Abstract
The two-stage anaerobic digestion (AD) is gaining popularity because of the process stability and possibility of recovering multiple-resources such as biohydrogen and organic acids from the first stage dark fermentation (DF) and methane in the AD as the second stage while treating the organic waste. As the performance of two-stage processes is influenced by the type of substrate and operational conditions, there have been several experiments at laboratory and pilot scales to determine the optimum conditions. The main objective of this review is to provide an updated overview of advancements in biohythane and organic acids production from food waste (FW) in the two-stage DF-AD process. Likewise, this work also provides an insight into the economic and future prospective of utilizing organic acids for different biochemicals such as polyhydroxyalkanoates, polylactate, and microalgal biomass production. The integration of optimum operational parameters, pretreatment methods, types of bioreactors is essential in combined DF-AD processes. The parameters and reactor configuration have to be optimized depending upon the targeted end-products. More research into the techno-economic analysis of different bioreactor configurations for long-term operations in an integrated DF-AD process with FW as a feedstock is needed to realize its viability for commercial application.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increase in energy demand, depletion of fossil fuel, and growing concern over global warming, the interest in green technologies for resource recovery is increasing. The current global energy demand is expected to double by 2050. More than 80% of the current energy demand is fulfilled by fossil fuels which are limited resources. Moreover, the by-products from burning fossil fuels are the major source of greenhouse gas emissions. The need for renewable sources to produce bioenergy and biochemicals that have positive environmental impacts is driving the bio-based economy (Cherubini et al. 2009; Bastidas-Oyanedel et al. 2019). The bio-based economy promotes the technologies that recover energy and chemicals from biomass through the biological degradation process.
Anaerobic digestion (AD) is one of the widely used biological processes for the production of renewable energy as biogas and nutrients from organic waste (Jain 2019). The benefits of converting the combustible methane (CH4) gas to heat and electricity through combined heat and power (CHP) plant and the use of digestate as fertilizer has made AD a popular technology. However, these factors may not upgrade both material recovery efficiency and the economic viability of commercial biogas facilities (Kaparaju et al. 2009; Sawatdeenarunat et al. 2015).
The pursuit of optimization of the AD process for multiple-resource recovery has led to realizing a two-stage AD process. The two-stage AD process differs from the single-stage AD in terms of microbial pathways, growth kinetics, and environmental condition for acidogens and methanogens which is carried out in two separate bioreactors. Besides, several studies show that the two-stage process is much more beneficial than single-stage processes in terms of performance, efficiency, and stability (Liu et al. 2006; Park et al. 2010; Luo et al. 2011; Ghimire et al. 2021). Moreover, the fermentation time and reactor volume are also reduced in a two-stage AD process with possibilities for multiple-resource recovery. As reported by Roy and Das (2016), the total gaseous energy recovery from the starchy wastewater for the two-stage process was 53.6% whereas only 28% of gaseous energy was recovered from the single-stage biohydrogen (H2) production. The two-stage AD process also shows stability in the long run which opens up possibilities for upscaling (Cavinato et al. 2012; Massanet-Nicolau et al. 2015).
The two-stage AD process simultaneously produces H2 in the first stage and CH4 in the second stage. The combination of H2 (10–25% v/v) and CH4 (75–90% v/v) is termed biohythane which has been considered a high-grade fuel, better than methane in terms of flammability, flame speed, and easier ignition with less energy input (Bolzonella et al., 2019). In addition, the by-products from the two-stage AD process such as Volatile Fatty Acid (VFA), Lactic Acid (LA), and alcohol are useful biochemicals (O-Thong et al. 2018). The two-stage AD has a good potential to become a sustainable biorefinery approach for multiple fuels and biochemicals production. Various processes such as dark fermentation (DF), photo-fermentation (PF) within the two-stage biorefinery process are available for H2 production. In particular, DF is the most studied and promising technology because it has the potential to produce H2 along with biochemicals from a wide variety of feedstock (Nasr et al. 2012; Alibardi and Cossu 2016). Furthermore, combining DF with AD can be an appropriate option to create H2 and CH4, thus, biohythane (Meena et al. 2020).
Current research interests in “biorefinery” are growing as shown by an increasing number of publications of 2,153 scientific articles and book chapters in the last decade (2010–2020) compared to 402 numbers (before the decade) in Scopus. The research and commercial interest in anaerobic biorefinery is increasing due to the versatility of the process for the production of multiple fuels and chemicals (K. C. et al. 2015; Agler et al. 2011; Tedesco and Stokes 2017). As such DF-based biochemical process can be a promising route for achieving the bio-based products and energy (Clomburg and Gonzalez, 2013; Bastidas-Oyanedel et al. 2015; Motte et al. 2015; Sarma et al. 2015; Luongo et al. 2017; Nizami et al. 2017; Moscoviz et al. 2018).
Conversion of Food waste (FW) to produce bioenergy (biofuels) and by-products (fertilizers, chemicals) has been considered as one of the efficient approaches for not only energy production but also solid waste management (Dahiya et al. 2018; Paritosh et al. 2017; Strazzera et al. 2018). About 57% and 53% of the waste composition is organic waste in low and middle-income countries, respectively (Kaza et al. 2018). The major portion of organic waste is FW. Globally, FW amounts to 1.6 billion tonnes and much of it ends up in landfill sites leading to the emission of greenhouse gases (GHGs). FW is estimated to produce 3.3 billion tonnes of carbon dioxide (CO2) emissions per year (FAO 2013). The theoretical CH4 yield and electricity production potential of FW is 0.300 m3/kg VS and 473.8 kWh, respectively (Suhartini et al. 2019). Therefore, tapping the energy potential of FW via a biorefinery framework could be a sustainable approach for organic waste management and the production of multiple-resources for commercial use.
This paper aims to provide an extended overview of the DF and AD process for biohythane and biochemical production (particularly VFA) from FW within the biorefinery approach. This paper discusses how pretreatment, different operational parameters, and the choice of bioreactors in the DF-AD affect the substrate degradation and metabolic pathways for H2, CH4 and organic acids production from FW. Likewise, the optimum conditions for biohythane and VFA production, economic and future prospective of utilizing the VFA for different biochemical such as polyhydroxyalkanoates, polylactate, and microalgal biomass has been discussed.
FW as a feedstock for biohythane and biochemical production
The FW which is generally rich in carbohydrates has been widely used for H2 and biochemical production. FW comprises mostly carbon chains and has a high energy content (Fatima et al. 2020). The average energy content in the mixed food sample (meat, fruits, and vegetables) was found to be 14.31 MJ/kg (Tanai 2016). Every year, approximately one-third of the food produced for human consumption gets wasted which is likely to increase with the global population (Gustavsson et al. 2011). However, the characteristics of FW vary depending upon the consumption pattern of different countries (Paritosh et al. 2017). Globally, the highest FW constituent is cereal (more than 80,000 million tonnes) whereas considering only Asia, vegetable (60,000-kilo tonnes) contributes to the maximum FW (Paritosh et al. 2017). Thus, this abundant FW can be extensively used in two-step DF-AD (Bolzonella et al. 2019; Dahiya et al. 2018).
Generally, carbohydrate-rich FW is regarded as the most ideal substrate for H2 and CH4 production in a two-stage process, whereas lipids and protein-rich foods are the least preferred (Alibardi and Cossu 2016). A 20 fold increase in biological H2 production was observed when using carbohydrate-rich substances compared to lipid and protein-rich substances (Fatima et al. 2020).
Cieciura-Włoch & Borowski (Cieciura-Włoch and Borowski 2019) found out that plant-based waste such as fruit and vegetable waste is most suitable for hydrogen production with the highest yield of 280 L H2/kg VS. They also found out that slaughter house and kitchen waste have high methane yield but unfavorable for hydrogen production because of high protein and fat. The protein-rich substrate with a low C: N ratio increases ammonia concentration during the AD process leading to inhibition of methanogenic microorganisms (Bolzonella et al. 2019). Similarly, the lipid is degraded into long-chain fatty acids (LCFA) which have an inhibitory effect on acetogens and methanogens (Dasa et al. 2016).
Besides the nutrients, the high total solid (TS) content of around 20–30% in FW makes it favorable for operating the first stage reactor under dry conditions (Capson-Tojo et al. 2017). According to the TS content of the FW was also found to be adjusted to 4.3% and 10% in tests carried out by Algapani et al. (2018) and Akhlaghi et al. (2019), respectively. The TS content greater than 15% can result in a decrease in substrate conversion and lactate is produced as a major fermentation product (Ghimire et al. 2017). Similarly, methane yield was found to be higher in the FW of TS 15–20% than in the TS of 5–10% (Chen et al. 2014). FW has a high VS content (21 to 27% VS) and is particularly suitable for the co-production of biohydrogen and platform molecules such as short organic acids and/or alcohols (Uçkun Kıran et al. 2015).
FW can be co-digested with substrates such as anaerobic sludge (can function as inoculum), waste activated sludge, wheat straw, chicken manure, and grass to enhance biohythane and VFA production (Ghimire et al. 2017; Show et al. 2018; Wang et al. 2014; Esteban-Gutiérrez et al. 2018). According to Wang et al. (2014), the VFA production from FW with the addition of aerobic and anaerobic activated sludge was recorded to be 0.482 g/g VSSremoval and 0.918 g/g VSSremoval, respectively. Similarly, the co-digestion of FW with brown water (feces without urine) formed acetic and butyric acids as major metabolites which are the most appropriate for methe and useable by-products production (Rajagopal et al. 2014).
FW is rich in organic content due to which there is a possibility of hydrolysis inhibition by the accumulation of ammonia, rapid acidification, and formation of LCFA (Menzel et al. 2020). This inhibits the optimum energy recovery from FW in single-stage AD. Therefore, besides co-digestion, two-stage AD, and pretreatment have also been considered as the efficient approach for enhancing the solubilization and hydrolysis of FW (Uçkun Kiran et al. 2015; Rodríguez-Valderrama et al. 2020; Menzel et al. 2020).
Two-stage AD process as a pathway for biohythane and biochemicals
Generally, the conversion of organic waste during AD consists of four steps: (i) hydrolysis (ii) acidogenesis (iii) acetogenesis, and iv) methanogenesis. In the two-stage AD process, these processes are physically separated by two reactors under controlled operating conditions (Fig. 1).
Stage I: dark fermentation (DF) process
Hydrolysis and acidogenesis phases are carried out in the first stage reactor. In this reactor, the complex organic compounds are hydrolyzed and further degraded by acidogenic microorganisms to produce C2-C5-based VFA, H2, CO2, and alcohols in the DF process (Camacho et al. 2019). Even though FW is readily biodegradable, hydrolysis is the rate-limiting step during the AD process (Zhang et al. 2014). However, for the two-stage AD process of organic waste, methanogenesis is the rate-limiting step (Meena et al. 2020) because of the slower methanogenic kinetics and longer lag phase in the second-stage reactor (De Gioannis et al. 2014).
During the DF process, most of the energy contained in the organic substrate is converted into VFAs whereas only 7.5–15% of the total energy is converted to H2 (Bolzonella et al. 2019). The possible pathways for H2 production are presented in Table 1 Eqs. (1) to (4), which shows that the maximum theoretical yield from the DF process can be 4 mol H2/mol glucose (Saratale et al. 2019). However, this cannot be achieved due to the complex nature of the FW which may contain a different fraction of carbohydrates, proteins, and fat.
Stage II-anaerobic digestion (AD) process
The DF effluent from the first stage can be fed into the second stage in which acetogenesis and methanogenesis occur. The VFAs with higher molecular weight and other intermediates are further oxidized by H2-producing acetogens as shown in Eqs. 5–7 (Van et al. 2020).
Approximately, 25% of acetate and 11% of H2 are formed in acetogenesis (Anukam et al. 2019). Though H2 is produced during acetogenesis, it is not recommended to extract H2 from this stage (Van et al. 2020). Because acetogenic microorganisms are compatible with methanogens (Roy and Das 2016). And, this acid phase product (acetate and H2 and CO2) is consumed by methanogenic microorganisms for CH4 production (Meegoda et al. 2018). The undigested or unhydrolyzed food residues can also be converted to CH4 in the second-stage reactor. In the two-stage AD process, AD is mostly used for the recovery of CH4 rather than organic acids.
In acetoclastic methanogenesis, acetate is converted into CH4 and CO2 which is accountable for two-thrid of CH4 production (Eq. 8). Alternatively, reduction or CO2 to CH4 can also take place, which is termed as hydrogenotrophic methanogenesis which is accountable for the remaining one-third CH4 (Eq. 9) (Fenchel et al. 2012).
The physical separation benefits the growth of required microorganisms within that particular process. For example, hydrolysis is limited by fermentative bacteria whose optimum pH is 5.5, whereas the pH of 7.5–8.5 is needed for methanogenic microorganisms (Sivagurunathan et al. 2018). In DF, the hydrolysis and acidogenesis process is maintained by lowering the hydraulic loading rate (HRT), controlling the pH around 5.5—6.5, and inoculum pretreatment to enrich H2 producers (Chu et al. 2008; Xia et al. 2016; Rafieenia et al. 2017).
Operational parameters affecting the two-stage AD process producing biohythane.
The optimization of operational parameters such as pH, temperature, hydraulic retention time (HRT), substrate loading rates, pretreatment methods, recirculation of effluent from the second stage to the first stage (recirculation ratio), types of bioreactor is essential in the integrated two-stage DF-AD process. The operational parameters affecting the biohythane production in the two-stage AD process are shown in Fig. 2. The effect of varying operating parameters in a two-stage AD process has been summarized and presented in Table 2.
pH
The pH is one of the most important parameters in biohythane production because it determines the subsistence of acidogenic or methanogenic microorganisms. It directly affects the metabolic pathways involved in the process of anaerobic fermentation and digestion. The formation of undissociated organic acids when pH is lower than 4 ceases H2 production in DF (Bolzonella et al. 2019).
While operating a two-stage process for biohythane production from FW, Cavinato et al. (2011) reported 15% of H2 production in the first phase and 65% of CH4 in the second phase at pH of 4.32 and 7.68, respectively. Moreover, around 35% of H2 production was observed at pH 3.51. Maintaining pH at acidic and neutral alkaline conditions is suitable for DF and AD reactors, respectively. This creates a favorable growth environment for H2 producing microorganisms in DF and methanogens in AD (Meena et al. 2020).
Anaerobic fermentation (AF) or DF process have been used by the different researchers for VFA production under controlled pH varying from pH of 6 to 8 (Table 3). However, the VFA production was improved in alkaline conditions (pH 9) in comparison to acidic conditions (pH 6) for FW in batch tests (Cheah et al. 2019). The certain value of pH also affects the concentration of targeted VFAs. The culture pH of 6 was found to be suitable for the production of acetate whereas, pH 7 facilitated the production of butyrate from FW (Hussain et al. 2017).
The pH inside the first stage keeps on fluctuating due to the formation of VFAs. This inhibits the H2 production pathway as H2 and CO2 is consumed by acetogens to produce acetate (Cavinato et al. 2011). Therefore, the pH of the first stage reactor can be controlled by external chemical addition (Micolucci et al. 2014), the use of co-substrate with high alkalinity or buffering capacity (Yeshanew et al. 2016; Ghimire et al. 2016), and continuous operation for CH4 production could also avoid pH drop as high VFA concentration is buffered by high total ammonia concentration (Capson-Tojo et al. 2016). However, these external methods and continuous processes result in high costs and instability of operation. Therefore, a cost-effective solution in two-stage AD could be recirculating the digestate of the CH4 reactor to the H2 reactor (Cavinato et al. 2012, 2011; Micolucci et al. 2014).
Temperature
An optimum temperature plays a vital role in the growth of microorganisms as it influences biochemical reactions and their metabolism. Microorganisms that are responsible for H2 production in the first stage and the CH4 production in the second stage are often found to be grown at mesophilic (30–35 °C) to, thermophilic (55–60 °C) to higher thermophilic condition (70–90 °C) (O-Thong et al. 2018).
Most of the researchers have kept the optimum temperature at 55 °C for biohythane production as evident in Table 2. According to O-Thong et al. (2018), higher thermophilic temperatures are more favorable for H2 production during DF than thermophilic and mesophilic conditions. Microorganisms such as Caldicellulosiruptor sp. survive in extreme temperatures and have hydrolytic enzymes which can utilize various substrates such as cellulose for more H2 production. Moreover, higher temperature accelerates their metabolism and degradation efficiency that reduces the retention time for biohythane production. Furthermore, thermophilic condition decreases the solubility of CH4 and CO2 along with the destruction of pathogens (O-Thong et al. 2018). However, Ghimire et al. (2021) recorded the highest H2 (53.5 mL H2/g VSadded) and CH4 (307.5 L CH4/kg VSadded) production from FW in the DF-AD process at mesophilic and thermophilic temperature, respectively. The probable reason for this distinction is the quicker adaptation of heat-treated inoculum at the mesophilic condition as it is sourced from the mesophilic digester (Ghimire et al. 2021).
The most commonly used process temperature for VFA production from the AF process is 35℃ (Table 3). According to Cho et al. (2015), the VFA concentration from AF increases with the increase in temperature. VFA produced at 55 °C was 3.2 times greater than the VFA produced at 35 °C. Similarly, the concentration of propionic acid increased by 15% but acetic acid decreased to 65.8% with the increase in temperature. However, Jiang et al. (2013) found 45℃ as the most favorable temperature for the highest VFA production (47.89 g/L). The value was only slightly higher than the VFA (41.34 g/l) produced at 35 ℃. According to Jiang et al. (2013), the higher temperature leads to higher solubilization but lower acidogenesis of FW thus, leading to lower VFA production.
Hydrogen partial pressure (HPP)
The hydrogen partial pressure (HPP) in the liquid phase is an essential parameter for the DF (Ding and Zhao 2018; Beckers et al. 2012). Because high HPP (> 60 Pa) shifts the equilibrium reaction to the H2 producing reaction (Ding and Zhao 2018) by deactivating the enzymes such as hydrogenase and NADH (Ramírez-Morales et al. 2015). However, no correlation was reported between HPP, liquid, and the NADH/NAD+ ratio (De Kok et al. 2013). But, lowering HPP affected pH and microbial growth rate in DF (Ding and Zhao 2018). The H2 and VFA production in DF decreases with the increase in HPP (Ramírez-Morales et al. 2013). Besides the concentration, HPP also influences the composition of VFAs though propionic acid concentration is not directly affected by ppH2 (Ding and Zhao 2018).
A study conducted by Beckers et al. (2012) presented an increase of 7% of biohydrogen yields on the decrease total pressure 0.11 bar and a decrease of 19.5% yield on the lowering of pressure by 0.18 bar. The researchers experimented on an anaerobic biodisc reactor (ABR) that fixes the biomass and favors liquid to gas transfer leading to efficient H2 production (Beckers et al. 2012). An identical study by Cazier et al. (2015) altered HPP from 0 to 1.557 bar which led to a substantial decrease in CH4 yield and substrate degradation. It was seen that high HPP led to a decrease in degradation of the substrate as well as accumulation of metabolites.
The HPP can be controlled by venting out excess H2 gas. Another solution could be introducing different gases such as N2, CO2, CH4, or even biogas and creating a vacuum on the headspace of the reactor (De Kok et al. 2013; Dionisi et al. 2019). However, this leads to diluted H2 production. Ramírez-Morales et al. (Ramírez-Morales et al. 2015) suggested integrating membrane technology to remove excess H2 during a continuous fermentation process. The most suitable option would be to vent out the H2 produced at regular intervals or continuously.
Hydraulic retention time (HRT)
In bioreactor systems without sludge recirculation, HRT can refer to the time length that the biomass remains in the bioreactor (Strazzera et al. 2018) which depends upon the volume of the reactor and the flow rate of the feed. Therefore, it is also associated with capital cost. The optimum HRT for the first reactor is 1–3 days whereas for the second reactor is 10–15 days (O-Thong et al. 2018). This is because the H2 producing bacteria have a faster growth rate compared to methanogenic bacteria.
Similarly, the longer HRT for the second reactor allows enough time for substrate degradation. The highest methane production rate of 2041.7 L CH4/m3/day from an anaerobic fixed bed reactor (AFBR) was possible even at the shortest HRT of 1.5 days given the maximum organic loading rate (OLR) of 6.1 kg COD/m3.day (Yeshanew et al. 2016). This implies that the use of different biofilm reactor technologies such as AFBR that prevent washing out methanogenic organisms. It helps in the reduction of HRT without compromising the reactors ‘performance. Though methanogens such as Clostridiaceae are highly reduced at low HRT (4–8 h), other homoacetogens like Clostridium ljungdahlii, were difficult to remove due to the granular structure of Up-flow Anaerobic Sludge Blanket (UASB) and packed bed reactor (PBR) (Si et al. 2015).
Vo et al. (2019) found out that the optimum H2 (714 mL/L.d) and CH4 (254 mL/L.d) were produced at an HRT of 2 days. The study experimented with a single-stage AF reactor which consists of two compartments each for H2 and CH4 production. The effect of HRT and OLR along with pH on VFA production from biowaste has been explored. Lim et al. (2008) studied the effect of 3 different HRTs (4, 8, and 12 days) at controlled pH 5.5 and OLR of 5 g TS/ L.d. There was an increase in the VFAs concentration (5.5 g/L at 4 days, 13 g/L at 8 days, 22 g/L at 12 days) with an increase in HRT. At shorter HRTs, acetate was the dominant product while at longer HRT (12 days) propionic acid was higher.
Han and Shin (2002) also studied the effect of HRT (0.25, 0.33, 0.50, and 1 d) on VFA production from FW on Leachate bed reactor (LBR) under controlled temperature (35℃) and pH. At an HRT of 1 day, the maximum VFAs concentration of 202 and 181 mmol/L was determined with rumen and anaerobic bacteria, respectively. However, at 0.25 days of HRT, the least VFAs concentration was detected. According to Strazzera et al. (2018), lower HRT (< 10 days) favors VFA production because the longer HRT facilitates methanogenic microorganisms to convert VFA to CH4.
Organic loading rate (OLR)
Organic loading rate (OLR) refers to the amount of substrate fed into the reactor in a day per unit of working volume. It is a deterministic parameter for the failure of the system due to bulking and acidifications. For CH4 production from vegetable waste, the optimum OLR for a CH4 production rate of 0.26m3 CH4/kg VS was 1.4 kg VS/m3.d, and as the OLR increased VS degradation and biogas yield decreased (Jalil et al. 2019). High OLR reduces the hydrolysis and acidogenesis process due to imbalanced osmotic pressure and increased viscosity that limits mass transfer and metabolic activities (Tang et al. 2016).
However, some experiments have also proved better biohythane production even at high OLR under the condition of low HRT (Cavinato et al. 2011; O-Thong et al. 2018; Yeshanew et al. 2016). A stable H2 (56.6 mL H2/g VS) and CH4 (248 mL CH4/g VS) production were observed under high OLR (> 10 g VS/ L.d) for a two-phase thermophilic continuous stirred tank reactor (CSTR). But under similar OLR the one-phase thermophilic CSTR failed because of acetate and propionate accumulation in the methane reactor (Luo et al. 2010). Another example from the pilot-scale (50L) two-stage AD process, the gas production from FW increased up to 13,000–15,000 L/d due to an increase in OLR at a low HRT of 3.9 days (Lee and Chung 2010).
Jiang et al. (2013) studied the VFA production from FW at 3 different OLR (5, 11, and 16 g TS/L) and constant temperature (35 °C), pH (6.0), and HRT (5 days). The researcher found that the VFA production increased sharply at the initial days (7–14 days) then remained stable for OLR of 5 and 16 g TS/L. However, at OLR of 16 g TS/L, the VFA production increased by 12 days then decreased sharply. Acetate and Butyrate were the dominant (60%) VFA produced at all OLR. Though there is an insignificant change in VFA fraction at high OLR (Paudel et al. 2016; Cheah et al. 2019), Amha et al. (2019) also recorded an increase in VFA production while increasing OLR. Because increasing OLR is attributed to the diverse microbial community (Srisowmeya et al. 2020).
Nevertheless, the two-stage AD process for FW can operate at high OLR but within controlled HRT and pH (Lim et al. 2008; Srisowmeya et al. 2020). High OLR provides excess carbon for degradation inducing acidic conditions in the reactor in the long-term. Maintaining the pH (5–6.5) for DF and low HRT (2–3 days) that helps to wash-out methanogenic microorganisms provides an optimum condition for biohythane and VFA production at high OLR in a two-stage AD process (Cavinato et al. 2016; Paudel et al. 2016).
Recirculation ratio (RR) of second stage-effluents
The recirculation ratio (RR) refers to the ratio of the returned volume of the second-stage reactor effluent to the volume of the first stage reactor influent. The digestate from the methanogenic stage is recirculated to maintain the pH of the first phase reactor (Algapani et al. 2019). This innovative strategy utilizes the residual buffer capacity of digestate to supply nutrients in the first stage reactor, i.e., DF reactor. This has proved to enhance the biohythane production for two-stage reactors and improves its cost-effectiveness (Micolucci et al. 2014; Cavinato et al. 2011).
In a study conducted by Yeshanew et al. (2016), the supernatant effluent from AFBR was fed to CSTR at a RR ranging from 0.24–0.48, 0.5–0.8, and 0.6–1 for three different periods, respectively. An improved and stable hydrogen production rate of 178.2, 253.5, and 391.7 L H2/m3.d in periods I, II, and III, respectively was obtained (Yeshanew et al. 2016). Similarly, Algapani et al. (Algapani et al. 2019) stated that the RR reduces the need for external chemicals to maintain the pH in the H2 reactor by 54%. It also increased the H2 by 8% and decreased the CH4 production by 3%. However, there was no difference in the total energy production (Algapani et al. 2019).
According to Chinellato et al. (2013), the first stage reactor produced CH4 at a high OLR of 20 kg TVS/m3/d instead of H2. However, with the RR of 2.9, the highest H2 production from FW was obtained at 20 kg TVS/m3/day. Similarly, for the VFA production from LBR, improving the leachate recirculation rate improved the hydrolysis performance by 10–16% and the acidification yield increased to 340 g COD/kg TVS added (Hussain et al. 2017).
Although many such experiments have proved to enhance the efficiency of biohythane production (Chu et al. 2008; Chinellato et al. 2013; Cavinato et al. 2016), the increased ammonia concentration in the first reactor could confine the performance of anaerobic microorganisms known for both H2 and CH4 production (Cavinato et al. 2012; Algapani et al. 2019). Therefore, it is recommended to daily observe the ammonia concentration and remove the recirculation of the second reactor effluent once the ammonia concentration reaches its maximum stable value (Cavinato et al. 2012).
Based on the literature, the optimum conditions in the two-stage AD process for H2, VFA, and CH4 production from the FW are summarized in Table 4. H2 production from DF was found to be suitable within the acidic range unlike for VFA and CH4 production. The selection of pH value for VFA also depends upon the targeted organic acid. Similarly, the temperature range is within the mesophilic to thermophilic conditions. From the limited available studies, a low range of HPP (below atmospheric pressure) is suitable for the DF-AD process. The OLR and RR vary depending upon the HRT and the reactor size.
DF-AD process integration
The coupling of DF and AD as two-staged biorefinery is observed to be more productive in terms of energy recovery not only because of the production of biohydrogen but also because of higher yields of CH4 compared to an uncoupled system (Ruggeri et al. 2015; Ghimire et al. 2021). This is seen as a result of biological pretreatment offered by DF by breaking the nutrients in the substrate down to VFAs (Malave’ et al. 2015; Malavè et al. 2018). The reactor type and configuration strongly influence the coupled system, especially through biomass retention (Show et al. 2011).
It is seen that the DF-AD system is integrated widely in CSTRs connected in series with a batch, continuous or semi-continuous feeding, and effluent extraction mechanism (Table 2). In batch mode, the substrate is added to the digester and inoculated for complete digestion, whereas, in continuous feeding mode, the substrate is fed continuously or semi-continuously, and biogas is collected continuously (Stalin and Prabhu 2007). The CSTR is suitable for FW with a TS of 2–12% (Liu et al. 2013). Moreover, two CSTRs have been combined vertically that produced maximum H2 and CH4 content of 8.6% and 48%, respectively, from the FW at HRT of 2 days (Vo et al. 2019). The popularity of CSTR is attributed to its simplicity, uniform mixing, and suitability with any kind of substrate (Saratale et al. 2019). However, at HRTs lower than 2.5 days, CSTR enabled mass cell wash-out from the reactor (Kongjan and Angelidaki 2010).
For the two-stage process stability and better performance, CSTR has also been combined with high state reactors like AFBR, UASB, Up-flow Anaerobic Packed Bed (UAPB), and Expanded Granular Sludge Bed (EGSB) (Lay et al. 2010; Liu et al. 2013; Cisneros-Pérez et al. 2017). These wet reactors are suitable for liquid substrate (TS < 2%) and facilitate biomass retention for a longer time which is appropriate for slow-growing methanogenic organisms; hence, mostly used as the second-stage reactors in the two-stage AD process. (Liu et al. 2013; Nualsri et al. 2016; Van et al. 2020).
A study by Ren et al. (2010) has concluded that attached sludge CSTR is more stable than suspended sludge CSTR. A study comparing CSTR and AFBR in immobilized and suspended cell systems concluded that DF is enhanced in reactors that aid biofilms (Qureshi et al. 2005). The recirculation strategy in these reactors' configurations allows maximum H2 production from DF even at HRT of less than 2 days (Yeshanew et al. 2016).
A settler tank can also be added in between CSTR and UASB, or similar reactors for solid–liquid separation. Lee and Chung (2010) developed the first pilot-scale system consisting of CSTR (500 L) as DF, UASB (2300 L) as AD fed with FW liquid using anaerobic sludge as the inoculum, and a fuel cell fed with the purified biohydrogen. However, CSTR and UASB have been successfully integrated for biohythane recovery from sugarcane without the need for a separation tank in between (Nualsri et al. 2016).
Another novel two-stage AD process has been an integration of dry reactors like LBR with UASB for biohythane (Han and Shin 2004) and VFA production (Browne et al. 2013; Yan et al. 2019) from FW. The dry reactor is suitable for FW with TS ≥ 15%(Liu et al. 2013; Van et al. 2020). Han and Shin (2004) used BIOCELL (LBR-UASB) which has VS conversion efficiency to H2 was 28.2% and CH4 69.9%, respectively, from FW. The BIOCELL demonstrated stability through the resource recovery process. LBR has several advantages to CSTR as LBR doesn’t require substrate dilution reducing process water, no stirrer for mixing that saves energy and the leachate can be recycled without passing it through the solid–liquid separation unit (Browne et al. 2013; Hussain et al. 2017). Similarly, the bacterial dynamics in LBR can degrade even the resistant dietary fibers present in FW (Xiong et al. 2019).
Various types of membrane filtration reactors (MFR) such as microfiltration, nanofiltration, evaporation, electro-dialysis, and ultrafiltration have been used to separate the VFAs (Zacharof and Lovitt 2014; Sasiradee et al. 2017). Short Chain Fatty Acids (SCFA) yield of 7453 mg COD/ L was recorded from the integration of alkaline sludge fermenter and membrane separation unit (Longo et al. 2015). The combination of anaerobic membrane filtration technologies is more efficient than the single step in recovering the high quality of targeted organic acid (Sasiradee et al. 2017; Sikder et al. 2012). Besides, the appropriate selection of adsorption media is essential in membrane filtration technology as it affects the removal efficiency (Uslu 2009). Out of the four different ion exchange resins (IRA-900, IRA-400, IRA-96, and IRA-67), Luongo et al. (2019) recorded IRA-67 recovered 97% of LA in batch test whereas the desorption efficiency in fixed bed reactor was only 68%. Similarly, Amha et al. (2019)found ceramic membrane favorable for FW treatment in long-term without irreversible fouling.
The working volume and flow rates of DF and AD systems vary according to the operating mechanism and the speed of reactions. The difference between the equivalent working volumes of the different stages of anaerobic is symbolized by the coupling ratio. Contorted coupling ratio could result in reactor failure or inefficient operation (Srisowmeya et al. 2020). From Table 2, it can be observed that the size of the second reactor is either equal to or greater than that of the first reactor in all the cases. As DF has the potential to process higher OLR than AD, the reactor size for AD is correspondingly seen to be higher than that of DF (Ren et al. 2011).
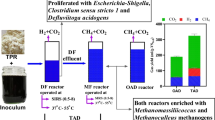
The DF-AD reactor configurations for FW experimented at laboratory or pilot scale have been shown in Fig. 3. The second-stage reactor configuration depends upon the characteristics of effluent from the first stage reactor (Liu et al. 2013). Similarly, the performance of each reactor along with its drawback is summarized in Table 5.
Overall, the selection of DF-AD reactor configuration depends upon the TS content of the feedstock, targeted VFA, and process stability required. In addition to a combination of two-stage reactors, an additional unit like aerobic denitrification tank (Lee et al. 2010) and zeolite adsorption unit (Petracchini et al. 2018) for further treatment of leachate before recycling to the first reactor. This is done to avoid the inhibition by ammonia in the first reactor and ensure long-term stability.
Pretreatment of FW and inoculum
FW composition is heterogeneous which may cause technical instability in the two-stage AD process (Cesaro and Belgiorno, 2014), especially during the start-up phase. The carbohydrate-rich FW is favorable for DF whereas, protein and lipid are difficult to degrade. To catalyze the decomposition process in DF, the inoculum is added. The inoculum obtained from the sludge of other waste treatment plants and breweries contains mixed culture (Alibardi and Cossu 2015; Cappai et al. 2018; Algapani et al. 2019). The mixed culture induces ecological interactions and competition between various H2 consuming and H2 producing microorganisms for the same organic substrate which ultimately reduces the performance of the reactor. The H2 producing microorganisms have a better chance to survive the harsh conditions during the pretreatment of inoculum than the non-spore-forming bacteria such as methanogens and can germinate when favorable conditions are provided to them (Li and Fang 2007).
The pretreatment of FW is usually done to improve the hydrolysis of hardly biodegradable compounds, while inoculum pretreatment is done to enrich H2 producing microorganisms and inactivate the consumers like methanogens during the start-up of the DF process (Wang and Yin 2017) and improve H2-CH4 yield in two-stage AD process (Lee and Chung 2010; Yin et al. 2014). The inoculum pretreatment may be only necessary for the start-up phase while the FW pretreatment is a continuous process. The common pretreatment measures adopted by the researchers for the FW and the enrichment of H2 producers are explained in the following sections.
Heat treatment
Heat treatment of inoculum for the enrichment appears to be common, simple, inexpensive, and effective (Li and Fang 2007; Wang and Wan 2008). During the heat-shock treatments, the inoculum is heated at 100° C for 30 min under atmospheric pressure (Jariyaboon et al. 2015; Akhlaghi et al. 2019; Sun et al. 2019). The acidogenic bacteria can form spores and ensure their survival whereas methanogens are deactivated in those conditions (Li and Fang 2007). The results after heat treatment did not show any traces of CH4 in the gas composition obtained after DF. Wang and Wan (2008) found out that heat-shock treated digested sludge increased the substrate degradation efficiency by 97.2%. It further improved H2 yield (221.5 mL/g glucose) and biomass concentration (2739 mg/L). In DF, Ghimire et al. (2016) reported that H2 yield from heat-shock treated activated sludge increased by two folds than the anaerobic sludge.
The substrates can be pretreated to enhance the conversion of substrates to intended products. During the heat pretreatment such as steam explosion, the substrate is heated under high temperature (160–260 °C) and pressure (7–50 bar) for a short duration (30 s–20 min) (Keskin et al. 2019). Then, the pressure is quickly released which causes an explosive effect. This pretreatment is suitable for substrates with high lignocellulose (Eg: wheat straw, corn starch) that is commonly found in food industry waste. The explosion improves its digestibility by 90% than steam pretreatment without explosion (Pielhop et al. 2016). This method has been commonly used in the solubilization of sewage sludge (Donoso-Bravo et al. 2015; Dereix et al. 2006) but can be also utilized for the FW pretreatment. However, the steam explosion pretreatment of FW makes it more dilute decreasing the acetic acid and lactic acid concentration (Svensson et al. 2018).
Another similar heat pretreatment method under high temperature and pressure is known as hydrothermal pretreatment. In this method, at high temperature and pressure, the ionized products of water are increased which hydrolyze the macromolecules resulting in a noticeable improvement in FW fermentation (Yin et al. 2014). The suitable temperature was reported to be 160 °C, which gave a VFA yield of 0.908 g/g VSremoval, 47.6% higher than the control. However, higher temperatures can result in the production of toxic compounds that inhibit microbial activities. Similarly, Ding et al. (2017) recorded 140 °C as the optimum temperature for hydrothermal heat pretreatment of FW as it favored efficient solubilization of carbohydrates and protein. The H2 and CH4 yield from two-stage AD was recorded to be 43.0 mL/g VS and 511.6 mL/g VS. The energy conversion efficiency of pretreated FW increased by 31.7% than the untreated FW.
Chemical treatment
Methanogens are strict anaerobes and are very sensitive to many chemicals. It has been found that CH4 production drops sharply at a pH of below 6.3 or above 7.8 (Li and Fang 2007). The chemical treatment involves the introduction of acidic/alkali compounds to maintain the desired pH of the reactor. Acid pretreatment is efficient in solubilizing carbohydrates whereas alkali treatment is suitable for solubilizing protein, lipids, and lignin (Parthiba Karthikeyan et al. 2018).
Jang et al. (2015) also recorded the highest H2 production by alkali-shock treatment of FW with 6 N KOH at pH 11 and 12 and without using any co-substrate. Vavouraki et al. (2013) also recorded that pretreatment of a kitchen waste under 1.12% HCl for 94 min or 1.17% HCl for 86 min (at 100 °C) increased the soluble sugar concentration by 120% compared to the untreated kitchen waste. According to Lamaison et al. (2015), acid treatment of the anaerobically digested sludge from sugarcane vinasse treatment plant is better than the heat-treated sludge in long-term. Because the acid pretreated sludge favored the growth of Clostridiaceae microbes which consumes the lactate concentration that is known for H2 inhibition. Likewise, the alkaline pretreatment of raw rice straw using 4% and 8% NaOH at 55 °C for 24 h, increased the H2 yield by 26 and 57-fold, respectively (Ghimire et al. 2016).
The chemicals like sodium 2-bromoethasulfonic acid (BESA), iodopropane, chloroform, and acetylene are commonly used as methanogenic chemical inhibitors (Wang et al. 2011; Li and Fang 2007; Zhu and Beland 2006; Venkata et al. 2008; O-Thong et al. 2009). BESA inhibits the activity of the co-enzyme M reductase complex which is a chief component for methanogenesis (Venkata et al. 2008; Zhu and Beland 2006). According to Zhu and Beland (2006), BESA is the most cost-effective pretreatment method for inhibiting methanogens without impacting the H2 producing microorganism in digested sludge.
Another chemical pretreatment with ozone is quite popular for waste-activated sludge (Carrère et al. 2010) but it is least explored with FW. Ariunbaatar et al. (2014) obtained a negligible increase in CH4 production from the ozone-treated FW. This could be mainly because a high amount of fermentable sugar lost at a high ozone dose.
The chemical pretreatment has been combined with heat and ultrasonic treatment for the effective conversion of FW to H2 and CH4. Monlau et al. (2012) compared seven different types of thermo-chemical pretreatment of sunflower stalk. Chemicals (NaOH, H2O2, Ca (OH)2, HCl, and FeCl3) were used to vary the pH from 2.3 to 12.2, and the temperature was maintained at 55 °C and 170 °C. Alkaline pretreatment (4% NaOH) at 55℃ for 24 h is recommended for the lignocellulose substrate (Monlau et al. 2012). Similarly, Elbeshbishy et al. (2011) found that the combination of ultrasonic sound and acid produced the highest H2 yield of 118 mL/g VSinitial and VFA concentration 16,900 mg COD/L.
The addition of chemicals is found to be quite an expensive pretreatment method and hence, least preferred. Moreover, concentrated acid pretreatment is required for the efficient hydrolysis of biomass with high cellulose content. As acids are highly corrosive, specific materials are needed to construct the reactor. This adds up to the capital cost making it an expensive option (Keskin et al. 2019). Similarly, the FW or inoculum needs to be neutralized after the chemical treatment which makes it complicated (Banu et al. 2020).
Other pretreatment methods
In the AD process, oxygen is avoided as it inhibits the activity of anaerobic fermentation microorganisms. However, aeration pretreatment of FW in single-stage AD process has been proven to enhance microbial diversity leading to improved hydrolysis and acidogenesis process (Lim and Wang 2013; Girotto et al. 2016). Even in a two-stage process, pre-aeration of FW has increased VFA production (Xu et al. 2014). According to Rafieenia et al. (2017), the pre-aerated FW produced less H2 than the non-aerated FW. But the CH4 production increased in the second reactor, especially the protein-rich FW produced the highest CH4 (351 ml/gV). The effect of aeration pretreatment depends upon the FW composition, aeration time, intensity, and air introduction method (Girotto et al. 2016; Rafieenia et al. 2017).
Moreover, researchers have also studied the effect of aeration pretreatment of inoculum on H2 production (Wang and Wan 2008; Zhu and Beland 2006). The comparison of the results of pretreatment methods (i.e., acid, base treatment, heat shock, aeration, and chloroform) showed that the inoculum pretreated with aeration has higher cumulative H2 production than the inoculum treated with chloroform and untreated inoculum (Wang and Wan 2008).
Load shock as a pretreatment was also carried out to obtain high glucose loading of 50 g/L at the beginning of fermentation (Jariyaboon et al. 2015). Different pretreatment methods of inocula for optimizing hydrogen production conducted by (Chang et al. 2011) consisted of acid, base, heat-shock, aeration, chloroform, and 2-bromoethanesulfonate treatment. Acid pretreatment at pH 3 yielded the best result with maximum hydrogen production of 22.81 mmolH2/gVSS from activated waste sludge.
Cisneros-Pérez et al. (2017) compared a cell wash-out treatment and heat pretreatment of the inoculum in an anaerobic fluid bed reactor (AFBR). This study showed that cell wash-out treatment had higher H2 volumetric production rates (7 L H2/L-d) and yields than thermal treatment (3.5 mol H2/mol hexose). The effect of pretreatment on H2 production is different based on different sources of inoculum. However, the selection of the pretreatment method should be based on the use of effluent from dark fermentation. For example, the selection of chemical treatment methods such as using BESA could create a problem when the effluents from dark fermentation are meant to be used for methane production in the second stage.
For FW with a high TS loading rate, ultrasonic pretreatment could be a good option (Gadhe et al. 2014). Ultrasonic pretreatment mechanically disrupts the physical, biological, and chemical properties of a substrate/ inoculum under varying frequency (Pilli et al. 2011). Gadhe et al. (2014) recorded 75% enhanced H2 production by ultrasonically treating the FW of TS content72,500 mg/L at 1200 W for 5, 10, and 15 min depending upon the specific energy input. Elbeshbishy and Nakhla (2011) also recorded increased efficiency in FW degradation along with an increase in H2 and CH4 production rates. But, ultrasonic pretreatment is energy-intensive and expensive hence, requires optimization of operational parameters to set up a full-scale plant (Pilli et al. 2011).
Moreover, commercial enzymes (glucoamylase, proteases, viscozyme, and lipases) have also been used to enhance hydrolysis (Moon and Song 2011; Uçkun Kiran et al. 2015; Donoso-Bravo et al. 2015). According to a study, a mixture of enzymes resulted in higher VFA production than the use of a single enzyme (Kim et al. 2006a). But, the cost of treating 1 ton of FW using glucoamylase is around USD 120 which is expensive (Uçkun Kiran et al. 2015). Therefore, microorganisms such as fungal biomass that is rich in glucoamylase and protease have been used for the efficient production of biomethane from FW (Uçkun Kiran et al. 2015).
Several researchers have reviewed and compared various pretreatment technologies for substrate and inoculum within the biorefinery framework (Galbe and Wallberg 2019; Bhatia et al. 2020; Karthikeyan et al. 2018; Banu et al. 2020). Although thermal pretreatment of FW has been commonly practiced because of its simplicity and cost-effectiveness, not a single pretreatment method could be termed as the “best”(Galbe and Wallberg 2019). The choice of pretreatment technologies depends upon the desired end product and the FW composition (Banu et al. 2020). Some of the researchers have also integrated more than two pretreatment methods (such as acid-thermal) to optimize the performance of the two-stage AD process.
Utilization of VFAs for polyhydroxyalkanoates (PHAs), polylactate, and microalgal biomass production.
With the increasing concern for sustainability and the environment, bio-plastics are becoming popular. By 2025, global demand for bio-based plastics will increase to 2.87 million tonnes from 2.11 million tonnes in 2020 (European Bioplastics 2018). One of the precursor chemicals used for biodegradable plastic production is polyhydroxyalkanoates (PHAs). PHAs are a type of bio-polyesters accumulated by different bacterial cells in the form of granules inside the cytoplasm. The unique thermal and mechanical behavior makes it a completely biodegradable and suitable alternative to plastics (Reis et al. 2011). Producing PHAs from VFA under low temperature, HRT conditions to prevent methanogenic activity has been known to be an affordable process (Beccari et al. 2009). Besides, using mixed microbial culture and VFA rich substrates like FW from AF and sewage sludge reduces the PHA production cost while optimizing PHA yield at the industrial level (Van Aarle et al. 2015; Serafim et al. 2004). Perez-Zabaleta et al. (2021) obtained the highest PHA concentration (3.3 g/L) and content (43.5% w/w) from VFA effluent rich in caprionic and acetic acid, respectively.
Within VFA, butyrate was recorded to produce 57% of polyhydroxybutyrate (PHB) which is higher than with acetate at 54% in sequence batch reactor (SBR), making it the desirable substrate for PHBs (Marang et al. 2013). The production of PHA from organic acid derived from the OFMSW has also been studied for a pilot-scale anaerobic percolation bio cell reactor (100 L). The study showed a PHA production of 223 ± 28 g/kg with a hydroxybutyrate/hydroxyvalerate (%) ratio of 53/47 under optimized acidogenic fermentation (Colombo et al. 2017). Though FW is a potential source to produce PHA, it could be an expensive method considering the pretreatment of FW, transportation cost, and microbial strain selection (Nielsen et al. 2017).
The DF effluents containing VFA can be also used for the production of microalgal biomass (Fei et al. 2015; Turon et al. 2015; Ren et al. 2014). Microalgae are unicellular microorganisms that can be a rich source of carbon compounds. The use of microalgae is increasing especially in the industrial sector as biofuels, by-products, health supplements, pharmaceuticals, and cosmetics (Chalima et al. 2017). They have also been applied for wastewater treatment and CO2 sequestration (Krishna et al. 2012). According to Lacroux et al. (2020), the growth of a mixed group of microalgae depends upon the concentration of VFA determined in the ranges of 71–207 mg/L and 13–25 mg/L as threshold concentrations for microalgae growth in acetic acid and butyric acid, respectively.
VFA is a byproduct derived from the fermentation of FW and thus, is known to be a cost-effective carbon source. This could be a possible alternative to expensive refined glucose for large-scale microalgal production. Fei et al. (2011) carried out a preliminary cost analysis on using VFA for cultivating C. albidus for biodiesel production. The study showed that VFAs-based biodiesel production was affordable compared to the use of agricultural products for lipid accumulation. In another study, Fei et al. (2015) used VFA as a carbon source to yield 0.187 g/g lipid coefficient and 48.7% lipid content of Chlorella protothecoides.
Moreover, the microalgae can directly convert the VFA into acetyl-CoA by acetyl coenzyme-A synthetase which could be used for fatty acid biosynthesis and lipid accumulation. Therefore, the use of VFA for microbial mass production could be favorable in terms of time and money. However, limited researches are available on the use of VFA derived from organic waste for microbial cell growth and especially lipid production. Most of the researches has focused on VFA production from microalgae. Therefore, there is a huge scope for further process development on using VFA for microbial-derived protein production for animal feed and energy biomass.
Economic prospects of biohythane and biochemicals
Besides the optimization of the two-stage AD process, some studies covered the economic viability for the production of biohythane and biochemical (Urbaniec and Grabarczyk 2014; Han, et al. 2016a, b; Bastidas-Oyanedel and Schmidt 2018). Bastidas-Oyanedel and Schmidt (2018) compared the economic advantage of combining DF with LA fermentation technologies for the conversion of 50 tonnes/day of FW into CH4, power generation, LA, PLA, H2, acetic acid, and butyric acid. Besides the power generation, all other alternatives were profitable. The highest profit of 296 $/tonne VS was obtained from DF with the separation and purification of butyric acid.
Similarly, the novel biohythane process (pretreatment, DF with Caldicellulosiruptor saccharolyticus and AD) resulted in high H2 productivity with 6.1 L/L/d of pure biohythane and 69% of energy recovery from the sugar fraction of the wheat straw. But, the sparging of DF has high energy demand leading to the increased operational cost. Moreover, the addition of nutrients for high H2 productivity also added to the total production cost of 160 Euro/GJ biofuel (Willquist et al. 2012). A two-stage AD of sugarcane (vinnase) showed 20–30% better economic performance than single-stage AD (Fuess et al. 2018). The net present value (NPV) was found to be $208.58–219.86 million. Besides, optimizing the alkalization of methanogenic systems improves both the economic and environmental performance of AD plants by reducing operating costs and the risks associated with human toxicity and freshwater eutrophication (Fuess et al. 2018).
Another large scale (50 m3) DF was found to be economically feasible as its return on investment was higher than the DF of 10 m3. The net present value, payback period, and internal rate of return with a scale of 50 m3 were USD 526,551, 6.9 years, and 9.25%, respectively. However, the major drawback of DF was the low hydrolysis rate and nutrient conversion efficiency (Han, et al. 2016a, b). Besides AD, DF has been integrated with solid-state fermentation (SSF) reactor to produce H2 from FW at a large scale. Considering the waste from the food industry, the H2 production cost is comparatively low (Table 6). The unit cost of an H2 production plant with a capacity of 10 tonnes/day and a lifetime of 10 years was USD 2.29/m3 (25.45 USD/kg) which was less than the market price of USD 2.7/m3 (30.0 USD/kg) (Han, et al. 2016a, b).
Similarly, Urbaniec and Grabarczyk (2014) compared the H2 production cost under three different scenarios: base, update, and optimistic whose values are based on knowledge, research, and considering two-stage will be fully developed in future, respectively. The H2 production cost (9.3 Euro/kg) was found lowest in the optimistic case.
According to Ljunggren and Zacchi (2010), the main contributors to the H2 production cost are the capital costs and the nutrients added. However, with cheap FW as substrate and effluent recirculation in two-stage AD can remove these hurdles. Recently, Li et al. (2020) demonstrated that a large-scale two-stage AD process that treats 216 tons of FW daily is technically and economically feasible. The research team estimated the total investment cost of large scale to be 24.34 million Yuan (1 Yuan is equivalent to 0.15 USD). And, with the selling price of biogas as 0.46 USD/m3, the total investment can be paid back in 1.5 years. Similarly, the advantage of the biorefinery system converting waste to multiple products (energy, biochemical, & nutrients) and its environmental benefit, trade-off the cost in long run (Krishnan et al. 2019). Nevertheless, more research on the techno-economic study of the two-stage conversion of FW has to be realized in future.
Currently, the industrial production of VFAs is mainly conducted using chemical processes like the oxidation or carboxylation of precursors such as aldehyde and alkenes. However, the continual use of energy-intensive processes contributes to CO2 emissions and ecological imbalances (Sivagurunathan et al. 2018). The feasibility study of DF conducted in Abu Dhabi showed DF as a promising biorefinery technology not only for the treatment of OFMSW but also for VFA production. The VFA is a versatile chemical as it can be further developed into biofuel or bioplastic in the downstream process. Given a waste tipping fee of 22 USD/tonnes of OFMSW, VFA could be used in downstream processes without any charge. This could make advancement and investment in technology a viable option. The maximum cost of VFA production from DF was calculated to be 15 USD/m3 effluent (Bonk et al. 2015).
The market size of organic acids in 2013 was reported to be 2.9 million tonnes with a market size of 3.5 billion USD. The market annual growth rate (AAGR) until 2020 is 8.8% (Sun et al. 2019). Table 7 shows the bulk market price and size of the direct DF products and value-added products that can be produced utilizing the DF effluents. Likewise, the bio-polymers (polylactic acid/PLA, PHA) are showing moderate market growth of 9–30% per annum with good future demand for the year 2023 (Sun et al. 2019).
The microbial pathway for biochemical production also influences the cost and yield (Manandhar and Shah 2020; Francois et al. 2020). A study for large-scale (10,000 metric tons per annum) production of lactic acid (LA) from corn determined the production cost of $1181, $1251, and $844 through bacteria, fungi, and yeast pathways were, respectively. The lowest production cost from yeast was due to the lower requirement of chemicals, equipment, and utilities for neutralization and recovery of LA. Moreover, the influencing factors for LA production cost are sugar-to-lactic-acid conversion rates, grain price, plant size, annual operation hours, and gypsum use (Manandhar and Shah 2020). Similarly, the chemical structure of feedstock is another factor affecting the production cost. Based on the study by Daful and Görgens (2017), the cellulose fraction of sugarcane bagasse and brown leaves (SCBL) is economically and environmentally more viable than the hemicellulose fraction of SCBL.
Although FW is a potential source to produce organic acids and biohydrogen, it could be an expensive method considering the pretreatment of food waste, transportation cost, and microbial strain selection (Nielsen et al. 2017; Yun et al. 2018). However, Monlau et al. (2015)suggested that alkaline pretreatment could be economically sustainable if it is applied at a high substrate concentration and/or maximum heat is recovered during pretreatment. Similarly, Colombo et al. (2017) recommended that optimizing the acidic fermentation process (Eg: recirculating the digestate) could support the organic acid formation from OFMSW at a large scale. Also, DF consumes less energy than other conventional technologies for H2 and VFA production (Bonk et al. 2015). Therefore, the two-stage AD biorefinery process from FW can be a sustainable route to achieving economic benefits with the least environmental impact (Dahiya et al. 2018).
Conclusion
Biorefinery framework considering two-stage AD process is a promising technology especially DF for H2 production along with biochemicals that have economic values. The selection of operational parameters depends upon the targeted VFA. Acetate and butyrate are the most abundant VFA produced from FW. Other parameters, OLR and RR also affect the stability of the reactor, biohythane and biochemical production. Generally, OLR and HRT are relative which means low HRT and high OLR enhance the biohythane and VFA production. To enhance the performance of two-stage AD, FW can be pretreated. However, the choice of pretreatment methods and optimum operational conditions depend upon the desired product. The DF-AD process integration also depends upon the TS content of the substrate, type of bioreactors, and coupling ratio.
Despite the high capital investment in two separate bioreactors systems, the DF-AD process can be economically viable if multiple fuels and biochemicals are recovered. Moreover, the market size for VFA production is expanding with an 8.8% growth rate. The two-step process can be a sustainable route to produce biohythane and biochemicals such as butyrate and PHA that have high economic values trades off the cost. Besides the use of biohythane in the industrial sector, the VFA can also be used to produce bioplastic and cultivate microalgae for further energy recovery. Furthermore, various bioreactors configuration within the DF-AD process for FW that can withstand the fluctuation in operational parameters for a longer-term needs to be explored. Similarly, a techno-economic analysis of biohythane and biochemicals recovery from the different FW compositions while optimizing the two-stage AD process needs to be conducted. This would help to determine the viability of a two-stage AD process with FW as a feedstock at a commercial scale.
Abbreviations
- BESA:
-
2-Bromoethasulfonic acid
- AD:
-
Anaerobic digestion
- AF:
-
Anaerobic fermentation
- AMFR:
-
Anaerobic membrane filtration reactor
- CO2 :
-
Carbon dioxide
- CSTR:
-
Continuous stirred tank reactor
- DF:
-
Dark fermentation
- EGSB:
-
Expanded granular sludge bed
- FW:
-
Food waste
- HRT:
-
Hydraulic retention time
- HCl:
-
Hydrochloric acid
- H2 :
-
Hydrogen
- HPP:
-
Hydrogen partial pressure
- LA:
-
Lactic acid
- LBR:
-
Leachate bed reactor
- LCA:
-
Life cycle assessment
- LCFA:
-
Long-chain fatty acid
- CH4 :
-
Methane
- NPV:
-
Net present value
- OFMSW:
-
Organic fraction of municipal solid waste
- OLR:
-
Organic loading rate
- PBR:
-
Packed bed reactor
- PHB:
-
Polyhydroxy butyrate
- PHA:
-
Polyhydroxyalkanoates
- PLA:
-
Polylactic acid
- KOH:
-
Potassium hydroxide
- RR:
-
Recirculation ratio
- SBR:
-
Sequence batch reactor
- SCFA:
-
Short chain fatty acid
- SRT:
-
Solids retention time
- NaOH:
-
Sodium hydroxide
- SCBL:
-
Sugarcane bagasse and leaves
- TS:
-
Total solid
- USD:
-
United state dollars
- UAPB:
-
Up-flow anaerobic packed bed
- UASB:
-
Up-flow anaerobic sludge blanket
- VFA:
-
Volatile fatty acid
- WAS:
-
Waste-activated sludge
References
Agler MT, Wrenn BA, Zinder SH, Angenent LT (2011) Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol 29(2):70–78
Akhlaghi M, Boni MR, Polettini A, Pomi R, Rossi A, De Gioannis G, Muntoni A, Spiga D (2019) Fermentative H2 production from food waste: Parametric analysis of factor effects. Bioresour Technol 276:349–360
Algapani DE, Qiao W, di Pumpo F, Bianchi D, Wandera SM, Adani F, Dong R (2018) Long-term bio-H2 and bio-CH4 production from food waste in a continuous two-stage system: Energy efficiency and conversion pathways. Biores Technol 248:204–213
Algapani DE, Qiao W, Ricci M, Bianchi D, Wandera M, S., Adani, F. and Dong, R. (2019) Bio-hydrogen and bio-methane production from food waste in a two-stage anaerobic digestion process with digestate recirculation. Renewab Energy 130:1108–1115
Alibaba (2020) Chinese e-commerce. Organic chemical bulk prices
Alibardi L, Cossu R (2015) Composition variability of the organic fraction of municipal solid waste and effects on hydrogen and methane production potentials. Waste Manag (New York, NY) 36:147–55
Alibardi L, Cossu R (2016) Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Manag 47:69–77
Amha YM, Corbett M, Smith AL (2019) Two-phase improves performance of anaerobic membrane bioreactor treatment of food waste at high organic loading rates. Environ Sci Technol 53(16):9572–9583
Anukam A, Mohammadi A, Naqvi M, Granström K (2019) A Review of the chemistry of anaerobic digestion: methods of accelerating and optimizing process efficiency. Processes 7(8):504
Ariunbaatar J, Panico A, Frunzo L, Esposito G, Lens PNL, Pirozzi F (2014) Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. J Environ Manage 146:142–149
Banu JR, Merrylin J, Usman TMM, Kannah RY, Gunasekaran M, Kim SH, Kumar G (2020) Impact of pretreatment on food waste for biohydrogen production: a review. Int J Hydrogen Energy 45(36):18211–18225
Bastidas-Oyanedel JR, Schmidt JE (2018) Increasing profits in food waste biorefinery-a techno-economic analysis. Energies, 11(6)
Bastidas-Oyanedel J-R, Bonk F, Thomsen MH, Schmidt JE (2015) Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev Environ Sci Bio/technol 14(3):473–498
Bastidas-Oyanedel JR, Bonk F, Thomsen MH, Schmidt JE (2019) The future perspectives of dark fermentation: moving from only biohydrogen to biochemicals. Biorefinery. Springer, Cham, pp 375–412
Beccari M, Bertin L, Dionisi D, Fava F, Lampis S, Majone M, Valentino F, Vallini G, Villano M (2009) Exploiting olive oil mill effluents as a renewable resource for production of biodegradable polymers through a combined anaerobic-aerobic process. J Chem Technol Biotechnol 84(6):901–908
Beckers L, Hiligsman S, Masset J, Hamilton C, Thonart P (2012) Effects of hydrogen partial pressure on fermentative biohydrogen production by a chemotropic Clostridium bacterium in a new horizontal rotating cylinder reactor. Energy Procedia 29:34–41
Bhatia SK, Jagtap SS, Bedekar AA, Bhatia RK, Patel AK, Pant D, Rajesh Banu J, Rao CV, Kim YG, Yang YH (2020) Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour Technol 300:122724
IEA Bioenergy. (2020). Biobased Chemicals A 2020 Update.
European Bioplastics (2018) Bioplastics market development update 2019.
Bolzonella D, Battista F, Cavinato C, Gottardo M, Micolucci F, Pavan P (2019) Biohythane production from food wastes. Elsevier B.V, New York
Bonk F, Bastidas-Oyanedel J-R, Schmidt JE (2015) Converting the organic fraction of solid waste from the city of Abu Dhabi to valuable products via dark fermentation – Economic and energy assessment. Waste Manage 40:82–91
Browne JD, Allen E, Murphy JD (2013) Improving hydrolysis of food waste in a leach bed reactor. Waste Manage 33(11):2470–2477
Camacho CEG, Ruggeri B, Mangialardi L, Persico M, Malavé ACL (2019) Continuous two-step anaerobic digestion (TSAD) of organic market waste. Int J Energy Environ Eng 10:413–427
Cappai G, De Gioannis G, Muntoni A, Spiga D, Boni MR, Polettini A, Pomi R, Rossi A (2018) Biohydrogen production from food waste: Influence of the inoculum-to-substrate ratio. Sustainability (switzerland) 10(12):1–15
Capson-Tojo G, Rouez M, Crest M, Steyer JP, Delgenès JP, Escudié R (2016) Food waste valorization via anaerobic processes: a review. Rev Environ Sci Biotechnol 15(3):499–547
Capson-Tojo G, Rouez M, Crest M, Trably E, Steyer JP, Bernet N, Delgenès JP, Escudié R (2017) Kinetic study of dry anaerobic co-digestion of food waste and cardboard for methane production. Waste Manage 69:470–479
Carrère H, Dumas C, Battimelli A, Batstone DJ, Delgenès JP, Steyer JP, Ferrer I (2010) Pretreatment methods to improve sludge anaerobic degradability: a review. J Hazard Mater 183(1–3):1–15
Cavinato C, Bolzonella D, Fatone F, Giuliano A, Pavan P (2011) Two-phase thermophilic anaerobic digestion process for biohythane production treating biowaste: Preliminary results. Water Sci Technol 64(3):715–721
Cavinato C, Giuliano A, Bolzonella D, Pavan P, Cecchi F (2012) Bio-hythane production from food waste by dark fermentation coupled with anaerobic digestion process: a long-term pilot scale experience. Int J Hydrogen Energy 37(15):11549–11555
Cavinato C, Bolzonella D, Pavan P, Cecchi F (2016) Two-phase anaerobic digestion of food wastes for hydrogen and methane production. In: pp 75–90
Cazier EA, Trably E, Steyer JP, Escudie R (2015) Biomass hydrolysis inhibition at high hydrogen partial pressure in solid-state anaerobic digestion. Biores Technol 190:106–113
Chalima A, Oliver L, De Castro LF, Karnaouri A, Dietrich T, Topakas E (2017) Utilization of volatile fatty acids from microalgae for the production of high added value compounds. Fermentation 3(4):1–17
Chang S, Li J, Liu F (2011) Evaluation of different pretreatment methods for preparing hydrogen-producing seed inocula from waste activated sludge. Renew Energy 36(5):1517–1522
Cheah YK, Vidal-Antich C, Dosta J, Mata-Álvarez J (2019) Volatile fatty acid production from mesophilic acidogenic fermentation of organic fraction of municipal solid waste and food waste under acidic and alkaline pH. Environ Sci Pollut Res 26(35):35509–35522
Chen Y, Luo J, Yan Y, Feng L (2013) Enhanced production of short-chain fatty acid by co-fermentation of waste activated sludge and kitchen waste under alkaline conditions and its application to microbial fuel cells. Appl Energy 102:1197–1204
Chen X, Yan W, Sheng K, Sanati M (2014) Comparison of high-solids to liquid anaerobic co-digestion of food waste and green waste. Biores Technol 154:215–221
Cherubini F, Bird ND, Cowie A, Jungmeier G, Schlamadinger B, Woess-Gallasch S (2009) Energy- and greenhouse gas-based LCA of biofuel and bioenergy systems: Key issues, ranges and recommendations. Resour Conserv Recycl 53:434–447
Chinellato G, Cavinato C, Bolzonella D, Heaven S, Banks CJ (2013) Biohydrogen production from food waste in batch and semi-continuous conditions: evaluation of a two-phase approach with digestate recirculation for pH control. Int J Hydrogen Energy 38(11):4351–4360
Cho HU, Kim YM, Choi Y-N, Kim HG, Park JM (2015) Influence of temperature on volatile fatty acid production and microbial community structure during anaerobic fermentation of microalgae. Biores Technol 191:475–480
Chu CF, Li YY, Xu KQ, Ebie Y, Inamori Y, Kong HN (2008) A pH- and temperature-phased two-stage process for hydrogen and methane production from food waste. Int J Hydrogen Energy 33(18):4739–4746
Cieciura-Włoch W, Borowski S (2019) Biohydrogen production from wastes of plant and animal origin via dark fermentation. J Environ Eng Landsc Manag 27(2):101–113
Cisneros-Pérez C, Etchebehere C, Celis LB, Carrillo-Reyes J, Alatriste-Mondragón F, Razo-Flores E (2017) Effect of inoculum pretreatment on the microbial community structure and its performance during dark fermentation using anaerobic fluidized-bed reactors. Int J Hydrogen Energy 42(15):9589–9599
Clomburg JM, Gonzalez R (2013) Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol 31(1):20–28
Colombo B, Favini F, Scaglia B, Sciarria TP, D’Imporzano G, Pognani M, Alekseeva A, Eisele G, Cosentino C, Adani F (2017). Enhanced polyhydroxyalkanoate (PHA) production from the organic fraction of municipal solid waste by using mixed microbial culture. Biotechnology for Biofuels, 10(1)
Daful AG, Görgens JF (2017) Techno-economic analysis and environmental impact assessment of lignocellulosic lactic acid production. Chem Eng Sci 162:53–65
Dahiya S, Kumar AN, Shanthi Sravan J, Chatterjee S, Sarkar O, Mohan SV (2018) Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour Technol 248:2–12
Dasa KT, Westman SY, Millati R, Cahyanto MN, Taherzadeh MJ, Niklasson C (2016) Inhibitory effect of long-chain fatty acids on biogas production and the protective effect of membrane bioreactor. BioMed Res Int
De Gioannis G, Friargiu M, Massi E, Muntoni A, Polettini A, Pomi R, Spiga D (2014) Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int J Hydrogen Energy 39(36):20930–20941
De Gioannis G, Muntoni A, Polettini A, Pomi R, Spiga D (2017) Energy recovery from one- and two-stage anaerobic digestion of food waste. Waste Manage 68:595–602
De Kok S, Meijer J, Van Loosdrecht MCM, Kleerebezem R (2013) Impact of dissolved hydrogen partial pressure on mixed culture fermentations. Appl Microbiol Biotechnol 97(6):2617–2625
Dereix M, Parker W, Kennedy K (2006) Steam-explosion pretreatment for enhancing anaerobic digestion of municipal wastewater sludge. Water Environ Res 78(5):474–485
Ding L, Cheng J, Qiao D, Yue L, Li YY, Zhou J, Cen K (2017) Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Biores Technol 241:491–499
Ding J, Zhao XL (2018) Effect of hydrogen partial pressure control on fermentative hydrogen production from organic wastewater. IOP Conf Ser Earth Environ Sci 188(1):012021
Dionisi D, Igwe V, Paton G (2019) Effect of the process conditions on the anaerobic fermentation of glucose for the production of chemicals. Biomass Conversion Biorefinery
do Carmo Lamaison, F, de Andrade PAM, Bigaton AD, Andreote FD, Antônio RV, Reginatto V (2015) Long-term effect of acid and heat pretreatment of sludge from a sugarcane vinasse treatment plant on the microbial community and on thermophilic biohydrogen production. Int J Hydrogen Energy 40(41):14124–14133
Donoso-Bravo A, Ortega-Martinez E, Ruiz-Filippi G (2015) Impact of milling, enzyme addition, and steam explosion on the solid waste biomethanation of an olive oil production plant. Bioprocess and Biosystems Engineering, 2015.
Elbeshbishy E, Nakhla G (2011) Comparative study of the effect of ultrasonication on the anaerobic biodegradability of food waste in single and two-stage systems. Biores Technol 102(11):6449–6457
Elbeshbishy E, Hafez H, Dhar BR, Nakhla G (2011) Single and combined effect of various pretreatment methods for biohydrogen production from food waste. Int J Hydrogen Energy 36(17):11379–11387
Esteban-Gutiérrez M, Garcia-Aguirre J, Irizar I, Aymerich E (2018) From sewage sludge and agri-food waste to VFA: Individual acid production potential and up-scaling. Waste Manage 77(2018):203–212
FAO, - Food and Agriculture Organization of the United Nations. (2013). Food wastage footprint.
Fatima A, Basak B, Ganguly A, Chatterjee PK, Dey A (2020) Biohydrogen production through dark fermentation of food wastes by anaerobic digester sludge mixed microbial consortium. Springer Singapore.
Fei Q, Chang HN, Shang L, dal Choi J, rae, Kim, N.J. and Kang, J.W. (2011) The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Biores Technol 102(3):2695–2701
Fei Q, Fu R, Shang L, Brigham CJ, Chang HN (2015) Lipid production by microalgae chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioprocess Biosyst Eng 38(4):691–700
Fenchel T, King GM, Blackburn TH (2012) Bacterial metabolism.
Francois JM, Alkim C, Morin N (2020) Engineering microbial pathways for production of bio-based chemicals from lignocellulosic sugars: current status and perspectives. Biotechnol Biofuels 13(1):1–23
Fuess LT, Klein BC, Chagas MF, Rezende AF, M.C., Garcia, M.L., Bonomi, A. and Zaiat, M. (2018) Diversifying the technological strategies for recovering bioenergy from the two-phase anaerobic digestion of sugarcane vinasse: An integrated techno-economic and environmental approach. Renewable Energy 122:674–687
Gadhe A, Sonawane SS, Varma MN (2014) Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int J Hydrogen Energy 39(15):7721–7729
Galbe M, Wallberg O (2019) Pretreatment for biorefineries: a review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol Biofuels 12(1):1–26
Ghimire A, Sposito F, Frunzo L, Trably E, Escudié R, Pirozzi F, Lens PNL, Esposito G (2016) Effects of operational parameters on dark fermentative hydrogen production from biodegradable complex waste biomass. Waste Manage 50:55–64
Ghimire A, Trably E, Frunzo L, Pirozzi F, Lens PNL, Esposito G, Cazier EA, Escudié R (2017) Effect of total solids content on biohydrogen production and lactic acid accumulation during dark fermentation of organic waste biomass. Bioresource Technology, 2017.
Ghimire A, Luongo V, Frunzo L, Lens PNL, Pirozzi F, Esposito G (2021) Biohythane production from food waste in a two-stage process: assessing the energy recovery potential. Environ Technol (United Kingdom), 0(0), pp.1–7.
Girotto F, Peng W, Rafieenia R, Cossu R (2016) Effect of aeration applied during different phases of anaerobic digestion. Waste and Biomass Valorization, 2016.
Gustavsson J, Cederbery C, Sonesson U, van Otterdijk RV, Meybeck A (2011) Global food losses and food waste-Extent, causes and prevention. Rome
Han SK, Shin HS (2002) Enhanced acidogenic fermentation of food waste in a continuous-flow reactor. Waste Manage Res 20(2):110–118
Han S, Shin H (2004) Performance of an innovative two-stage process converting food waste to hydrogen and methane performance of an innovative two-stage process converting food waste to hydrogen and methane. J Air Waste Manag Assoc 2004:242–249
Han W, Hu YY, Li SY, Li FF, Tang JH (2016a) Biohydrogen production from waste bread in a continuous stirred tank reactor: A techno-economic analysis. Biores Technol 221:318–323
Han W, Liu Z, Fang J, Huang J, Zhao H, Li Y (2016b) Techno-economic analysis of dark fermentative hydrogen production from molasses in a continuous mixed immobilized sludge reactor. J Clean Prod 127:567–572
Hussain A, Filiatrault M, Guiot SR (2017) Acidogenic digestion of food waste in a thermophilic leach bed reactor: Effect of pH and leachate recirculation rate on hydrolysis and volatile fatty acid production. Biores Technol 245(July):1–9
Jain S (2019) Global potential of biogas. World Biogas Assoc 2019:1–56
Jalil A, Karmaker S, Basar S, Hoque S (2019) Anaerobic digestion of vegetable wastes for biogas production in single chamber and double chamber reactors. Int J Waste Resour 09(01):1–6
Jang S, Kim DH, Yun YM, Lee MK, Moon C, Kang WS, Kwak SS, Kim MS (2015) Hydrogen fermentation of food waste by alkali-shock pretreatment: microbial community analysis and limitation of continuous operation. Biores Technol 186:215–222
Jariyaboon R, O-Thong, S. and Kongjan, P. (2015) Bio-hydrogen and bio-methane potentials of skim latex serum in batch thermophilic two-stage anaerobic digestion. Biores Technol 198:198–206
Jiang J, Zhang Y, Li K, Wang Q, Gong C, Li M (2013) Volatile fatty acids production from food waste: effects of pH, temperature, and organic loading rate. Biores Technol 143:525–530
Kaparaju P, Serrano M, Thomsen AB, Kongjan P, Angelidaki I (2009) Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Biores Technol 100(9):2562–2568
Kaza S, Yao L, Bhada-Tata PW, Van F (2018) What a Waste 2.0: a global snapshot of solid waste management to 2050. World Bank Group, Washington, DC
Keskin T, Nalakath Abubackar H, Arslan K, Azbar N (2019) Biohydrogen production from solid wastes. Elsevier B.V, New York
Kim HJ, Kim SH, Choi YG, Kim GD, Chung TH (2006a) Effect of enzymatic pretreatment on acid fermentation of food waste. J Chem Technol Biotechnol 81(May):974–980
Kim S-H, Han S-K, Shin H-S (2006b) Effect of substrate concentration on hydrogen production and 16S rDNA-based analysis of the microbial community in a continuous fermenter. Process Biochem 41(1):199–207
Kongjan P, Angelidaki I (2010) Extreme thermophilic biohydrogen production from wheat straw hydrolysate using mixed culture fermentation: Effect of reactor configuration. Biores Technol 101(20):7789–7796
Krishnan S, Din MFM, Taib SM, Ling YE, Puteh H, Mishra P, Nasrullah M, Sakinah M, Wahid ZA, Rana S, Singh L (2019) Process constraints in sustainable bio-hythane production from wastewater: Technical note. Bioresour Technol Rep 5:359–363
Krishna AR, Dev L, Thankamani V (2012) An integrated process for Industrial effluent treatment and Biodiesel production using Microalgae. Res Biotechnol 3(1):47–60
Lacroux J, Trably E, Bernet N, Steyer JP, van Lis R (2020) Mixotrophic growth of microalgae on volatile fatty acids is determined by their undissociated form. Algal Res 47:101870
Lay CH, Wu JH, Hsiao CL, Chang JJ, Chen CC, Lin CY (2010) Biohydrogen production from soluble condensed molasses fermentation using anaerobic fermentation. Int J Hydrogen Energy 35(24):13445–13451
Lee DY, Ebie Y, Xu KQ, Li YY, Inamori Y (2010) Continuous H2 and CH4 production from high-solid food waste in the two-stage thermophilic fermentation process with the recirculation of digester sludge. Biores Technol 101(1 SUPPL.):S42–S47
Lee YW, Chung J (2010) Bioproduction of hydrogen from food waste by pilot-scale combined hydrogen/methane fermentation. Int J Hydrogen Energy 35(21):11746–11755
Li C, Fang HHP (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed Cultures. Crit Rev Environ Sci Technol 37(1):1–39
Lim JW, Wang JY (2013) Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manage 33(4):813–819
Lim SJ, Kim BJ, Jeong CM, Choi JDR, Ahn YH, Chang HN (2008) Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Biores Technol 99(16):7866–7874
Liu D, Liu D, Zeng RJ, Angelidaki I (2006) Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res 40(11):2230–2236
Liu Z, Zhang C, Lu Y, Wu X, Wang L, Wang L, Han B, Xing XH (2013) States and challenges for high-value biohythane production from waste biomass by dark fermentation technology. Biores Technol 135:292–303
Li W, Loh KC, Zhang J, Tong YW, Dai Y (2018) Two-stage anaerobic digestion of food waste and horticultural waste in high-solid system. Appl Energy 209:400–408
Li K, Wang K, Wang J, Yuan Q, Shi C, Wu J, Zuo J (2020) Performance assessment and metagenomic analysis of full-scale innovative two-stage anaerobic digestion biogas plant for food wastes treatment. J Clean Prod 264
Ljunggren M, Zacchi G (2010) Techno-economic analysis of a two-step biological process producing hydrogen and methane. Biores Technol 101(20):7780–7788
Ljunggren M, Wallberg O, Zacchi G (2011) Techno-economic comparison of a biological hydrogen process and a 2nd generation ethanol process using barley straw as feedstock. Biores Technol 102(20):9524–9531
Longo S, Katsou E, Malamis S, Frison N, Renzi D, Fatone F (2015) Recovery of volatile fatty acids from fermentation of sewage sludge in municipal wastewater treatment plants. Biores Technol 175:436–444
Luongo V, Ghimire A, Frunzo L, Fabbricino M, d’Antonio G, Pirozzi F, Esposito G (2017) Photofermentative production of hydrogen and poly-Β-hydroxybutyrate from dark fermentation products. Biores Technol 228:171–175
Luongo V, Palma A, Rene ER, Fontana A, Pirozzi F, Esposito G, Lens PNL (2019) Lactic acid recovery from a model of Thermotoga neapolitana fermentation broth using ion exchange resins in batch and fixed-bed reactors. Sep Sci Technol (Philadelphia) 54(6):1008–1025
Luo G, Xie L, Zou Z, Wang W, Zhou Q, Shim H (2010) Anaerobic treatment of cassava stillage for hydrogen and methane production in continuously stirred tank reactor (CSTR) under high organic loading rate (OLR). Int J Hydrogen Energy 35(21):11733–11737
Luo G, Xie L, Zhou Q, Angelidaki I (2011) Enhancement of bioenergy production from organic wastes by two-stage anaerobic hydrogen and methane production process. Biores Technol 102(18):8700–8706
Malave’, A.C.L., Bernardi, M., Fino, D. and Ruggeri, B. (2015) Multistep anaerobic digestion (MAD) as a tool to increase energy production via H2 + CH4. Int J Hydrogen Energy 40(15):5050–5061
Malavè ACL, Fino D, Gómez Camacho CE, Ruggeri B (2018) Experimental tests on commercial Sweet Product Residue (SPR) as a suitable feed for anaerobic bioenergy (H2 + CH4) production. Waste Manage 71:626–635
Manandhar A, Shah A (2020) Techno-economic analysis of bio-based lactic acid production utilizing corn grain as feedstock. Processes 8(2):199
Marang L, Jiang Y, van Loosdrecht MCM, Kleerebezem R (2013) Butyrate as preferred substrate for polyhydroxybutyrate production. Biores Technol 142:232–239
Massanet-Nicolau J, Dinsdale R, Guwy A, Shipley G (2015) Utilising biohydrogen to increase methane production, energy yields and process efficiency via two stage anaerobic digestion of grass. Biores Technol 189:379–383
Meegoda JN, Li B, Patel K, Wang LB (2018) A review of the processes, parameters, and optimization of anaerobic digestion. Int J Environ Res Public Health 15(10):2224
Meena RAA, Rajesh Banu J, Yukesh Kannah R, Yogalakshmi KN, Kumar G (2020) Biohythane production from food processing wastes – challenges and perspectives. Bioresour Technol 298:122449
Menzel T, Neubauer P, Junne S (2020) Role of microbial hydrolysis in anaerobic digestion. Energies 13(21):5555
Micolucci F, Gottardo M, Bolzonella D, Pavan P (2014) Automatic process control for stable bio-hythane production in two-phase thermophilic anaerobic digestion of food waste. Int J Hydrogen Energy 39(31):17563–17572
Monlau F, Barakat A, Steyer JP, Carrere H (2012) Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Biores Technol 120:241–247
Monlau F, Kaparaju P, Trably E, Steyer JP, Carrere H (2015) Alkaline pretreatment to enhance one-stage CH4 and two-stage H2/CH4 production from sunflower stalks: Mass, energy and economical balances. Chem Eng J 260:377–385
Moon HC, Song IS (2011) Enzymatic hydrolysis of foodwaste and methane production using UASB bioreactor. Int J Green Energy 8(3):361–371
Moscoviz R, Trably E, Bernet N, Carrère H (2018) The environmental biorefinery: State-of-the-art on the production of hydrogen and value-added biomolecules in mixed-culture fermentation. Green Chem 20(14):3159–3179
Motte J-C, Sambusiti C, Dumas C, Barakat A (2015) Combination of dry dark fermentation and mechanical pretreatment for lignocellulosic deconstruction: an innovative strategy for biofuels and volatile fatty acids recovery. Appl Energy 147:67–73
Nasr N, Elbeshbishy E, Hafez H, Nakhla G, Hesham El Naggar M (2012) Comparative assessment of single-stage and two-stage anaerobic digestion for the treatment of thin stillage. Biores Technol 111:122–126
Nielsen C, Rahman A, Rehman AU, Walsh MK, Miller CD (2017) Food waste conversion to microbial polyhydroxyalkanoates. Microb Biotechnol 10(6):1338–1352
Nizami AS, Shahzad K, Rehan M, Ouda OKM, Khan MZ, Ismail IMI, Almeelbi T, Basahi JM, Demirbas A (2017) Developing waste biorefinery in Makkah: a way forward to convert urban waste into renewable energy. Appl Energy 186:189–196
Nualsri C, Kongjan P, Reungsang A (2016) Direct integration of CSTR-UASB reactors for two-stage hydrogen and methane production from sugarcane syrup. Int J Hydrogen Energy 2016:1–12
O-Thong S, Mamimin C, Prasertsan P (2018) Biohythane production from organic wastes by two-stage anaerobic fermentation technology. Adv Biofuels Bioenergy
O-Thong, S., Prasertsan, P. and Birkeland, N.-K. (2009) Evaluation of methods for preparing hydrogen-producing seed inocula under thermophilic condition by process performance and microbial community analysis. Biores Technol 100(2):909–918
REN21 (2019) Renewables 2019 global status report. REN21 Secretariat, Paris
Paritosh K, Kushwaha SK, Yadav M, Pareek N, Chawade A, Vivekanand V (2017) Food waste to energy: an overview of sustainable approaches for food waste management and nutrient recycling. BioMed Research International, 2017.
Park MJ, Jo JH, Park D, Lee DS, Park JM (2010) Comprehensive study on a two-stage anaerobic digestion process for the sequential production of hydrogen and methane from cost-effective molasses. Renewable Energy 35(12):6194–6202
Parthiba Karthikeyan O, Trably E, Mehariya S, Bernet N, Wong JWC, Carrere H (2018) Pretreatment of food waste for methane and hydrogen recovery: a review. Biores Technol 249:1025–1039
Paudel S, Kang Y, Yoo Y-S, Seo GT (2016) Effect of volumetric organic loading rate (OLR) on H2 and CH4 production by two-stage anaerobic co-digestion of food waste and brown water. Waste Management
Perez-Zabaleta M, Atasoy M, Khatami K, Eriksson E, Cetecioglu Z (2021) Bio-based conversion of volatile fatty acids from waste streams to polyhydroxyalkanoates using mixed microbial cultures. Bioresource Technol 323:124604
Petracchini F, Liotta F, Paolini V, Perilli M, Cerioni D, Gallucci F, Carnevale M, Bencini A (2018) A novel pilot scale multistage semidry anaerobic digestion reactor to treat food waste and cow manure. Int J Environ Sci Technol 15(9):1999–2008
Pielhop T, Amgarten J, Von Rohr PR, Studer MH (2016) Steam explosion pretreatment of softwood: The effect of the explosive decompression on enzymatic digestibility. Biotechnol Biofuels 9(1):1–13
Pilli S, Bhunia P, Yan S, LeBlanc RJ, Tyagi RD, Surampalli RY (2011) Ultrasonic pretreatment of sludge: a review. Ultrason Sonochem 18(1):1–18
Qureshi N, Annous BA, Ezeji TC, Karcher P, Maddox IS (2005) Biofilm reactors for industrial bioconversion process: Employing potential of enhanced reaction rates. Microb Cell Fact 4:1–21
Rafieenia R, Girotto F, Peng W, Cossu R, Pivato A, Raga R, Lavagnolo MC (2017) Effect of aerobic pre-treatment on hydrogen and methane production in a two-stage anaerobic digestion process using food waste with different compositions. Waste Manage 59:194–199
Rajagopal R, Ahamed A, Wang JY (2014) Hydrolytic and acidogenic fermentation potential of food waste with source segregated feces-without-urine as co-substrate. Biores Technol 167:564–568
Ramírez-Morales JE, Tapia-Venegas E, Toledo-Alarcón J, Ruiz-Filippi G (2015) Simultaneous production and separation of biohydrogen in mixed culture systems by continuous dark fermentation. Water Sci Technol 71(9):1271–1285
Ramírez-Morales JE, Tapia-Venegas E, Nemestóthy N, Bakonyi P, Bélafi-Bakó K, Ruiz-Filippi G (2013) Evaluation of two gas membrane modules for fermentative hydrogen separation. Int J Hydrogen Energy 38(32):14042–14052
Reis M, Albuquerque M, Villano M, Majone M (2011) Mixed culture processes for polyhydroxyalkanoate production from agro-industrial surplus/wastes as feedstocks. Elsevier B.V, New York
Ren NQ, Tang J, Liu BF, Guo WQ (2010) Biological hydrogen production in continuous stirred tank reactor systems with suspended and attached microbial growth. Int J Hydrogen Energy 35(7):2807–2813
Ren N, Guo W, Liu B, Cao G, Ding J (2011) Biological hydrogen production by dark fermentation: challenges and prospects towards scaled-up production. Curr Opin Biotechnol 22(3):365–370
Ren HY, Liu BF, Kong F, Zhao L, Xing D, Ren NQ (2014) Enhanced energy conversion efficiency from high strength synthetic organic wastewater by sequential dark fermentative hydrogen production and algal lipid accumulation. Biores Technol 157:355–359
Rodríguez-Valderrama S, Escamilla-Alvarado C, Rivas-García P, Magnin JP, Alcalá-Rodríguez M, García-Reyes RB (2020) Biorefinery concept comprising acid hydrolysis, dark fermentation, and anaerobic digestion for co-processing of fruit and vegetable wastes and corn stover. Environ Sci Pollut Res 27(23):28585–28596
Roy S, Das D (2016) Biohythane production from organic wastes: present state of art. Environ Sci Pollut Res 23(10):9391–9410
Ruggeri B, Tommasi T, Sanfilippo S (2015) BioH2 & BioCH4 through anaerobic digestion. Biohydro Biomethane Anaerobic Digest 2015:230
Saratale GD, Saratale RG, Chang JS (2019) Biohydrogen from renewable resources. Biohydrogen 2019:185–221
Sarma SJ, Pachapur V, Brar SK, Le Bihan Y, Buelna G (2015) Hydrogen biorefinery: potential utilization of the liquid waste from fermentative hydrogen production. Renew Sustain Energy Rev 50:942–951
Sasiradee J, Kienberger M, Nuttakul MS, M. (2017) Potential and assessment of lactic acid production and isolation – a review. J Chem Technol Biotechnol 92(12):2885–2893
Sawatdeenarunat C, Surendra KC, Takara D, Oechsner H, Khanal SK (2015) Anaerobic digestion of lignocellulosic biomass: challenges and opportunities. Biores Technol 178:178–186
Serafim LS, Lemos PC, Oliveira R, Reis MAM (2004) Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol Bioeng 87(2):145–160
Show KY, Lee DJ, Chang JS (2011) Bioreactor and process design for biohydrogen production. Biores Technol 102(18):8524–8533
Show KY, Yan Y, Ling M, Ye G, Li T, Lee DJ (2018) Hydrogen production from algal biomass – advances, challenges and prospects. Bioresour Technol 257:290–300
Sikder J, Roy M, Dey P, Pal P (2012) Techno-economic analysis of a membrane-integrated bioreactor system for production of lactic acid from sugarcane juice. Biochem Eng J 63:81–87
Sivagurunathan P, Kuppam C, Mudhoo A, Saratale GD, Kadier A, Zhen G, Chatellard L, Trably E, Kumar G (2018) A comprehensive review on two-stage integrative schemes for the valorization of dark fermentative effluents. Crit Rev Biotechnol 38(6):868–882
Si B, Li J, Li B, Zhu Z, Shen R, Zhang Y, Liu Z (2015) The role of hydraulic retention time on controlling methanogenesis and homoacetogenesis in biohydrogen production using upflow anaerobic sludge blanket (UASB) reactor and packed bed reactor (PBR). Int J Hydrogen Energy 40(35):11414–11421
Srisowmeya G, Chakravarthy M, Nandhini Devi G (2020) Critical considerations in two-stage anaerobic digestion of food waste – a review. Renew Sustain Energy Rev 119:109587
Stalin N, Prabhu HJ (2007) Performance evaluation of partial mixing anaerobic digester. J Eng Appl Sci 2(3):1–6
Strazzera G, Battista F, Garcia NH, Frison N, Bolzonella D (2018) Volatile fatty acids production from food wastes for biorefinery platforms: a review. J Environ Manage 226:278–288
Suhartini S, Lestari YP, Nurika I (2019) Estimation of methane and electricity potential from canteen food waste. In: IOP conference series: earth and environmental science, 230(1)
Sun C, Xia A, Fu Q, Huang Y, Lin R, Murphy JD (2019) Effects of pre-treatment and biological acidification on fermentative hydrogen and methane co-production. Energy Convers Manage 185:431–441
Surendra KC, Sawatdeenarunat C, Shrestha S, Sung S, Khanal SK (2015) Anaerobic digestion-based biorefinery for bioenergy and biobased products. Ind Biotechnol 11(2):103–112
Svensson K, Paruch L, Gaby JC, Linjordet R (2018) Feeding frequency influences process performance and microbial community composition in anaerobic digesters treating steam exploded food waste. Biores Technol 269(July):276–284
Tanai YS (2016) Food waste energy analysis: characterizing energy content as a function of proximate analysis Factors 2016:2
Tang J, Wang X, Hu Y, Zhang Y, Li Y (2016) Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manage 52:278–285
Tedesco S, Stokes J (2017) Valorisation to biogas of macroalgal waste streams: a circular approach to bioproducts and bioenergy in Ireland. Chem Pap 71(4):721–728
Thauer RK, Jungermann K, Decker K, Pi, P.P.H.–. (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41(3):809
Turon V, Baroukh C, Trably E, Latrille E, Fouilland E, Steyer J-P (2015) Use of fermentative metabolites for heterotrophic microalgae growth: yields and kinetics. Biores Technol 175:342–349
Uçkun Kiran E, Trzcinski AP, Liu Y (2015) Enhancing the hydrolysis and methane production potential of mixed food waste by an effective enzymatic pretreatment. Biores Technol 183:47–52
Uçkun Kıran E, Trzcinski AP, Liu Y (2015) Platform chemical production from food wastes using a biorefinery concept. J Chem Technol Biotechnol 90(October):1364–1379
Urbaniec K, Grabarczyk R (2014) Hydrogen production from sugar beet molasses - a techno-economic study. J Clean Prod 65:324–329
Uslu H (2009) Adsorption equilibria of formic acid by weakly basic adsorbent Amberlite IRA-67: equilibrium, kinetics, thermodynamic. Chem Eng J 155(1–2):320–325
Van Aarle IM, Perimenis A, Lima-Ramos J, de Hults E, George IF, Gerin PA (2015) Mixed inoculum origin and lignocellulosic substrate type both influence the production of volatile fatty acids during acidogenic fermentation. Biochem Eng J 103:242–249
Van DP, Fujiwara T, Tho BL, Toan PPS, Minh GH (2020) A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ Eng Res 25(1):1–17
Vavouraki AI, Angelis EM, Kornaros M (2013) Optimization of thermo-chemical hydrolysis of kitchen wastes. Waste Manage 33(3):740–745
Venkata SM, Babu VL, Sarma PN (2008) Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Biores Technol 99(1):59–67
Vo TP, Lay CH, Lin CY (2019) Effects of hydraulic retention time on biohythane production via single-stage anaerobic fermentation in a two-compartment bioreactor. Bioresour Technol 292:121869
Wang J, Yin Y (2017). Principle and application of different pretreatment methods for enriching hydrogen-producing bacteria from mixed cultures. Int J Hydrogen Energy
Wang J, Wan W (2008) Comparison of different pretreatment methods for enriching hydrogen-producing bacteria from digested sludge. Int J Hydrogen Energy 33(12):2934–2941
Wang Y-Y, Ai P, Hu C-X, Zhang Y-L (2011) Effects of various pretreatment methods of anaerobic mixed microflora on biohydrogen production and the fermentation pathway of glucose. Int J Hydrogen Energy 36(1):390–396
Wang K, Yin J, Shen D, Li N (2014) Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: effect of pH. Biores Technol 161:395–401
Wang Q, Jiang J, Zhang Y, Li K (2015) Effect of initial total solids concentration on volatile fatty acid production from food waste during anaerobic acidification. Environ Technol 36(15):1884–1891
Willquist K, Nkemka VN, Svensson H, Pawar S, Ljunggren M, Karlsson H, Murto M, Hulteberg C, Van Niel EWJ, Liden G (2012) Design of a novel biohythane process with high H 2 and CH 4 production rates. Int J Hydrogen Energy 37(23):17749–17762
Wu Y, Ma H, Zheng M, Wang K (2015) Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Biores Technol 191:53–58
Xia A, Cheng J, Murphy JD (2016) Innovation in biological production and upgrading of methane and hydrogen for use as gaseous transport biofuel. Biotechnol Adv 34(5):451–472
Xiong Z, Hussain A, Lee J, Lee HS (2019) Food waste fermentation in a leach bed reactor: reactor performance, and microbial ecology and dynamics. Biores Technol 274:153–161
Xu S, Selvam A, Wong JWC (2014) Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manage 34(2):363–369
Yan BH, Selvam A, Wong JWC (2019) Bio-hydrogen and methane production from two-phase anaerobic digestion of food waste under the scheme of acidogenic off-gas reuse. Bioresour Technol
Yeshanew MM, Frunzo L, Pirozzi F, Lens PNL, Esposito G (2016). Production of biohythane from food waste via an integrated system of continuously stirred tank and anaerobic fixed bed reactors. Bioresource Technol, 2016, p.(In press).
Yin J, Wang K, Yang Y, Shen D, Wang M, Mo H (2014) Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Biores Technol 171:323–329
Yun YM, Lee MK, Im SW, Marone A, Trably E, Shin SR, Kim MG, Cho SK, Kim DH (2018) Biohydrogen production from food waste: Current status, limitations, and future perspectives. Biores Technol 248:79–87
Zacharof MP, Lovitt RW (2013) Complex effluent streams as a potential source of volatile fatty acids. Waste and Biomass Valorization 4(3):557–581
Zacharof MP, Lovitt RW (2014) Recovery of volatile fatty acids (VFA) from complex waste effluents using membranes. Water Sci Technol 69(3):495–503
Zhang C, Su H, Baeyens J, Tan T (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renew Sustain Energy Rev 38:383–392
Zhu H, Beland M (2006) Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int J Hydrogen Energy 31(14):1980–1988
Funding
The research is funded by EnergizeNepal Project (PID: ENEP-RENP-II-19–01).
Author information
Authors and Affiliations
Contributions
SD: conceptualization, Formal analysis, writing original draft; AG: conceptualization, formal analysis, supervision, funding acquisition, contribution in writing the original draft, writing-reviewing, and editing; BT: formal analysis, project administration, supervision, funding acquisition, and visualization; LS: project administration, supervision, and funding acquisition; ST: resources compilation, writing-reviewing; AK: writing-reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
About this article
Cite this article
Dangol, S., Ghimire, A., Tuladhar, S. et al. Biohythane and organic acid production from food waste by two-stage anaerobic digestion: a review within biorefinery framework. Int. J. Environ. Sci. Technol. 19, 12791–12824 (2022). https://doi.org/10.1007/s13762-022-03937-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-03937-y