Abstract
Interspecific potato somatic hybrids between Solanum tuberosum L. (di)haploid C-13 and 1 endosperm balance number non-tuberous wild species S. etuberosum Lindl. were produced by protoplasts electrofusion. The objective was to transfer virus resistance from this wild species into the cultivated potatoes. Post-fusion products were cultured in VKM medium followed by regeneration of calli in MS13 K medium at 20°C under a 16-h photoperiod, and regenerants were multiplied on MS medium. Twenty-one somatic hybrids were confirmed by RAPD, SSR and cytoplasm (chloroplast/mitochondria) type analysis possessing species-specific diagnostic bands of corresponding parents. Tetraploid nature of these somatic hybrids was determined through flow cytometry analysis. Somatic hybrids showed intermediate phenotypes (plant, leaves and floral morphology) to their parents in glass-house grown plants. All the somatic hybrids were male-fertile. ELISA assay of somatic hybrids after artificial inoculation of Potato virus Y (PVY) infection reveals high PVY resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The wild Solanum species of the cultivated potato (S. tuberosum L.) are an important source of resistance for various biotic and abiotic stresses (Helgeson and Haberlach 1999). Their introgression into potato using classical breeding methods is time-consuming and may lead to difficulties due to various sorts of sexual incompatibilities and particularly differences in the ploidy level or in the endosperm balance number (EBN) (Johnston et al. 1980; Jackson and Hanneman 1999). Therefore, somatic hybridization is one of the techniques used to overcome these limitations. Somatic hybridization can provide new opportunities for producing pre-breeding materials with increased genetic variability and transferring desirable agronomic traits into cultivated potatoes. In comparison to other parallel techniques of chromosomal and genetic engineering, it has a unique potential to transfer simultaneously both nuclear and cytoplasmic genes (Zhou et al. 2001). It bypasses the gene segregation and enables the transfer of both mono- and polygenic traits among the sexually incompatible species (Thieme et al. 1997, 2004; Gavrilenko et al. 2003). Symmetric protoplasts fusion between potato and S. brevidens was used to integrate virus and aphids resistance (Valkonen et al. 1992a, b; Polgar et al. 1999). In many experiments, somatic hybrids have been produced between cultivated potato and wild species such as S. tarnii (Thieme et al. 2008), S. etuberosum (Novy and Helgeson 1994a, b; Chavez et al. 1988; Gavrilenko et al. 2003; Novy et al. 2002, 2007; Gillen and Novy 2007; Thompson et al. 2007), S. pinnatisectum (Szczerbakowa et al. 2005; Greplova et al. 2008), S. bulbocastanum (Szczerbakowa et al. 2001; Greplova et al. 2008), S. berthaultii (Bidani et al. 2007), S. nigrum (Szczerbakowa et al. 2003; Zimnoch-Guzowska et al. 2003), S. circaeifolium (Oberwalder et al. 2000), and S. acaule (Rokka et al. 1998) for transferring various biotic and abiotic resistance traits. In future, because of the continuous threat of phytopathogens, the introduction of new resistant germplasm to increase genetic diversity of potato gene pool is of vital need. Therefore, development of more interspecific potato somatic hybrids would provide a broad spectrum of resistant potato lines against major diseases such as late blight and viruses.
The S. etuberosum (1 EBN; Solanum sect. Etuberosum) is characterized as a diploid wild, native to Chile, non-tuberous species having E-genome distinct from A-genome of tuber-bearing cultivated potato S. tuberosum (4 EBN; Solanum sect. Petota) (Spooner and Hijmans 2001). The distinct genomic and taxonomic differences between S. etuberosum and cultivated potato have made sexual hybridization difficult. Interestingly, S. etuberosum was shown to have high levels of resistance to the potato leaf roll virus (PLRV), potato virus Y (PVY), potato virus X (PVX), and green peach and potato aphid (Valkonen et al. 1992a, b; USDA ARS National Genetic Resources Program 2003). Consequently, the virus resistance trait could be transferred into the cultivated potato gene pool by somatic hybridization.
In the present paper, we report the production of tetraploid interspecific potato somatic hybrids between cultivated potato and Solanum etuberosum Lindl. The results of protoplasts electrofusion and regeneration, confirmation of somatic hybrids through random amplified polymorphic DNA (RAPD), simple sequence repeat (SSR), cytoplasm (mitochondrial/chloroplast) type, flow cytometry (FC) analysis, phenotypic characterization, male fertility and PVY resistance assessment are discussed here.
Materials and methods
Plant material
The androgenic (di)haploid C-13 (2n = 2x = 24) of Indian tetraploid (2n = 4x = 48) potato (Solanum tuberosum L. subsp. tuberosum) cv. Kufri Chipsona 2 was used in protoplast-fusion experiments with 1 EBN non-tuberous wild species S. etuberosum (CGN No.: 23066) (2n = 2x = 24). The (di)haploid C-13 was developed at the Central Potato Research Institute, Shimla, India (Chanemougasoundharam et al. 2004; Sharma et al. 2010). True potato seeds (TPS) of wild species were obtained from the Centre for Genetic Resources, the Netherlands (CGN), Wageningen University, and Research Centre, Wageningen, the Netherlands. Seeds of wild species were germinated in vitro and subsequently multiplied by sub-culturing leafy nodes on MS (Murashige and Skoog 1962) medium (pH 5.8) supplemented with sucrose (20 g l−1) and solidified with gelrite (2 g l−1). Cultures were grown at 20°C under a 16-h photoperiod (light intensity 50–60 μmol m−2 s−1).
Protoplast isolation and electrofusion
Protoplasts were isolated from 3-week-old in vitro plants following highly optimized protocols: in vitro plants were grown in the dark (covered with dark-black muslin cloths) in a culture room at 20°C for 48 h under 16-h photoperiod to integrate cell cycles (Binding et al. 1978). The enzymatic mixtures of 1% cellulose ‘Onozuka’ RS (Yakult Pharmaceuticals, Tokyo) and 0.5% macerozyme R 10 (Yakult) solutions were used for cell wall degradation. Young leaf tissues were minced in 90 × 15 mm Petri dish containing protoplast digestion solution (PDS) (10 ml PDS g−1 tissue) followed by incubation in the dark at 25°C for 16 h without shaking. After incubation, 0.3 M KCl (sterile) was added in a 1:1 ratio after gentle shaking of PDS containing released protoplast. Subsequently, suspension was filtered through a 60-μm nylon sieve and collected in centrifuge tubes. Purification of protoplasts was performed as: filtrates were centrifuged at 50 RCF for 5 min and then resuspended the pellets in 9 ml of 0.6 M sucrose (sterile) followed by 1 ml of 0.3 M KCl layer onto it and centrifuged as above. Protoplasts were recovered from sucrose: KCl interface, diluted with 5 ml of 0.3 M KCl, centrifuged as above and finally suspended in 0.5 M Mannitol to a final density of 1 × 106 protoplast ml−1. The protoplasts were electrofused in a 3.0-mm microslide using the BTX Electro Cell Manipulator ECM 2001 (Harvard Apparatus, Mass., USA). An AC field of 100–120 V cm−1 and high frequency (1 MHz) was first applied for 20–30 s to align the protoplasts. Two square DC pulses 800–1,250 V cm−1 were applied for 40–60 μs to achieve protoplast fusion, with a 10-s post-fusion AC field for compacting the homokaryons/heterokaryons.
Protoplast culture and plant regeneration
Fused protoplasts were cultured in VKM liquid medium (Binding and Nehls 1977) supplemented with glucose (90 mg ml−1) at 20°C in the dark. Following the cell wall development, regenerating cells were cultured on MS13 K medium (Behnke 1975) at 20°C under a 16-h photoperiod for the calli development. Newly regenerated shoots from the calli were cultured in vitro on MS medium for subsequent growth and multiplication. Identification of hybrid regenerants was based on culture of entire fusion products. Subsequently, first shoot per callus was regenerated and multiplied for further studies.
DNA analysis
The hybrid nature of the somatic hybrids was confirmed by RAPD and SSR analysis. Further, cytoplasm type (mitochondrial and chloroplast genome) was also analyzed using mitochondrial and chloroplast specific primers. Total genomic DNA was isolated from in vitro leaf samples following a modified CTAB-dichloromethane protocol of Saghai-Maroof et al. (1984). Leaf samples (~300 mg) were vigorously homogenized in CTAB extraction buffer (1 ml) at room temperature. DNA was precipitated with isopropanol, washed with 70% ethanol, air dried and diluted in 100 μl TE buffer (pH 8.0) after RNase A (100 μg ml−1) treatments.
RAPD analysis was performed using 15 random decamer primers, viz., OPAC-06, OPAC-09, OPAC-13, OPAC-14, OPAQ-02, OPAQ-14, OPAQ-15, OPAQ-16, OAPQ-20, OPAT-03, OPAT-06, OPAT-09, OPD-03, OPG-09 and OPK-06 (Operon Technology), for the confirmation of somatic hybrids. The polymerase chain reaction (PCR) was carried out in a Mastercycler Gradient (Eppendorf, Hamburg, Germany) in a total volume of 25 μl and consisted of 50 ng DNA templates in 1× PCR buffer, 2.5 mM MgCl2, 200 mM dNTP, 0.2 μM of primer, 1 Unit Taq Polymerase (Qiagen). The PCR procedure included: one cycle of 4 min at 94°C, 1 min at 36°C and 2 min at 72°C followed by 43 cycles of 1 min at 94°C, 1 min at 36°C, and 2 min at 72°C, with a final extension of 8 min at 72°C. The amplified RAPD products were separated by electrophoresis on 1.6% agarose gel stained with ethidium bromide (0.5 μg ml−1) in 0.5× TBE buffer (Tris–borate-EDTA, pH 8) using horizontal gel electrophoresis system Sub-Cell GT (Bio-Rad, USA) at room temperature. The gels were visualized under Gel Doc System (Alpha Innotech, San Leandro, CA, USA) and compared with 100 bp DNA ladder (Fermentas, Burlington, Canada).
Twenty-five potato SSR markers, viz., STG0001, STG0010, STG0016, STG0025, STl0001, STI0003, STI0004, STI0012, STI0014, STI0030, STI0032, STI0033, STM0019a, STM0019b, STM0031, STM0037, STM1052, STM1053, STM1064, STM1104, STM1106, STM5114, STM5121, STM5127 and STPoAc58 (Ghislain et al. 2009), and 11 tomato SSR markers, viz. SSR 22, SSR 31, SSR 67, SSR 76, SSR 111, SSR 128, SSR 136, SSR 146, SSR 188, SSR 310 and SSR 350 (Van der Hoeven et al. 2001), were used for the confirmation of somatic hybrids. Annealing temperature (Ta) was decided using Mastercycler Gradient (Eppendorf) and PCR was performed as described by Ghislain et al. (2009). Amplified products were resolved on 4% high resolution agarose (Sigma–Aldrich, St Louis, MO, USA) and 8% polyacrylamide gel electrophoresis (PAGE) followed by gel documentation as above.
Cytoplasm type was analyzed by using mitochondrial specific primers pair ALM4/ALM5 and ALM 6/ALM7, and chloroplast type was determined using primer pair ALCP1/ALCP3. The PCR condition was followed at optimized annealing temperatures (Ta = 55°C for mitochondrial specific primers and Ta = 45°C for chloroplast specific primers) as described by Lössl et al. (2000). Amplified products were analyzed by electrophoresis on 1.6% agarose gel in 0.5× TBE buffer and stained with ethidium bromide (0.5 μg ml−1) as above. As a result of RAPD, SSR and mitochondrial DNA analysis, somatic hybrids were confirmed on the basis of presence/absence of species-specific diagnostic bands of the corresponding parents in the hybrids profiles.
Flow cytometry analysis
The hybrid nature of somatic hybrids was also proven by ploidy determination through flow cytometric analysis following the protocol described by Arumuganathan and Earle (1991b), and Sharma et al. (2010). Fresh leaf samples (~100 mg) from 3- to 4-week-old in vitro plants were macerated in 1 ml nuclei isolation buffer [MgSO4 buffer (MgSO4∙7H2O, 10 mM; KCl, 50 mM; HEPES, 5 mM), 14.325 ml; Dithiothretol (15 mg); Triton X (375 μl)] on ice. Macerates were filtered through a 41-μm nylon sieve followed by centrifugation at 5,000g for 5 min. White pellets (nuclei) were resuspended in MgSO4 buffer and treated with 2 μl RNAse at 37°C for 15 min. Propidium iodide-stained nuclei were used to measure relative fluorescence in a FACSCalibur flow cytometer (Becton–Dickinson, San Jose, USA) using CRBC (chicken red blood cell) (2C value of DNA = 2.33 pg; Galbraith et al. 1983) as an internal standard. The materials were analyzed for forward (FSC) versus side (SSC) scatter signals for at least 10,000 nuclei in each sample. The peak corresponding to the CRBC nuclei was adjusted to around channel 250 set on a linear scale of fluorescence intensity. The nuclear DNA amount (2C value in pg) was estimated by direct comparison of the mean position of nuclear peak of somatic hybrids to that of CRBC (Arumuganathan and Earle 1991a). For each sample, there were three independent replicated measurements.
Potato virus Y (PVY) resistance assay
In vitro raised plants were mechanically inoculated using PVYo isolates maintained in tobacco (Nicotiana glutinosa L. and N. tabacum L.) plants at CPRI, Shimla. Infected fresh tobacco leaf tissue (~2.5 g) was ground in 25 ml of cold 1 mM potassium phosphate (pH 8) buffer. Two to three young leaves of each plant were dusted with carborandrum powder and then lightly rubbed with cheesecloth dipped in virus inoculum. Fresh tissue from the third compound leaf (from the top) of artificially inoculated plants was collected and ground in grinding buffer using a hand crushing machine. Plants were tested by das-ELISA at 20 days after PVY inoculation using the method described by International Potato Centre, Lima-Peru (Anonymous 2007). Two negative and two positive controls maintained at CPRI, Shimla were used in each 96-well ELISA plate. A popular and PVY susceptible variety Kufri Chandramukhi was used as standard check in PVY assay. Absorbance values were measured at 405 nm (A405 nm) using an automated ELISA reader (BioTek, Winooski, VT, USA). The absorbance threshold for susceptible plants was set at absorbance levels two times greater than means of negative controls (Sutula et al. 1986). The antibody (1 μg/μl) used for the coating on the 96-well ELISA plates was produced at CPRI, Shimla.
Phenotypic characterization
The morphology of in vitro regenerated somatic hybrids was assessed in the earthen pot-grown plant. Somatic hybrids and parents were planted in the glass-house in the summer season (May–August) at Shimla (31.06°N, 77.10°E, 2,202 m above msl), as potato plants typically flower only under long-day conditions of Shimla hills (mid-Himalayas) in northern India. The in vitro plants (5 plants per clone) were planted in earthen pots in a randomized complete block design (RCBD) with three replications. Fertilizer schedules and cultural practices were followed using a recommended package of practices. Morphological characters for general canopy characteristics: plant vigor, plant height, foliage structure, foliage color and foliage gloss; stem morphology: solidity, predominant coloration, secondary coloration and wings type; leaf morphology: leaf structure, anthocyanin coloration of rachis, length, width, shape and waviness of margin were recorded at 50 days after planting. Observations on days to first flowering, inflorescence size, corolla size, corolla color, anthocyanin coloration of bud, outer and inner side of corolla, anther color, stylar length and stigma shape were recorded after initiation of flowering. Plant maturity type was recorded at 90 days after planting whereas tuber characters: shape, skin color, skin type, flesh color and depth of eyes, were recorded after harvesting (at 115 days after planting). All the non-parametric categories of the traits were taken from the DUS characterization of potato crop as described by Gopal et al. (2008).
Male fertility determination
Male fertility of the somatic hybrids was determined by acetocarmine (1%) staining of pollens collected from the glass-house grown plants. Stained viable and non-viable pollen was examined through an inverted florescence microscope (Olympus, Tokyo). Counts were recorded from at least ten different flowers from five different locations on slide and mean value was estimated.
Statistical analysis
Prior to univariate ANOVA analysis, homogeneity and normality assumption were tested for the independent variables. Accordingly, data were analyzed followed by all pairwise multiple comparisons of mean values using the post-hoc Tukey’s honestly significant difference (HSD) test (MSTAT-C; Michigan State University, Michigan, USA). The term significant has been used to indicate differences for which P ≤ 0.05. Verification ability of flow cytometry and DNA analysis (RAPD, SSR and mitochondrial DNA) was tested by McNemar Chi-square test to assess the null hypothesis (H0) that no difference exists among flow cytometry, DNA analysis for the hybridity.
Results
Protoplast isolation, electrofusion, culture and regeneration
Protoplasts were isolated from the mesophyll tissue of the parents and 257 electrofusion attempts were made followed by successful culture of post-fusion products. Formation of cell wall was observed for a minimum of 21 days in the fused products; this varies from product to product taking more than a year for final regeneration into plantlets. As a result, 142 calli were recovered from the post-fused products. Subsequently, 84 in vitro somatic hybrids plants were regenerated and the most vigorously growing one was multiplied, while the rest were discarded because of growth abnormalities. Finally, 40 in vitro somatic hybrids plants were grown successfully for the molecular, cytogenetic and phenotypic studies (Table 1).
Identification of somatic hybrids
RAPD analysis
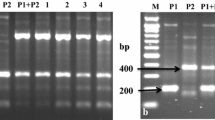
Somatic hybrids were confirmed by using 15 RAPD decamer primers. All primers showed high polymorphic bands in the somatic hybrids and their parents. The presence of species-specific RAPD bands of their corresponding parents in the somatic hybrids confirmed the hybridity. Figures 1 and 2 show RAPD profiles of somatic hybrids and parents generated by primer OPAC-13, where somatic hybrids showed four diagnostic bands, two of each parent: 630 and 996 bp of C-13, and 1,138 and 1,605 bp of S. etuberosum. Similar trends of highly polymorphic bands of species-specific diagnostic bands were revealed by the other 14 RAPD primers and confirmed the 21 clones, namely E 1 to E 12, E 18 to E 20, E 22, E 24 to E 26, E 32 and E 33, as somatic hybrids (Table 2). Thus, based on the RAPD fingerprinting patterns, these 21 somatic hybrids were confirmed, whereas 19 somatic hybrids, E 13 to E17, E 21, E 23, E 27, E 28, E 29 to E 31, E 34 to E 40, showed only S. etuberosum bands, and therefore they failed as somatic hybrids.
RAPD profiles generated by primer OPAC-13 on 1.6% agarose gel. M = 100-bp ladder, P 1 (Parent 1) = C-13, P 2 (Parent 2) S. etuberosum P 1 + P 2 = pooled parental DNA, 15 clones (nos. 1–12 and 18–20) were confirmed as somatic hybrids, whereas 6 clones (nos. 13–17 and 21) were not somatic hybrids (continued on Fig. 2)
RAPD profiles generated by primer OPAC-13 on 1.6% agarose gel. M = 100-bp ladder, P 1 (Parent 1) = C-13, P 2 (Parent 2) S. etuberosum P 1 + P 2 = pooled parental DNA, 6 clones (nos. 22, 24–26, 32, 33) were confirmed as somatic hybrids, whereas 13 clones (nos. 23, 27–31, 34–40) were not somatic hybrids
SSR analysis
Analysis of somatic hybrids’ plant DNA was assayed by 36 SSR primers out of which only 4 SSR, viz., STG0016, SSR 136, SSR 188 and SSR 350, confirmed the somatic hybrids nature. Since, they possessed species-specific diagnostic bands of their corresponding parents. For example, primer STG0016 showed distinct amplification at Ta = 63.1°C whereas the other three, SSR 136, SSR 188 and SSR 350, amplified at Ta = 55°C. Primer STG0016 produced amplification products of 137 and 148 bp in parent C-13, and 160 bp in parent S. etuberosum as described by Ghislain et al. (2009). In addition, primer SSR 136 amplified at 147 and 158 bp in C-13 and S. etuberosum, respectively; SSR 350 produced 242 bp and 262 products in C-13 and S. etuberosum, respectively; whereas the profile obtained by SSR 188 was 146 and 157 bp in C-13 and 168 bp in S. etuberosum; expected sizes as described by Gillen and Novy (2007). As a result, combined amplification of their corresponding parents was produced in the somatic hybrids’ profile (Fig. 3). Other primers showed the amplification with some common bands among the parental lines (data not shown) which could not be characterized as species-specific diagnostic bands for the confirmation of hybridity of plants.
Cytoplasm type analysis
The cytoplasmic genome (mitochondrial and chloroplast DNA) analysis was based on the primers described by Lössl et al. (2000). Amplification with mitochondrial primers ALM4/ALM5 and ALM6/ALM7 and chloroplast primer ALCP1/ALCP3 showed a distinct polymorphism between parental profiles and consequently confirmed the hybridity. The amplification profile obtained by ALM4/ALM5 primers showed a band of 2,400 bp in C-13, a unique band of 780 bp in S. etuberosum and, as a result, both parental bands (780 and 2,400 bp) were produced in the somatic hybrids E 2-3 and E 2-6 profile while all other showed only a 780-bp band. The use of primer ALM6/ALM7 also revealed a polymorphism between parental lines, and somatic hybrids led to profiles comprised of their parental PCR products: 470 bp of C-13 and 2,400 bp of S. etuberosum. Somatic hybrids E 2-1, E 2-2, E 2-8, E 6-1, E 6-2, E 6-3, E 8 and E 10 showed products of 2,400 bp while all others produced 2,000-bp products by ALM6/ALM7. The chloroplast primer ALCP1/ALCP3 produced amplicon of 622 bp in both the parents while an additional band of 815 bp was also obtained in S. etuberosum. Consequently, all the somatic hybrids produced only product size of 622 bp. Data suggest that the somatic hybrids having amplification products of 2,400 bp using ALM4/ALM5 and ALM6/ALM7, and 622 bp by ALCP1/ALCP3 possess W/α type cytoplasm (W: mitochondria type and α: chloroplast type) (Lössl et al. 2000) while all others differed from these amplified products have unique and undetermined cytoplasm type (Figs. 4, 5).
Flow cytometry analysis
All regenerated somatic hybrids of C-13 (+) S. etuberosum were tested for ploidy level by FC analysis. It revealed the co-efficient of variation of the nDNA content means varied between 1.51 and 3.82% among the somatic hybrids (Fig. 6). The nDNA content of somatic hybrid plants ranged from 1.61 to 3.82 pg. For the tetraploid somatic hybrids, the value ranged between 3.34 and 3.82 pg, whereas for diploid clones it ranged between 1.61 and 2.03 pg. Based on nDNA content, 21 somatic hybrids (E1 to E12, E18 to E20, E22, E24 to E26, E32 and E33) were observed as tetraploid, whereas 19 somatic hybrids (E 13 to E17, E21, E23, E27, E28, E29 to E31, E34 to E40) were found to be diploid (Table 3). A highly significant difference in nDNA content was estimated in multiple pairwise comparisons between diploid and tetraploid, according to Tukey’s HSD test at P ≤ 0.01, whereas an insignificant difference was observed in the paired comparison of either diploid–diploid or tetraploid–tetraploid following the same test.
PVY resistance assessment
Somatic hybrids showed vigor as they derived from a PVY-resistant line (S. etuberosum), and the effects of virus infection of these lines was investigated. It was also performed for hybrids coming from two contrasting parental lines. Hybrid plants and their parents were planted in the glass-house and mechanically inoculated with PVYo. However, one parent, C-13, displayed sensitivity to the virus with clear symptoms of progressive leaf discoloration and decrease in plant growth and symptom appearance. The hybrids showed resistance to PVY compared to parents. They displayed resistance to PVY consisting of the absence of virus multiplication and symptoms compared to parent C-13. Twenty-one somatic hybrids showed resistance to PVY as a result of das-ELISA. The ELISA value (A405 nm) of susceptible line C-13 was more than double (>0.06) that of the negative healthy control (0.03). In all the somatic hybrids, the das-ELISA value was lower than the threshold value of healthy control (0.03); consequently, they were confirmed as somatic hybrids. Other plants which failed to be somatic hybrids were also found to have PVY resistance as they possess the wild component S. etuberosum as revealed by molecular data.
Phenotypic assessment
A distinct phenotypic variation was observed in the somatic hybrids of C-13 (+) S. etuberosum and parental genotypes in the glass-house under Shimla conditions. The recorded data are presented in the Table 4. All the somatic hybrids displayed intermediate phenotypes of their parents (Figs. 7, 8 and 9), except 19 hybrids (E13 to E17, E21, E23, E27, E28, E29 to E31, E34 to E40) which showed wild parental type phenotypes. Interestingly, leaf shape of the somatic hybrids was ovate-lanceolate type with weak margins in contrast to ovate-lanceolate type and strong margins of C-13, and ovate type and weak margins of S. etuberosum. Intermediate type leaf structure was appeared in the somatic hybrids in contrary to closed type in both the parents. Phenotype of the wild parent was dominant in the somatic hybrids particularly with respect to foliage color (mid-green), foliage gloss (medium), secondary stem color (purple), flower intensity (low), presence of anthocyanin color in rachis, bud and corolla, anther color (yellow), stigma shape (round), and non-tuberous characters. Parent C-13 was dominant in the somatic hybrids for foliage structure (semi-compact). The corolla size of somatic hybrids was medium (3.56 cm) compared to the small size of C-13 (2.38 cm) and S. etuberosum (2.84 cm). Flowers of somatic hybrids were white with purple shade while C-13 had white with yellow stripes. All the somatic hybrids including parents were of late maturing type (>120 days). There was no tuber formation in the 1 EBN wild parent S. etuberosum and somatic hybrids.
Male fertility determination
Flower intensity was high in somatic hybrids of C-13 (+) S. etuberosum grown in the glass-house under Shimla conditions in the months August–September. The mean value of the pollen fertility of the somatic hybrids was estimated to be about 81.24%. In the parents C-13 and S. etuberosum, pollen fertility was estimated at 75.42 and 78.54%, respectively.
Discussion
The results reported here show that the fusion protocol used was highly efficient, and as a result, 21 somatic hybrids were produced with an average frequency of 15% success over regenerated calli. Similar report has been documented on electrofusion which is a widely used technique associated with a relatively high frequency of heterokaryons and somatic hybrids (Fish et al. 1988). It even more effective than chemical fusion (Pehu et al. 1989). In our experiment, somatic hybrids C-13 (+) S. etuberosum were confirmed on the different criteria, as in earlier reports, such as RAPD (Rokka et al. 1994; Thieme et al. 1997; Naess et al. 2001; Barone et al. 2002; Szczerbakowa et al. 2003; Greplova et al. 2008), SSR (Nouri-Ellouza et al. 2006; Gillen and Novy 2007; Thieme et al. 2008), flow cytometric nDNA content (Thieme et al. 1997; Maciejewska et al. 1999; Oberwalder et al. 2000; Horsman et al. 2001; Szczerbakowa et al. 2003; Greplova et al. 2008), and phenotypic assessment (Novy and Helgeson 1994a; Thieme et al. 2008; Szczerbakowa et al. 2003; Greplova et al. 2008). Confirmation of somatic hybrids was revealed by species-specific diagnostic bands (RAPD and SSR) from both the parental components. Based on RAPD and SSR data, we confirmed 21 interspecific potato somatic hybrids. However, the most common problem with RAPD is its low reproducibility (Devos and Gale 1992). To check the minor differences in amplified products, it can be optimized with PCR reaction to obtain reproducible and interpretable results (Caetano-Anolles et al. 1992). The use of RAPD for routine screening of somatic hybrids could be insufficient if the amount of DNA introgressed from one of the fusion partner is too low (i.e., below 8%; Oberwalder et al. 1998). We confirmed the hybridity by standardization of PCR cycles which resulted in distinct ‘species-specific diagnostic bands’. Additionally, the use of SSR primers also made it possible to confirm the hybridity and validate the results of RAPD. Nevertheless, out of 36 SSR primers, only 4 validated the RAPD findings which further suggests that somatic hybrid plants are the result of recombination between C-13 and S. etuberosum genomes. Our findings of STG0016 agree with Ghislain et al. (2009), while SSR 136, SSR 188 and SSR 350 also concur with Gillen and Novy (2007) by producing expected PCR products. Other SSRs did not confirm the hybridity due to some shared bands between the parents which could not be characterized in the hybrids from which they derived. Cytoplasm type data suggest that the somatic hybrids possess W/α type cytoplasm (Lössl et al. 2000) while other hybrids which did not show specific bands have undetermined cytoplasm type. These cytoplasmic type data confirmed the somatic hybrids and suggest that the unique hybrids may likely be the result of different recombination within the parental genome, and are potential materials for future study.
Flow cytometry analysis for ploidy estimation reveals the tetraploid nature of somatic hybrids and has also been successfully used earlier. The nDNA content also gives preliminary information on genome size of the somatic hybrids. Nevertheless, cytogenetic and chromosomal counts enable estimation of completeness and proportion of parental genomes in the somatic hybrids. Phenotypic evaluation of somatic hybrids validated the RAPD, SSR and FC results. The somatic hybrids are possible because of good cytoplasmic and nuclear fusion in heterokaryon. Probably, the 19 somatic hybrids failed due to the high regeneration potential of individual calli and shoot development thereof from one parent only. Finally, studies on male fertility assessment of somatic hybrids revealed relatively higher fertility, thus indicating their wider application for transferring virus resistance to the cultivated potatoes. In the past, researchers have used the fertile pollen and transferred the virus resistance characters from S. etuberosum-derived somatic hybrids into cultivated potatoes and their subsequent progenies (Gavrilenko et al. 2003; Novy et al. 2002, 2007; Gillen and Novy 2007; Thompson et al. 2007).
Taking into account the intermediate phenotypes, the ploidy level, and the analysis of nuclear and cytoplasmic (mitochondrial/chloroplast) genomes, we conclude that the 21 plants are somatic hybrids. It suggests that both parental genomes expressed well at molecular, cytogenetic and morphological levels in the somatic hybrids. The response of the hybrids to inoculation with PVY showed resistance to virus in comparison to parent C-13. These data clearly show that interspecific protoplast fusion can lead to an improved resistance to PVY in the resulting regenerated plants, whereas parent C-13 is highly sensitive and the other one, S. etuberosum, is resistant. Valkonen and Rokka (1998) reported a loss of parental resistance characters in the interspecific hybrids resulting from protoplast fusion between S. brevidens and S. tuberosum. Other studies (Gibson et al. 1988; Valkonen et al. 1994) described symmetric somatic hybrids with intermediate titer of PVY compared to parental lines. These results suggest that parental genome recombination and somaclonal variation events (Polgar et al. 1999) may lead to somatic hybrid lines harboring different kinds of responses to phytopathogens. Resistance genes can be entirely or partially inactivated or lost, as they can be activated by recombination of parental genomes.
These encouraging results demonstrate that interspecific somatic hybridization could be considered as an alternative and effective means of creating resistant cultivated potatoes. It has the potential to supplement conventional breeding methods for potato improvement. However, its inheritance and durability require more detailed assessment for their stability in subsequent progenies. In addition, such somatic hybrids would be of particular interest for more analysis at the molecular level for genomic compositions of the E- and A-genomes. Additionally, combining resistance genes into the cultivated potatoes and their validation though molecular markers are a future need.
Abbreviations
- CRBC:
-
Chicken red blood cell
- CTAB:
-
Cetyltrimethylammonium bromide
- nDNA:
-
Nuclear DNA
- DUS:
-
Distinctness, uniformity and stability
- EBN:
-
Endosperm balance number
- FC:
-
Flow cytometry
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- PVY:
-
Potato virus Y
- RAPD:
-
Random amplified polymorphic DNA
- RCBD:
-
Randomized complete block design
- SSR:
-
Simple sequence repeat
- TPS:
-
True potato seed
References
Anonymous (2007) Procedures for standard evaluation trials of advanced potato clones. An International Cooperators’ Guide. International Potato Center (CIP), Lima-Peru, pp 75–94
Arumuganathan K, Earle ED (1991a) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9:208–218
Arumuganathan K, Earle ED (1991b) Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol Biol Rep 9:229–241
Barone A, Li J, Sebastiano A, Cardi T, Frusciante L (2002) Evidence for tetrasomic inheritance in a tetraploid Solanum commersonii (+) S. tuberosum somatic hybrid through the use of molecular markers. Theor Appl Genet 104:539–546
Behnke M (1975) Regeneration in Gewebekulturen einiger dihaplider Solanum tuberosum-Klone. Z Pflanzenziicht 75:262–265
Bidani A, Nouri-Ellouz O, Lakhoua L, Sihachakr D, Cheniclet C, Mahjoub A, Drira N, Gargouri-Bouzid R (2007) Interspecific potato somatic hybrids between Solanum berthaultii and Solanum tuberosum L. showed recombinant plastome and improved tolerance to salinity. Plant Cell Tiss Organ Cult 91:179–189
Binding H, Nehls R (1977) Regeneration of isolated protoplast to plant Solanum dulcamara L. Z Pflanzenphysiol 85:279–280
Binding H, Nehls R, Schieder O, Sopory SK, Wenzel C (1978) Regeneration of mesophyll protoplast isolated from dihaploid clones of Solanum tuberosum L. Plant Physiol 43:52–54
Caetano-Anolles G, Bassam BJ, Gresshoff PM (1992) Primer template interactions during DNA amplification fingerprinting with single arbitrary oligonucleotides. Mol Gen Genet 235:157–165
Chanemougasoundharam A, Sharma S, Sarkar S, Pandey SK, Khurana SMP, Thakur KC, Manivel P (2004) Induction of andrognesis and regeneration of androgenic plants from tetraploid Indian potato (Solanum tuberosum L. subsp. tuberosum) cultivars. Potato J 31:59–65
Chavez R, Brown CR, Iwanaga M (1988) Application of interspecific sesquiploidy to introgression of PLRV resistance from non-tuber-bearing Solanum etuberosum to cultivated potato germplasm. Theor Appl Genet 76:497–500
Devos KM, Gale MD (1992) The use of random amplified polymorphic DNA markers in wheat. Theor Appl Genet 84:567–572
Fish N, Karp A, Jones MGK (1988) Production of somatic hybrids by electrofusion in Solanum. Theor Appl Genet 76:260–266
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051
Gavrilenko T, Thieme R, Heimbach U, Thieme T (2003) Fertile somatic hybrids of Solanum etuberosum + dihaploid Solanum tuberosum and their backcrossing progenies: relationships of genome dosage with tuber development and resistance to potato virus Y. Euphytica 131:323–332
Ghislain M, Jorge N, Herrera MR, Pignataro J, Guzman F, Bonierbale M, Spooner DM (2009) Robust and highly informative microsatellite-based genetic identity kit for potato. Mol Breed 23:377–388
Gibson RW, Jones MGK, Fish N (1988) Resistance to potato leaf roll virus and potato virus Y in somatic hybrids between dihaploid Solanum tuberosum and S. brevidens. Theor Appl Genet 76:113–117
Gillen AM, Novy RG (2007) Molecular characterization of the progeny of Solanum etuberosum identifies a genomic region associated with resistance to potato leafroll virus. Euphytica 155:403–415
Gopal J, Kumar V, Pandey SK, Kumar R, Pandey PC, Singh SV (2008) Morphological descriptors for DUS testing of potato varieties. Plant Genet Res News 154:40–47
Greplova M, Polzerova H, Vlastnkova H (2008) Electrofusion of protoplasts from Solanum tuberosum, S. bulbocastanum and S. pinnatisectum. Acta Physiol Plant 30:787–796
Helgeson JP, Haberlach GT (1999) Somatic hybrids of Solanum tuberosum and related species. In: Altman A, Ziv M, Izhar S (eds) Plant biotechnology and in vitro biology in the 21st century. Kluwer, Dordrecht, pp 151–154
Horsman K, Gavrilenko T, Bergervoet JEM, Huigen DJ, Joe ATW, Jacobsen E (2001) Alteration of the genomic composition of Solanum nigrum (+) potato backcross derivatives by somatic hybridization: selection of fusion hybrids by DNA measurements and GISH. Plant Breed 120:201–207
Jackson SA, Hanneman RE Jr (1999) Crossability between cultivated and wild tuber- and non-tuber-bearing Solanums. Euphytica 109:51–67
Johnston SA, den Nijs APM, Peloquin SJ, Hannemann RE (1980) The significance of genetic balance to endosperm development in interspecific crosses. Theor Appl Genet 57:5–9
Lössl A, Götz M, Braun A, Wenzel G (2000) Molecular markers for cytoplasm in potato: male sterility and contribution of different plastid-mitochondrial configurations to starch production. Euphytica 116:221–230
Maciejewska U, Skierski JS, Szczerbakowa A (1999) Nuclear DNA content of Solanum species grown in vitro as determined by flow cytometry. Acta Physiol Plant 21:37–43
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Naess SK, Braden JM, Wielgus SM, Haberlach GT, McGrath JM, Helgeson JP (2001) Analysis of introgression of Solanum bulbocastanum DNA into potato breeding lines. Mol Genet Genomics 265:694–704
Nouri-Ellouza O, Gargouri-Bouzid R, Sihachakr D, Triki MA, Ducreux G, Drira N, Lakhoua L (2006) Production of intraspecific somatic hybrids with improved tolerance to PVY and Pythium aphanidermatum. J Plant Physiol 163:1321–1332
Novy RG, Helgeson JP (1994a) Resistance to potato virus Y in somatic hybrids between Solanum etuberosum and S. tuberosum × S. berthaultii hybrid. Theor Appl Genet 89:783–786
Novy RG, Helgeson JP (1994b) Somatic hybrids between Solanum etuberosum and diploid, tuber bearing Solanum clones. Theor Appl Genet 89:775–782
Novy RG, Nasruddin A, Ragsdale DW, Radcliffe EB (2002) Genetic Resistances to Potato Leafroll Virus, Potato Virus Y, and Green Peach Aphid in Progeny of Solanum etuberosum. Am J Potato Res 79:9–18
Novy RG, Gillen AM, Whitworth JL (2007) Characterization of the expression and inheritance of potato leafroll virus (PLRV) and potato virus Y (PVY) resistance in three generations of germplasm derived from Solanum etuberosum. Theor Appl Genet 114:1161–1172
Oberwalder B, Schilde-Rentschler L, Ruoβ B, Wittemann S, Ninnemann H (1998) Asymmetric protoplast fusions between wild species and breeding lines of potato–effect of recipients and genome stability. Theor Appl Genet 97:134–1354
Oberwalder B, Schilde-Rentschler L, Loffelhardt-Ruoβ B, Ninnemann H (2000) Differences between hybrids of Solanum tuberosum L. and Solanum circaeifolium Bitt. obtained from symmetric and asymmetric fusion experiments. Potato Res 43:71–82
Pehu E, Karp A, Moore K, Steele S, Dunckley R, Jones MGK (1989) Molecular, cytogenetic and morphological characterization of somatic hybrids of dihaploid Solanum tuberosum and diploid S. brevidens. Theor Appl Genet 78:696–704
Polgar Z, Wielgus SM, Horvath S, Helgeson JP (1999) DNA analysis of potato + Solanum brevidens somatic hybrid lines. Euphytica 105:103–107
Rokka VM, Xu YS, Kankila J, Kuusela A, Pulli S, Pehu E (1994) Identification of somatic hybrids of dihaploid Solanum tuberosum lines and S. brevidens by species specific RAPD patterns and assessment of disease resistance of the hybrids. Euphytica 80:207–217
Rokka VM, Tauriainen A, Pietilä L, Pehu E (1998) Interspecific somatic hybrids between wild potato Solanum acaule Bitt. and anther-derived dihaploid potato (Solanum tuberosum L.). Plant Cell Rep 18:82–88
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal locations, and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Sharma S, Sarkar D, Pandey SK (2010) Phenotypic characterization and nuclear microsatellite analysis reveal genomic changes and rearrangements underlying androgenesis in tetraploid potatoes (Solanum tuberosum L.). Euphytica 171:313–326
Spooner DM, Hijmans R (2001) Potato systematics and germplasm collecting, 1989–2000. Am J Potato Res 78:237–268
Sutula CL, Gillett JM, Morrissey SM, Ramsdell DC (1986) Interpreting ELISA data and establishing the positive-negative threshold. Plant Dis 70:722–726
Szczerbakowa A, Maciejewska U, Pawlowski P, Skierski JS, Wielgat B (2001) Electrofusion of protoplasts from Solanum tuberosum, S. nigrum and S. bulbocastanum. Acta Physiol Plant 2:169–179
Szczerbakowa AU, Maciejewska E, Zimnoch-Guzowska E, Wielgat B (2003) Somatic hybrids Solanum nigrum (+) S. tuberosum: morphological assessment and verification of hybridity. Plant Cell Rep 21:577–584
Szczerbakowa A, Boltowicz D, Lebecka R, Radomski P, Wielgat B (2005) Characteristics of the interspecific somatic hybrids Solanum pinnatisectum (+) S. tuberosum H-8105. Acta Physiol Plant 3:265–273
Thieme R, Darsow U, Gavrilenko T, Dorokhov D, Tiemann H (1997) Production of somatic hybrids between S. tuberosum L. and late blight resistant Mexican wild potato species. Euphytica 97:189–200
Thieme R, Darsow U, Rakosy-Tican L, Kang Z, Gavrilenko T, Antonova O, Heimbach U, Thieme T (2004) Use of somatic hybridization to transfer resistance to late blight and potato virus Y (PVY) into cultivated potato. Plant Breed Seed Sci 50:113–118
Thieme R, Rakosy-Tican E, Gavrilenko T, Antonova O, Schubert J, Nachtigall M, Heimbach U, Thieme T (2008) Novel somatic hybrids (Solanum tuberosum L. + Solanum tarnii) and their fertile BC1 progenies express extreme resistance to potato virus Y and late blight. Theor Appl Genet 116:691–700
Thompson AL, Taylor RJ, Pasche JS, Novy RG, Gudmestad NC (2007) Resistance to Phytophthora erythroseptica and Pythium ultimum in a Potato Clone Derived from S. berthaultii and S. etuberosum. Am J Potato Res 84:149–160
USDA ARS National Genetic Resources Program (2003) Germplasm Resources Information Network-(GRIN). [Online Database] http://www.ars-grin.gov/cgi-bin/npgs/html/obs.pl?1189587 National Germplasm Resources Laboratory, Cited 23 Sept 2003
Valkonen JPT, Rokka V-M (1998) Combination and expression of two virus resistance mechanisms in interspecific somatic hybrids of potato. Plant Sci 131:85–94
Valkonen JPT, Brigneti G, Salazar LF, Pehu LF, Gibson RW (1992a) Interactions of the Solanum spp. of the Etuberosa group and nine potato-infecting viruses and viroid. Ann Appl Biol 120:301–313
Valkonen JPT, Brigneti G, Pehu E (1992b) Resistance to Myzus persicae (Suls.) in wild potatoes of the series Etuberosa. Acta Agric Scand, Sectt B. Soil Plant Sci 42:118–127
Valkonen JPT, Slack SA, Plaisted RL (1994) Use of the virus strain group concept to characterize the resistance to PVX and PVYo in the potato cv. “Allegany.” Am Potato J 71:507–516
Van der Hoeven R, Fulton T, Ilut DC, Tanksley SD (2001) Development of a Solanaceae genome database: SGN. Plant and animal genome IX conference, 2001, San Diego, CA. Abstract #W54_06. Available: http://sgn.cornell.edu
Zhou A, Xia G, Zhang X, Chen H, Hu H (2001) Analysis of chromosomal and organellar DNA of somatic hybrids between Triticum aestivum and Haynaldia villosa Schur. Mol Genet Genomics 265:387–393
Zimnoch-Guzowska E, Lebecka R, Kryszczuk A, Maciejewska U, Szczerbakowa A, Wielgat B (2003) Resistance to Phytophthora infestans in somatic hybrids of Solanum nigrum L. and diploid potato. Theor Appl Genet 107:43–48
Acknowledgments
Authors are indebted to Dr. PS Ahuja, Director, Institute of Himalayan Bioresource and Technology (IHBT), Palampur, Himachal Pradesh, for the facility provided for FC analysis. The authors thank Mr. Sheeshram Thakur for the in vitro multiplication and field management. Hearty thanks are also extended to Mr. Akshay and Sanjay, IHBT, for help rendered in FC analysis. The financial assistance received in the form of an ad-hoc research project (F. No. 8-45/2004-Hort. II) from the Indian Council of Agricultural Research (ICAR), New Delhi, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiwari, J.K., Poonam, Sarkar, D. et al. Molecular and morphological characterization of somatic hybrids between Solanum tuberosum L. and S. etuberosum Lindl.. Plant Cell Tiss Organ Cult 103, 175–187 (2010). https://doi.org/10.1007/s11240-010-9765-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9765-x