Abstract
A water quality investigation was carried out in the Deoria district, Ganga plain, to assess the suitability of surface and groundwaters for domestic, agricultural, and industrial purposes. As much as 50 representative samples from river and groundwater were collected from various stations to monitor the water chemistry of various ions, comprising Ca2+, Mg2+, Na+, K+, HCO3 −, SO4 2−, NO3 −, Cl−, F−, and trace metals, such as Fe, Cu, Mn, Zn, Cd, and Pb. The results showed that electrical conductance (EC), total dissolved solids (TDS), HCO3 −, Mg2+, Na+, and total hardness (TH) are above the maximum desirable limit, and apart from Fe and Mn all other trace metals are within the maximum permissible limit for drinking water. The calculated values for sodium absorption ratio (SAR), salinity, residual sodium carbonate (RSC), and permeability index (PI) indicate well to permissible use of water for irrigation. High values of Na%, RSC, and Mg-hazard (MH) at some stations restrict its use for agricultural purpose. Anthropogenic activities affect the spatial variation of water quality. Economic and social developments of the study area is closely associated with the characteristics of the hydrological network.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Ganga alluvial plain is one of the most densely populated regions in the world. This region has uniformly distributed urban centers and agricultural land cover. The interaction between the environment and human activities are complex, important, and poorly understood. However, human activities are continuously modifying the physical, chemical, and biological composition of the environment. It is, therefore, essential to understand them as the existence, and survival of human beings depend upon the appropriate interactions with the environment (Butler et al. 2005; UNESCO 1995; Gibbs 1970).
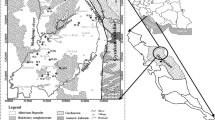
Deoria district is located in the eastern part of the Ganga plain, which is situated on both banks of the Chhoti Gandak River (Fig. 1). It occupies an estimated area of 2,489.41 km2. The total population of the area is 2.73 millions with population density of 1,062 persons per square kilometer according to 2001 census. Population growth rate of the area is about 25% in the last two decades. Groundwater is the only source for regular supply of domestic water. The residents have their own groundwater wells and hand pumps installed within the vicinity of the settlements. River water is generally used for irrigation, livestock, and local industries. Groundwater studies in Deoria are very important because of increasing pressure of population on resources. The urban and agricultural expansions have caused an ever-growing need for freshwater which is provided through hand dug or drilled wells and rivers. The water quality of river at any point reflects several major influences, including the lithology of the basin, atmospheric inputs, climatic conditions, and anthropogenic inputs (Bricker and Jones 1995). The weathering process and major ion chemistry of the Ganga–Brahmaputra river system has been analyzed (Galy and France-Lanord 1999; Sarin et al. 1989). The quality of water is identified in terms of its physical, chemical, and biological parameters (Sargaonkar and Deshpande 2003) and is important for sustainable development of an urban area (Subba Rao 2008). Present paper deals with the physical and chemical parameters of the surface and groundwater of the Deoria district which have direct societal impact.
Study area
The study area is situated in east part of the Ganga plain between 26°06′ N–26°44′ N and 83°30′ E–84°12′ E (Fig. 1). The elevation varies between 65 and 106 m above mean sea level. Physiographically this area is a fertile plain, with gentle slope toward southeast.
The Chhoti Gandak with its tributaries, Rapti and Ghaghara are the main drainage system of this region. The drainage pattern is subdendritic to dendritic. The main river Chhoti Gandak is 250-km long which drains the entire district from north to south. It originates in the terai area of Ganga Plain near Desopool in Maharajgan district of Uttar Pradesh. The slope gradient is 0.021% and the sinuosity is 2.05 and so the river is meandering in nature. Channel widths (25–200 m) and valley width (0.1–0.5 m) are increasing simultaneously toward middle reaches. This river joins the Ghaghara near Gothini in Siwan district of Bihar. In this area upland terrace surface, river valley terrace surface, and present day river channel with narrow flood plains made up of older alluvium and newer alluvium can be identified (Singh et al. 2009).
Climate is sub-humid and the vegetation is composed of deciduous trees and grassland. Maximum rain occurs in July, average annual rainfall is 1,128 mm and the annual potential evapo-transpiration is about 1,955 mm. May and June are hot with mean maximum temperature of 39.2°C, whereas December and January are cold with mean minimum temperature of 9.5°C. Humidity is maximum (84%) in August and minimum (43%) in April (Statistical Diary of Uttar Pradesh 2009; Indian Meteorology Department 2009).
The Section line X-X′ which runs in SSW–NNE direction is about 40 km in length (Fig. 1) and the depth of the boreholes ranges from 70.10 to 103.63 m (Fig. 2). The borehole data of national and state groundwater agencies indicate that the subsurface consists of thick deposits of alluvium made up of clay, sand, calcrete (kankar), and gravel mixed in different proportions at different depth (Bhardwaj et al. 2010). Sedimentation on the inter-channel areas takes place mainly by sheet flow (Kumar et al. 1995) and interfluve deposits (Singh et al. 1999), whereas in the channel areas by rivers in the form of channel bar deposits (Singh and Singh 2005). Geomorphologically, the interchannel areas are differentiated into broad low lying areas with small ponds, lakes, creeks, abandoned channels, and small ridges. There is no proper land-use planning. The upland terrace surfaces are used for settlement to minimize the effect of flooding. The river valley terrace surfaces are used for agriculture and for new settlements due to increasing demographic pressure and poor land-use planning. The present day channel during low discharge periods are used for Zayad crops. The waste land is used for grazing.
Materials and methods
Samples of surface and groundwater were collected from different stations in Deoria District during summer (June), 2006. The samples were collected and stored in acid-cleaned, high-density polyethylene (HDPE) bottles (1,000 ml), which were carefully rinsed three times before use. The use of HDPE bottles minimizes container pollution and promotes sample preservation (Hall 1998). The surface water samples were collected from rivers at 10 stations designated as SW-1 to SW-10. The groundwater samples are designated as A to O (Fig. 1). Two sets of samples were collected at each station.
Analyses of water samples were carried out immediately after their collection. Samples were stored in a refrigerator at 4°C prior to analysis. Temperature, pH, conductivity, and total dissolved solids were determined in the field by Cyber Scan 510, potassium, sodium and calcium by Systronics Flame photometer 128, nitrate by Perkin–Elmer, UV/VIS spectrometer, Lambda 40. Perkin–Elmer Atomic Absorption Spectrometer was used for the analysis of Mg, Fe, Cu, Mn, Zn, Cd, and Pd. Other parameters (Cl−, F−, SO4 2−, and TH) were also done according to APHA (1992, 1998) in Geochemical Laboratory of Centre of Advanced Study in Geology, University of Lucknow, Lucknow. The reproducibility of the analytical procedures was checked by carrying out a duplicate analysis. Duplicate results did not differ by more than 5% of the mean.
Results and discussion
Quality assessment
The water quality data for sampling stations are summarized in Tables 1 and 2. A range of variation exists in the parameters of water. The data obtained by chemical analyses were evaluated in terms of suitability for domestic, agricultural, and industrial uses.
Suitability for domestic use
To assess the suitability for drinking and public health, the hydrochemical parameters of the groundwater were compared with the prescribed specification of WHO (1997), Indian standard for drinking water (BIS 1991, ISI 1983). Surface water data are not included in this section because it is not used for drinking and general domestic purposes in the study area. Table 3 shows that many of the parameters exceed the desirable limits of WHO (1997) and BIS (1991), though it is within the maximum permissible limit. The EC and concentration of TDS is more than the desirable limit of 750 μScm−1 and 500 mgL−1, respectively, in 13 and 60% of the total groundwater samples. The higher EC and TDS values may cause a gastrointestinal irritation in the consumers (Howard and Bartram 2003). The TH of the analyzed water samples varies between 53.5 and 250 mgL−1 (average 134.2 mgL−1) in groundwater samples indicating soft to hard types of water. Hardness of the water is attributable to the presence of alkaline minerals primarily Ca and Mg and sometimes bicarbonates. The data indicate that 80% of the samples have TH beyond the desirable limit of 100 mgL−1 for drinking water. Hardness has no known adverse effect on health, but it can prevent formation of lather and increase the boiling point of water. The high TH may cause encrustation on water supply distribution systems. There is some suggestive evidence that long-term consumption of extremely hard water might lead to an increased incidence of urolithiasis, anencephaly, parental mortality, some types of cancer, and cardio-vascular disorders (Durvey et al. 1991). The recommended limit for sodium concentration in drinking water is 200 mgL−1. A higher sodium intake may cause hypertension, congenial heart diseases, and kidney problems (Dahl 1960). Concentrations of sodium are within the prescribed limit of 200 mgL−1 in the analyzed groundwater samples. Concentration of Ca, Mg, and K is also found within the highest permissible limit. The contents of HCO3 and Cl have no known adverse health effects; however, it should not exceed the safe limit of 200 and 250 mgL−1, respectively for drinking water. The analytical data show that HCO3 exceeds the desirable limit in about 6% and Cl is below limit. Higher concentration of Cl in drinking water causes a salty taste and has a laxative effect in people not accustomed to it. Concentration of sulfate was found within the desirable limits of 200 mgL−1. Higher concentration of sulfate in drinking water is associated with respiratory problems (Maiti 1982; Subba Rao 1993). High sulfate concentration may have a laxative effect with excess of Mg in water. Sulfate may also cause corrosion of metals in the distribution system, particularly in waters having low alkalinity. High concentration of nitrate has undesirable effects when present in drinking water. High concentrations of nitrates can cause methemoglobinemia, gastric cancer, goiter, birth malformations, and hypertension (Majumdar and Gupta 2000). All groundwater samples have nitrate concentration below the recommended level of 45 mgL−1 (BIS 1991). Fluoride is an essential element for maintaining normal development of teeth and bones, but higher concentration causes fluorosis problems. Concentration of fluoride also was found to be below the permissible limits of 1.5 mgL−1.
The concentration of Fe ranges from 107.6 to 1,834 μgL−1 and that of Mn from 28 to 950 μgL−1 (Table 2). Some of the stations of groundwater, like A, G, H, and J reveal fairly high concentrations of Fe. As much as 7 out of 15 groundwater samples exceed the maximum permissible value of 300 μgL−1 (WHO 1997). For two samples Mn exceeds the WHO limit of 500 μgL−1. Higher Fe concentrations in the aquifers might have resulted from interaction of oxidized Fe minerals and organic matter and subsequent dissolution of Fe2CO3 at a comparatively lower pH. This type of water is clear when first drawn from the well, but soon becomes cloudy and then brown by the precipitation of Fe(OH)3, which is a common problem in some parts of the study area. The other reasons for the high concentration of this element may be the removal of dissolved oxygen by organic matter leading to reduced conditions. Under reducing conditions, the solubility of Fe bearing minerals (siderite, marcasite, etc.) increases leading to enrichment of dissolved iron in the groundwater (White et al. 1991; Applin and Zhao 1989). The concentrations of Cu varied from 5.8 to 33.5 μgL−1. All the samples are much below the maximum permissible WHO value of 2,000 μgL−1. The concentration of Zn varies between 97.5 to 327.7 μgL−1. Occurrence of Zn concentration in the groundwater might be indicative of leaching of this element through percolation into the groundwater from the overlying soil. Among the biologically important trace elements, Zn appears to have an important role next to Fe, since it is present in many enzymes necessary for metabolism of carbohydrates, lipids, and proteins.
Suitability for livestock
Water for livestock should be of high quality to prevent them from various diseases, salt imbalance, or poisoning by toxic constituents. Most of the water quality variables for livestock are same as for human drinking-water although the total permissible levels of total suspended solids and salinity may be higher. Irrigation canals, ponds, rivers, reservoirs, and groundwater may serve as water resources for livestock. The data in Ayers and Wescot (1985) indicate that water having salinity <1,500 mgL−1 and Mg <250 mgL−1 is suitable for drinking for most livestock. The water resources of the area meet these standards and are suitable for livestock. The common livestock in the area are cattle, goat, and chickens, whereas horses, sheep, and asses are also present. Excessive salinity in livestock drinking water can upset the animal’s water balance and cause death. High levels of salinity and specific ions like Mg in water can cause animal health problems and death. Environmental Studies Board (1972) offers upper limits for toxic substances in water and provides the guidelines for use of saline waters for livestock. Unsafe levels of salts and ions depend on the amount of water consumed each day and the weight of the animal.
Suitability for irrigation uses
Salinization is the major cause of loss of production and is one of the most prolific adverse environmental impacts associated with irrigation. Saline conditions severely restrict the choice of crops, adversely affect the crop germination and yields, and can pollute the soils. Careful management can reduce the rate of salinity build up and minimizes the effects on crops. Management strategies include: leaching, altering irrigation methods and schedules, installing sub-surface drainage, changing tillage techniques, adjusting crop patterns, and incorporating soil ameliorates. All such actions, which are costly, would require careful study to determine their local suitability. All evaluation regarding irrigation water quality should be linked to the evaluation of the soils to be irrigated (Ayers and Wescot 1985). Low-quality water for irrigation might be hazardous for clayey soils, while the same water could be used satisfactorily on sandy and/or permeable soils. The important hydrochemical properties of surface and subsurface water used to determine its suitability for irrigation are: EC and sodium percentage (Na%). EC and sodium concentration are very important in classifying irrigation water. The salts, besides affecting the growth of plants directly, also affect the soil structure, permeability, and aeration, which indirectly affect the growth of plant. The sodium percentage (Na%) in the water samples is calculated by the following equation:
The sodium percentage (Na%) in the area ranges between 16.12 and 70.55% (avg. 32.39%) in groundwater samples and 22.02–65.75% (avg. 34.9%) in surface water samples. A high sodium percent causes deflocculation and impairment of the tilth and permeability of soils (Karanth 1987). As per the Indian Standard (BIS 1991), maximum sodium recommended for irrigation water is 60%. The plot of analytical data on the Wilcox (1955) diagram, relating EC and sodium percent shows that the quality of water in study area is excellent to good. Only one sampling station of groundwater fall in the category of good to permissible and one in the permissible to doubtful quality. It suggests that waters may be used for irrigation purposes (Fig. 3).
Alkali and salinity hazard
The total concentration of soluble salts in irrigation water can be categorized as low (EC ≤ 250 μScm−1), medium (250–750 μScm−1), high (750–2,250 μScm−1), and very high (2,250–5,000 μScm−1). High salt concentration in water leads to formation of saline soil and high sodium concentration leads to development of an alkaline soil. Excessive solutes in irrigation water are common problem in semi-arid areas, where water loss through evaporation is high. Salinity problems are common where drainage is poor, and water table is close to the root zone of plants. The sodium salts accumulate in the soil through capillary rise and evaporation. The sodium or alkali hazard in the use of water for irrigation is determined by the absolute and relative concentration of cations and is expressed in terms of sodium adsorption ratio (SAR). It can be estimated by the formula:
There is a significant relationship between SAR values of irrigation water and the extent to which sodium is adsorbed by the soils. If water used for irrigation is high in sodium and low in calcium, the cation-exchange complex may become saturated with sodium. This can destroy the soil structure due to dispersion of clay particles. The calculated value of SAR in the study area ranges from 0.47 to 3.48 in surface water and 0.51–1.37 in groundwater samples. The plot of data on the US salinity diagram (USSL 1954), in which the EC is taken as salinity hazard and SAR as alkalinity hazard, shows that all of the water samples fall in the category C1S1 and C2S1, indicating low to medium salinity and low alkali water (Fig. 4). It can be used for irrigation for most soil and crops with less negative impact (Richards 1954; Fig. 4).
Residual sodium carbonate
The quantity of bicarbonate and carbonate in excess of alkaline sediments (Ca and Mg) also influence the suitability of water for irrigation purposes. When the sum of carbonates and bicarbonates is in excess of calcium and magnesium, there may be possibility of complete precipitation of Ca and Mg (Raghunath 1987). To quantify the effects of carbonate and bicarbonate, residual sodium carbonate (RSC) has been computed by the equation:
A high value of RSC in water leads to an increase in absorption of sodium in soil (Eaton 1950). Waters having RSC values greater than 5 meqL−1 are considered harmful to the growth of plants, while waters with RSC values above 2.5 meqL−1 are not considered suitable for irrigation purpose. In most of the analyzed water samples, RSC values are below 2.5 meqL−1; only one sample exceeds 2.5 meqL−1 limits. This indicates that water is suitable for irrigation uses.
Permeability index
Permeability is the ease with which water can flow into the medium. This parameter is very important for retaining the water at a station. Doneen (1964) classified irrigation waters based on the permeability index (PI). PI is defined by
As much as 76% of the water samples fall in Class-I and 20% in Class-II in the Doneen’s chart (Domenico and Schwartz 1990), implying that the water is of good quality for irrigation purposes with 75% or more of maximum permeability. However, one surface water sample belongs to Class-III and so unsuitable for the irrigation (Fig. 5).
Magnesium hazard
Magnesium constitutes about 2% of the Earth’s crust by mass, which makes it the eighth most abundant element in the crust. It is the 11th most abundant element by mass in the human body; its ions are essential to all living cells, where they play a major role in manipulating important biological polyphosphate compounds. Magnesium ions are essential for plant growth, but they may associate with soil aggregation and friability. Szabolcs and Darab (1964) proposed magnesium hazard (MH) value for irrigation water as:
MH > 50 is considered harmful and unsuitable for irrigation use. All groundwater samples and six surface water samples have MH > 50 and hence it is not suitable for irrigation. Plants have an additional use for magnesium in that chlorophylls are magnesium-centered porphyrins. Magnesium deficiency in plants causes late-season yellowing between leaf veins, especially in older leaves, and can be corrected by applying Epsom salts (which is rapidly leached), or else crushed dolomitic limestone to the soil.
Suitability for industrial use
The quality of water required for industries depends on the type and processes of industries. One useful parameter to assess quality of water for industrial purpose is the SI of minerals (Rhades and Bernstein 1971).
where IAP is the ion activity product, K sp is the solubility product at a given temperature.
The SI value is positive for calcite and dolomite for most of the stations, which indicate the possibility of precipitation (Table 4). The supersaturation restricts the safe use of water for industrial purpose, particularly in electrical power stations, industrial boiler houses, etc. The high values of TDS, hardness, and sulfate concentration at some stations also make this water unsafe for textiles, paper, and allied industries. To use this water for food industries disinfections and treatment should be done before use.
Chemical classification and relationships
The trilinear diagrams of Piper (1953) are useful in determining chemical relationships in groundwater. This method is more definite than other possible plotting methods (Walton 1970). Piper’s trilinear diagram method is also used to classify the surface and subsurface water on the basis of constituent ionic concentrations. The Piper’s diagram for the groundwater samples are shown in Fig. 6.
Hydrochemical facies
Two hydrochemical facies are identified from the Piper diagrams (Fig. 6).
-
Facies (I) Na + K–SO4 + Cl facies represents 13.33% of the total groundwater samples and 20% of total surface water samples. Its source is believed to be mainly by gypsum or anhydrite dissolution, as Na+ and SO42− are the main ions that form this facies.
-
Facies (II) Ca–Mg–HCO3 represents 33.33% of the total groundwater samples and 20% of total surface water samples. It is interpreted as a result of dissolution of carbonate minerals (calcite, dolomite, and aragonite).
-
No pair down facies represents none of the water samples, while the no pair up represents 53.33% of the total groundwater samples and 60% of the total surface water samples.
Ion-exchange processes
Dissolution of undesirable constituents in subsurface water cannot be controlled; however, it is important to know the changes undergone by water during surface runoff and infilteration (Pojasek 1977; Johnson 1979; Sastri 1994). The ion exchange between the groundwater and its environment during residence or percolation can be inferred by analyzing the chloro-alkaline indices (Schoeller 1965, 1967), i.e.
Na and K ions in water are exchanged with Mg and Ca ions. Positive values of CA indices indicate base-exchange reaction, whereas negative values indicate disequilibrium. The reaction is known as a cation–anion exchange reaction. CA indices value for the groundwater samples (Table 2) reveal that cation–anion exchange (chloro-alkaline disequilibrium) exists all over the area, except at seventh station, where the values are positive, indicating a base-exchange reaction (chloro-alkaline equilibrium). Groundwater with a base-exchange reaction in which the alkaline earths have been exchanged for sodium ions (HCO3 > Ca + Mg) may be referred to as base-exchange-soft water, and those in which the sodium ions have been exchanged for the alkaline earths (Ca + Mg > HCO3) may be referred to as base exchange hard water (Handa 1969). In the study area the alkaline earths have higher concentrations than bicarbonate, which indicates the exchange of sodium ions for alkaline earths, and the water as base-exchange hard water.
Water classification
Water samples can be divided into five divisions on the basis of the percentage of Na and the EC value (Wilcox 1955, Table 5). Based on the Na%, about 4% of water samples are within permissible limit, 8% are doubtful, 80% are good, and 8% are excellent. On the basis of EC, 8% of water samples are permissible and 56% are good. Richards (1954) classified water quality on the basis of sodium absorption ratio (SAR) as shown in Table 5. According to Richard’s classification, 100% of water samples are excellent. Stuyfzand (1989) classified water on the basis of Cl ion concentration into eight divisions as shown in Table 5. According to Stuyfzand, 28% of water samples are brackish, 20% are fresh brackish, 16% are fresh, and 36% are brackish-salt. USSL (1954) classification is based on the concentration of total dissolved solids as shown in Table 5. According to USSL classification, 36% of water samples showed total dissolved solids concentrations in the range of 500–1,500 mgL−1, 24% are in the range of 200–500 mgL−1, and 40% are in the range of <200 mgL−1. Eaton (1950) also classified water quality on the basis of percentage of Na in water. According to the Eaton classification, 92% of water samples are safe, while 8% are unsafe for use.
Downstream profiles of monitored parameters
The spatial variation in the chemical composition of river water is due to the minerals dissolved in it. However, the composition is modified by ion-exchange equilibria, precipitation of compounds during evaporation, mixing and dilution, the uptake and recycling of nutrient elements, exchange with the gasses of the atmosphere, and by discharge of municipal, agricultural, and industrial wastes (Bhardwaj et al. 2009). Conductivity, which is a good measure of the total dissolved solids (viz., Ca, Mg, Na, K, bicarbonate, sulfate, chloride, phosphate, etc.) fall within the maximum permissible limit for all the stations. The maximum value was observed at station SW-5. Stations near small catchment areas showed higher values of conductivity, which could be due to dilution by low runoff. Higher levels of ionic concentration during the season with low volume of water could be an indication of contribution from the urban waste as well as subsurface flow, which could be rich in ionic concentration. Total dissolved solids (TDS) indicate the general nature of water quality or salinity. TDS values were below the maximum permissible limit of 1,500 mgL−1. Locations near the maximum urbanization showed higher values, which indicate dilution with salt-free water. In case of other location urban runoff, and agricultural runoff passing through several wetlands in the study area might have reduced the dissolved load as the wetlands act as spongy filters and take up the ions present, thereby reducing the dissolved salts concentration. The total hardness values are below the maximum permissible value of 500 mgL−1, but most of the stations showed high values than maximum desirable value 100 mgL−1. Majority of the stations are observed to have total hardness values that exceed the desirable value of 100 mgL−1 and the maximum was for the station SW-3 (370 mgL−1), which is due to the presence of urban area. Chloride concentrations are below the desirable level of 250 mgL−1, maximum values being at station SW-3. Chloride penetrates into natural waters from soil, natural layers of salt, municipal, and industrial sewage. The points around SW-3, SW-6, and SW-9, which drain the urban area, show comparatively higher values (121, 60, and 85 mg L−1) that are indication of organic waste in higher quantities compared to other stations. Sulfate values are far below the permissible value of 600 mgL−1. Sampling near the urban areas, SW-6 and SW-9, show the maximum value. Source of increased levels of sulfate can also get into natural water due to chemical weathering of rocks and soil in the upstream and biochemical oxidation of sulfur compounds in the downstream. Sulfur is widely distributed in nature. In this region it is found in many minerals and ores, e.g., pyrites, gypsum, epsom salts, and also the fertilizers. Sulfur is a component of all living cells. The amino acids cysteine, methionine, homocysteine, and taurine contain sulfur as do some common enzymes; it is a component of most proteins. All metals except gold and platinum form sulfides and many ores are sulfides.
Analysis show that the nitrate concentration varies in the range of 14–38 mgL−1, which is below permissible value 50 mgL−1. Highest value of nitrate at station SW-6 is attributed to decaying organic matter and sewage water in the urban area. The concentration of sodium was found to be below the permissible limit of 200 mgL−1 for all the stations. The downstream increase in concentration indicates the anthropogenic contribution. Main source of potassium in natural water is similar to that of sodium. Concentration of potassium also shows a similar trend as that of sodium, with a maximum concentration of 116 mgL−1 at station SW-9, which is, however, below the maximum permissible level of 200 mgL−1.
Metal concentration show variability in downstream direction. Except Fe, none of the metals show higher values than WHO standards for river water. Variability of metals shows more or less similar trends. All metals show the influences of smaller catchment areas, urban areas, sewage sludge, municipal refuges and comparatively high organic matter presence.
Conclusions
The quality assessment of groundwater shows that in general, the water is suitable for domestic purposes. However, high values of EC, TDS, HCO3 −, Mg2+, Na+, and Fe at some stations make it unsafe for drinking and demand detailed regional groundwater investigation. Investigations of ground and surface water meet the standards for livestock, and show suitability for livestock consumption. Wilcox diagram, US salinity diagram, Permeability index and Residual Sodium Carbonate values indicate that the ground and surface water can be used for irrigation for most of the soil. However, many of the sampling stations show high vales of Magnesium hazard, which may indicate restricted use of water for irrigation. The saturation indices for dolomite, calcite, and aragonite demonstrate that most of the waters are supersaturated and hence not useful for industrial purposes. Downstream variability in chemical composition of river water is mainly governed by the land-use pattern and amalgamation of natural sources and anthropogenic activity near the river.
References
APHA (American Public Health Association) (1992) Standard methods for the examination of water and wastewater, 19th edn. Washington, DC
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater, 20th edn. Washington, DC
Applin KR, Zhao N (1989) The kinetics of Fe(II) oxidation and well screen encrustation. Groundwater 27:168–174
Ayers RS, Wescot DW (1985) Water quality for irrigation. FAO irrigation and drainage paper No. 20, Rev 1, FAO, Rome
Bhardwaj V, Singh DS, Singh AK (2009) Environmental repercussions of cane-sugar industrial sets of Chhoti Gandak river basin, Ganga plain, India. Environ Monit Assess. doi:10.1007/s10661-009-1281-2)
Bhardwaj V, Singh DS, Singh AK (2010) Hydrogeochemistry of groundwater and anthropogenic control over dolomitization reactions on alluvial sediments of the Deoria district—Ganga plain, India. Environ Earth Sci 59:1099–1109. doi:10.1007/s12665-009-0100-y
BIS (1991) Bureau of Indian Standards-Indian standard specification for drinking water IS: 10500
Bricker OP, Jones BF (1995) Main factors affecting the composition of natural waters. In: Salbu B, Steinnes E (eds) Trace elements in natural waters. CRC Press, Boca Raton, pp 1–5
Butler CD, Carlos FC, Koren HS (2005) Human health, well-being, and global ecological scenarios. Ecosystems 8:135–162
Dahl LK (1960) Possible role of salt intake in the development of essential hypertension. In: Cottier P, Bock KD (eds) Essential hypertension: an international symposium. Springer, Heidelberg, pp 53–65
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York
Doneen LD (1964) Notes on water quality in agriculture. Water science and engineering paper 4001. Department of Water Sciences and Engineering, University of California, California
Durvey VS, Sharma LL, Saini VP, Sharma BK (1991) Handbook on the methodology of water quality assessment. Rajasthan Agriculture University, India
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 39:123–133
Environmental Studies Board (1972) Water quality criteria. National Academy of Science, National Academy of Engineering
Galy A, France-Lanord C (1999) Weathering processes in the Ganga–Brahmaputra basin and the riverine alkalinity budget. Chem Geol 159(1–4):31–60
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Hall GEM (1998) Relative contamination levels observed in different types of bottles used to collect water samples. Explore 101:1–7
Handa BK (1969) Description and classification of media for hydrochemical investigations: In: Mithal RS, Singhal BBS (eds) Proceedings of groundwater studies in arid and semi arid regions. Department of Geology, University of Roorkee, India, pp 319–337
Howard G, Bartram J (2003) Domestic water quality, service level and health. World Health Organization, Geneva
Indian Meteorology Department data (2009). http://www.imd.gov.in
ISI (1983) Drinking water standards, Table-1. Substances and characteristics affecting the acceptability of water for domestic use 18, 10500. Indian Standard Institution, New Delhi
Johnson CC (1979) Land application of water—an accident waiting to happen. Groundwater 17(1):69–72
Karanth KR (1987) Groundwater assessment, development and management. Tata McGraw Hill, New Delhi
Kumar S, Singh IB, Singh M, Singh DS (1995) Depositional pattern in upland surface of Central Ganga Plain near Lucknow. J Geol Soc India 46:545–555
Maiti TC (1982) The dangerous acid rain. Sci Rep 9:360–363
Majumdar D, Gupta N (2000) Nitrate pollution of groundwater and associated human health disorders. Indian J Environ Health 42:28–39
Piper AM (1953) A graphic procedure in the chemical interpretation of water analysis. US Geological Survey Groundwater Note 12
Pojasek RB (1977) Drinking water quality enhancement through protection. Ann Arbor Science, Ann Arbor
Raghunath HM (1987) Groundwater. Wiley Eastern Ltd, Delhi
Rhades JD, Bernstein L (1971) Chemical physical and biological characteristics of irrigation and soil water. In: Ciaccio LL (ed) Water and water pollution. Marcel Dekker Inc, New York
Richards LA (1954) Diagnosis and improvement of saline and alkali soils, US Department of Agriculture, Hand Book, No. 60
Sargaonkar A, Deshpande V (2003) Development of an overall index of pollution for surface water based on a general classification scheme in Indian context. Environ Monit Assess 89:43–67
Sarin MM, Krishnaswami S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga–Brahamputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009
Sastri JCV (1994) Groundwater chemical quality in river basins. Hydrogeochemical modeling lecture notes. Refresher Course School of Earth Sciences, Bharathidasan University, Tiruchipalli
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development. UNESCO, pp 54–83
Schoeller H (1967) Geochemistry of groundwater. An international guide for research and practice. UNESCO, chap 15, pp 1–18
Singh DS, Singh IB (2005) Facies architecture of the Gandak Megafan, Ganga Plain, India. Special Publication of the Palaeontological Society of India, No. 2, pp 125–140
Singh IB, Srivastava P, Sharma S, Sharma M, Singh DS, Rajagopalan G, Shukla U (1999) Upland interfluve (Doab) deposition: alternative model to muddy overbank deposits. Facies 40:197–210
Singh DS, Awasthi A, Bhardwaj V (2009) Control of tectonics and climate on Chhoti Gandak River Basin, East Ganga Plain, India. Himalayan geology, vol 30, no 2, pp 147–154
Statistical Diary of Uttar Pradesh (2009) State Planning Institute, Uttar Pradesh, Lucknow, 295 pp
Stuyfzand PJ (1989) Nonpoint source of trace element in potable groundwaters in Netherland. In: Proceedings of the 18th TWSA Water Working, Testing and Research Institute. KIWA, Nieuwegein, The Netherlands
Subba Rao N (1993) Environmental impact of industrial effluents in groundwater regions of Visakhapatnam Industrial Complex. Indian J Geol 65:35–43
Subba Rao N (2008) Groundwater quality monitoring in an urban area for sustainable development. Environ Geosci 15(2):63–73
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils. In: Proceedings of 8th international congress of ISSS, Trans, vol II, pp 803–812
UNESCO (1995) A global geochemical database for environmental and resource management. Earth Science, vol 19. UNESCO Publication, Paris, 122 pp
USSL (1954) Diagnosis and improvement of saline and alkali soils. USDA Handbook 60:147
Walton WC (1970) Groundwater resource evaluation. McGraw-Hill, New York
White AF, Benson SM, Yee AW, Woolenberg HA, Flexser S (1991) Ground water contamination at the Kesterson reservoir, California—Geochemical parameters influencing selenium mobility. Water Resour Res 27:1085–1098
WHO (1997) Guidelines for drinking-water quality, vol 1, recommendations. World Health Organisation, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. US Department of Agriculture, Cir 969, Washington DC
Acknowledgments
The financial assistance from Department of Science and Technology, Government of India, New Delhi is highly acknowledged (Project No – SR/54/ES-21/Ganga Plain/P3). We are thankful to Prof. N.L. Chhabra, Head, Centre of Advanced Study in Geology, University of Lucknow, for providing the working facilities. The Directors of Central and State Ground Water Department are thanked for providing the subsurface data. People of Ropan Chhapra, Lar, Deoria district are thanked for logistic support during field work. Mr. Amit Awasthi is thanked for his help in the field.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhardwaj, V., Singh, D.S. Surface and groundwater quality characterization of Deoria District, Ganga Plain, India. Environ Earth Sci 63, 383–395 (2011). https://doi.org/10.1007/s12665-010-0709-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-010-0709-x