Abstract
Organelle genome diversity was analysed in interspecific potato somatic hybrids using chloroplast (cp)- and mitochondrial (mt)-specific molecular markers. Out of total 25 markers (15 cpDNA and 10 mtDNA) tested in total 16 samples, only four mtDNA primers (rpS14/cob, Nsm2, ALM4/ALM5 and ALM6/ALM7) detected polymorphism, whereas other primers were monomorphic. Cluster analysis showed higher genetic diversity among the genotypes by mtDNA profiles than that by cpDNA. Ten haplotypes were grouped by cluster analysis comprised of maximum seven genotypes in haplotype no. 3. Monomorphic markers did not reveal variability in our samples and suggest highly conserved organelle genomic regions. New genomic arrangements were observed in the somatic hybrids for mt polymorphic loci. Our study suggests that somatic hybrids are comprised of diverse cytoplasm types consisting predominantly of T-, W-, and C-, with a few A- and S-type chloroplast, and α-, β- and γ-type mitochondrial genome, and have unique potential to widen the cultivated potato gene pool by breeding methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic hybridization has been used extensively for interspecific gene transfer across the sexual barriers in potato [14, 17, 18]. In contrast to sexual crosses, somatic (protoplast) fusion can induce genetic variability within cytoplasmic organelle genomes [chloroplast (cp) and mitochondrial (mt) DNA] and novel cytoplasmic–nuclear interactions [10]. Considering the importance of organelle genomes, their analysis is immensely useful for introgression of desirable agronomic and/or stress (biotic and abiotic) response traits. In sexual crosses, organelle genomes are normally maternally inherited and exhibit very low mutation rates as compared to nuclear genome [4]. In potato, five basic cpDNA types (A, C, S, T and W) and mtDNA types (α, β, γ, δ and ε) and thus five main cytoplasm types (cp/mt), i.e. W/α, T/β, W/γ, W/δ and S/ε, have been identified [10]. Importantly, the T-type chloroplast coexists with the β-type mitochondrial genome, while mt types α, γ and δ were found in combination with W-type chloroplast in wild species; and mt type ε was found in A- and S-types in cultivated species [11]. In general, most cultivated Chilean potato (Solanum tuberosum ssp. tuberosum) has T-type chloroplast and β-type mitochondrial genomes (hence referred to T/β cytoplasm), which is lacking in Andean potato (S. tuberosum ssp. andigena) and wild species. Moreover, the T/β cytoplasm is predominant in the common potato, so sterility problem is unavoidable when T/β cytoplasm is present [11].
The wild Solanum species represent a reservoir of genes for genetic improvement and have been used for both sexual and somatic hybridization. For example, S. tuberosum ssp. andigena has wide diversity, and tuberosum (female) × andigena (male) cross results in increased heterotic vigour and yield but high male sterility, while reciprocal cross is male fertile indicating an important role of cytoplasm in potato breeding [6]. Previous researchers have investigated the recombinant cytoplasmic genome types in the cultivated gene pool by introgression from wild species through somatic hybridization and highlighted their importance for better characterization of genetic resources and utilization in potato breeding. Cytoplasm types have been analysed earlier by cpDNA and/or mtDNA markers in potato somatic hybrids, for example, S. tuberosum (+) S. etuberosum [17], S. bulbocastanum (+) S. tuberosum [2, 9] and Solanum spp. [1, 13, 15].
The aim of this study was to analyse organelle genome diversity using chloroplast and mitochondrial genome-specific primers in our previously developed interspecific potato somatic hybrids. These somatic hybrids were produced between the dihaploid of cultivated Solanum tuberosum ‘C-13’ and the diploid wild species S. pinnatisectum via protoplast fusion for late blight resistance to widen the cultivated potato gene pool.
Materials and Methods
Plant Materials
In the present study, 14 interspecific potato somatic hybrids (P1, P2, P3, P4, P5, P6, P7, P8, P9, P10, P11, P12, P13 and P14) and two parents (S. tuberosum dihaploid parent ‘C-13’ and diploid wild species S. pinnatisectum) developed earlier [14] were used. The late blight-resistant potato somatic hybrids were developed at the Cell and Molecular Biology Laboratory, Division of Crop Improvement, Central Potato Research Institute, Shimla, India. In vitro propagated plants were used for plastome and chondriome genome analyses.
DNA Analysis
Leaf samples from in vitro propagated plants were used for genomic DNA isolation using DNeasy Plant DNA extraction kit (Qiagen). DNA quality and quantity were determined with NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, USA). Genomic DNA was used in PCR amplification using cpDNA- and mtDNA-specific primer pairs, amplified genomic regions at annealing temperature (Ta) as detailed in Table 3. The polymerase chain reaction (PCR) was performed in a Mastercycler Gradient (Eppendorf, Hamburg, Germany) in a total volume of 25 µl and consisted of 50 ng DNA templates in 1 × PCR buffer, 2.5 mM MgCl2, 200 µM dNTP, 0.5 µM of primer, 1 unit Taq polymerase (Qiagen). The PCR procedure included: 5 min at 94 °C followed by 33 cycles of 1 min at 94 °C, 1 min at Ta and 1 min at 72 °C, with a final extension of 7 min at 72 °C. Gel electrophoresis and documentation were followed as described in Tiwari et al. [17]. SSR type cp- and mtDNA primers were analysed on ‘3500 Genetic Analyzer’ (Applied Biosystems, California, USA). Fragment analysis of SSR data was performed using GeneMapper® Software version 4.1 (ABI). A 500-bp ‘GS 500 ROX’ standard was used to estimate the molecular size of the fragments.
Data Analysis
All reactions were repeated at least twice, and only distinct, reproducible and well-resolved bands across the run were considered for analysis. A data matrix was constructed on the basis of the presence (1) or absence (0) of bands of the amplified DNA fragments. Missing data were scored as ‘9’. In the SSR fragment analyses, higher peak intensity (≥1000) and band size (≥100 bp) were considered for analysis, which were scorable. Number of alleles, allele size, frequencies and polymorphic information content (PIC) of each marker were calculated for 16 samples. The PIC of each SSR marker was calculated according to the formula: PIC = 1 − ∑(Pi 2), where Pi is the frequency of the ith allele of a marker detected in all accessions. Genetic diversity analysis was performed with the program NTSYS-PC 2.21 [12]. A similarity matrix was calculated by Jaccard’s similarity coefficient, and the dendrogram was generated using unweighted pair group method with arithmetic mean (UPGMA) clustering method.
Results
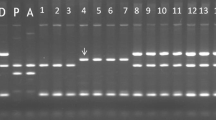
In the present study, organelle genome diversity was analysed in potato somatic hybrids using cpDNA and mtDNA-specific markers. Detected polymorphism, number of alleles, allelic absolute frequencies and PIC values of all 25 markers amplified in total 16 samples are presented in Table 1. Fifteen cpDNA markers resulted into one to two scorable bands per primer and generated total of 28 monomorphic bands ranging from 120 to 900 bp, whereas 10 mtDNA markers amplified one to five scorable bands per primer in the samples with total of 24 bands ranging from 146 to 3000 bp, from which 16 mtDNA bands were polymorphic (Tables 2, 3). The PIC value for markers rpS14/cob, NSm2, ALM4/ALM5 and ALM6/ALM7 was 0.65, 0.93, 0.67 and 0.80, respectively, whereas other markers were monomorphic. Based on the classification of cytoplasm types as given in Table 4, somatic hybrids showed five chloroplast types (A, C, S, T and W) and three mitochondrial types (α, β and γ). In particular, amplification patterns of the ALC1/ALC3, ALM1/ALM3, ALM4/ALM5 and ALM6/ALM7 markers showed mixed cytoplasm types (W/α, W/γ and T/β) in the somatic hybrids. Monomorphic loci indicated the presence of conserved organelle genomic regions in the hybrids. To illustrate, organelle genome profiles of the hybrids using selected polymorphic mtDNA markers ALM4/ALM5 and ALM6/ALM7 are shown in Fig. 1. Moreover, amplification product of SSR type mtDNA primers A14-1 and T11-2 in the hybrids, for example P1, analysed on the ‘3500 Genetic Analyser’ (ABI) is shown in Fig. 2.
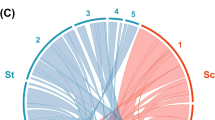
Cluster analysis based on the Jaccard’s similarity coefficient values of cp- and mtDNA primers amplified in the samples could be grouped into 10 different haplotypes (Fig. 3). Each haplotype comprising genotype(s) was characterized by similar amplification patterns in which only one haplotype no. 3 was comprised of seven genotypes (P3, P5, P6, P7, P13, P8 and P9) and other haplotypes had single genotype. Analysis revealed that among the haplotype no. 3 included genotypes comprised of common genomic regions, viz. rps14/cob, NSm2 (rps14–rps10), ALM4/ALM5 (cob and rps10) and ALM6/ALM7 (cob) (Table 2).
Discussion
Variations among the chloroplast and mitochondrial genomic regions using organelle-specific primers (cpDNA and mtNDA) were detected among the somatic hybrids. Out of total 25 markers (15 cpDNA and 10 mtDNA), only mtDNA primers detected the polymorphism in the hybrids. Based on the classification of cytoplasm types using mitochondrial and chloroplast types, somatic hybrids had five chloroplast types (A, C, S, T and W), three mitochondrial types (α, β and γ) and overall mixed cytoplasm types (W/α, W/γ and T/β). Our amplification patterns of cpDNA and mtDNA markers matched with the earlier reports [3, 7, 8, 15, 17]. In many plant species, a high genetic diversity in mtDNA profiles has been known to that in cpDNA, which is explained by slow evolution and low mutation rates of mtDNA [8]. The difference between cytoplasmic genomes variability depends on their various mechanisms of molecular evolution, coding and non-coding regions, and patterns of transmission to progenies. The known mtDNA and cpDNA markers were characterized earlier in wild and cultivated potatoes [15, 17]. The genomic region analysed was previously found to be involved in the rearrangement of the potato plastome and chondriome in somatic hybrids [9, 10].
Past studies highlighted the narrow genetic base of modern Indian potato cultivars predominantly T/β type with a few W/α and A/ε cytoplasm types [5], and also German varieties had mt types β, α and γ [11]. Breeding constraints imposed by the pollen sterility and the T/β-type cytoplasm in potato may suggest the need for a change in breeding practices involving more diverse material, either from within the cultivated gene pool or from wild species [10]. Therefore, researchers have highlighted the importance of cytoplasmic diversity and stressed the need to increase levels of cytoplasmic variability found in the modern potato gene pool from wild sources. Present study shows that somatic hybrids possess diverse cytoplasm predominantly of T/β, W/α and W/γ types. In another study, Hosaka and Sanetomo [8] showed phylogenetic relationship of the cultivated potatoes and closely related wild Solanum species using cpDNA and mtDNA markers. Further, Scotti et al. [15] studied evolutionary patterns by mitochondrial DNA variation in cultivated and wild potato species and highlighted importance of mtDNA for better characterization of potato genetic resources. Organelle genomes diversity analysis in our potato somatic hybrids revealed divergence among the genotypes. Somatic hybrids derived from wild and cultivated potato species have potential to widen the cultivated gene pool by using as parental lines in breeding. Germplasm analysis with more polymorphic markers, high-throughput fragment analysis and genome sequencing can allow a more efficient selection of cytoplasmic donors and the characterization of hybrids providing a novel useful tool to enlarge the gene pool of cultivated potato.
References
Ames M, Spooner DM (2008) DNA from herbarium specimens settles a controversy about origins of the European potato. Am J Bot 95:252–257
Aversano R, Savarese S, De Nova JM, Frusciante L, Punzo M, Carputo D (2009) Genetic stability at nuclear and plastid DNA level in regenerated plants of Solanum species and hybrids. Euphytica 165:353–361
Bryan GJ, Mcnicoll J, Ramsay G, Meyer RC, De Jong WS (1999) Polymorphic simple sequence repeat markers in chloroplast genomes of Solanaceous plants. Theor Appl Genet 99:859–867
Cardi T, Bastia T, Monti L, Earle ED (1999) Organelle DNA and male fertility variation in Solanum spp. and interspecific somatic hybrids. Theor Appl Genet 99:819–828
Chimote VP, Chakrabarti SK, Pattanayak D, Pandey SK, Naik PS (2008) Molecular analysis of cytoplasm type in Indian potato varieties. Euphytica 162:69–80
Grun P (1990) The evolution of cultivated potatoes. Econ Bot 44(suppl):39–55
Hosaka K (2003) T-type chloroplast DNA in Solanum tuberosum L. ssp. tuberosum was conferred from some populations of S. tarijense Hawkes. Am J Potato Res 80:21–32
Hosaka K, Sanetomo R (2009) Comparative differentiation in mitochondrial and chloroplast DNA among cultivated potatoes and closely related wild species. Genes Genet Syst 84:371–378
Iovene M, Savarese S, Cardi T, Fruscinate L, Scotti N, Simon PW, Carputo D (2007) Nuclear and cytoplasmic genome composition of Solanum bulbocastanum (+) S. tuberosum somatic hybrids. Genome 50:443–450
Lössl A, Adler N, Horn R, Frei U, Wenzel G (1999) Chondriome-type characterization of potato: mt α, β, γ, δ, ε and novel plastid-mitochondrial configurations in somatic hybrids. Theor Appl Genet 98:1–10
Lössl A, Götz M, Braun A, Wenzel G (2000) Molecular markers for cytoplasm in potato: male sterility and contribution of different plastid-mitochondrial configurations to starch production. Euphytica 116:221–230
Rohlf F (2006) NTSYSpc: numerical taxonomy system (ver. 2.2). Exeter Publishing, Ltd, Setauket
Sanetomo R, Hosaka K (2011) A maternally inherited DNA marker, descended from Solanum demissum (2n = 6x = 72) to S. tuberosum (2n = 4x = 48). Breed Sci 61:426–434
Sarkar D, Tiwari JK, Shruti Sharma, Poonam Sharma Sanjeev, Gopal J, Singh BP, Luthra SK, Pandey SK, Pattanayak D (2011) Production and characterization of somatic hybrids between Solanum tuberosum L. and S. pinnatisectum Dun. Plant Cell Tissue Organ Cult 107:427–440
Scotti N, Cozzolino S, Cardi T (2007) Mitochondrial DNA variation in cultivated and wild potato species (Solanum spp.). Genome 50:706–713
Sukhotu T, Hosaka K (2004) Origin and evolution of Andean potatoes revealed by chloroplast and nuclear DNA markers. Genome 49:636–647
Tiwari JK, Chandel P, Singh BP, Bhardwaj V (2014) Analysis of plastome and chondriome genome types in potato somatic hybrids of Solanum tuberosum (+) S. etuberosum. Genome 57:1–7
Tiwari JK, Poonam Sarkar D, Pandey SK, Gopal J, Kumar SR (2010) Molecular and morphological characterization of somatic hybrids between Solanum tuberosum L. and S. etuberosum Lindl. Plant Cell Tissue Organ Cult 103:175–187
Acknowledgments
The authors are grateful to the ICAR-Central Potato Research Institute, Shimla, and the DBT, Government of India (BT/PR4291/AGR/2/838/2011), for providing financial supports and necessary facilities. Hearty thanks are extended to Mr. Seeshram Thakur for in vitro maintenance of somatic hybrids plants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Authors contribution
J.K. Tiwari, S. Devi, P. Chandel and N. Ali have performed the original research work. J.K. Tiwari has planned the work, analysed the data and prepared the manuscript. V. Bhardwaj and B.P. Singh have contributed in language correction of the manuscript.
Rights and permissions
About this article
Cite this article
Tiwari, J.K., Devi, S., Chandel, P. et al. Organelle Genome Analysis in Somatic Hybrids Between Solanum tuberosum and S. pinnatisectum Revealed Diverse Cytoplasm Type in Potato. Agric Res 5, 22–28 (2016). https://doi.org/10.1007/s40003-015-0197-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-015-0197-z