Abstract
In the management of water resources, quality of water is just as important as its quantity. In order to know the quality and/or suitability of groundwater for domestic and irrigation in upper Gunjanaeru River basin, 51 water samples in post-monsoon and 46 in pre-monsoon seasons were collected and analyzed for various parameters. Geological units are alluvium, shale and quartzite. Based on the analytical results, chemical indices like percent sodium, sodium adsorption ratio, residual sodium carbonate, permeability index (PI) and chloroalkaline indices were calculated. The pre-monsoon waters have low sodium hazard as compared to post-monsoon season. Residual sodium carbonate values revealed that one sample is not suitable in both the seasons for irrigation purposes due the occurrence of alkaline white patches and low permeability of the soil. PI values of both seasons revealed that the ground waters are generally suitable for irrigation. The positive values of Chloroalkaline indices in post-monsoon (80%) and in pre-monsoon (59%) water samples indicate absence of base-exchange reaction (chloroalkaline disequilibrium), and remaining samples of negative values of the ratios indicate base-exchange reaction (chloroalkaline equilibrium). Chadha rectangular diagram for geochemical classification and hydrochemical processes of groundwater for both seasons indicates that most of waters are Ca–Mg–HCO3 type. Assessment of water samples from various methods indicated that majority of the water samples in both seasons are suitable for different purposes except at Yanadipalle (sample no. 8) that requires precautionary measures. The overall quality of groundwater in post-monsoon season in all chemical constituents is on the higher side due to dissolution of surface pollutants during the infiltration and percolation of rainwater and at few places due to agricultural and domestic activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent times, there has been a tremendous increase in demand for fresh water due to population growth and intense agricultural activities. The hydrogeologic factors controlling recharge and hydrogeochemical reactions are so important for the chemical constituents to reach the groundwater uses. Land use for agricultural purpose in Gunjanaeru River basin has increased alarmingly during the last two decades. Scant attention has been paid so far on groundwater quality of the study area. The upper Gunjaneru catchment is formed by four ephemeral streams and lies between north latitudes 13°43′45′′ and 14°1′44′′ and east longitudes 79°15′16′′ and 79°27′38′′. The climate of the river basin is hot and semi-arid. Shales of Cuddapah formation with recent alluvial deposits mostly cover the study area. The shale is mainly composed of silt and clay with calcareous and argillaceous cementing material. In the absence of any perennial rivers, infiltration during the period of precipitation is the only source of recharge to the shale aquifers.
One of the most interesting aspects of hydrochemistry is the occurrence of water bodies with different water chemistries in very close proximity to each other. This has been variously attributed to the surface and subsurface geology (Stallard and Edmand 1983), small climatic differences (Petrovic 1980) and the flow direction (Lyons et al. 1992). Water quality assessment in Akpabuyo, Southeastern Nigeria indicated that the waters are acidic, soft and characterized by low sodium adsorption ratio, and the waters are also classified into four chemical facies: Ca–Cl, Na–Cl, Ca–SO4 and Ca–HCO3 (Offiong and Edet 1998). Studies conducted on the chemical quality of groundwater of Mangalore City in Karnataka State and Madras in Tamil Nadu, India revealed that the groundwater quality has been deteriorated due to overexploitation of groundwater (Narayana and Suresh 1989; Ramesh et al. 1995). Sreedevi (2004) has studied the groundwater quality of Pageru River basin in Cuddapah district and the assessment of water samples from various methods proved that majority of the water samples are good either for drinking or for agriculture in post- and pre-monsoon seasons.
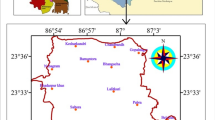
In order to study the quality variations of the groundwater in the upper Gunjaneru River basin 51 samples during post-monsoon and 46 samples during pre-monsoon season are collected (Janardhana Raju 1991) from dug wells in the vicinity of heavily cultivated agricultural land, dug wells in densely populated area and some abandoned wells (Fig. 1). These were analyzed for different geochemical constituents using standard methods of analysis (Hem 1985). In this paper an attempt has been made to evaluate the quality indices of groundwater to understand the hydrogeochemical relationships of the water quality parameters for the suitability of groundwater resources. There is no documented evidence of the chemical quality of the groundwater in this basin. In view of this, an extensive survey has been conducted in order to know the quality of water for domestic, irrigation and industrial uses. The author has referred several aspects of chemical data interpretation and discussed the main features of groundwater quality classifications for domestic and irrigation purposes.
Results and discussion
The classical use of water analyses in groundwater hydrology is to produce information concerning the water quality. The water quality may yield information about the environments through which the water has circulated. The chemical indices derived from hydrogeochemical parameters of post- and pre-monsoon groundwater samples of upper Gunjanaeru River basin are presented in Table 1. The total dissolved solids (TDS) estimated by residue on evaporation method range from 87 to 1,126 mg/l with a mean of 444 mg/l and from 95 to 1,009 mg/l with a mean of 375 mg/l for post- and pre-monsoon water samples, respectively. The increase of TDS in post-monsoon season is on the higher side than the pre-monsoon season due to mixing of surface pollutants during the infiltration and percolation of rainwater. Salts, which held back in the interstice or pores in clay/shale while groundwater is evaporated or water table falls, get leached back to the groundwater during the rainy period. Hence the post-monsoon waters have higher TDS levels when compared to pre-monsoon season. The analytical results have been evaluated to ascertain the suitability of groundwater of the study area for human consumption, by comparing with the specifications of TDS set by the WHO (1984). According to WHO specification TDS up to 500 mg/l is highest desirable and up to 1,000 mg/l is maximum permissible. Based on this classification it indicates that 65% of samples and 85% belong to highest desirable category and remaining samples belong to maximum permissible category in both post- and pre-monsoon seasons, respectively. Davis and De Wiest (1966) have also classified ground waters on the basis of TDS, up to 500 mg/l (desirable for drinking); 500–1,000 mg/l (permissible for drinking) and up to 3,000 mg/l (useful for agricultural purposes). Based on his classification, it is observed that out of 51 samples collected 33 are desirable for drinking, 16 are permissible for drinking, and out of 46 samples collected 39 are desirable for drinking, 6 samples are permissible for drinking and all are suitable for agricultural purposes in post- and pre-monsoon, respectively.
The electrical conductivity (EC) ranges from 150 to 1,850 μmhos/cm at 25°C with a mean of 752 μmhos/cm at 25°C and 140–1,515 μmhos/cm at 25°C with a mean of 550 μmhos/cm at 25°C for post- and pre-monsoon seasons, respectively. The US Salinity Laboratory (1954) classified ground waters on the basis of electrical conductivity (Table 2). Based on this classification, 2 and 4 of samples belongs to the excellent category, 28 and 35 belongs to good category, 21 and 7 belongs to fair category for post- and pre-monsoon seasons, respectively. Day and Nightingale (1984) reported the value of multiplying factor as 0.527 to 0.570 for ground waters of Fresnol Wisalie and Bakersfield in USA. Janardhana Raju et al. (1990) have reported the multiplying factor for post-monsoon water samples is 0.58 for upper Gunjanaeru River basin, Cuddapah district in Andhra Pradesh, India. The multiplying factor is 0.65 for pre-monsoon samples of upper Gunjaneru River basin. The differences between the values may reflect the wide variation in the activities and processes prevailing in the surface and subsurface environments (Amadi and Amadi 1990).
Sodium content
Sodium concentration is important in classifying irrigation water because sodium reacts with soil to reduce its permeability. Sodium content is usually expressed in terms of percent sodium or soluble-sodium percentage (%Na). As per the Indian Standards, maximum of 60% sodium is permissible for irrigation water (Table 1). The pre-monsoon waters have low sodium hazard as compared to post-monsoon season in the upper Gunjaneru River basin (Janardhana Raju et al. 1992). Wilcox (1948) proposed a method for rating irrigation waters to be used, based on percent sodium and electrical conductivity. The diagram consists of five distinct areas such as excellent to good, good to permissible, permissible to doubtful, doubtful to unsuitable and unsuitable. The percent sodium is obtained by the equation
where all ionic concentrations are expressed in epm.
Wilcox diagram has revealed that out of 51 samples 31 fall under excellent to good, 19 fall under good to permissible and one under permissible to doubtful, and out of 46 samples 40 fall under excellent to good, 5 fall under good to permissible and one under permissible to doubtful for post- and pre-monsoon seasons (Fig. 2), respectively. The agricultural yields are observed to be generally low in lands irrigated with waters belonging to permissible to doubtful category. This is probably due to the presence of sodium salts, which cause osmotic effects in soil plant system. While a high salt content (high EC) in water leads to formation of saline soil, high sodium content (SAR) leads to development of an alkaline soil. The sodium or alkali hazard in the use of water for irrigation is expressed by determining the sodium adsorption ration (SAR) and it can be estimated by the formula
where all ionic concentrations are expressed in epm.
Rating of groundwater samples on the basis of electrical conductivity and percent sodium (after Wilcox 1948)
As per the Richards (1954) classification based on SAR values (Table 2), 51 samples in post-monsoon and 46 samples in pre-monsoon are excellent category because none of the samples exceeded the value of SAR = 10 (Table 1). SAR can indicate the degree to which irrigation water tends to enter into cation-exchange reactions in soil. Sodium replacing adsorbed calcium and magnesium is a hazard as it causes damage to the soil structure and becomes compact and impervious.
Residual sodium carbonate (RSC) has been calculated to determine the hazardous effect of carbonate and bicarbonate on the quality of water for agricultural purpose (Eaton 1950) and has been determined by the formula:
where concentration of cations and anions are expressed in epm.
The US Salinity Laboratory Diagram (1954) proposed that waters containing more than 2.5 meq/l of RSC are not suited for irrigation, while those having 1.25–2.5 meq/l are marginally suitable and those with less than 1.25 meq/l are probably safe for irrigation. From the residual sodium carbonate values of the study area, it is observed that out of 51 post-monsoon water samples, 48 samples are safe, 2 samples are marginal and 1 sample is not suitable for irritation. But out of 46 pre-monsoon water samples, 42 samples are safe, 1 sample is marginally suitable and 3 are not suitable for irrigation. The RSC in ground waters of post- and pre-monsoon varies from 0 to 13.14 meq/l and from 0 to 11.42 meq/l, respectively. From these analyses, it is found that the sample no. 8 only is not suitable in both the seasons. This is clearly established by the field studies of the occurrence of alkaline white patches and of the low permeability of the soil. The samples 5 and 13 during pre-monsoon are found to be unsuitable, even though the water table is occurring at greater depths. The poor quality of water in these two wells is due to localized pollution. Hence, continued usage of high residual sodium carbonate waters affects the yields of crop.
Permeability index (PI)
The soil permeability is affected by long-term use of irrigation water and is influenced by sodium, calcium, magnesium and bicarbonate contents of the soil. Doneen (1964) has evolved a criterion for assessing the suitability of water for irrigation based on PI (Table 1), and PI has been calculated by using the formula
where all the ions are expressed in epm.
According to PI values, the ground waters of both seasons in the study area can be designated as class II (25–75%) excepting the five samples in post-monsoon and seven samples in pre-monsoon seasons, which classified as class I (>75%). As suggested by Raghunath (1982), on the basis of US salinity diagram and Doneen’s chart the groundwater in the study area is in general suitable for irrigation purposes.
Chloroalkaline indices (CAI)
Knowledge of the changes brought about in the chemical composition of the groundwater during its travel underground is essential (Sastri 1994). Control on the dissolution of undesirable constituents in waters is impossible during the subsurface run off but it is essential to know the various changes undergone by waters during the travel (Johnson 1979). The ion exchange between the groundwater and its host environment during residence or travel can be understood by studying the chloroalkaline indices: CAI-I = [Cl − (Na + K)]/Cl and CAI-II = [Cl − (Na + K)]/(SO4 + HCO3 + CO3 + NO3) (Schoeller 1967). CAI are negative when there is exchange between sodium and potassium (Na + K) in water with calcium and magnesium (Ca + Mg) in rocks. If the ratio is positive, then there is no base exchange. CAI (Table 1) indicate that 41 (80%) and 27 (59%) water samples show positive ratios in the post- and pre-monsoon, respectively, while 10 (20%) and 19 (41%) of the respective seasons belong to negative ratios depicting the type of base exchange. The positive values are indicating absence of base exchange and the reaction as a cation–anion exchange reaction. The negative values of the ratio indicate base exchange between sodium and potassium in water with calcium and magnesium in the rocks.
Chemical relationships
Hydrochemical diagrams are aimed at facilitating interpretation of evolutionary trends, particularly in groundwater systems, when they are interpreted in conjunction with distribution maps and hydrochemical sections. Chadha (1999) has proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. The proposed diagram is a modification of Hill Piper diagram with a view to extend its applicability in representing water analysis in the simplest way possible. In the proposed diagram (Fig. 3), the difference in milliequivalent percentage between alkaline earths (Ca + Mg) and alkali metals (Na + K), expressed as percentage reacting values, is plotted on the x-axis, and the difference in milliequivalent percentage between weak acidic anions (CO3 + HCO3) and strong acidic anions (Cl + SO4) is plotted on the y-axis. The milliequivalent percentage differences between alkaline earths and alkali metals, and between weak acidic anions and strong acidic anions, would plot in one of the four possible sub-fields of the rectangle diagram. The rectangular field describes the overall character of the water. In order to define the primary character of water, the rectangular field is divided into eight sub-fields, each of which represents a water type: (1) alkaline earths exceed alkali metals; (2) alkali metals exceed alkaline earths; (3) weak acidic anions exceed strong acidic anions; (4) strong acidic anions exceed weak acidic anions; (5) alkaline earths and weak acidic anions exceed both alkali metals and strong acidic anions, respectively; such water has temporary hardness; (6) alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions; such water has permanent hardness; (7) alkali metals exceed alkaline earths and strong acidic anions exceed weak acidic anions; (8) alkali metals exceed alkaline earths and weak acidic anions exceed strong acidic anions.
Diagram showing geochemical classification and hydrochemical parameters of groundwater (after Chadha 1999)
Results of analyses were plotted on the proposed diagram to test its applicability for geochemical classification of groundwater (Table 3) and to study hydrochemical processes of post- and pre-monsoon are shown in Fig. 3. From the Table 3, it is evident that majority of samples in post-monsoon (73%) and pre-monsoon (78%) seasons fall under the subdivision of alkaline earths and weak acidic anions that exceed both alkali metals and strong acidic anions (Ca2+–Mg2+–HCO− 3 type); such waters have temporary hardness. Eighteen percent of post-monsoon and 16% of pre-monsoon samples fall under the subdivision of alkaline earths exceeding alkali metals and strong acidic anions exceeding weak acidic anions (Ca2+–Mg2+–Cl− type), such waters have permanent hardness and do not deposit RSC in irrigation use. Four percent of the water samples in post-monsoon fall under the subdivision of Na+–Cl− type; such waters generally create salinity problems both in irrigation and domestic uses. Remaining 4% of water samples of both the seasons fall under the category of Na+–HCO −3 type; such waters deposit residual sodium carbonate in irrigation and cause foaming problems in domestic use. The water quality data are plotted on an anion–cation balance control chart for assessing the accuracy of results (Fig. 4). The anionic sum in milliequivalents per liter should equal the cationic sum in milliequivalents per liter. The solution should be electrically neutral, although they are seldom equal in practice. This inequality increases as the ion concentration increases.
Conclusions
The water quality data from the study area for both the seasons revealed that all the samples except one in Yanadipalle (no. 8) are suitable for various purposes. Based on TDS, out of 51 samples collected in post-monsoon, 33 are desirable for drinking, 16 are permissible for drinking and all are suitable for agricultural purposes; in pre-monsoon, out of 46 samples collected 39 are desirable for drinking, 6 samples are permissible for drinking, and all are suitable for agricultural purposes. Irrigation waters classified based on SAR has indicated that both post- and pre-monsoon are excellent. Wilcox classification has revealed that most of the samples fall under the excellent to permissible category and one sample fall under the permissible to doubtful category for both post- and pre-monsoon seasons. As per the PI values, the ground waters of both seasons are classified as good to excellent. The positive values of chloroalkaline indices are indicating as a cation–anion exchange reaction and negative values indicating that the host rocks are primary sources of dissolved solids in the water. Distribution of the groundwater samples in different subdivisions of rectangular diagram reveals that about 75% of the groundwater samples in both seasons fall under the calcium–magnesium–bicarbonate category (such water has temporary hardness) and remaining samples fall under the calcium–magnesium–chloride (such water has permanent hardness). The seasonal variation in groundwater quality is due to agricultural and domestic activities through infiltration and percolation during monsoon. The overall quality of groundwater in upper Gunjanaeru River basin is controlled by lithology apart from other local environmental conditions.
References
Amadi UMP, Amadi PA (1990) Salt water migration in the coastal aquifers of southern Nigeria. J Min Geol 26:35–44
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Davis SN, De Wiest RJM (1966) Hydrogeology, vol 463. Wiley, New York
Day BA, Nightingale HI (1984) Relationship between groundwater silica, total dissolved solids and specific electrical conductivity. Groundwater 22(1):80–85
Doneen LD (1964) Notes on water quality in Agriculture. Published as a Water Science and Engineering Paper 4001, Department of Water Sciences and Engineering, University of California
Eaton FM (1950) Significance of carbonate in irrigation water. Soil Sci 69(2):123–133
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. US Geol Surv Water Supply Paper—2254, 264
Janardhana Raju N (1991) Hydrogeology of the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, India. Unpublished PhD Thesis, SV University, Tirupati, 177p
Janardhana Raju N, Reddy TVK, Reddy SS, Nayudu PT (1990) Regression models between total dissolved solids (TDS), silica (SiO2) and specific electrical conductivity (SEC) for well waters of upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh. Indian Water Works Assoc XXII(1):123–126
Janardhana Raju N, Reddy TVK, Kotaiah B, Nayudu PT (1992) A study on seasonal variations of groundwater quality in upper Gunjanaeru River basin, Cuddapah district, Andhra Pradesh. Fresenius Envir Bull 1:98–103
Johnson CC (1979) Land application of Waste—an accident waiting to happen. Groundwater 17(1):69–72
Lyons W, Lent RM, Djukic N, Maletin S, Pujin V, Carey AE (1992) Geochemistry of surface waters of Vojvodina, Yugoslavia. J Hydrol 137:33–55
Narayana A, Suresh G (1989) Chemical quality of groundwater of Mangalore City, Karnataka. Indian J Environ Health 31(3):228–236
Offiong OE, Edet AE (1998) Water quality assessment in Akpabuyo, Cross River basin, South-Eastern Nigeria. Environ Geol 34:167–174
Petrovic G (1980) Physo-chemical aspects of alkaline ponds in Yugoslavia. In: Dokuli SM, Metz M, Jewson D (eds) Development in Hydrobiology, pp 89–95
Raghunath HM (1982) Groundwater, vol 456. Wiley Eastern Limited, New Delhi
Ramesh R, Shiv Kumar K, Eswaramoorthy S, Purvaja GR (1995) Migration and contamination of major and trace elements in groundwater of Madras City, India. Environ Geol 25:126–136
Richards LA (1954) Diagnosis and improvement of saline alkali soils: Agriculture, vol 160. Handbook 60, US Department of Agriculture, Washington DC
Sastri JCV (1994) Groundwater chemical quality in river basins, hydrogeochemical facies and hydrogeochemical modeling. Lecture notes—refresher course conducted by school of Earth Sciences. Bharathidasan University, Thiruchirapalli, Tamil Nadu, India
Schoeller H (1967) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigation and development. Water Research, Series-33, UNESCO, pp 44–52
Sreedevi PD (2004) Groundwater quality of Pageru River basin, Cuddapah District, Andhra Pradesh. J Geol Soc India 64:619–636
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon 2. The influence of geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
US Salinity Laboratory (1954) Diagnosis and improvement of saline and alkali soils. Agricultural Handbook No. 60. USDA, 160 p
WHO (1984) Guidelines for drinking water quality, vol 1. Recommendations, World Health Organisation, Geneva, 130 p
Wilcox LV (1948) The quality of water for irrigation use, vol 40. US Department of Agriculture Technology Bulletin 962, Washington DC
Acknowledgments
The author is highly thankful to the Alexander von Humboldt Foundation, Germany, for equipment donation for carrying out the research studies of this kind. Author is also grateful to Prof. T.V. Krishna Reddy, Department of Geology, S.V. University, Tirupati, for his help and valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raju, N.J. Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ Geol 52, 1067–1074 (2007). https://doi.org/10.1007/s00254-006-0546-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0546-0