et al. 2012). High concentrations of TDS and EC in irrigation water may increase the soil salinity, which affects the plant salt intake.
pH
Results of the chemical analysis of groundwaters of the selected area show a wide variation in different individual parameters (Table 2). The pH decides the acidic or basic condition of water and if the pH is not within the prescribed limit of 6.5–8.5, it damages mucous membrane present in eyes, nose, mouth, abdomen, anus, etc. The pH values of groundwaters in the Nilakottai block ranged between 7.11 and 8.57, with an average of 7.83 (Table 2). The groundwater of the study area is generally neutral to slightly alkaline in nature and is within the permissible limit of 6.5–8.5 (Table 3) prescribed for drinking water by BIS (2012) and WHO (2004). The spatial distribution of pH in ground water samples of the study area is given in Fig. 2a. The northeastern and western portion showed alkaline nature of ground water.
Dissolved oxygen (DO)
Dissolved oxygen is one of the most important factors in water quality assessment. It reflects the physical and biological process prevailing in natural water. The values of DO are in the range of 4.95–7.40 mg/L (Table 2) for all the groundwater samples. The spatial distribution of DO % of ground water in the area is given in Fig. 2b. DO values suggest that samples are well oxygenated with less contamination by organic matter.
Electrical conductivity (EC)
EC is a measure of a material’s ability to conduct an electric current and higher EC values indicate enrichment of salts in the groundwater. Higher EC may be attributed to high salinity and high mineral percentage in groundwater samples, which are generally due to geochemical process like ion exchange, evaporation, silicate weathering and solubilization process taking place within the aquifers (Sanchez-Perez and Tremolieres 2003; Ramesh and Elango 2012; Bozdag and Gocmez 2013). Spatial distribution of EC shows a higher concentration in the central and eastern part of the study area (Fig. 2c). In natural waters, dissolved solids mainly consist of carbonates, bicarbonates, chlorides, sulfates, phosphates and nitrates of calcium, magnesium, sodium and potassium with traces of iron, manganese and other minerals. Organic matter and various dissolved gases are also present in small amounts (Jain et al. 2010). EC ranges from 374 to 7028 μS/cm at 25º C, with an average of 2333.4 μS/cm (Table 2). EC was above the maximum permissible limit (1500 μS/cm) in 69.09% of samples as per WHO (2004) standards (Table 3). The higher EC may cause a gastrointestinal irritation in human beings. According to the WHO (2004) classification of EC, it can be classified as type I, permissible (EC < 1500 μS/cm); type II, not permissible (EC: 1500 and 3000 μS/cm); and type III, hazardous (EC > 3000 μS/cm) (Table 4). In the study area, 30.91% of the total groundwater samples comes under the type I (permissible), 41.82% under the type II (not permissible), and 27.27% under the type III (hazardous) (Table 4).
Total dissolved solids (TDS)
To ascertain the suitability of groundwaters for any purpose, it is essential to classify them depending on their hydrochemical properties based on TDS values (Davis and DeWiest 1966). The spatial distribution of TDS in ground water in the selected area is given in Fig. 2d. The TDS of the water samples range from 276.90 to 4910.5 mg/L with an average value of 1693.3 mg/L (Table 2). According to Davis and De Wiest (1996) classification, 3.64% of the groundwater samples are desirable for drinking, 23.64% of the samples are permissible for drinking, 65.45% of samples are useful for irrigation and 7.27% of the samples are unfit for drinking and irrigation (Table 5). Water having high TDS values if used for drinking purpose may induce an unfavorable physiological reaction in the transient consumer and gastrointestinal irritation (Shankar et al. 2008). It has also a laxative effect, and prolonged intake of water with the higher TDS can cause kidney stones, which are widely reported from different parts of India (Garg et al. 2009).
Total alkalinity (TA) and total hardness (TH)
The alkalinity is referred to as the acid neutralizing capacity (ANC) of water. This is an expression of buffering capacity. The basic species responsible for alkalinity in water are bicarbonate ions [HCO3 −], carbonate ions [CO3 2−] and hydroxide ions [OH−]. TA values are observed to be between 27.53 and 675.24 mg/L with an average of 174.34 mg/L (Table 2). The TA is a measure of HCO3 − and CO3 2− ions. The spatial distribution of TA in the groundwaters of the selected study area is shown in Fig. 3a. Phenolphthalein alkalinity was completely absent in all the samples, it indicates TA is caused by HCO3 − ion only, as the pH is between 7.11 and 8.57. Bicarbonate is a major element in the human body, which is necessary for digestion. When ingested, for example, with mineral water, it helps buffer lactic acid generated during exercise and also reduces acidity of dietary components. It has a preventive effect on dental cavities. The chemical data show that only 3.64% of the water samples exceeded the desirable limit of 350 mg/L in all the groundwater samples (Table 3).
The principal cations that impart hardness are Ca2+ and Mg2+ ions. The anions responsible for hardness are mainly carbonate, bicarbonate, sulfate, chloride, and nitrate. Hardness is an important criterion for determining the usability of water for drinking as well as for other domestic use, as it causes unpleasant taste and reduces the ability of soap to produce lather. The total hardness (TH) values of the water samples range from 180.47 to 2305.47 mg/L with an average value of 730.03 mg/L (Table 2). Groundwater exceeding the limit of 150 mg/L of hardness is considered to be hard (Sawyer and McCartly 1967). The classification of groundwater (Table 6) based on TH showed that a majority of the groundwater samples are very hard water. Most of the samples with higher TH are distributed in the south east part of the selected study area (Fig. 3b). According to the classification of TH, approximately 10.91% of the groundwater samples come under the hard category and the remaining 89.09% of the groundwater samples fall in the very hard category (Table 6). The high amount of hardness in the study area samples is due to the presence of carbonate minerals (calcite, dolomite, and aragonite) and evaporates (gypsum, anhydrite). High values of TH above 1500 mg/L may cause kidney problems (Jain 1996). High values of both TA and TH are distributed in the northern part of the study area (Fig. 3a, b). There is some suggestive evidence that long-term consumption of extremely hard water might lead to an increased incidence of urolithiasis, anecephaly, pre-natal mortality, some types of cancer and cardiovascular disorders (Ramesh and Elango 2012).
Sodium (Na+) and potassium (K+)
Major cations present in high concentrations in most of the groundwaters are calcium, magnesium, sodium and potassium (Younger 2007; Singh et al. 2012). The concentration of sodium varies from 19.23 to 761.4 mg/L with an average value of 169.91 mg/L (Table 2). In 34.55% of the analyzed samples (Table 3), Na+ exceeded the WHO (2004) standard and distributed itself in the middle part of the selected area (Fig. 4a). It makes the water unsuitable for drinking, because it causes severe health problems like hypertension, congenital diseases, kidney disorders and nervous disorders in the human body (Holden 1970; Subba Rao et al. 2012). Na+ is the dominant ion present in water among the other cations. This is because of the silicate weathering and/or dissolution of soil salts stored by the influences of evaporation and anthropogenic activities (Subba Rao 2002; Subba Rao et al. 2012), in addition to the agricultural activities and poor drainage conditions. Moreover, the solubility of Na+ salts is generally high. The higher contribution of Na+ than that of Ca2+ to the total cations is expected due to the influence of ion exchange. Groundwater in most of the study area comes under non-safe category for drinking, with reference to the concentration of Na+, which is more than 200 mg/L. Therefore, sodium restricted diet is suggested for the patients who suffer from heart diseases and also from kidney problems. Potassium contents range from 1.25 to 124.90 mg/L with an average value of 21.43 mg/L in the samples (Table 2). The maximum value is observed at southwest part of the selected area (Fig. 4b), which is surrounded by agricultural fields. The excessive use of potassium fertilizers and manures in cultivation might have percolated into the groundwater.
Calcium (Ca2+) and magnesium (Mg2+)
Calcium and magnesium contents of the analyzed samples are within the limit of WHO (2004) standard. The spatial distribution of Ca2+ and Mg2+ in the groundwaters of the study area is given in Fig. 4c, d. The concentrations of calcium range from 7.71 to 194.95 mg/L with an average value of 46.57 mg/L (Table 2). Ca2+ can be derived from the dissolution of carbonate and evaporitic minerals (e.g., calcite, dolomite, aragonite, gypsum, and anhydrite). The concentration of Mg2+ ranges from 5.68 to 56.27 mg/L with an average of 22.89 mg/L (Table 2), which is below the prescribed limit of 150 mg/L for potable water. Mg2+ is an essential ion for the functioning of cells in enzyme activation, etc. (Garg et al. 2009). The principal source of Mg2+ in natural water is ferromagnesian minerals (olivine, augite, diopside biotite, hornblend) in igneous and metamorphic rocks and magnesium carbonate (dolomite) in sedimentary rocks (Singh et al. 2012). In contrast to the concentrations of Ca2+, Mg2+, and Na+ ions among the cations, lower concentration of K+ was observed from the groundwaters (Table 2), as the potash feldspars are more resistant to chemical weathering and is fixed on clay products. Generally, the concentration of K+ is less than 10 mg/L in drinking waters. It maintains fluid balance in the body. The K+ exceeds 20 mg/L in 25.45% of the total groundwater samples (Table 3).
Fluoride (F−)
Optimum concentration of fluoride (about 1 mg/L) in drinking water is good for dental health and for good bone development. Intake of excess fluoride (beyond 1.5 mg/L, WHO guideline) (WHO 2004) for long periods can result in the incidence of fluorosis. High fluoride concentration in groundwater above the permissible limit of 1.5 mg/L has been a major toxicological and geo-environmental problem in India. Fluoride in groundwater generally occurs as a natural constituent. In this study area, groundwaters have F− from 1.40 to 3.60 mg/L (Table 2), and majority of the samples have F− higher than the standard limit of 1.50 mg/L (Table 3). This may be due to leaching of fluoride ions from natural sources of fluoride bearing rocks present in these areas. Samples with F− above permissible limit are located throughout the study area as indicated in the spatial distribution map (Fig. 5a). High intake of fluoride (over 1.5 mg/L) results in physiological disorders, dental, skeletal and non-skeletal fluorosis, thyroxine changes and kidney damages (Latha et al. 1999; Xiong et al. 2007; BIS 2012). Raja Reddy (1979) has stated that higher intake of F− may change the metabolic activities of soft tissues (thyroid, reproductive organs, brain, liver, and kidney).
Nitrate (NO3 −) and Sulfate (SO4 2−)
Nitrate concentrations in the study area range from 0.89 to 25.42 mg/L with an average of 4.43 mg/L (Table 2). High nitrate concentrations in groundwaters are reported in many parts of India because of intensive agricultural practices which utilize nitrogenous fertilizers. All the samples are within the permissible limit of 45 mg/L as per WHO and this is shown in the spatial distribution map (Fig. 5b). Higher concentration of NO3 − can cause methaemoglobinaemia (commonly known as blue baby syndrome, which affects the infants), gastric cancer, goiter, birth malformation and hypertension (Jain et al. 2010; Tank and Chandel 2010).
The concentrations of SO4 2− ions range from 20.42 to 92.81 mg/L with an average of 39.3 mg/L (Table 2) and all the samples are well within the desirable limit of 250 mg/L (WHO 2004). The spatial distribution of SO4 2− in groundwater samples of the study area is shown in (Fig. 5c). The sulfates in groundwater generally occur as salts of calcium, magnesium, and sodium. High concentration of sulfate in water is due to the accumulation of soluble salts in soil, anthropogenic activity, addition of excessive sulfate fertilizer, etc. The major source of SO4 2− in groundwater is due to weathering of sulfur-bearing minerals such as gypsum, anhydrite, magnesium and sodium sulfate minerals. Higher concentration of SO4 2− in drinking water is associated with respiratory problems (Subba Rao 1993). Sulfate when present in excess of >400 mg/l with sodium or magnesium may lead to gastrointestinal irritations (Shankar et al. 2008).
Chloride (Cl−)
The drinking and irrigation water classifications are subdivided into five groups with respect to each anion concentration as: very good (I), good (II), usable (III), usable with caution (IV) and harmful (V). The classification limits are illustrated in Table 7. High concentrations of Cl− in drinking water cause a salty taste and have a laxative effect (Bhardwaj and Singh 2011) in people not accustomed to it. In the study area, Cl− varies from 17.75 to 1148.43 mg/L with an average of 281.68 mg/L (Table 2). The desirable limit of Cl− for drinking water is specified as 200 mg/L as per WHO (2004) and 60% of the samples are above this limit (Table 3). Most of the samples with higher values of Cl− are distributed in the middle and southern part of the study area (Fig. 5d).The concentration of Cl− in groundwater is relatively high due to evaporation, enriched irrigation, return flow and poor sanitary conditions. Soil porosity and permeability also play a key role in building up the chloride values (Jain et al. 2003, 2010; Jameel and Hussain 2011).
Water quality index (WQI)
Water quality index was calculated to determine the suitability of water for drinking purpose. The WQI values revealed that all groundwater samples are above 100, which is the permissible limit and, therefore, cannot be used for human consumption. The special distribution map of WQI is shown in Fig. 6 and it is clearly indicated that throughout the study area all the water sources are not fit for human consumption.
Correlation coefficient
The interrelations among the chemical parameters are evaluated, using a correlation coefficient (r) model, to assess the sources of dissolved salts in the groundwater (Table 8). The EC and TDS are positively correlated with salinity (r = 0.989 and 0.993), TH (r = 0.664 and 0.637), Ca2+ (r = 0.609 and 0.590), Mg2+ (r = 0.483 and 0.489), Na+ (r = 0.804 and 0.814), Cl− (r = 0.837 and 0.825), and SO4 2− (r = 0.169 and 0.140), indicating that the groundwater is mainly controlled by Ca2+, Mg2+, Na+, Cl−, and SO4 2− ions, which depend on the mineral dissolution, mineral solubility, ion exchange, evaporation, topographical features, anthropogenic activities and water flow path conditions. The TA as HCO3 − has good correlation with Na+ (r = 0.532), F− (r = 0.400) and salinity (r = 0.325); the TH with Ca2+ (r = 0.763) and Mg2+ (r = 0.639), salinity (r = 0.635) with Na+ (r = 0.517), Cl− (r = 0.802), and SO4 2− (r = 0.258). The Ca2+ is in good positive correlation with Na+ (r = 0.424) and Cl− (r = 0.765) while the Mg has significant positive correlation with Na+ (r = 0.414), Cl− (r = 0.678), and SO4 2− (r = 0.156; Table 6), reflecting the influences of Ca2+, Mg2+, and Na+-bearing minerals, in addition to the anthropogenic source. Ca2+ and SO4 2− ions are positively correlated (r = 0.323), reflecting dissolution of sulfate minerals, especially gypsum and anhydrite. The salinity shows highly positive correlation with Cl− (r = 0.822), Na+ (r = 0.818), Ca2+ (r = 0.586) and Mg2+ (r = 0.477) which depends on the mineral dissolution, mineral solubility, ion exchange, evaporation, anthropogenic activities and water flow path conditions. The positive correlations among chemical variables suggest that there are geochemical and biochemical process between these dissolved species, contributing to the water chemistry within the study area (Subba Rao et al. 2012). Na+ and Cl− exhibit a high correlation coefficient (r = 0.830). The high concentration of these ions indicates the predominance of chemical weathering and leaching of chloride salts (mostly halide) (Esmaeili and Moore 2012). As the HCO3 − is a key factor to the enrichment of F− concentration, these two parameters have good positive correlation (r = 0.400). Moreover, groundwater with high HCO3 − and Na+ contents are usually in alkaline conditions. F− and OH− are negatively charged and possess similar ionic radii 1.33 Å F− for and 1.40 Å for OH−. Therefore, OH− can replace the exchangeable F− from F-bearing minerals and enhance the F− concentration in groundwater (Jacks et al. 2005; Dar et al. 2011).
Hydro-geochemical classification of groundwater
The quality of groundwater in its natural state indicates the hydro-geochemical nature of aquifers. Piper tri-linear diagrams (Piper 1953) have been widely used since it is helpful in understanding the hydro-geochemistry of groundwates. In Nilakottai block as seen from (Fig. 7), the left side of the triangle type is dominated with Na+ + K+ cations over the study period. Ca2+ or Mg2+ ions were not identified. Similarly for anion concentrations (right side of the triangle), Cl− ions predominated, followed by the HCO3 − ions. SO4 2− containing samples were not detected. The diamond-shaped field can be classified into six types: (1) Ca–HCO3; (2) Na–Cl; (3) mixed Ca–Na–HCO3 (4) mixed Ca–Mg–Cl; (5) Ca–Cl; (6) Na–HCO3 (Fig. 2). Most samples fell into zones 2, 4 and 5 of the diamond-shaped field, indicating a predominance of sodium chloride, mixed and calcium chloride types. The plot shows that most of the groundwater samples fall in the field of alkaline metals (Na+, K+) over the alkaline earth metals (Ca2+, Mg2+) and strong acid ions (Cl−, SO4 2−) exceed the weak acid (HCO3 −) ions. The dominant anion is Cl− and HCO3 −, followed by SO4 2−; Na+ is the major cation and followed by Mg2+ and Ca2+ in the groundwater. The relative concentrations of the ions occur in the order of Na+ > Mg2+ > Ca2+ and Cl− > HCO3 − > SO4 2− (Fig. 8).
Piper (1953) diagram of the groundwater samples
Evaluation of irrigational quality of groundwater
Excessive concentrations of dissolved ions in the irrigation water affect plants and agricultural soil physically and chemically through lowering of osmotic pressure in the plant structural cells (Ramesh and Elango 2012). Water quality criteria can be used as guidelines by farmers for selecting appropriate management practice to overcome potential hazards that would pose problems for irrigation and to maintain existing soil productivity as well as high crop yield. The suitability of groundwater for irrigation purposes depends on the effect of mineral constituents of water on both plants and soils. The salts besides affecting growth of plant directly also affect soil structure, soil permeability, aeration, texture and make soil hard (Trivedy and Geol 1984).
The general criteria for assessing the irrigation water quality are: total salt concentration as measured by EC, relative proportions of Na+ as expressed by Percent Na (%Na), Sodium adsorption ratio (SAR), Kelly’s Index (KI) and Magnesium hazard (MH). The spatial distribution map of %Na, SAR, KI and MH in ground water samples of the selected study area is given in Fig. 9.
Percent sodium (%Na)
The classification of groundwater samples with respect to the %Na is shown in Table 9. The %Na in the study area ranged between 32.13% and 85.21%, with an average of 63.63% (Table 9). It is observed that most of groundwater samples fall in the category of doubtful (63.64%) followed by permissible (27.27%), unsuitable (5.45%) and a few samples fall under good (3.64%) category. As per the Indian standards (BIS 2012), a maximum Na content of 60% is allowed for irrigation water. The agricultural yields were observed to be generally low in fields irrigated with water high in sodium salts, which cause osmotic effects in soil–plant systems (Raju 2007).
Sodium adsorption ratio (SAR)
Irrigation water quality criteria developed by US Salinity Laboratory (USSL 1954) have received wide acceptance in many countries. Salinity and sodium hazards were proposed to assess irrigation water quality; salinity hazard is based on electrical conductivity (EC) measurement. The salts present in water, besides affecting the growth of plants directly, also affect the soil structure, permeability and aeration, which indirectly affect the plant growth. Sodium or alkali hazard of water for irrigation is determined by the absolute and relative concentration of cations and is expressed in terms of sodium adsorption ratio (SAR). There is a significant relationship between SAR values of irrigation water and the extent to which sodium is absorbed by the soils (Richards 1954). The calculated value of SAR in the study area ranges from 1.05 to 19.50 with an average of 5.11 in the groundwaters. In the given data in US salinity diagram (Fig. 10), EC is taken as salinity hazard and SAR as alkalinity hazard. In C1S1 class, salinity and sodicity are low and suitable for irrigation with most crops on most soils. Classes C1S2, C2S2, and C2S1 can be used for irrigation if a moderate amount of leaching occurs. Classes C1S3, C2S3, C3S1, C3S2, and C3S3 require special management like good drainage, high leaching, and selecting plants with good salt tolerance. In classes C1S4, C2S4, C3S4, C4S4, C4S3, C4S2, and C4S1, salinity and/or sodicity are very high and the water is not suitable for irrigation under ordinary condition, but may be used under very special circumstances. Figure 10 shows that majority of the water samples fall in the category of C3S1 and C4S1. About 58.18% of the groundwater samples fall in the field of C3S1 indicating high salinity and low alkaline water; this can be used for irrigation in almost all types of soil with little danger of exchangeable sodium. Salt-tolerant plants/crops should be selected for high saline regions and irrigated with excess water to provide considerable leaching. About 38.18% of the ground water samples fall in the fields of C4S1, indicating very high salinity and low to medium sodium content. This water is suitable for plants with good salt tolerance, but the suitability is restricted for irrigation of soils with limited drainage. Very few of the groundwater samples (3.64%) fall in the field of C2S1, indicating medium salinity and low sodium content. These waters can be used for irrigation with little danger of harmful exchangeable Na+.
Magnesium hazard (MH)
Szabolcs and Darab (1964) have proposed a magnesium hazard for assessing the suitability of water quality for irrigation. Generally, Ca2+ and Mg2+ maintain a state of equilibrium in ground water. More Mg2+ present in waters affects the soil quality converting it to alkaline and decreases crop yield. MH values >50 are considered harmful and unsuitable for irrigation purposes. If MH exceeds the value of 50, water associated with such a value is considered to be harmful and hence is unsuitable for irrigation, because it adversely affects the crop yield. In the present study, MH varied from 22.37 to 64.40 with an average of 45.35 (Table 3). MH of 36.36% of the groundwater samples exceeds the value 50 and is not suitable for irrigation.
Conclusions
This study deliberated the hydrochemical characteristics of groundwater of Nilakottai block, Tamil Nadu, South India. The water quality was assessed with respect to its suitability for human consumption and irrigational activities. pH values of the groundwater samples in this block show that they are generally neutral to slightly alkaline in nature. EC values of 30.91% of the total groundwater samples come under permissible levels, 41.83% under not permissible levels, and 27.27% under hazardous levels. Of the samples, 72.72% have a higher TDS value than the standard limit. The classification of groundwater based on TH showed that approximately 10.91% of the groundwater samples come under the hard category and the remaining 89.09% of the groundwater samples fall in the very hard category. In the study area, most of the samples of groundwater come under the non-safe category for drinking, with reference to concentration of Na+ and Cl−, which are more than 200 mg/L. High concentrations of both the ions in drinking water impart a salty taste.
Hydro-geochemical classification of groundwater indicates the predominance of Na–Cl, mixed Ca–Mg–Cl and Ca–Cl type. Most of the groundwater samples fall in the category of alkaline metals (Na+, K+) over the alkaline earth metals (Ca2+, Mg2+). Strong acid ions (Cl−, SO4 2−) exceed the weak acid ions (HCO3 −). The relative concentrations of the ions occur in the order of Na+ > Mg2+ > Ca2+ and Cl− > HCO3 − > SO4 2−. Groundwater samples have F− in the range of 1.40–3.60 mg/L and almost all the water samples have higher F− than the standard limit of 1.50 mg/L. Slight alkalinity, high TDS, higher HCO3 − and Na+, and lower Ca2+ contents are favorable for F− to be enriched in groundwater. The presence of high HCO3 − concentration could facilitate F− dissolution from fluoride-rich minerals. The mechanisms also include F−/OH− anion exchange processes as well as evapotranspiration, both of which increase fluoride concentration.
The suitability of groundwater for irrigation purposes depends on the effect of mineral constituents of water on both plants and soils. Calculated values of % of Na, SAR and MH indicate that most of the ground water samples are not suitable for irrigation of crops. The overall pattern of the water quality index of the present study area revealed higher WQI values indicating the poor water quality. GIS data of this study evident that the overall water quality of the study area is very poor and unsuitable for drinking as well as for irrigation purposes.
Water quality management is very essential in the study area, which implies utilization and development of water in a way that maintains its quality at optimum level for the present and potential future users. If rainwater is harvested and conserved properly, it is possible to reduce the concentrations of TDS, TH, Na+, Cl−, NO3 −, and F− well below the unsafe limits for drinking and irrigational purposes. Public awareness programs on the consequences of inferior water quality on human health and agricultural fields and rain water conservation methods are key factors for the success of water quality management for sustainable development.
References
Aghazadeh N, Mogaddam A (2011) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, northwest of Iran. Environ Monit Assess 176:183–195
Ahmad Z, Qadir A (2011) Source evaluation of physicochemically contaminated groundwater of Dera Ismail Khan area, Pakistan. Environ Monit Assess 175:9–21
Alexakis D (2011) Assessment of water quality in the Messolonghi-Etoliko and Neochorio region (West Greece) using hydrochemical and statistical analysis methods. Environ Monit Assess 82:397–413
Amalraj A, Pius A (2013) Health risk from fluoride exposure of a population in selected areas of Tamil Nadu South India. Food Sci Human Wellness 2:75–86
APHA (1995) Standard methods for the examination of water and wastewater. American public Health Association, 19th edn. Washington, DC
Bhardwaj V, Singh DS (2011) Surface and groundwater quality characterization of Deoria District, Ganga Plain, India. Environ Earth Sci 63:383–395
BIS (2012) Bureau of Indian standards for drinking water. New Delhi IS 10500–2012. Second revision. https://law.resource.org/pub/in/bis/S06/is.10500.2012.pdf
Bozdag A, Gocmez G (2013) Evaluation of groundwater quality in the Cihanbeyli basin, Konya, Central Anatolia, Turkey. Environ Earth Sci 69:921–937
Brahman KD, Kazi TG, Afridi HI, Naseem S, Arain SS, Ullah N (2013) Evaluation of high levels of fluoride, arsenic species and other physicochemical parameters in underground water of two sub districts of Tharparkar, Pakistan: a multivariate study. Water Res 47:1005–1020
Central Ground Water Board (2008) Ministry of water resources. South Eastern Coastal Region, Chennai
Chenini I, Mammou AB, May ME (2010) Groundwater recharge zone mapping using GIS-based multi-criteria analysis: a case study in Central Tunisia (Maknassy Basin). Water Resour Manag 24:921–939
Dar MA, Sankar K, Dar IA (2011) Fluorine contamination in groundwater: a major challenge. Environ Monit Assess 173:955–968
Davis SN, DeWiest RJ (1966) Hydrogeology. Wiley, NewYork
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York
Esmaeili A, Moore F (2012) Hydrogeochemical assessment of groundwater in Isfahan province. Iran. Environ Earth Sci 67:107–120
Frengstad B, Banks D, Siewers U (2001) The chemistry of Norwegian groundwater: IV. The pH dependence of element concentrations in crystalline bedrock groundwaters. Sci Total Environ 227:101–117
Garg VK, Suthar S, Singh S, Sheoran A, Garima M, Jai S (2009) Drinking water quality in villages of southwestern Haryana, India: assessing human health risks associated with hydrochemistry. Environ Geol 58:1329–1340
Holden WS (1970) Water treatment and examination. J & Churchill Publishers, London
Hu S, Luo T, Jing C (2013) Principal component analysis of fluoride geochemistry of groundwater in Shanxi and Inner Mongolia, China. J Geochem Explor 135:124–129
Jacks G, Bhattacharya P, Chaudhary V, Singh KP (2005) Controls on the genesis of some high-fluoride groundwaters in India. Appl Geochem 20:221–228
Jain PK (1996) Hydrochemistry and groundwater quality of Singhari river Basin district, Chattapur (MP). Pollut Res 15:407–409
Jain CK, Bhatia KKS, Kumar SR (2003) Groundwater quality in Mala Prabha sub-basin, Karnataka. Indian J Environ Prot 23:321–329
Jain CK, Bandyopadhyay A, Bhadra A (2010) Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environ Monit Assess 166(1–4):663–676
Jameel AA, Hussain AZ (2011) Monitoring the quality of groundwater on the bank of Uyyakondan channel of river Cauvery at Tiruchirappalli, Tamil Nadu-India. Environ Monit Assess 183:103–111
Jha MK, Chowdhury A, Chowdary VM, Peiffer S (2007) Groundwater management and development by integrated remote sensing and geographic information systems: prospects and constraints. Water Resour Manag 21:427–467
Jiang Y, Yan J (2010) Effects of land use on hydrochemistry and contamination of Karst groundwater from Nandong underground river system, China. Water Air Soil Poll 210:123–141
Kelly WP (1946) Permissible composition and concentration of irrigation waters. In: Proceeding American Society of Civil Engineering, 607
US Salinity Laboratory (1954) Diagnosis and improvement of saline and alkali soils. Agricultural handbook. USDA No. 60
Latha SS, Ambika SR, Prasad SJ (1999) Fluoride contamination status of groundwater in Karnataka. Curr Sci 76:730–734
Lee S, Kimb Y, Ohc H (2012) Application of a weights-of-evidence method and GIS to regional groundwater productivity potential mapping. J Environ Manage 96:91–105
Li P, Wu J, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69:2211–2225
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ Monit Assess 171:289–308
Naik PK, Awasthi AK, Anand AVSS, Behera PN (2009) Hydrogeochemistry of the Koyna River basin, India. Environ Earth Sci 59:613–629
National Research Council (NRC) (1993) Ground water vulnerability assessment: contamination potential under conditions of uncertainty, Committee on Techniques for Assessing Ground Water Vulnerability, Water Science and technology Board, Commission on Geosciences, Environment, and Resources. National Academy Press, Washington, DC
Orion Star and Star Plus Meter User Guide (2008) Thermo Fisher Scientific Inc
Paliwal KV (1967) Effect of gypsum application on the quality if irrigation waters. Madras Agric J 59:646–647
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analyses. USGS Groundwater, Note 12, Washington D.C
Pius A, Jerome C, Sharma N (2012) Evaluation of groundwater quality in and around Peenya industrial area of Bangalore, South India using GIS techniques. Environ Monit Assess 184:4067–4077
Prasanna MV, Chidambaram S, Senthil Kumar G, Ramanathan AL, Nainwal HC (2010) Hydrogeochemical assessment of groundwater in Neyveli Basin, Cuddalore District, South India. Arab J Geosci 4:319–330
Raja Reddy D (1979) Hand book of neurology. North Holland Publishing Company, Amsterdam
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ Geol 52:1067–1074
Ramachandran M, Sabarathinam C, Ulaganthan K, Paluchamy A, Sivaji M, Hameed S (2012) Mapping of fluoride ions in groundwater of Dindigul district, Tamilnadu, India-using GIS technique. Arab J Geosci 5:433–439
Ramesh K, Elango L (2012) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environ Monit Assess 184:3887–3899
Ravikumar P, Somashekar RK, Angami M (2011) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environ Monit Assess 173:459–487
Reghunath R, Sreedhara Murthy TR, Raghavan BR (2002) The utility of multivariate statistical techniques in hydrogeochemical studies: an example from Karnataka, India. Water Res 36:2437–2442
Richards LA (1954) Diagnosis and improvement of saline alkaline soils, US Department of Agriculture. HandBook 60:160
Sanchez-Perez JM, Tremolieres M (2003) Change in groundwater chemistry as a consequence of suppression of floods: the case of the Rhine floodplain. J Hydrol 270:89–104
Sawyer GN, McCartly DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw Hill, New York
Shankar BS, Balasubramanya N, Reddy MTM (2008) Impact of industrialization on groundwater quality—a case study of Peenya industrial area, Bangalore, India. Environ Monit Assess 142:263–268
Singh VK, Bikundia DS, Sarswat A, Mohan D (2012) Groundwater quality assessment in the village of Lutfullapur Nawada, Loni, District Ghaziabad, Uttar Pradesh, India. Environ Monit Assess 184:4473–4488
Subba Rao N (1993) Environmental impact of industrial effluents in groundwater regions of Visakhapatnam Industrial Complex. Indian J Geol 65:35–43
Subba Rao N (2002) Geochemistry of Groundwater in Parts of Guntur District, Andhra Pradesh, India. Environ Geol 41:552–562
Subba Rao N, Surya Rao P, Venktram Reddy G, Nagamani M, Vidyasagar G, Satyanarayana NLVV (2012) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environ Monit Assess 184:5189–5214
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Szabolcs I (ed) Proceedings 8th international congress of the International Society of Soil Science, Research Institute of Soil Science and Agrochemistry, Hungarian Academy Science 803–812
Tank DK, Chandel CPS (2010) A hydrochemical elucidation of the groundwater composition under domestic and irrigated land in Jaipur City. Environ Monit Assess 166:69–77
Tiwari TN, Mishra M (1985) A preliminary assignment of water quality index of major Indian rivers. Indian J Environ Prot 5:276–279
Trivedy RK, Geol PK (1984) Chemical and biological methods for water pollution studies. Environ Publications, Karad
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2013) Identification of groundwater contamination zones and its sources by using multivariate statistical approach in Thirumanimuthar sub-basin. Tamil Nadu, India. Environ Earth Sci 68:1783–1795
WHO (2004) Guidelines for drinking water quality, vol 1. Recommendations, 3rd edn. WHO, Geneva
Wilcox LV (1955) Classification and use of irrigation water. USDA, Circular, Washington
Xiong X, Liu J, He W, Xia T, He P, Chen X, Yang K, Wang A (2007) Dose-effect relationship between drinking water fluoride levels and damage to liver and kidney functions in children. Environ Res 103:112–116
Young SM, Pitawala A, Ishiga H (2011) Factors controlling fluoride contents of groundwater in north-central and northwestern Sri Lanka. Environ Earth Sci 63:1333–1342
Younger PL (2007) Groundwater in the environment: an introduction. Blackwell Publishing, Oxford
Zhang B, Song X, Zhang Y, Han D, Tang C, Yu Y, Ma Y (2012) Hydrochemical characteristics and water quality assessment of surface water and groundwater in Songnen plain, Northeast China. Water Res 46:2737–2748
Acknowledgements
The authors would like to thank the University Grants Commission, Government of India for the financial support [F. No.39-806/2010(SR)]. The first author (AA) is grateful to the UGC for the fellowship under Research Fellowship in Sciences for Meritorious Students and also wishes to thank Mr. N. Shenbagaraj, Research Scholar, Department of Rural Development, GRI, Gandhigram for his active help and cooperation. The authors also thank the authorities of GRI for the encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amalraj, A., Pius, A. Assessment of groundwater quality for drinking and agricultural purposes of a few selected areas in Tamil Nadu South India: a GIS-based study. Sustain. Water Resour. Manag. 4, 1–21 (2018). https://doi.org/10.1007/s40899-017-0113-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-017-0113-8