Abstract
Groundwater quality in five catchment areas in Isfahan province of Iran is assessed by measuring physicochemical parameters including major cation and anion compositions, pH, total dissolved solid, electrical conductivity and total hardness. For this purpose, 567 piezometric well samples were collected in October 2007. The abundance of major ions in four of the catchment areas including Gavkhuni, Ardestan, Salt lake and Central Iran desert basins is similar and follows Cl− > SO4 2− > Na+ > HCO3 − > Ca2+ > Mg2+ > K+ > CO3 2− trend, while in the fifth basin (Karoon), the trend changes into HCO3 − > Ca2+ > Cl− > SO4 2− > Mg2+ > Na+ > K+ >CO3 2−. In general, four water facies are determined and it is shown that alkali elements and strong acids are dominating over alkaline earth and weak acids. Statistical analysis including Mann–Whitney U test indicate that physicochemical parameters in three of the five investigated basins [Gavkhuni, Ardestan and Central Iran desert (CID)] are similar, while Karoon and salt lake basins display different characteristics. The result indicate that groundwater west of the province is suitable for irrigation, while in the central and eastern parts of the province the groundwater loses its quality for this purpose. It is concluded that mineral dissolution and evapotranspiration are the main processes that determine major ion compositions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the ever-increasing demand for potable and irrigation water and inadequacy of available surface water, the importance of groundwater is increasing exponentially (Nagarajan et al. 2010). Groundwater quality is an essential factor in the suitability of water for various purposes. Quality of groundwater is a function of physical, chemical and biological parameters (Joshi and Seth 2010). Moreover, groundwater quality, i.e. of dissolved ions content, is mostly affected by either natural geochemical characteristics including climate, lithology, mineral weathering, nature of geochemical reactions, solubility of salts, dissolution/precipitation reactions, ion exchange, wet and dry deposition of atmospheric salt and evapotranspiration; or various anthropogenic activities such as agriculture, sewage disposal, mining and industrial wastes (Nisi et al. 2008; Singh and Chandel 2006; Jiang and Yan 2010; Tatawat and Chandel 2008). Over the last few decades, competition for economic development, associated with rapid growth in population and urbanization, has further affected the groundwater quality due to over exploitation and increasing demand for agriculture, domestic and industrial water supply, as well as improper sewage flow and solid waste disposal (Jameel and Sirajudeen 2006; Marghade et al. 2010). Intensive cultivating and urban development has caused a high demand on groundwater resources in arid and semiarid regions of the world and Iran while putting these resources at greater risk to contamination (Asghari Moghaddam and Najib 2006; Giridharan et al. 2008; Jalali 2007; Tayfur et al. 2008; Aghazadeh and Moghaddam 2010). Hence, to utilize and protect valuable water sources effectively and predict the change in groundwater environments, it is necessary to understand the hydrochemical parameters of groundwater such as pH, electrical conductivity (EC), total dissolved solids (TDS), sodium absorption ratio (SAR), total hardness (TH), major anions (CO3 2−, HCO3 −, Cl− and SO4 2−) and major cations (Ca2+, Mg2+, Na+ and K+) concentrations (Prasanna et al. 2010; Guendouz et al. 2003; Edmunds et al. 2006). In Iran, groundwater is the primary source of water for irrigation as well as human consumption and industrial use. The Isfahan province with a surface area of approximately 107,090 km2 is located in the center of Iran. It is an important industrial center and contains several metalliferous and non-metalliferous ore deposits. The provincial capital (Isfahan city) is the third populated city in Iran and because of its historic sites is an important tourism center that is visited by thousands of national and foreign tourists. In the second half of the twentieth century, rapid population growth and increasing demand for groundwater has led to depleting groundwater supply. The intense withdrawal of groundwater in many areas has resulted in land subsidence.
The main objectives of this research are to determine geochemical characteristics of Isfahan groundwater, evaluate groundwater quality, and asses its suitability for drinking and irrigation. Geochemical processes responsible for the groundwater chemistry in Isfahan province will also be looked upon.
Study area
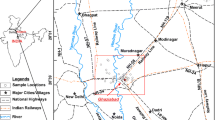
Isfahan province is located in central Iran and lies between north latitude 30°43′–34°27′ and east longitude 49°38′–55°32′. It includes 21 townships. The surface of the study area which is mostly desert is 107,090 km2. In 2008, the population of Isfahan province was reported to be 4,559,256, of which 83% is urban and 17% is rural. Isfahan province climate is highly variable, i.e. arid and semi-arid in eastern and central parts, and semi-humid in western parts. The annual mean temperature is 16.7°C, ranging from minimum 10.6°C in winter to maximum 40.6°C in summer. Average annual rainfall in eastern, central and western parts, is 90, 177 and 513 mm, respectively. Rainfall mainly occurs in winter and spring season (Fig. 1b). Groundwater is an important water resource for drinking, agriculture and industrial uses in the study area. Low precipitation and overexploitation of groundwater resources in recent years have caused an extensive groundwater level decline in this plain prohibiting further development of the aquifer.
Geology and hydrogeology
Geologically Isfahan province contains part of different geological zones of Iran, including Central Iran, Sahand-Bazman volcano-magmatic belt, Sanandaj-Sirjan metamorphic belt and Zagros folded belt (Nabavi 1976). The basement of the study area is mostly windblown sand deposits (Loess), alluvial fan deposits, marl, shale, gypsum and halite in the east (Lut block and center zone of Iran); anhydrite, halite, marl (Center zone of Iran), andesitic and dacitic tuff (Sahand–Bazman magmatic belt) in the center, and phyllite, slate, metasandstone (Sanandaj-Sirjan zone) and thick bedded limestone (Zagros fold belt) in the west. Structural and tectonic history of Isfahan indicates several episodes of deformation, resulting in repeated folding, faulting, jointing and fracturing (Fig. 2).
Hydrologically Isfahan is located in Kavir-e-Markazi catchment area and can be divided into five zones based on drainage basins and major aquifers including Gavkhuni (central), Salt lake (north), Ardestan (central to eastern), Karoon (western) and CID (eastern) (Fig. 1a). Table 1 represents the hydrogeological characteristics of the five catchment areas. The aquifers in the study area mostly unconfined and occur in alluvial plains. The average alluvium thickness is about 76.1 m while maximum thickness is 225 in the middle of Gavkhuni catchment area (Isfahan Regional Water Authority 2007). The average depth of wells in the Gavkhuni, Ardestan, CID, Salt lake and Karoon catchments is 58.27, 65.25, 56.5, 61.31 and 66.5 m, respectively. Average discharge in the five catchments areas is also 12.65, 14.4, 12.47, 14.56 and 15.4 l/s, respectively. The current annual rate of groundwater withdrawal is about four million cubic meters. Ninety-three percent of exploited groundwater is used for agriculture, 6% for drinking and 1% is used by the industry. The piezometric map of water table on the basis of 567 measurements in October 2007 is presented in Fig. 1c. This map shows the main direction of groundwater flow in the five investigated catchment areas. Eight ephemeral streams flow in the area, however, the linear recharge is insignificant, except for Zayandehrud river in the west. Therefore, rainfall is the main source of recharge, particularly in the eastern regions. Comparison between the present piezometric map and an older one prepared 30 years ago, reveals significant water table drop in the five catchment areas. For example, in Zayandehrud catchment area the piezometric level has dropped from 17 to 30 m. The reason is said to be intense over-pumping and rapid development of agriculture and industry, especially in Gavkhuni basin.
Materials and methods
Sampling was carried out by Isfahan regional water organization from 567 piezometric wells during October 2007, including 33 samples from Karoon basin, 298 samples from Gavkhuni basin, 124 samples from Salt lake basin, 84 samples from Ardestan and finally 28 samples from CID basin (Fig. 1a). Physicochemical parameters including temperature, EC and pH were measured in situ using portable measuring instruments immediately after sampling. Dry, clean and sterilized polyethylene bottles (1 l) were used for water sampling. The bottles were triple washed by the water to be sampled before sampling. The collected samples were labeled, sealed and transported to the laboratory and preserved in refrigerator at a temperature of about 4°C until analysis. Water samples were analyzed for chemical constituents such as sodium, potassium, calcium, magnesium, chloride, bicarbonate, carbonate, sulfate and TDS in the laboratory using standard methods within 48 h after sampling (Table 2). TDS were measured by evaporating a pre-filtered sample to dryness. The accuracy of the chemical analyses was verified by calculating ion-balance errors. The errors were generally around ±5%. TH, SAR, sodium percent (Na%) and residual sodium carbonate (RSC) were calculated using equations in Table 3. The PHREEQC computer software was used to calculate the distribution of aqueous species and mineral saturation indices (SI). Correlation coefficient, factor analysis (FA) and Mann–Whitney U test were determined to reveal the relationship between parameters, using SPSS 17 and Minitab 13 software packages.
Groundwater chemistry
The quality of groundwater is important because it determines the suitability of water for drinking, domestic, agricultural and industrial purposes (Tatawat and Chandel 2008). Table 4 illustrates the physicochemical parameters of Isfahan groundwaters in all basins indicating minimum, maximum, median and mean values. The chemical composition of groundwater samples (n = 567) covers a wide range. pH values of Isfahan groundwater varies from 7 to 9 with a mean of 8.14, indicating a neutral to slightly alkaline water. Nearly 7% of the samples display pH values above WHO standard (2004). Water conductivity in the study area varies from 167 to 19,600 μs/cm with an average of 2,755 μs/cm (Fig. 3). maximum allowable limit for EC in drinking water is 1,500 μs/cm according to WHO standard. Therefore, EC values of 50% of the samples is above the standard value. TDS values range from 109 to 13,720 mg/l, with a mean value of 1,913 mg/l. Groundwater with high TDS is not suitable for both irrigation and drinking purposes (Fetters 1990). TDS in 48% of the samples exceed the desirable limit given by WHO (Fig. 3). The mean concentration of cations (mg/l) indicates that Na+ > Ca2+ > Mg2+ > K+ in Gavkhuni, Ardestan, Salt lake and CID basins, while in the Karoon basin the trend changes to Ca2+ > Na+ > Mg2+ > K+. Similarly, the mean concentration of anions (mg/l) displays the following decreasing trend: Cl− > SO4 2− > HCO3 − >> CO3 2− in the first four basins, while in Karoon basin it changes to HCO3 − > Cl− ≥ SO4 2− >> CO3 2−. Sodium content in 45% of the analyzed samples exceeds the desirable limit of 200 mg/l (WHO 2004). The high concentration of Na+ is probably the result of weathering of rock forming minerals such as halite and sodium plagioclase along with anthropogenic sources including industrial, domestic and animal waste (Freeze and Cherry 1979). Calcium content in 19% of the analyzed samples exceed WHO standard (Fig. 3). The concentrations of calcium ranges from 12 to 1,182 mg/l. Calcium can be derived from either weathering of carbonate minerals (calcite, dolomite and aragonite) or leakage of chemical fertilizers (Prasanna et al. 2010). Magnesium and potassium contents range from 2.4 to 874 mg/l and 0.39 to 54 mg/l, respectively. Twelve percent of the magnesium content is above WHO standard, while potassium concentration is below the WHO guideline value (Fig. 3). The major source of Mg2+ in groundwater is probably Mg-bearing minerals and anthropogenic wastes (Marghade et al. 2010). Chloride content varies from 3.54 to 6,664 mg/l (Fig. 3). Nearly 43% of the analyzed samples display chloride content above the standard value (250 mg/l; WHO 2004). Sulfate content ranges from 0.48 to 3,666 mg/l whereby 48% of samples exceed WHO standard (Fig. 3). The major source of sulfate in groundwater is weathering of sulfur-bearing minerals such as gypsum, anhydrite and sulfide minerals along with industrial and mining waste (Fianko et al. 2009). Most of the sulfate in water samples comes from anhydrite- and halite-bearing sediments. Such sediments are more abundant and exposed in the eastern parts of Isfahan (CID, Ardestan and east parts of Gavkhuni and salt lake catchments). Carbonate content ranges between 0 to 35 mg/l whereas bicarbonate ranges from 48 to 1,115 mg/l (Fig. 3). The principal source of HCO3 − is dissolution of carbonate minerals, and secondary sources can result from dissolution of CO2 by anoxic biodegradation of organic matter from anthropogenic sources such as industrial and domestic sewage and buried waste in landfills (Subrahmanyam and Yadaiah 2000).
Water type
Major cations and anions in meq/l were plotted on a trilinear Piper diagram (Piper 1944), using rockwork14 software to evaluate the hydrochemistry of groundwater in the five investigated basins in Isfahan province (Fig. 4). The results show that the majority of groundwater samples fall in the field of Na+ + K+ − Cl− + SO4 2− and Na+ + K+ − Cl water type (48.24 and 21.92%), while 13.15% plot in the Ca2+ − HCO3 −, 9.12% in mixed-HCO3 − and 7.57% in the Ca2+ + Mg2+ − HCO3 − fields (Table 5). Chloride and sulfate groundwater mostly occurs in CID, Ardestan and the east part of Gavkhuni and Salt lake catchments, while bicarbonate groundwater mainly fall in Karoon, and west of Gavkhuni and Salt lake catchments. It is clear that in the center and east of Isfahan province, alkali elements (Na+ and K+) content is significantly higher than alkaline earth elements (Ca2+ and Mg2+). Strong acid anions (Cl− and SO4 2−) also exceed weak acid anions (CO3 2− and HCO3 −). Abundance of alkali elements is probably the result of dissolution of gypsum, halite, anhydride and alkali feldspar minerals in the aquifer matrix. Alkaline earth content is significantly higher in the west of the study area, reflecting dissolution of calcite, dolomite and other Ca-bearing minerals.
Mann–Whitney U test
The Mann–Whitney U test is used to compare the measured physicochemical parameters in the five investigated aquifers. This test is nonparametric and is based on ranked sums that compare the medians of two independent samples (Moore and Cobby 1998). The mean result of Mann–Whitney U test for the measured physicochemical parameters (EC, TDS, HCO3 −, SO4 2−, Cl−, Ca2+ and Na+) in Table 6 shows that Gavkhuni, Ardestan and CID basins have p value >0.05 and thus have similar physicochemical parameters, while Karoon and Salt lake basin are different.
Correlation coefficient
Correlation analysis estimates the relationship between any pair of variables (ions). The distribution of ions in groundwater samples are non-parametric, therefore spearman rank correlation is used in this study to measure correlation between ions. The correlation coefficients for 11 quality parameters are presented in Table 7. The highest correlation occurs between EC and TDS in p value <0.01 (0.981). EC, TDS and TH display good positive correlation with Ca2+, Mg2+, Na+, Cl− and SO4 2−. The correlation between Na+ and Cl− is generally used to identify the mechanism of salinity in arid and semi-arid regions (Ganyaglo et al. 2010). Na+ and Cl− exhibit high correlation coefficient in p value <0.01 (0.964). The observed high concentration of these ions measured in the majority of samples, indicate the predominance of chemical weathering and leaching of chloride salts (mostly halite). Calcium and sulfate ions are positively correlated in p value <0.01 (0.791) reflecting dissolution of sulfate minerals, specially gypsum and anhydrite. The SO4 2−/Ca2+ molar ratio is greater than 1, indicating that calcium and sulfate do not follow a similar geochemical pathway. In fact, sulfate remains dissolved while calcium is precipitated as calcite. The significant correlation between SO4 2− and Mg2+ (0.79) and SO4 2− and Na+ (0.86) suggests that part of these ions is derived from the weathering of magnesium and sodium sulfate minerals. Correlation coefficient between Calcium and bicarbonate in Karoon basin and west of Gavkhuni basin is significant (0.38). These regions consist of carbonatic formations and calcite is the probable sources of Ca2+. Correlation between Ca2+ and HCO3 − east of Gavkhuni, Ardestan, Salt lake and CID basins is poor indicating that in addition to calcite, dissolution of other mineral phases is also important.
Factor analysis
The goal of FA is reduction of dimensionality and is to explain as much information contained in the data as possible in as few components as possible (Harman 1976; Johnson and Wichern 2002; Reimann et al. 2002). FA was carried out using SPSS version 17 software with principal component analysis (PCA) method and varimax rotation in order to determine the optimum number of factors. The solution using the eigenvalue 1 in three components explain 82.32% of the total variance (Table 8). Component 1 indicate that EC, TDS, TH, Na+, Ca2+, Mg2+, Cl− and SO4 2− explain 57.80% of the total variance. It represents halite and evaporites from the underlying geologic formations (sulfate and chloride water types). The constituents in component 2 are pH and CO3 2− and explain 14.58% of total variance. Component 3 is explains 9.66% of the total variance defined by bicarbonate and probably indicates weathering of carbonate minerals (Fig. 5).
Irrigational quality of groundwater
Excessive concentration of dissolved ions in water can affect plants and physicochemical properties of soils, and lead to lower productivity and destruction of soil structure (Ravikumar et al. 2010; Yidana et al. 2008a, b; Dar et al. 2010). The chemical effects disrupt plant metabolism while the physical effects lower permeability in soil and reduce osmotic pressure in the plant cell structure, therefore preventing water from reaching branches and leaves (Sahinci 1991). In Isfahan province, surface water is limited (especially in central and eastern regions), therefore, irrigation mainly depends on groundwater. Irrigation suitability of groundwater in Isfahan province is evaluated by measuring EC, SAR, %Na, TH, TDS and RSC. The results are presented in Table 9.
The EC (salinity hazard) and Na concentration (sodicity or alkalinity hazard) are usually considered important in classifying irrigation water. High EC in groundwater leads to formation of saline soil, whereas high Na content causes alkaline soil (Nagarajan et al. 2010). According to Richards (1954), based on EC 1.94% of samples have excellent quality, 29.8% of samples have good quality and 28.39% of samples fall in permissible quality. The remaining 40% belong to high salinity category, indicating that the water exceeds the permissible quality and is not suitable for irrigation. The majority of saline groundwater samples belong to the central part of Gavkhuni basin, Ardestan and CID basins. The calculated values of SAR in the study area (Eq. a in Table 3) varies from 0.06 to 33.89 meq/l. The correlation between SAR and EC are also plotted on the US salinity diagram (Fig. 6). It is found that 31.73% of samples fall in the C1S1 and C2S1 fields, indicating low to medium salinity and low alkalinity, which are suitable for irrigation of most crops and majority of soils with little danger of exchangeable sodium (mostly in Karoon catchment). 44.61% of groundwater samples fall in the fields of C3S1, C3S2 and C4S2, indicating high salinity and low to medium alkalinity, suitable for plants with good salt tolerance and also restricted suitability for irrigation of soils with restricted drainage (west of Gavkhuni, Salt lake and Ardestan catchments). 23.66% of water samples fall in C4S3 and C4S4 fields and are not suitable for irrigation (in Central Gavkhuni, east of Ardestan and Salt lake, and in CID catchments).
Processes regulating groundwater chemistry
Reaction between groundwater and aquifer minerals play a significant role in water quality, which is also useful in understanding the genesis of groundwater (Prasanna et al. 2010). Hence, Gibbs diagram were employed to asses hydrochemical processes such as precipitation, rock weathering and evaporation on water chemistry in the five investigated aquifers in Isfahan provinces. Figure 7 illustrates that all groundwater samples in Karoon basin, 70% of the samples in Salt lake basin and 50% of samples in Gavkhuni basin (central and western parts) fall in the rock weathering field, suggesting weathering of minerals such as carbonates and silicates. 90, 80 and 50% of samples in CID, Ardestan and Gavkhuni (east) plot in the evaporation zone. The climate east of Isfahan province is arid, hence groundwater evaporation is a common phenomenon. Evaporation increases salinity through increasing Na+ and Cl− content and hence TDS. High Na+ and Cl− content detected in samples collected from central and eastern regions, suggest chloride salt dissolution. Na+/Cl− ratio has often been used to identify the mechanisms for acquiring salinity and saline intrusion in semi-arid regions (Magaritz et al. 1981; Dixon and Chiswell 1992; Sami 1992; Jalali 2005). If evaporation is the dominant process Na+/Cl− ratio should remain constant with increasing EC (Jankowski and Acworth 1997). The plotting of groundwater samples in Na+/Cl− versus EC scatter diagram, shows that the trend line is horizontal (Fig. 8) revealing that evapotranspiration is responsible for high Na+ and Cl− content in the groundwater. Anthropogenic activity such as the use of agriculture fertilizers and irrigation return flow, also influence the evaporation by increasing Na+, Cl− and thus TDS.
It is obvious that, mineral dissolution and evaporation are the major processes that regulate water chemistry in Isfahan aquifers. Therefore, Saturatin indices of groundwater samples with respect to evaporites (gypsum anhydrite and halite) and carbonates (calcite, dolomite and aragonite) minerals, as well as activity of ion species were calculated using PHREEQC computer software version 2 (Appelo and Postma 1999) via the following equation:
where IAP and K S are ion activity product and solubility product constant of minerals at the sample temperature, respectively. PHREEQC uses ion activity and Debye-Hückel expression to compute the coefficient activity. This type of aqueous model is adequate for low ionic strength (lower than sea water), and hence is suitable for groundwater in the study area (Parkhurst and Appelo 1999). Saturation indices of carbonate and evaporate minerals vary within groundwater catchment areas (Fig. 9). Groundwater samples are classified into three categories: oversaturated (SI > 0), saturated (0 < SI < −1) and undersaturated (SI < −1). The average SI values for minerals in the five investigated aquifers are shown in Table 10. Nearly all water samples are oversaturated with respect to calcite, dolomite and aragonite (SI > 0). Therefore, water chemistry in the study area seems to be largely affected by the dissolution of carbonate minerals. Saturation indices of halite varies between −8.06 and −1.87 (Fig. 9) while SI values of anhydrate and gypsum ranges from −4.37 to −0.18 and −4.15 to 0.04, respectively, revealing that halite does not play an important role in the groundwater chemistry. 83 groundwater sample in Gavkhuni and 147 samples in CID, Salt lake and Ardestan basins are saturated with respect to gypsum and anhydrate. Therefore, except for Karoon basin which is oversaturated with Carbonate minerals, the chemical composition of groundwater in other aquifers is determined by the dissolution of carbonate and sulfate minerals.
Conclusion
Ionic concentration, TDS, EC and other groundwater quality parameters in Isfahan province suggest that climate, lithological units and residence time are major controlling factors of groundwaters chemistry. The quality of groundwater in alluvial fan deposits, and gypsiferous and halite-bearing sediments in central Gavkhuni, eastern parts of Ardestan, Salt lake, and CID catchment basins is not suitable for drinking and irrigation due to high chloride and sulfate ion content, and can result in adverse affects in crops, soils and human. Groundwater in carbonatic formations (Karoon basin) and igneous rocks (between Gavkhuni and Ardestan basins) have good quality and is suitable for various purposes. Due to arid to semi-arid climate, the indiscriminate exploitation of groundwater, and recent drought, salinity of groundwater in Isfahan province (especially in eastern parts) has remarkably increased. Salinity has reached a point where sulfate minerals are close to the saturation boundary. Considering the important role played by groundwater in the development of Isfahan province, particularly east of the province, urgent management plans are needed to supervise the exploitation of groundwater resources in this province and prevent further deterioration of groundwater quality.
References
Aghazadeh N, Moghaddam AA (2010) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environ Monit Assess. doi:10.1007/s10661-010-1575-4
Appelo CAJ, Postma D (1999) A consistent model for surface complexation on birnessite (–MnO2) and its application to a column experiment. Geochim Cosmochim Acta 63:3039–3048
Asghari Moghaddam A, Najib A (2006) Hydrogeologic characteristics of the alluvial tuff aquifer of northern Sahand Mountain slopes, Tabriz, Iran. Hydrogeol J 14:1319–1329
Dar IA, Sankar K, Dar MA (2010) Spatial assessment of groundwater quality in Mamundiyar basin, Tamil Nadu, India. Environ Monit Assess. doi:10.1007/s10661-010-1702-2
Dixon W, Chiswell B (1992) The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queensland, Australia. J Hydrol 130:299–338
Edmunds WM, Ma JZ, Aeschbach-Hertig W, Kipfer R, Darbyshire DPF (2006) Groundwater recharge history and hydrogeochemical evolution in the Minqin basin, North West China. Appl Geochem 21:2148–2170
Fetters CW (1990) Applied hydrogeology. CBS, New Delhi
Fianko JR, Osae S, Adomako D, Achel DG (2009) Relationship between land use and groundwater quality in six districts in the eastern region of Ghana. Environ Monit Assess 153:139–146. doi:10.1007/s10661-008-0344-0
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs, p 604
Ganyaglo SY, Banoeng-Yakubo B, Osae S, Dampare SB, Fianko JR (2010) Water quality assessment of groundwater in some rock types in parts of the eastern region of Ghana. Environ Earth Sci. doi:10.1007/s12665-010-0594-3
Giridharan L, Venugopal T, Jayaprakash M (2008) Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environ Monit Assess 143:161–178
Guendouz A, Moulla AS, Edmunda WM, Zouari K, Shand P, Mamou A (2003) Hydrogeochemical and isotopic evolution of water in the complex terminal aquifer in the Algerian Sahara. Hydrogeol J 11:483–495
Harman HH (1976) Modern factor analysis, 3rd edn. University of Chicago press, Chicago, p 487
Jalali M (2005) Major ion chemistry of groundwaters in the Bahar area, Hamadan, Western Iran. Environ Geol 47:763–772. doi:10.1007/s00254-004-1200-3
Jalali M (2007) Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah Basin in Western Iran. Environ Monit Assess 130:347–364
Jameel A, Sirajudeen J (2006) Risk assessment of physicochemical contaminants in groundwater of Pettavaithalai area, Tiruchirappalli, Tamilnadu, India. Environ Monit Assess 123:299–312. doi:10.1007/s10661-006-9198-5_cSpringer2006
Jankowski J, Acworth RI (1997) Impact of depris-flow deposits on hydrogeochemical processes and the development of dryland salinity in the Yass River catchment, New South Wales. Aust Hydrogeol J 5(4):71–88
Jiang Y, Yan J (2010) Effects of land use on hydrochemistry and contamination of Karst groundwater from Nandong underground river system, China. Water Air Soil Pollut 210:123–141. doi:10.1007/s11270-009-0229-z
Johnson RA, Wichern DW (2002) Applied multivariate statistical analysis, 5th edn. Prentice Hall, New Jersey, p 767
Joshi A, Seth G (2010) Hydrochemical profile for assessing the groundwater quality of Sambhar lake City and its Adjoining area. Environ Monit Assess. doi:10.1007/s10661-010-1477-5
Magaritz M, Nadler A, Koyumdjisky H, Dan N (1981) The use of Na/Cl ratio to trace solute sources in a semiarid zone. Water Resour Res 17:602–608
Marghade D, Malpe DB, Zade AB (2010) Geochemical characterization of groundwater from northeastern part of Nagpur urban, Central India. Environ Earth Sci. doi:10.1007/s12665-010-0627-y
Moore P, Cobby J (1998) Introductory statistic for environmentalist. Prentice Hall, Europe
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ Monit Assess. doi:10.1007/s10661-009-1279-9
Nabavi MH (1976) Preface geology of Iran (in Persian). Geology Survey, Iran
Nisi B, Buccianti A, Vaselli O, Perini G, Tassi F, Minissale A et al (2008) Hydrogeochemistry and strontium isotopes in the Arno River Basin (Tuscany, Italy): constraints on natural controls by statistical modeling. J Hydrol 360:166–183
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2)—A computer program for speciation, batch-reaction, onedimensional transport, and inverse geochemical calculations. USGS Water-Resources Investigations Report, 99–4259
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25:914–923
Prasanna MV, Chidambaram S, Senthil Kumar G, Ramanathan AL, Nainwal HC (2010) Hydrogeochemical assessment of groundwater in Neyveli Basin, Cuddalore District, South India. Arab J Geosci. doi:10.1007/s12517-010-0191-5
Ravikumar P, Venkatesharaju K, Prakash KL, Somashekar RK (2010) Geochemistry of groundwater and groundwater prospects evaluation, Anekal Taluk, Bangalore Urban District, Karnataka, India. Environ Monit Assess. doi:10.1007/s10661-010-1721-z
Reimann C, Filzmoser P, Garrett RG (2002) Factor analysis applied to regional geochemical data: problems and possibilities. Appl Geochem 17:185–206
Richards LA (1954) Diagnosis and improvement of saline alkali soils: agriculture, vol 160. Handbook 60, US Department of Agriculture, Washington DC
Sahinci A (1991) Geochemistry of natural waters (in Turkish). Reform Printing Office, Izmir, p 548
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J Hydrol 139:27–48
Singh V, Chandel CPS (2006) Analysis of wastewater of Jaipur City for agricultural use. Res J Chem Environ 10(1):30–33
Subrahmanyam K, Yadaiah P (2000) Assessment of the impact of industrial effluents on water quality in Patancheru and environs, Medak district, Andhra Pradesh, India. Hydrogeol J 9(3):297–312. doi:10.1007/s100400000120
Tatawat RK, Chandel SCP (2008) A hydrochemical profile for assessing the groundwater quality of Jaipur City. Environ Monit Assess 143:337–343
Tayfur G, Kirer T, Baba A (2008) Groundwater quality and hydrogeochemical properties of Torbali Region, Izmir, Turkey. Environ Monit Assess 146:157–169
WHO (World Health Organization) (2004) Guidelines for drinking water quality, vol 1, 3rd edn. WHO, Geneva, p 515
Yidana SM, Ophori D, Banoeng-Yakubo B (2008a) A multivariate statistical analysis of surface water chemistry—The Ankobra Basin, Ghana. J Environ Manag 86:80–87
Yidana SM, Ophori D, Banoeng-Yakubo B (2008b) Hydrogeological and hydrochemical characterization of the Voltaian Basin: the Afram Plains area, Ghana. Environ Geol 55:1213–1223
Acknowledgments
The authors would like to thank Isfahan Regional Water Authority and Shiraz University Research Committee for making this study possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmaeili, A., Moore, F. Hydrogeochemical assessment of groundwater in Isfahan province, Iran. Environ Earth Sci 67, 107–120 (2012). https://doi.org/10.1007/s12665-011-1484-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1484-z