Abstract
In the light of progressive depletion of groundwater reservoir and water quality deterioration of the Neyveli basin, an investigation on dissolved major constituents in 25 groundwater samples was performed. The main objective was detection of processes for the geochemical assessment throughout the area. Neyveli aquifer is intensively inhabited during the last decenniums, leading to expansion of the residential and agricultural area. Besides semi-aridity, rapid social and economic development stimulates greater demand for water, which is gradually fulfilled by groundwater extraction. Groundwaters of the study area are characterized by the dominance of Na + K over Ca + Mg. HCO3 was found to be the dominant anion followed by Cl and SO4. High positive correlation was obtained among the following ions: Ca–Mg, Cl–Ca,Mg, Na–K, HCO3–H4SiO4, and F–K. The hydrochemical types in the area can be divided into two major groups: the first group includes mixed Ca–Mg–Cl and Ca–Cl types. The second group comprises mixed Ca–Na–HCO3 and Ca–HCO3 types. Most of the groundwater samples are within the permissible limit of WHO standard. Interpretation of data suggests that weathering, ion exchange reactions, and evaporation to some extent are the dominant factors that determine the major ionic composition in the study area.
تجريدي
في ضوء نضوب التدريجي لخزان المياه الجوفية وتدهور نوعية المياه في حوض Neyveli ، تحقيقا بشأن حل المكونات الرئيسية في عينات المياه الجوفية 25 قد أنجز. وكان الهدف الرئيسي من عمليات الكشف عن تقييم الجيوكيميائية في جميع أنحاء المنطقة. ويسكنها بشكل مكثف Neyveli طبقة المياه الجوفية خلال decenniums الماضي ، مما أدى إلى التوسع في المنطقة السكنية والزراعية. وبالاضافة الى الجفاف نصف النهائي ، سريع التنمية الاجتماعية والاقتصادية ويحفز زيادة الطلب على المياه ، التي تؤدى تدريجيا عن طريق استخراج المياه الجوفية. وتتميز المياه الجوفية في منطقة الدراسة عن طريق هيمنة نا + ك + الكالسيوم على المغنيسيوم. تم العثور على HCO3 أن تكون مهيمنة أنيون يليه الكلورين وSO4. تم الحصول على العلاقة الإيجابية بين السامية الأيونات التالية : الكالسيوم ، الماغنسيوم ، الكالسيوم ، الكلور ، الماغنسيوم ، الصوديوم ، البوتاسيوم ، HCO3 - H4SiO4 وكيه. ويمكن تقسيم أنواع الهيدروكيميائية في المنطقة إلى مجموعتين رئيسيتين : المجموعة الأولى تضم مختلطة الكالسيوم ، الماغنسيوم ، الكالسيوم وأنواع الكلور ، كلور. المجموعة الثانية تضم أنواع مختلطة الكالسيوم ، الصوديوم والكالسيوم ، HCO3 HCO3. معظم العينات الجوفية تقع ضمن الحد المسموح به من منظمة الصحة العالمية القياسية. تفسير البيانات تشير إلى أن العوامل الجوية التبادل الأيوني ، وردود الفعل والتبخر الى حد ما هي العوامل المسيطرة التي تحدد تركيبة الأيونية الرئيسية في منطقة الدراسة.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many areas, particularly in arid and semi-arid zones, groundwater quality limits to supply of potable fresh water. Hence, to utilize and protect valuable water resources effectively and predict the change in groundwater environments, it is necessary to understand the hydrochemical characteristics of the groundwater and its evolution under natural water circulation processes (Guendouz et al. 2003; Wen et al. 2005, 2008; Edmunds et al. 2006; Taheri Tizro and Voudouris 2008). In many coastal towns or cities, groundwater seems to be the only source of freshwater to meet domestic, agricultural, and industrial needs. However, groundwater is under constant threat of saline water intrusion, which seems to have become a worldwide concern (Amer 1995; Melloul and Goldenberg 1998; Ozler 2003; Terzic et al. 2008; Adepelumi et al. 2009).
The aquatic environment gets contaminated with a variety of pollutants generated from diverse sources (agriculture, industrial, and domestic). There has been tremendous increase in demand for fresh water due to population growth and intense agriculture activities. Land use for agricultural purpose in Neyveli region has increased alarmingly during the last decenniums. Variation of groundwater quality in an area is a function of physicochemical parameters that are greatly influenced by geological formations and anthropogenic activities. Rao (2006a) has studied the nitrate pollution and its distribution in the groundwater of Srikakulam District, Andhra Pradesh, India. Fianko et al. (2009) have studied the various sources of contaminants as well as assess the physical and chemical quality of the groundwater in Tema District, Ghana. Jalali (2010) has reported the major hydrogeochemical processes that control the observed groundwater chemistry in the Alisadr, Hamadan, western Iran. Earlier studies reported the importance of hydrogeochemical studies of groundwater in a particular region (Sikdar et al. 2001; Apodaca et al. 2002; Tesoriero et al. 2004; Moller et al. 2007).
Neyveli basin lies within one of the semi-arid areas in India representing the southeastern part of Tamilnadu state. Water resources in such areas are mainly derived from groundwater and infrequent surface runoff, where water conservation projects are applied. In Cuddalore district, groundwater is considered as a major source of fresh water that is used in different purposes. It is currently exploited for two main reasons: (1) Neyveli open cast mining of Lignite requires heavy pumping at the rate of 9,000–10,000 m3 h−1, as water table condition has to be brought down below the level of mining (Anandhan 2005). (2) Water from the Veeranam Lake is supplied through transmission line to Chennai City. To augment the supply during summer season, 48 deep bore wells were drilled, operations are done alternatively to pump the groundwater from deep aquifer, and the pumped water is connected to the New Veeranam Scheme pipelines (Prasanna et al. 2009).
The Neyveli basin has met the total water requirement of the region for agriculture, industry (thermal power plant), drinking water, and other miscellaneous uses. Pumping for strata depressurization has been a continuous process by Neyveli Lignite Corporation (NLC) for the last five decades. Detailed analysis of the pumping scenarios at the NLC mines revealed that optimal pumping for mining lies at around 170 (million cubic meters per annum) MCM/annum. Extraction from the Neyveli basin by NLC for depressurization of the mines and use by the thermal plants and the township is around 130 MCM/annum. Groundwater extractions by other agencies for purposes like irrigation, drinking water for towns and villages, and industrial use totals 110 MCM/annum (NLC 1970–2007). The latter represents the most important renewable water resource in Cuddalore district, which is used for irrigation and domestic purposes and drinking in many areas. The increasing exploitation due to farming frequently causes deterioration in water quality. Therefore, variations in natural and human activities reflect spatial variations in the hydrochemical parameters of the groundwater. The differences of dissolved ions concentration in groundwater are generally, governed by lithology, velocity and quantity of groundwater flow, nature of geochemical reactions, solubility of salts, and human activities (Karanth 1997; Bhatt and Salakani 1996). Appropriateness of quantity and quality of groundwater become a more crucial alternative resource to meet the drastic increase in social and agricultural development and to avoid the expected deterioration of groundwater quality due to heavy abstraction for miscellaneous uses.
Some of the previous studies carried out in Neyveli basin focused on groundwater control techniques for safe exploitation of the Neyveli Lignite deposit (Anandhan et al. 2010a), delineation of recharge area and artificial recharge studies (Anandan et al. 2010b), and groundwater basin management (Ravi Kumar et al. 2010). Hence, it is apparent that the hydrogeochemical processes that control the groundwater chemistry of the basin have not been studied in great deal. Hence, the present work was carried out with the major objective of identifying the major hydrogeochemical processes that are responsible for groundwater chemistry in the study area. Assessment of groundwater quality for various purposes was also carried out. This kind of investigation will help to create suitable management plans to protect aquifer as well as remedial measures for contaminated groundwater by natural and manmade activities.
Study area
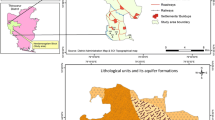
Neyveli basin is located in Cuddalore district of Tamilnadu, South India. The area is located between the geographical coordinates, latitudes of 11°21′–11°45′N and between longitudes of 79°30′–79°54′E (Fig. 1). The Neyveli hydrogeological basin is traversed with multiple aquifers and covers an area of about 3,500 km2. Relative humidity of the area was recorded in the eastern part of the district, i.e., at Cuddalore ranging between 60% and 83%, whereas in the western part of the district, i.e., at Lekkur ranging between 46.73% and 71.12%. Higher rates of relative humidity are observed during the months of northeast monsoon period, i.e., from October to November to December, whereas lower rates are observed during the summer months of April and May. The 70 years average annual rainfall of the district is 1,160 mm. More than 50% of the rainfall occurs during northeast Monsoon. About 30% of the rainfall was recorded during southeast monsoon and the balance from January to May (CGWB 1997). In urban area, the total population is 562,167, and rural population is 1,560,592. The density of population is 579 persons/km2.
The Neyveli area can be broadly divided into four physiographic divisions: two that are relatively high in the northwest and central portion, and the Gadilam-Ponniyar and Vellar-Manimuktha Nadhi alluvial plains. The northwestern high land lies in the western part of Neyveli passing between Senthanadu and Virdhachalam in a northeast–southwest direction. The elevation ranges between 30 and 100 m above mean sea level. The central high area stretches roughly north–northeast to south–southwest in the center of the Neyveli field (Ravi Kumar et al. 2010).
The general stratigraphy of the study area is shown in Table 1: the aquifer sands of this huge basin and the associated formations (including the lignite), which belong to the Upper Miocene age of the Tertiary era (Anandhan et al. 2010a). In the mines area, the typical stratigraphical sequence of the basin consists of an uppermost layer of 2–3 m of topsoil (lateritic loam) underlain by 40–50 m of argillaceous sandstone. The sandstone is underlain by a discontinuous seam of white sandy clay (1–2 m thick). The sandy clay is sometimes underlain by 2–10 m of semi-confined sand. Below this is the lignite seam, which ranges from 12 to 20 m in thickness. The lignite is generally underlain by a seam of ball clay 1–2 m thick. This is underlain by a confined sand formation that is about 400 m thick, which is the main aquifer of the basin. Clay seams ranging in thickness from 0.50 to 3.0 m occur within the aquifer formation.
The study area is underlined by the various geological formations ranging in age from Mio-pliocene to recent sediments. The lithology of the study area (Fig. 2) has two stratigraphic units as follows: (1) Tertiary Unit, argillaceous sandstone, pebble bearing grits, clays (variegated) with lignite seams, and pebble beds; and (2) Quarternary Unit, soils, alluvium and coastal sands, and laterite. There are three types of aquifers in this region, viz., phreatic aquifers, aquifer under semi-confined to confined conditions, and alluvial aquifers. Phreatic aquifers are found in the areas with the top Cuddalore sand stone. Along the river, shallow aquifers are available, which are also under water table conditions. Generally, thickness of the top Cuddalore sand stone extends up to a depth of about 100 m below ground level, where the alluvial formation along the river varies from 20 to 40 m below ground level (CGWB 1997). However, these aquifers are very shallow. The confined aquifers are deep in nature. These aquifers are available from 100 to 450 m below ground level. Generally, yield from the shallow aquifers are less than the deep aquifers.
Methodology
To assess the water chemistry, groundwater samples were collected during the period of February 2007 in the study area. Twenty-five samples of groundwater were collected from bore wells. After 15–20 min of pumping, water samples were collected in polyethylene bottles (1 L). Then, it was sealed and brought to the laboratory for analysis and stored properly (4°C) before analysis. The samples collected were analyzed for major cations like Ca and Mg by Titrimetry; Na and K by Flame photometer (CL 378); anions, Cl, and HCO3 by Titrimetry; SO4, PO4, and H4SiO4 by Spectrophotometer (SL 171 minispec); and nitrate and fluoride by ion electrodes. EC and pH were determined in the field using electrode (Eutech). The analyses were done by adopting standard procedures (APHA 1998). The analytical precision for the measurements of cations and anions is indicated by the ionic balance error, which was computed on the basis of ions expressed in milliequivalent per liter. The values were observed to be within a standard limit of ±5% (Domenico and Schwartz 1998). All concentration values were expressed in milligram per liter unless otherwise indicated. In this study, correlation analyses were applied using Statistical Package of Social Studies version 10.
Results and discussion
Major ions chemistry
Hydrochemical characteristics of groundwater in the study area are summarized in Table 2. The pH of groundwater varies within a range (6.6–8.1), which elaborates a trend of alkaline chemical reaction within the groundwater system. The EC varies from 354 to 1,596 μs/cm with an average of 827.7 μs/cm. Chemical data shows that 13 samples are fresh water (<500 μs/cm), while 12 samples are marginal waters (500–1,500 μs/cm) in the study area. The total dissolved solids (TDS) concentrations of the groundwater range from 247.8 to 1,117.2 mg/l with an average value of 571.17 mg/l. The concentrations of TDS in most groundwater samples are <1,000 mg/l, i.e., fresh water. The relative concentrations of the ions occur in the order of Na > Ca > K > Mg and HCO3 > Cl > SO4. The concentrations of major dissolved constituents were also quite variable. Such wide range of ionic concentrations also indicates the involvement of several hydrochemical processes influencing the water quality. Correlation between ions was carried out using correlation coefficient analysis (Chidambaram et al. 2007). Table 3 shows the correlation coefficients between the major ions in groundwater. High positive correlation (>0.5) has been obtained between Ca–Mg, Cl–Ca,Mg, Na–K, HCO3–H4SiO4, and F–K, indicating the predominance of chemical weathering along with leaching of secondary salts. SO4 and NO3 develop poor correlation with all the ions, indicating the anthropogenic impact from agricultural practices.
Groundwater types
Ionic concentrations were plotted in a piper diagram to evaluate the geochemical characteristics of the sampled groundwater. The projection of the points in the central diamond-shaped revealed the heterogeneity of the groundwater chemical composition in this area. On the basis of this diagram (Fig. 3), it is clear that in all the waters, alkali earth elements (Ca + Mg) are higher than alkali elements (Na + K). The abundance of the alkali earth elements is attributed to dissolution of Ca and Mg-rich silicate minerals in the aquifer matrix. The removal of Ca, through the precipitation of carbonate minerals, may cause a disturbance in chemical equilibrium, resulting in the dissolution of minerals containing calcium such as calcite and gypsum (Freeze and Cherry 1979). These minerals precipitated from irrigated water under the influence of high rate of evaporation and evapotranspiration. Weathering of rocks results in the release of sodium and potassium from K-feldspars and plagioclase into the environment, even though potassium has low mobility and remains associated with clay minerals. On the other hand, the position of the groundwater in the anions triangle indicates dominance of the Cl and HCO3, but a complete absence of sulfate in the waters. The distribution of points in the cations triangle reveals that majority of samples do not categorize in any dominant cation type.
As a consequence, two types of groundwater can be chemically distinguished (Fig. 3). In the first group, the position of data represents mixed Ca–Mg–Cl and Ca–Cl types. Cl is the major anion, and Ca + Mg are major cations in this groundwater. This facies is characterized by low concentration of HCO3, and relatively higher concentration of Cl and Ca, which are mainly distributed among the marine sediments, occurs in the intermediate zone of groundwater discharge area.
In the second group, the position of data represents mixed Ca–Na–HCO3 and Ca–HCO3 types. HCO3 is the major anion, and Ca is the major cation in this groundwater. This type of water occurs during rapid flow through times, which results in low ionic concentrations during high recharge in shallow zones. Na and HCO3 concentrations come mainly from weathering of alkali feldspars from rocks related with the recharge areas.
Geochemical evolution
The results from the chemical analyses were used to identify the geochemical processes and mechanisms in the Neyveli basin. The Na–Cl relationship has often been used to identify the mechanisms to acquire salinity and saline intrusions in semi-arid/arid regions (Sami 1992). In general, evaporation causes an increase in concentrations of all species in water. If the evaporation process is dominant, and no minerals species are precipitated, the Na–Cl ratio is unchanged (Jankowski and Acworth 1997). Hence, the plot Na/Cl versus Cl would give a horizontal line, which would indicate concentration by evaporation and transpiration (Fig. 4a). If halite dissolution is responsible for sodium, the Na/Cl molar ratio is approximately 1, whereas a ratio >1 is typically interpreted that Na is released from a silicate weathering reaction (Meybeck 1987). In the present study area, it generally ranges from 0.3 to 2.1 with an average of 0.8. When Na is plotted against Cl (Fig. 4b), most of the samples that lie below the 1:1 trend line show excess Cl, highlighting additional geochemical process. The high Na/Cl ratios are probably controlled by water–rock interaction, most likely by feldspar weathering. The high K/Cl ratios (>0.2) are associated with the dissolution of K-feldspar that suggests its weathering (Fig 4c).
The study of the Ca/Mg ratio of groundwater from this area suggests that greater calcite contribution (Maya and Loucks 1995), that is, if the ratio of Ca/Mg = 1, dissolution of dolomite should occur, whereas a higher ratio is indicative of greater calcite contribution. Katz et al. (1998) also explained that the higher Ca/Mg molar ratio (>2) is indicative of dissolution of silicate minerals, which contribute calcium and magnesium to groundwater. The groundwater samples have a ratio between 1 and 2, indicating the dissolution of calcite (Fig. 4d). The molar ratio is generally less than or equal to 1 in the study area, and some of them have this ratio more than 1, which indicates that the dissolutions of dolomite and calcite are the dominant geochemical processes followed by the dissolution of silicate minerals.
Calcium and magnesium are the dominant cations present in groundwater next to sodium in this region. Similarly, bicarbonate is also present in considerable amounts. Carbonate-rich rocks such as crystalline limestone, dolomitic limestone, calc-granulite, and kankar (lime-rich weathered mantle overlies carbonate rocks) are the major sources for carbonate weathering. The available carbonates in these rocks might have been dissolved and added to the groundwater system during irrigation, rainfall infiltration, and groundwater movement.
In Ca + Mg versus HCO3 + SO4 scatter diagram (Fig. 4e), the points falling along the equiline (Ca + Mg = HCO3 + SO4) suggest that these ions have resulted from weathering of carbonates and sulfate minerals (Datta and Tyagi 1996). Moreover, if the Ca and Mg solely originated from carbonate and silicate weathering, these should be balanced by the alkalinity alone. However, most of the points are placed in the Ca + Mg side, which indicates excess calcium and magnesium derived from other process such as reverse ion exchange reactions. The plot of Ca + Mg versus Cl (Fig. 4f) indicates that concentrations of the two items are roughly the same, as Ca and Mg do not increase with increasing salinity that could be the indication of reverse ion exchange in the clay/weathered layer.
The Ca + Mg/HCO3 ratio is used to explain the sources of Ca and Mg in groundwater. As this ratio increases with salinity, Mg and Ca are added to solution at a greater rate than HCO3. If Mg and Ca only originate from the dissolution of carbonates in the aquifer materials and from the dissolution of carbonates in the aquifer materials and from the weathering of accessory minerals, this ratio would be about 0.5 (Sami 1992). The plot of Ca + Mg versus HCO3 in Fig. 4g shows that most of the data fall above the 1:1 trend line, although some points approach this line. The low Ca + Mg/HCO3 ratio (<0.5) could be the result of either (Ca + Mg) depletion by cation exchange or HCO3 enrichment (Fig. 4h). However, high ratios cannot be attributed to HCO3 depletion; under the existing alkaline conditions, HCO3 does not form carbonic acid (H2CO3) (Spears 1986). High ratio suggests that an excess of alkalinity in these waters is balanced by the alkalis Na and K.
Molar Na/Ca ratios vary between 0.4 and 6, and some of them are of more than 2 (Fig. 4i). This indicated the reaction of silicate minerals and/or some cation exchange is occurring at the expense of some other cation (Wen et al. 2005).
Mechanisms controlling the groundwater chemistry
Reactions between groundwater and aquifer minerals have a significant role on water quality, which are also useful to understand the genesis of groundwater (Cederstorm 1946). Groundwater chemistry in the study region is regulated by diverse processes and mechanisms. Since the study region experiences dry and semi-arid climatic condition, evaporation may also contribute in water chemistry in the study region. Hence, Gibbs plot is employed in this study to understand and differentiate the influences of rock–water interaction, evaporation, and precipitation on water chemistry (Gibbs 1970). Figure 5 illustrates that most of the groundwater samples fall in the water–rock interaction field and few samples plotted on evaporation zone, which suggests that the weathering of rocks primarily controls the major ion chemistry of groundwater in this region.
Evaporation
Evaporation process is not only a common phenomenon in surface water but also in groundwater system. Na/Cl ratio can be used to identify the evaporation process in groundwater. Evaporation will increase the concentration of TDS in groundwater, and the Na/Cl ratio remains the same, and it is one of the good indicative factors of evaporation. If evaporation is the dominant process, Na/Cl ratio should be constant when EC rises (Jankowski and Acworth 1997). The EC versus Na/Cl scatter diagram of the groundwater samples (Fig. 6) shows that the trend line is inclined, and Na/Cl ratio decreases with increasing salinity (EC) which seems to be the removal of sodium by ion exchange reaction. This observation indicates that evaporation may not be the major geochemical process controlling the chemistry of groundwater in this study region or ion exchange reaction dominating over evaporation. However, the Gibbs diagrams (Fig. 5) justify that evaporation is not a dominant process in this basin.
Ion exchange
Ion exchange is one of the important processes responsible for the concentration of ions in groundwater. Chloro-alkaline indices 1 and 2 (CAI 1 and CAI 2) calculated for the groundwater samples of the basin strongly suggest the occurrence of ion exchange process.
All values are expressed in meq/l
When there is an exchange between Ca or Mg in the groundwater with Na and K in the aquifer material, both the above indices are negative, and if there is a reverse ion exchange, then both these indices will be positive (Schoeller 1965, 1967). CAI 1 and CAI 2 values of the study area vary with respect to samples, but they are positive in most of the samples, and few samples show negative values. This observation indicates that reverse ion exchange is the dominant process in the groundwater, whereas normal ion exchange is also noticed in very few wells during the study period.
Groundwater salinization
Since the study area fall near to the coastal zone, intensively pumped coastal aquifers lead to possible salinization in the groundwater. Figure 7 shows that Na, Ca, and Mg are enriched when compared to its ratio with Cl in seawater. The Na enrichment observed in Fig. 6a demonstrates that Na–Ca exchange, which is common in coastal aquifers intruded by seawater (Andersen et al. 2005), depleting Na content, is not the main process occurring in this area. In that case, the enrichment observed for Ca (Fig. 7b) is probably due to calcite dissolution, followed by an increasing pH (CaCO3 + H Ca + HCO3). The lowest enrichment was observed for SO4 (Fig. 7d), which may be related to the sulfate reduction process (Appelo and Postman 2005).
Groundwater quality
Both for drinking as well as irrigation purposes, substantial amount of groundwater is being used in this area. Further agriculture is economically important in the study area and mostly covered by agricultural lands and residents. Hence, groundwater contamination by irrigation return flow, fertilizer and farm manure application, leaching of soil-mineralized nitrogen, domestic sewage, etc. is also an important issue on groundwater contamination in the study area. The concentration of nitrate and chloride in the groundwater justifies the effect of surface contamination sources. The nitrate concentration varies from 1 to 67.9 mg/l with an average of 18.9 mg/l. However, four samples exceed WHO (2004) standard and are not fit for drinking, which may cause blue baby disease/methemoglobinemia in children and gastric carcinomas (Comly 1945; Gilly et al. 1984). Since most of the basin is intensively irrigated, higher concentration of nitrate in groundwater can be resulted from the agricultural activities (Subramani et al. 2005a). In Neyveli basin, most of the samples are within the permissible limits (WHO 2004) for all parameters except K. Higher concentration of K also implies the effect of surface contamination, especially irrigation return flow to the groundwater.
Saturation index (dissolution and deposition)
Using the saturation index (SI) approach, it is possible to predict the subsurface mineralogy from groundwater data without collecting the samples of the solid phase and analyzing the mineralogy (Deutsch 1997). In the present study area, to determine the chemical equilibrium between minerals and waters, saturation indices of carbonate minerals were calculated using the following equation (Lloyd and Heathcode 1985):
where IAP is the ion activity product and Ks is the solubility product of the minerals.
The calculated values of SI for calcite, dolomite, aragonite, and magnesite of the groundwater samples show that the calcite and aragonite fall near saturated to oversaturated state, indicating precipitation takes place (Fig. 8). Given the semi-arid climate, this is likely to be a result of evaporation. However, the SI values of dolomite and magnesite are less than zero, which shows that it remains under saturation.
Summary and conclusions
The results of this investigation provide an outline of the geochemical processes controlling the groundwater chemistry in the study area. The hydrochemical types in the area can be divided into two major groups. The first group includes mixed Ca–Mg–Cl and Ca–Cl types, indicating the intermediate zone of groundwater discharge area. The second group comprises mixed Ca–Na–HCO3 and Ca–HCO3 types, revealing the groundwater recharge area. The concentrations of TDS in most groundwater samples are <1,000 mg/l, i.e., fresh water. The relative concentrations of the ions occur in the order of Na > Ca > K > Mg and HCO3 > Cl > SO4. In the study area, rock weathering and ion exchange reactions are the major hydrogeochemical processes responsible for the concentration of major ions in groundwater. Higher concentration of nitrate in some groundwater can be resulted from the agricultural activities. Generally, most of the samples in the basin are within the permissible limits for all parameters except K. Calcite and aragonite have high SI values, which indicates precipitation as the result of evaporation, whereas dolomite and magnesite show undersaturation state. The information presented in this paper will be useful for sustainable management of this important groundwater resource according to the water chemistry and enables planners and policymakers to evolve a strategy to solve similar problems elsewhere.
References
Adepelumi AA, Ako BD, Ajayi TR, Afolabi O, Omotoso EJ (2009) Delineation of saltwater intrusion into the freshwater aquifer of Lekki Peninsula, Lagos, Nigeria. Environ Geol 56:927–933
Amer AM (1995) Saltwater intrusion in coastal aquifers. Water Resour Manag 2:521–529
Anandan KS, Sahay SN, Karthikeyan S (2010b) Delineation of recharge area and artificial recharge studies in the Neyveli hydrogeological basin. Mine Water Environ 29:14–22
Anandhan P (2005) Hydrogeochemical studies in and around Neyveli mining region, Tamilnadu, India. Ph.D Thesis, Department of Earth Sciences, Annamalai University, India, p 189
Anandhan KS, Sahay SN, Ramabadran TK, Shiv Prasad S (2010a) Groundwater control techniques for safe exploitation of the Neyveli Lignite deposit, Cuddalore District, Tamilnadu, India. Mine Water Environ 29:3–13
Andersen MS, Nyvang V, Jakobsen R, Postma D (2005) Geochemical processes and solute transport at the seawater/freshwater interface of a sand aquifer. Geochim Cosmochim Acta 69:3979–3994
APHA (1998) Standard methods for the examination of water and waste water, 19th edn. American Public Health Association, Washington
Apodaca LE, Jeffrey BB, Michelle CS (2002) Water quality in shallow alluvial aquifers, Upper Colorado River Basin, Colorodo. J Am Water Resour Assoc 38:133–143
Appelo CAJ, Postman D (2005) Geochemistry. Groundwater and pollution, 2nd edn. Balkema, Rotterdam, p 649
Bhatt KB, Salakani S (1996) Hydrogeochemistry of the upper Ganges river, India. J Geol Soc India 48:171–182
Cederstorm DJ (1946) Genesis of groundwater in the coastal plain of Virginia. Environ Geol 41:218–245
Centre for Groundwater Board (CGWB) (1997) Groundwater resources and development prospects in South Arcot-Vallalar District, Tamilnadu. South Eastern Coastal Region, Chennai (in press)
Chidambaram S, Ramanathan AL, Prasanna MV, Anandhan P, Srinivasamoorthy K, Vasudevan S (2007) Identification of hydrogeochemically active regimes in groundwaters of erode district, Tamilnadu-A statistical approach. Asian J Water Environ Pollut 5(3):93–102
Comly HH (1945) Cyanosis in infants caused by nitrates in well water. J Am Med Assoc 129:12–114
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater regime. J Geol Soc India 47:179–188
Deutsch WJ (1997) Groundwater geochemistry: fundamentals and application to contamination. CRC, Boca Raton
Domenico PA, Schwartz FW (1998) Physical and chemical hydrogeology, 2nd edn. Wiley, New York, p 506
Edmunds WM, Ma JZ, Aeschbach-Hertig W, Kipfer R, Darbyshire DPF (2006) Groundwater recharge history and hydrogeochemical evolution in the Minqin basin, North west China. Appl Geochem 21:2148–2170
Fianko JR, Nartey Vincent K, Donkor A (2009) The hydrochemistry of groundwater in rural communities within the Tema District, Ghana. Environ Monit Assess. doi:10.1007/s10661-009-1125-0
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall Inc, Englewood Cliffs, p 553
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci J 170:795–840
Gilly G, Corrao G, Favilli S (1984) Concentrations of nitrates in drinking water and incidence of gastric carcinomas. First descriptive study of the Piemonate Region, Italy. Sci Total Environ 34:35–37
Guendouz A, Moulla AS, Edmunda WM, Zouari K, Shand P, Mamou A (2003) Hydrogeochemical and isotopic evolution of water in the complex terminal aquifer in the Algerian Sahara. Hydrogeol J 11:483–495
Jalali M (2010) Groundwater geochemistry in the Alisadr, Hamadan, western Iran. Environ Monit Assess 166:359–369
Jankowski J, Acworth RI (1997) Impact of depris-flow deposits on hydrogeochemical processes and the development of dryland salinity in the Yass River catchment, New South Wales. Aust Hydrogeol J 5(4):71–88
Karanth KB (1997) Groundwater assessment, development and management. McGraw-Hill Publishers, New Delhi
Katz BG, Gopalan TB, Bullen TD, Davis JH (1998) Use of chemical and isotopic tracers to characterize the interaction between groundwater and surface water in mantled karst. Groundwat J 35:1014–1028
Lloyd JW, Heathcode JA (1985) Nature inorganic hydrochemistry in relation to groundwater. Oxford University Press, New York
Maya AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the Central Wasatch Range, Utah. J Hydrol 172:31–59
Melloul LC, Goldenberg AJ (1998) Early-indicator signals of groundwater contamination: the case of seawater encroachment. Environ Geol 33(4):279–288
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Moller P, Rosenthal E, Geyer S, Guttman J, Dulski P, Rybakov M (2007) Hydrochemical processes in the lower Jordan valley and in the Dead Sea area. Chem Geol 239:27–49
NLC (1970–2007) Various unpublished in-house technical reports on groundwater modeling of the Neyveli Basin. Neyveli Lignite Corporation Ltd, Neyveli
Ozler HM (2003) Hydrochemistry and salt-water intrusion in the Van aquifer, East Turkey. Environ Geol 43:759–775
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2009) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90
Rao NS (2006) Nitrate pollution and its distribution in the groundwater of Srikakulam district, Andhra Pradesh, India. Environ Geol 49:413–429
Ravi Kumar V, Sahay SN, Periasamy SP, Karthikeyan S (2010) Groundwater basin management at the Neyveli Lignite Mines. Mine Water Environ 29:23–28
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J Hydrol 139:27–48
Schoeller H (1965) Qualitative evaluation of groundwater resources. In methods and techniques of groundwater investigations and development. UNESCO, Paris, pp 54–83
Schoeller H (1967) Geochemistry of groundwater-an international guide for research and practice, Chapter 15. UNESCO, Paris, pp 1–18
Sikdar PK, Sarkar SS, Palcoudhury S (2001) Geochemical evolution of groundwater in the quaternary aquifer of Calcutta and Howrah, India. J Asian Earth Sci 19:579–594
Spears DA (1986) Mineralogical control of the chemical evolution of groundwater. In: Trudgill ST (ed) Solute processes. Wiley, Chichester, p 512
Subramani T, Elango L, Damodaraswamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River basin, Tamilnadu, India. J Environ Geol 47:1099–1110
Taheri Tizro A, Voudouris KS (2008) Groundwater quality in the semi-arid region of the Chahardouly basin, West Iran. Hydrol Process 22:3066–3078
Terzic J, Markovic T, Pekas Z (2008) Influence of sea-water intrusion and agricultural production on the Blato Aquifer, Island of Korcula, Croatia. Environ Geol 54:719–729
Tesoriero AJ, Spruill TB, Eimers L (2004) Geochemistry of shallow groundwater in coastal plain environments in the southeastern United States: implication for aquifer susceptibility. Appl Geochem 19:1471–1482
Wen X, Wu Y, Su J, Zhang Y, Liu F (2005) Hydrochemical characteristics and salinity of groundwater in the Ejina basin, northwestern China. Environ Geol 48:665–675
Wen XH, Wu YQ, Wu J (2008) Hydrochemical characteristics of groundwater in the Zhangye basin, northwestern China. Environ Geol 55:1713–1724
WHO (World Health Organization) (2004) Guidelines for drinking water quality, vol 1, 3rd edn. WHO, Geneva, p 515
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasanna, M.V., Chidambaram, S., Senthil Kumar, G. et al. Hydrogeochemical assessment of groundwater in Neyveli Basin, Cuddalore District, South India. Arab J Geosci 4, 319–330 (2011). https://doi.org/10.1007/s12517-010-0191-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-010-0191-5