Abstract
A new biorefinery conceptual process is proposed for biohydrogen and biomethane production from a combination of fruits and vegetable wastes (FVW) and corn stover (CS). The objective of this work was to perform the acid hydrolysis (HCl 0.5% v/v, 120 °C, 2 h) of the FVW and CS at 3:1 dry basis ratio, and to process its main physical phases, liquid hydrolyzates (LH) and hydrolyzed solids (HS), by mesophilic dark fermentation (DF) and anaerobic digestion (AD), respectively. In DF of LH as carbon source, hydrogen was produced at maximum rate of 2.6 mL H2/(gglucose h) and maximum accumulation of 223.8 mL H2/gglucose, equivalent to 2 mol H2/molglucose, in a butyric-pathway-driven fermentation. HS were digested to methane production assessing inoculum to substrate ratios in the range 2–4 ginoculum/gVS. The main results in AD were 14 mmol CH4/gvs. The biorefinery demonstrated the feasibility to integrate the acid hydrolysis as pretreatment and subsequently use the LH for hydrogen production, and the HS for methane production, with an energy yield recovery of 9.7 kJ/gvs, being the energy contribution from anaerobic digestion 8-fold higher than of dark fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mexico is committed to reducing its greenhouse gases (GHG) by the year 2030 to 22%, whereas emissions of short-lived climate pollutants (black carbon) are aimed to 51% reduction (Gobierno de México 2015). The main proposals for obtaining energy from renewable sources are bioenergy, solar energy, geothermal energy, hydroelectric energy, wind energy, and tidal energy. The biomass as a source for bioenergies, i.e., biohydrogen, biomethane, bioethanol, and biobutanol (Mahlia et al. 2019), is most promising in the energetic share it could reach; according to some estimates, in Mexico, it could supply ca. 46% of the annual energy, yet currently, only 5% is supplied (International Energy Agency 2016).
Residual biomass can be classified by its origin in forestry residues, agricultural residues, agro-industrial wastes, and organic fraction of municipal solid waste (Li et al. 2016). Residual biomass exhaustive use has led to the development of the biorefinery of residues.
The biorefinery concept is associated with the intensive conversion of organic matter into added-value products (Escamilla-Alvarado et al. 2016; Moncada-Botero et al. 2016). Biorefineries are outlined by the following sections: (i) pretreatment section to fractionate biomass, (ii) section of bioprocesses (e.g. fermentation, digestion), (iii) purification of bioproducts to discard impurities and pollutants, and (iv) energy conversion section (Giuliano et al. 2016; Hernández-Flores et al. 2017).
The pretreatment with the highest efficiency of fermentable sugars release is diluted acid hydrolysis (Gonzales et al. 2016; Joglekar et al. 2019). It employs high temperatures and acids in low concentrations to hydrolyze macromolecules such as cellulose and hemicellulose into monomeric sugars such as glucose, xylose, and arabinose (Kumar et al. 2015). Such soluble hydrolyzate is prone to any kind of fermentation, yet the insoluble fraction remaining from the pretreatment (hydrolyzed solids) is also susceptible for downstream processing. In the biorefinery perspective, the use of both hydrolyzates would be very attractive to increase the productivities of the installation.
Fermentable sugars in liquid hydrolyzates (HL) can be used as a carbon source to produce hydrogen through dark fermentation (DF) bioprocess (Roy and Das 2016). On the other hand, the insoluble biomass, or hydrolyzed solids (HS), may be converted into a bioenergetic such as methane through anaerobic digestion (AD) (Oliwit et al. 2019). Actually, only few works have dealt with the either DF or AD of hydrolyzates with interesting results. For instance, Datar et al. (2007) applied steam explosion hydrolysis followed by the separated fermentation of the liquid hydrolyzates and the solid hydrolyzates for hydrogen production. Curiously, the dark fermentation of solid hydrolyzates did not produce hydrogen during the first 21 days, which was attained only after 20 h since the addition of cellulases. On the other hand, Tapia-Rodríguez et al. (2019) evaluated the parallel production of hydrogen and methane from enzymatic hydrolyzates of agave bagasse; however, the HS were not included in the biorefining process.
The sources of biomass for biorefineries are numerous and in order to not compromise the food supply, biowastes should be mainly used as its substrates (Romero-Cedillo et al. 2016). Considering that Mexico was the 11th agriculture producer worldwide with an annual production of 210 million tons in 2018 (SADER 2019), its wastes generation along the food supply chains (production, postharvest, processing, distribution, consumption) are also considerable. Indeed, as corn is the second most important crop in Mexico, the wastes associated with the over 26 × 106 metric tons production (SADER 2019) are corn stover (CS) and corn cobs. Since close to 82% of the CS generated is used for animal feed, the remaining 18% does not have a defined use (ca. 4.7 × 106 metric tons in dry base) and is therefore underused (Hernández et al. 2019). Currently, CS is the most promising lignocellulosic waste for biofuels production due to its high cellulose (23–40%) and hemicellulose (12–32%) contents, and its low-cost abundant biomass (Kim et al. 2019; Tan et al. 2019).
Another underused biowaste in Mexico are the fruits and vegetable wastes (FVW), which compose ca. 44% of municipal solid wastes and that are disposed in landfills and open dumps (Taboada-González et al. 2011; Díaz et al. 2017; Gavilán et al. 2018).
Even though worldwide distribution of biowaste has raised attractiveness on biorefineries, some traits of these substrates such as its recalcitrant nature and seasonal availability are some of the most important challenges for the development of biorefineries at large scale (Sultana and Kumar 2011; Giuliano et al. 2016). It has been proposed that the combination of multiple lignocellulosic biomass would be a feasible solution as it also improves biofuels yields, complements the nutritional balance, and reduces delivery costs (Sultana and Kumar 2011; Wang et al. 2011). Therefore, this work aimed to evaluate a biorefinery concept for biohydrogen and biomethane production, as well as the resulting energy potential, from a combination of fruit and vegetable wastes (FVW) and corn stover (CS). The biorefinery concept (h-H-M) integrated the diluted acid hydrolysis (h) as pretreatment and the parallel production of hydrogen by DF (H) from liquid hydrolyzates and methane (M) from hydrolyzed solids.
Materials and methods
Biorefinery h-H-M set up
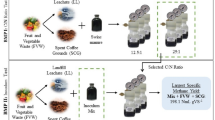
The FVW and CS were used as substrates to test the h-H-M biorefinery concept as shown in Fig. 1. The co-substrates were combined in dry basis (db) at the ratio 3:1 FVW:CS according to Rodríguez-Valderrama (2018). The co-substrates were subjected to hydrolysis pretreatment (h-stage) using HCl as the catalyst acid. After the dilute acid hydrolysis, the liquid and solid phases were separated by centrifugation. Liquid hydrolysates (LH) were limed to decrease the concentration of inhibitory compounds and used as a carbon source in an H2-producing dark fermentation stage (H-stage) at mesophilic temperature. The hydrolyzed solids (HS) were washed to remove the catalyst acid excess and used as carbon source in a methane producing anaerobic digestion bioprocess (M-stage). Each of the three stages will be described in detail in the following sections.
Co-substrates
Corn stover (collected from Cuencamé, Durango, Mexico) and FVW (collected from a local cafeteria Chemical Sciences Faculty, Universidad Autónoma de Nuevo León, Nuevo León, Mexico) were separately dried in an oven at 85 °C for 24 h, grinded to 180-μm particle size of using a manual mill, and stored in distinct tightly closed plastic bags at room temperature. FVW and CS were physico-chemically characterized (Table 1). The empirical molecular formulas and the heat power value were CH4.31O0.68N0.01, 3606 cal/gdb, and CH0.8O0.62N0.04, 2712 cal/gdb for CS and FVW, respectively.
Diluted acid hydrolysis and overliming
The acid hydrolysis was carried out using dilute HCl (0.5% v/v) in 0.5-L Schott bottles. The reaction volume was 0.3 L and the solid content was 6.6% of reaction volume. The co-substrate ratio was 3:1 FVW:CS (g:g). The hydrolysis reaction was performed in an autoclave (121 °C) for 120 min (Yan et al. 2009; Kumar et al. 2015). After the hydrolysis, liquid hydrolyzates (LH) were separated by centrifugation (10,000g, 10 min) and characterized in terms of reducing and monomeric sugars and the inhibitory compounds, i.e., acetic acid, formic acid, furfural, 5-hydroxymethyl-furfural (HMF), and total phenolic compounds (TPC).
Overliming treatment was applied to the LH in a two-step approach (Chang et al. 2011). Firstly, the pH of LH was adjusted to 10 by adding powder Ca(OH)2 in continuous stirring for 1 h. Secondly, the pH was reduced to 7 by 6 M HCl addition, following a centrifugation (10,000g, 15 min) and the separation of liming precipitates and supernatant. Sample was retrieved from the LH to analyze the removal of inhibitory compounds and sugars.
Dark fermentation
DF was carried out by duplicate in 0.5-L Schott bottles with 0.4 L of reaction volume. The methanogenic anaerobic sludge was heat-treated to inhibit methane-producing microflora in water bath at 96 °C for 2 h. The initial reducing sugars (RS) were adjusted to 13 g/L and the amount of substrate and inoculum was added according to the inoculum to substrate ratio of 1.2 (VS basis). The fermentation volume was supplemented with 4 mL of 200-fold mineral medium previously reported by Rodríguez-Valderrama et al. (2019). The medium was supplemented with nitrogen source (1 g NH4Cl/L) and buffer medium (3 g K2HPO4/L, 1.5 g KH2PO4/L). The experimental units were stirred at 150 rpm in a multiple magnetic stirrer inside an incubator at 35 °C.
Anaerobic digestion
HS from the separation of LH were washed twice with distilled water (0.03 L of water per 10 gwb of HS), vortexed, and sedimented for 10 min, to remove the residual acid catalyst. Afterwards, HS were recovered by centrifugation (15 min at 10,000g), dried at 80 °C, and characterized (Table 1). HS empirical molecular formula based on elemental composition was CH2.30O0.42N0.03.
AD was carried out in 0.120-L serum bottles with 0.08 L of work volume. The inoculum was anaerobic sludge from a semi-continuous digester fed with FVW at 30 days of hydraulic retention time. The inoculum was degassed during 3 days and then used for HS anaerobic digestion. The inoculum had the following characteristics: 7.81 pH, 93.64%wb moisture, 6.36%wb TS, 51.46%db VS, 48.54%db ashes. The alkalinity and total VFA were 12,300 mg CaCO3/L and 8040 mg VFA/L, respectively. Its empirical molecular formula based on elemental composition was CH1.34O2.02N0.10.
Inoculum to substrate ratios (ISR) were assayed in batch mode: 2, 2.5, 3, 3.5, and 4 gvs inoculum/gvs HS. A control was run with fresh FVW at ISR 3. A blank was loaded only with inoculum and water to determine the methane production by the organic matter present in the inoculum. The methane production (2.16 mmol CH4) from blanks was subtracted from the methane produced by HS. All the experimental units were kept constant in its inoculum content at 2.5 gvs, and the substrate amounts were adjusted according to each ISR. The anoxic environment in each bottle was promoted by flushing N2 during 3 min. Afterwards, the bottles were sealed with a rubber stopper and aluminum rings. The operation temperature and stirred velocity were 35 ± 1 °C and 150 rpm, respectively. All the experiments were carried out by duplicate.
Analytical methods
The pH was determined according to the procedure described by NMX-AA-25-1984 (1992). Solids profile was measured according to standard methods (APHA/AWWA/WEF 2005). Cellulose and acid-insoluble lignin contents were determined by the gravimetric method based on AOAC methods (AOAC 1992). Hemicellulose amount was determined subtracting the cellulose content from holocellulose content after by lignin oxidation by NaClO (Escamilla-Alvarado et al. 2015). The extractives in CS and FVW were determined by differential weight after extraction in a water bath at 60 °C 24 h (Sluiter et al. 2008). The elemental characterization (C, H, O, N) was determined by an elemental analyzer (Thermo Scientific Flash 2000, USA), O2 was used as combustion gas and He was used as carrier gas.
The reducing sugars (RS) in hydrolyzate were determined by the 3,5-dinitrosalicylicacid method (DNS) using dextrose for the calibration curve (Miller 1959). Glucose, xylose, and arabinose were quantified by high performance liquid chromatograph (LDC Analytical, USA) equipped with a Rezex RHM-Monosaccharide (300 mm × 7.8 mm) column and a refractive index detector (Varian Prostar, USA). The column temperature was 65 °C, whereas the mobile phase (H2O) flow rate was maintained at 0.6 mL/min.
The total amount of biogas produced in DF and AD was determined by the acid-brine displacement method (Escamilla-Alvarado et al. 2013). Hydrogen and methane gas contents were determined in a gas chromatograph (Thermo Scientific Trace 1310, USA); the gas chromatograph was equipped with a thermal conductivity detector and a molecular sieve column (TG-BOND Msieve 5A, 30 m × 0.33 mm). The operating temperatures were 100 °C, 150 °C, and 200 °C for the oven, injector, and detector, respectively. Nitrogen gas was used as a carrier gas with a flow rate of 3 mL/min.
The total phenolic compounds (TPC) were determined by the Folin-Ciocalteu method proposed by Blainski et al. (2013) using tannic acid as the standard. Furfural, HMF, formic acid, acetic acid, propionic acid, succinic acid, and lactic acid were demined by gas chromatography (Varian CP 3380, USA) with a column ZB-FFAP (15 m × 0.53 × 1 μm) and flame ionization detector. The injector and detector temperatures were 230 and 280 °C, respectively. The temperature program for the column initiated at 90 °C for 3 min, then increased to 200 °C at 20 °C/min rate and maintained for 3 min, and finally raised to 250 °C at 30 °C/min, which was maintained for 4 min.
Total volatile fatty acids (TVFA) and alkalinity concentration were determined by a titration method (Anderson and Yang 1992). Acetic acid, propionic acid, butyric acid, and ethanol were quantified using a gas chromatograph according to the method described in our previous work (Rodríguez-Valderrama et al. 2019).
Calculations
A set of response variables was calculated according to Table 2 to analyze the production of hydrogen, methane, and energy potential. The cumulative specific hydrogen production H(t) (mL H2/gglucose) was used for describing the accumulated production of hydrogen in time and to obtain kinetic parameters from fitting the results by the Gompertz equation (Eq. 1). Thus, the maximum cumulative specific hydrogen production Hmax (mL H2/gglucose), the maximum specific hydrogen production rate rmax,H (mL H2/(gglucose h)), and the adaptation time λ (h) were determined.
The hydrogen molar pseudoyield, Y’H2 (mol H2/molglucose) in Eq. 2, was obtained from the Hmax (mL H2/gglucose) as a means to compare the system to the maximum theoretical hydrogen yield (2 and 4 mol H2/gglucose for butyrogenic and acetogenic pathways, respectively).
Other variables in the equations listed in Table 2 used in Eq. 1 or Eq. 2 are t is fermentation time (h), e is 2.718, CRS,0 and CRS,f (g/L) are the concentration of RS at the beginning and at the end of DF, and MWglucose is the glucose molar weight (180.16 g/mol).
Regarding the AD equations and parameters (Table 2), the cumulative methane production B(t) (mmol CH4) and cumulative specific methane production b(t) (mmol CH4/gvs) were used to calculate the kinetic parameters by two methods: an adaptation of the Gompertz equation (Lo et al. 2010, Eq. 3 and Eq. 4), and the first-order model proposed by Hashimoto (1989, Eq. 5 and Eq. 6). Through Eq. 3 and Eq. 5, the maximum cumulative methane production Bmax is obtained, whereas through Eq. 4 and 6, the maximum cumulative specific methane production bmax is estimated. The other parameters estimated through these equations are the maximum methane production rate Rmax,M (mmol CH4/day), the maximum specific methane production rate rmax,M (mmol CH4/(gvs day)), λ, and the methane production rate k (1/day).
The specific gross energy potential Êp (kJ/gvs) was used to compare our h-H-M biorefinery against other biorefinery models in the literature, either serial where DF is followed by AD (Eq. 7), or in parallel DF and AD systems (Eq. 8). In these equation, the hydrogen high heating value HHVH2 is 282.8 kJ/mol, the methane high heating value HHVCH4 is 889.9 kJ/mol, VM is the molar volume of an ideal gas at standard conditions (22.4 L/mol), 1000 is the mL to L conversion factor, ηDF is the quotient of VS consumed and VS fed in the DF units, and ηAH is the quotient of glucose released and VS fed in acid hydrolysis experiments.
Results and discussion
Diluted acid hydrolysis and overliming
The RS concentration after co-substrate hydrolysis was 23.49 g/L, containing high amounts of glucose (10.36 g/L) followed by xylose (8.61 g/L), and arabinose (0.39 g/L). The RS production yield was 48.54% (calculated as the amount of RS produced divided by the sum of volatile solids added) for the 3:1 FVW:CS combination. This yield is comparable with those reported in the literature for acid hydrolysis of either FVW or CS. For instance, Datar et al. (2007) obtained a hydrolysis yield of 47% in the steam-explosion treatment of acid impregnated CS. Cao et al. (2009) managed to extract the 35.20% of sugars in the CS acid hydrolysis. On the other hand, Díaz et al. (2017) extracted 35.9% of the reducing sugars available in tomato wastes through acid hydrolysis. Additionally, one of the main benefits of the co-substrate acid hydrolysis is the improvement of the monomeric sugar distribution. For instance, in our 3:1 FVW:CS experiment, the monosaccharide distribution in the liquid hydrolyzates was 53.5% glucose, 44.5% xylose, and 2% arabinose (Table 1). In contrast, the main monomeric sugar distribution from hydrolysis of only CS was 9.08% of glucose, 83.08% of xylose, and 7.84% of arabinose (Datar et al. 2007), whereas FVW were only composed of 100% hexoses (Del Campo et al. 2006).
The overliming treatment of acid hydrolyzates successfully reduced the inhibitory compounds as TPC in 33.86%, although 10.05% RS were also lost (Table 3). RS loss is commonly expected in such treatments (Saha et al. 2005), for instance, Chang et al. (2011) reported 9% RS loss after the overliming of rice husk hydrolyzates, and Purwadi et al. (2004) had 8.42% RS loss from detoxification by Ca(OH)2 of Swedish forestry residues hydrolyzates. The concentration of TPC, HMF, furfural, and acetic acid did not surpass the concentrations known to inhibit hydrogen production, which are in the following ranges: 0.8–2.28 g/L for TPC, 0.86–1.89 g/L for HMF, 0.8–3.41 g/L for furfural, and 0.6–7.80 g/L for acetic acid (Ren et al. 2008; Gonzales et al. 2016).
Dark fermentation
After overliming pretreatment, the LH were used for hydrogen production at initial pH of 7 and 35 °C. After 150 h of fermentation, maximum experimental cumulative biogas and maximum cumulative specific hydrogen production were 2717 mL and 223.8 mL H2/gglucose, respectively (Fig. 2). The hydrogen average content in biogas was 50.89%, whereas methane was not detected. According to the Gompertz parameters (Table 4), the production of hydrogen from the acid hydrolyzates of the co-substrates mixtures (3:1 FVW:CS) showed a rmax,H of 2.60 (mL H2/(gglucose h)) and 19.25 h of adaptation time.
rmax,H is comparable with other studies presented in literature where hydrogen production from acidic hydrolyzates of organic waste has been studied. Zhang et al. (2015) reported a rmax,H of 0.92 (mL H2/(gglucose h)) in a 100-mL serum vials with acid hydrolyzates (1.7% v/v H2SO4, 120 min, 120 °C) from CS produced by activated sludge. The reason for their low rmax,H compared with our study might be attributed to the low initial sugar concentration (5 g/L against 13 g/L, respectively) and the low initial ISR (0.19 against 1.2, respectively), as such parameters are directly related to hydrogenogenic performance (Fan et al. 2006; Ozmihci et al. 2011). In addition, the hydrogen batch fermentation at ISR lower than 0.16 could have presented inhibition due to the high amounts of substrate (Wong et al. 2014).
The maximum productivity of hydrogen from LH was 2909 mL H2/Lreactor, which can be compared with other studies also using LH for hydrogen production. In the case of Datar et al. (2007), they reported hydrogen productivity of 3310 mL H2/Lreactor from CS hydrolyzates in a CSTR reactor using a pre-treated anaerobic sludge as an inoculum; the volumetric productivity reported in our work was 12.1% lower than theirs. However, the adaptation time reported by Datar et al. (2007) was 49.5% higher due to the initial concentrations of inhibitors present in their hydrolyzates.
The molar pseudoyield obtained at 35 °C was 2.02 mol H2/molglucose, comparable with those using pure and mixed cultures from acid hydrolyzates. Biohydrogen production from starch hydrolyzates led to a maximum YH2 of 1.28 and 0.85 mol H2/molglucose by either Clostridium pasteurianum CH5 or by consortium from dark fermentation sludge, respectively (Chen et al. 2008). Both experiments were carried out at 37 °C and initial sugar concentration of 26.7 g/L. Using acid hydrolyzates from wheat starch (121 °C, 15 min, pH 2.5, H2SO4), Cakr et al. (2010) obtained a Y’H2 of 2.4 mol H2/molglucose at 55 °C and initial sugar concentration of 18.5 g/L using heat-treated anaerobic sludge. This value was 1.2-fold higher than ours; however, the use of thermophilic temperatures may decrease the energy gains from biofuel production (Rodríguez-Valderrama et al. 2019).
The final pH in hydrogen production was 5.34 (Table 4), an adequate value for hydrogen production as reported in literature (Braguglia et al. 2018). In our experiments, the pH0 was adjusted to 7 as commonly done in free fermentation experiments. For instance, Liu et al. (2013) observed a considerable hydrogen yield increase from 0.02 mol H2/molsugars at pH0 5 to 0.44 mol H2/molsugars at pH0 7 when using rice straw acid hydrolyzates.
The main metabolites in DF were butyric acid (6.13 g/L), acetic acid (1.11 g/L), propionic acid (0.36 g/L), and a small amount of ethanol (0.07 g/L), which indicates that the fermentation was carried out in greatly by butyric acid metabolic pathway (Chen et al. 2006; Wang and Wan 2009). These results demonstrated the good performance of dark fermentation from acid hydrolyzates obtained from organic substrates mixtures by anaerobic sludge.
Anaerobic digestion
The cumulative methane production and specific methane production as a function of time for different ISR are shown in Fig. 3. The best results for each parameter were obtained at ISR 2.5 and 4, respectively. Indeed, all the experiments evaluated with HS presented methane productions and specific methane productions higher than the experiments with FVW (control) during the first 5 days due to the readily biodegradable organic matter in the HS. Still, at the end, the control produced as good results as most of them due to the RIS used and the good biodegradability of FVW at high digestion times (Li et al. 2011).
The cumulative methane production (Bmax) at the different studied ISR (2–4) was in the range 6.37 to 8.78 mmol CH4. The best results were obtained in the order ISR 2.5 > 4 > 3 > 2 > 3.5 (Table 5, Fig. 3a). On the other hand, the cumulative specific methane production (bmax) was in the range 5.84–13.64 mmol CH4/gvs, obtained from highest to lowest in the order ISR 4 > 3 > 3.5 > 2.5 > 2. This was in agreement to reports in literature that have ascribed this phenomenon to the fact that a greater inoculum to substrate ratio allows a more exhaustive conversion of biomass into methane (Hashimoto 1989).
The predicted maximum cumulative methane production (Bmax, 8.90 mmol CH4) and the lowest deviation in respect to the experimental value (1.30%) were presented with the first-order kinetic model for ISR = 2.5, whereas Gompertz prediction had a deviation of 3.36% respect to the measured production. The coefficient of determination for this experiment (ISR = 2.5) was 0.99, slightly higher than the obtained by the Gompertz model (0.99). The maximum methane rates (k) for each ISR evaluated were in the range of 0.19 to 0.28 (1/day). The maximum k (0.28 1/day) corresponded to ISR of 2.5, which could have reflected a positive interaction of a rapid substrate biodegradability due to pretreatment and an adequate inoculum load. Similarly, at 2.5 ISR, Moset et al. (2015) registered a k of 0.16 (1/day) in the anaerobic digestion of corn stover silage (1 year) and 0.08 (1/day) for wheat straw without any previous treatment.
The predicted specific methane productions (bmax) by the first-order kinetic and Gompertz models were 14 mmol CH4/gvs and 13.2 mmol CH4/gvs, respectively (Table 6). However, the first-order kinetic model presented the best fit to the experimental data since its error (2.62%) was lower than the respective of the Gompertz model (3.07%). These errors are usual when comparing the fits of mathematical models. As reported by Zhang et al. (2014) who obtained deviations from 1.5% for the first-order kinetic model and 3.7% for the Gompertz model when they evaluated the anaerobic digestion of dewatered sewage sludge at ISR of 1 and 37 °C of fermentation temperature.
The maximum predicted bmax (14 mmol CH4/gvs or 342.3 L CH4/kgvs) is comparable with the maximum methane specific productions in literature, only 8% lower than the value of 372.4 L CH4 /kgvs, from alkaline pretreated (5% NaOH) CS on solid-state (Zhu et al. (2010). This difference may be mainly due to the positive traits of alkaline treatment and its less severity compared with acid treatments. Besides lignin solubilization, alkaline pretreatments also provide neutralization of the different acids in lignocellulosic compound degradation. However, the maximum methane production rate (9.3 L CH4/(kgvs day)) found by Zhu et al. (2010) after 40 days was 6.2-fold lower than that obtained from HS during 15 days (2.35 mmol CH4/(gvs day); 57.4 L CH4/(kgvs day)), which indicates the rapid substrate degradation due to the acid pretreatment process.
The bmax and the rmax,M obtained in the control experiment with fresh untreated FVW were 10.01 mmol CH4/gvs (244.5 L CH4/kgvs) and 1.29 mmol CH4/(gvs day) (31.6 L CH4/(kgvs day)), respectively. This bmax was 28.63% lower than that obtained with HS (342.3 L CH4/kgvs), because when pretreating a substrate, either by alkaline pretreatment or acidic pretreatment, the methane production is raised, since soluble organic matter was increased and then easily used by microorganisms (Abudi et al. 2016). The increase in methane production (28.63%) due to substrate pretreatment was also observed in the literature. For instance, Abudi et al. (2016) registered the increment of 6.69% in the methane production from rice straw without pretreatment and 45.15% from alkali rice straw organic fraction of municipal solid wastes (OFMSW). On the other hand, both substrates (rice straw and pretreated rice straw) showed higher methane production when compared with OFMSW digestion, but this phenomenon seems to be related to the nature of the substrate rather than to the pretreatment applied.
Energy evaluation of the h-H-M concept biorefinery
The energy yield from h-H-M biorefinery model and its comparison with two-stage systems from different biomasses as substrates are shown in Table 7. The energy yield for h-H-M biorefinery based on gaseous biofuel production was 9.7 kJ/gvs. The stage with the greatest contribution to the energy yield was the methane production (M) stage with a contribution near to 89.4% (8.7 kJ/gvs), whereas the contribution of hydrogen produced in dark fermentation (H) was the remaining 10.6% (1.0 kJ/gvs). Ghimire et al. (2015) calculated a maximum energy yield of 4.4 kJ/gvs for hydrogen and methane production from food waste. Energy contribution of DF was 29.6% (1.3 kJ/gvs) when the food wastes were fermented in a 1.5-L semi-continuous reactor at 55 °C, whereas the maximum energy yield contribution was presented by AD (70.4%, 3.1 kJ/gvs). The relatively low energy contribution of DF is ascribed to the inherent energy loss in the form of metabolites in the effluents (Xia et al. 2013).
Conclusions
This work demonstrated that a new biorefinery approach coined as h-H-M was technically feasible for treating a preparation of fruit and vegetable wastes and corn stover through acid hydrolysis, dark fermentation, and anaerobic digestion. Acid hydrolysis of the preparation 3:1 fruit and vegetable wastes to corn stover produced hydrolyzates containing up to 10.4 g/L of glucose, 8.61 g/L of xylose, and 0.39 g/L of arabinose.
Dark fermentation from liquid hydrolyzates performed competitively at inoculum to substrate ratio 1.2, reaching productivity of 2909.7 mL H2/Lreactor and pseudoyield 2.02 mol H2/molglucose.
The hydrolyzed solids were adequately used as carbon source for anaerobic digestion, promoting higher initial methane production when compared with fresh substrate. The maximum cumulative methane production, 8.9 mmol CH4, and the highest methane specific production, 13.6 mmol CH4/gvs, were obtained at the inoculum to substrate ratios of 2.5 and 4, respectively.
The total energy potential from the h-H-M biorefinery concept in the form of hydrogen and methane reached 9.7 kJ/gvs, of which hydrogen contributed to 10.6%.
Using a combination of co-substrates such as FVW and CS opens up the possibility of interesting configurations for real biorefineries, as this approach may provide an alternative to mono-substrate drawbacks.
Abbreviations
- AD:
-
anaerobic digestion
- B(t) :
-
cumulative methane production (mmol CH4)
- B max :
-
maximum cumulative methane production (mmol CH4)
- b(t) :
-
cumulative specific methane production (mmol CH4/gvs)
- b max :
-
maximum cumulative specific methane production (mmol CH4/gvs)
- CS:
-
corn stover
- C RS,0 :
-
initial reducing sugars concentration (g/L)
- C RS,f :
-
final reducing sugars concentration (g/L)
- DF:
-
dark fermentation
- e :
-
Euler number (2.718)
- Ê p :
-
specific gross energy potential (kJ/gvs)
- FVW:
-
fruit and vegetable wastes
- h :
-
diluted acid pretreatment stage
- H :
-
hydrogen production stage by DF
- H(t) :
-
cumulative hydrogen specific production (mL H2/gglucose)
- H max :
-
maximum cumulative specific hydrogen production (mL H2/gglucose)
- h-H-M :
-
biorefinery model
- HHV H2 :
-
high hydrogen heating value (282.8 kJ/mol)
- HHV CH4 :
-
high methane heating value (889.9 kJ/mol)
- HMF:
-
5-hydroxymethylfurfural
- HS:
-
hydrolyzed solids
- ISR:
-
inoculum to substrate ratio
- k :
-
methane production rate (1/day)
- LH:
-
liquid hydrolyzates
- M :
-
methane production stage by AD
- MW glucose :
-
glucose molar weight (180.16 g/mol)
- ND:
-
not determined
- pH0 :
-
initial pH
- r max,M :
-
maximum specific methane production rate (mmol/(gvs day))
- r max,H :
-
maximum specific hydrogen production rate (mL/(gglucose h)),
- R max,M :
-
maximum methane production rate (mmol/day)
- R 2 :
-
coefficient of determination
- RS:
-
reducing sugars
- T :
-
operational temperature
- t :
-
time
- TPC:
-
total phenolic compounds
- TS:
-
total solids
- TVFA:
-
total volatile fatty acids
- V M :
-
molar volume at standard conditions (22.4 L/mol H2 or CH4)
- V O :
-
operational volume
- VS:
-
volatile solids
- Y’ H2 :
-
hydrogen molar pseudoyield (mol H2/molglucose)
- db:
-
dry basis
- wb:
-
wet basis
- λ :
-
adaptation time (h or day)
- η AH :
-
acid hydrolysis efficiency (gglucose/gvs)
- η DF :
-
dark fermentation efficiency (gvs consumed/gvs added)
References
Abudi ZN, Hu Z, Sun N, Xiao B, Rajaa N, Liu C, Guo D (2016) Batch anaerobic co-digestion of OFMSW (organic fraction of municipal solid waste), TWAS (thickened waste activated sludge) and RS (rice straw): influence of TWAS and RS pretreatment and mixing ratio. Energy 107:131–140. https://doi.org/10.1016/j.energy.2016.03.141
Anderson GK, Yang G (1992) Determination of bicarbonate and total volatile acid concentration in anaerobic digesters using a simple titration. Water Environ Res 64:53–59. https://doi.org/10.2175/WER.64.1.8
AOAC (1992) AOAC official methods of analysis. Assoc Off Agric Chem, Washington, DC
APHA-AWWA-WEF (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Blainski A, Lopes GC, Palazzo de Mello JC (2013) Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules 18:6852–6865. https://doi.org/10.3390/molecules18066852
Braguglia CM, Gallipoli A, Gianico A, Pagliaccia P (2018) Anaerobic bioconversion of food waste into energy: a critical review. Bioresour Technol 248:37–56. https://doi.org/10.1016/j.biortech.2017.06.145
Cakr A, Ozmihci S, Kargi F (2010) Comparison of bio-hydrogen production from hydrolyzed wheat starch by mesophilic and thermophilic dark fermentation. Int J Hydrog Energy 35:13214–13218. https://doi.org/10.1016/j.ijhydene.2010.09.029
Cao G, Ren N, Wang A, Lee DJ, Guo W, Liu B, Feng Y, Zhao Q (2009) Acid hydrolysis of corn stover for biohydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. Int J Hydrog Energy 34:7182–7188. https://doi.org/10.1016/j.ijhydene.2009.07.009
Chang ACC, Tu YH, Huang MH, Lay CH, Lin CY (2011) Hydrogen production by the anaerobic fermentation from acid hydrolyzed rice straw hydrolysate. Int J Hydrog Energy 36:14280–14288. https://doi.org/10.1016/j.ijhydene.2011.04.142
Chen S-D, Lee K-S, Lo Y-C, Chen W-M, Wu J-F, Lin C-Y, Chang J-S (2008) Batch and continuous biohydrogen production from starch hydrolysate by Clostridium species. Int J Hydrog Energy 33:1803–1812. https://doi.org/10.1016/j.ijhydene.2008.01.028
Chen X, Sun Y, Xiu Z, Li X, Zhang D (2006) Stoichiometric analysis of biological hydrogen production by fermentative bacteria. Int J Hydrog Energy 31:539–549. https://doi.org/10.1016/j.ijhydene.2005.03.013
Datar R, Huang J, Maness PC, Mohagheghi A, Czernik S, Chornet E (2007) Hydrogen production from the fermentation of corn stover biomass pretreated with a steam-explosion process. Int J Hydrog Energy 32:932–939. https://doi.org/10.1016/j.ijhydene.2006.09.027
Del Campo I, Alegría I, Zazpe M, Echeverría M, Echeverría I (2006) Diluted acid hydrolysis pretreatment of agri-food wastes for bioethanol production. Ind Crop Prod 24:214–221. https://doi.org/10.1016/j.indcrop.2006.06.014
Díaz AI, Laca A, Laca A, Díaz M (2017) Treatment of supermarket vegetable wastes to be used as alternative substrates in bioprocesses. Waste Manag 67:59–66. https://doi.org/10.1016/j.wasman.2017.05.018
Escamilla-Alvarado C, Poggi-Varaldo HM, Ponce-Noyola MT (2016) Bioenergy and bioproducts from municipal organic waste as alternative to landfilling: a comparative life cycle assessment with prospective application to Mexico. Environ Sci Pollut Res 24:25602–25617. https://doi.org/10.1007/s11356-016-6939-z
Escamilla-Alvarado C, Poggi-Varaldo HM, Ponce-Noyola T, Ríos-Leal E, Robles-Gonzalez I, Rinderknecht-Seijas N (2015) Saccharification of fermented residues as integral part in a conceptual hydrogen-producing biorefinery. Int J Hydrog Energy 40:17200–17211. https://doi.org/10.1016/j.ijhydene.2015.06.164
Escamilla-Alvarado C, Ponce-Noyola MT, Poggi-Varaldo HM, Ríos-Leal E, García-Mena J, Rinderknecht-Seijas N (2014) Energy analysis of in-series biohydrogen and methane production from organic wastes. Int J Hydrog Energy 39:16587–16594. https://doi.org/10.1016/j.ijhydene.2014.06.077
Escamilla-Alvarado C, Ponce-Noyola T, Ríos-Leal E, Poggi-Varaldo HM (2013) A multivariable evaluation of biohydrogen production by solid substrate fermentation of organic municipal wastes in semi-continuous and batch operation. Int J Hydrog Energy 38:12527–12538. https://doi.org/10.1016/j.ijhydene.2013.02.124
Fan YT, Zhang YH, Zhang SF, Hou HW, Ren BZ (2006) Efficient conversion of wheat straw wastes into biohydrogen gas by cow dung compost. Bioresour Technol 97:500–505. https://doi.org/10.1016/j.biortech.2005.02.049
Farhat A, Miladi B, Hamdi M, Bouallagui H (2018) Fermentative hydrogen and methane co-production from anaerobic co-digestion of organic wastes at high loading rate coupling continuously and sequencing batch digesters. Environ Sci Pollut Res 25:27945–27958. https://doi.org/10.1007/s11356-018-2796-2
Gavilán A, Escamilla-Alvarado C, Martínez MÁ, Ramírez T (2018) Chapter 8. Municipal solid waste management. In: Molina LT (ed) Progress and opportunities for reducing short-lived climate pollutants across Latin America and the Caribbean. United Nations Environment Programme and the Climate and Clean Air Coalition, pp 118–135
Ghimire A, Valentino S, Frunzo L, Trably E, Escudié R, Pirozzi F, Lens PNL, Esposito G (2015) Biohydrogen production from food waste by and residue post-treatment to anaerobic digestion : a synergy for energy recovery. Int J Hydrog Energy 40:16045–16055. https://doi.org/10.1016/j.ijhydene.2015.09.117
Giuliano A, Poletto M, Barletta D (2016) Process optimization of a multi-product biorefinery: the effect of biomass seasonality. Chem Eng Res Des 107:236–252. https://doi.org/10.1016/j.cherd.2015.12.011
Gobierno de México (2015) Documento de Posición de México en 21a Conferencia de las Partes de la Convención Marco de las Naciones Unidas sobre el Cambio Climático. 1–13
Gonzales RR, Sivagurunathan P, Parthiban A, Kim SH (2016) Optimization of substrate concentration of dilute acid hydrolyzate of lignocellulosic biomass in batch hydrogen production. Int Biodeterior Biodegrad 113:22–27. https://doi.org/10.1016/j.ibiod.2016.04.016
Hashimoto AG (1989) Effect of inoculum/substrate ratio and pretreatments on methane yield and production rate from straw. Biol Wastes 28:247–255. https://doi.org/10.1016/0961-9534(94)00086-9
Hernández-Flores G, Solorza-Feria O, Poggi-Varaldo HM (2017) Bioelectricity generation from wastewater and actual landfill leachates: a multivariate analysis using principal component analysis. Int J Hydrog Energy 42:20772–20782. https://doi.org/10.1016/j.ijhydene.2017.01.021
Hernández C, Escamilla-Alvarado C, Sánchez A, Alarcón E, Ziarelli F, Musule R, Valdez-Vazquez I (2019) Wheat straw, corn stover, sugarcane, and Agave biomasses: chemical properties, availability, and cellulosic-bioethanol production potential in Mexico. Biofuels Bioprod Biorefin 13:1143–1159. https://doi.org/10.1002/bbb.2017
International Energy Agency (2016) Mexico energy outlook. Int Energy Agency, Paris
Joglekar SN, Pathak PD, Mandavgane SA, Kulkarni BD (2019) Process of fruit peel waste biorefinery: a case study of citrus waste biorefinery, its environmental impacts and recommendations. Environ Sci Pollut Res 26:34713–34722. https://doi.org/10.1007/s11356-019-04196-0
Kim S, Dale BE, Jin M et al (2019) Integration in a depot-based decentralized biorefinery system: corn stover-based cellulosic biofuel. GCB Bioenergy 11:871–882. https://doi.org/10.1111/gcbb.12613
Kumar S, Dheeran P, Singh SP, Mishra IM, Adhikari DK (2015) Kinetic studies of two-stage sulphuric acid hydrolysis of sugarcane bagasse. Renew Energy 83:850–858. https://doi.org/10.1016/j.renene.2015.05.033
Kumari S, Das D (2019) Biohythane production from sugarcane bagasse and water hyacinth: a way towards promising green energy production. J Clean Prod 207:689–701. https://doi.org/10.1016/j.jclepro.2018.10.050
Li K, Liu R, Sun C (2016) A review of methane production from agricultural residues in China. Renew Sust Energ Rev 54:857–865. https://doi.org/10.1016/j.rser.2015.10.103
Li Y, Park SY, Zhu J (2011) Solid-state anaerobic digestion for methane production from organic waste. Renew Sust Energ Rev 15:821–826. https://doi.org/10.1016/j.rser.2010.07.042
Liu CM, Chu CY, Lee WY, Li YC, Wu SY, Chou YP (2013) Biohydrogen production evaluation from rice straw hydrolysate by concentrated acid pre-treatment in both batch and continuous systems. Int J Hydrog Energy 38:15823–15829. https://doi.org/10.1016/j.ijhydene.2013.07.055
Lo HM, Kurniawan TA, Sillanpää MET, Pai TY, Chiang CF, Chao KP, Liu MH, Chuang SH, Banks CJ, Wang SC, Lin KC, Lin CY, Liu WF, Cheng PH, Chen CK, Chiu HY, Wu HY (2010) Modeling biogas production from organic fraction of MSW co-digested with MSWI ashes in anaerobic bioreactors. Bioresour Technol 101:6329–6335. https://doi.org/10.1016/j.biortech.2010.03.048
Luo G, Talebnia F, Karakashev D, Xie L, Zhou Q, Angelidaki I (2011) Enhanced bioenergy recovery from rapeseed plant in a biorefinery concept. Bioresour Technol 102:1433–1439. https://doi.org/10.1016/j.biortech.2010.09.071
Mahlia TMI, Ismail N, Hossain N, Silitonga AS, Shamsuddin AH (2019) Palm oil and its wastes as bioenergy sources: a comprehensive review. Environ Sci Pollut Res 26:14849–14866. https://doi.org/10.1007/s11356-019-04563-x
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Moncada-Botero J, Aristizábal-Marulanda V, Cardona-Alzate CA (2016) Design strategies for sustainable biorefineries. Biochem Eng J 116:122–134. https://doi.org/10.1016/j.bej.2016.06.009
Moset V, Al-zohairi N, Moller HB (2015) The impact of inoculum source, inoculum to substrate ratio and sample preservation on methane potential from different substrates. Biomass Bioenergy 83:474–482. https://doi.org/10.1016/j.biombioe.2015.10.018
NMX-AA-25-1984 (1992) Norma Mexicana NMX-AA-25-1984. Protección al ambiente-Contaminación del suelo-residuos sólidos-determinación del pH-Método potenciométrico Norma Mex. 4–5
Oliwit AT, Cayetano RDA, Kumar G, Kim JS, Kim SH (2019) Comparative evaluation of biochemical methane potential of various types of Ugandan agricultural biomass following soaking aqueous ammonia pretreatment. Environ Sci Pollut Res:1–11. https://doi.org/10.1007/s11356-019-07190-8
Ozmihci S, Kargi F, Cakir A (2011) Thermophilic dark fermentation of acid hydrolyzed waste ground wheat for hydrogen gas production. Int J Hydrog Energy 36:2111–2117. https://doi.org/10.1016/j.ijhydene.2010.11.033
Purwadi R, Niklasson C, Taherzadeh MJ (2004) Kinetic study of detoxification of dilute-acid hydrolyzates by Ca(OH) 2. J Biotechnol 114:187–198. https://doi.org/10.1016/j.jbiotec.2004.07.006
Ren N, Cao G, Wang A, Lee DJ, Guo W, Zhu Y (2008) Dark fermentation of xylose and glucose mix using isolated Thermoanaerobacterium ihermosaccharolyticum W16. Int J Hydrog Energy 33:6124–6132. https://doi.org/10.1016/j.ijhydene.2008.07.107
Rodríguez-Valderrama S (2018) Enfoque de Biorrefienría para la Producción de Hidrógeno y Metano a partir de residuos Orgánicos. Universidad Autónoma de Nuevo León
Rodríguez-Valderrama S, Escamilla-Alvarado C, Amezquita-Garcia HJ, Cano-Gómez JJ, Magnin JP, Rivas-García P (2019) Evaluation of feeding strategies in upflow anaerobic sludge bed reactor for hydrogenogenesis at psychrophilic temperature. Int J Hydrog Energy 44:12346–12355. https://doi.org/10.1016/j.ijhydene.2018.09.215
Romero-Cedillo L, Poggi-Varaldo HM, Ponce-Noyola T, Ríos-Leal E, Ramos-Valdivia AC, Cerda-García Rojas CM, Tapia-Ramírez J (2016) A review of the potential of pretreated solids to improve gas biofuels production in the context of an OFMSW biorefinery. J Chem Technol Biotechnol 92:937–958
Roy S, Das D (2016) Biohythane production from organic wastes: present state of art. Environ Sci Pollut Res 23:9391–9410. https://doi.org/10.1007/s11356-015-5469-4
SADER (2019) Expectativas de producción agropecuaria y pesquera. Mayo 2019
Saha BC, Iten LB, Cotta MA, Wu YV (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem 40:3693–3700. https://doi.org/10.1016/j.procbio.2005.04.006
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of extractives in biomass. NREL.
Sultana A, Kumar A (2011) Optimal configuration and combination of multiple lignocellulosic biomass feedstocks delivery to a biorefinery. Bioresour Technol 102:9947–9956. https://doi.org/10.1016/j.biortech.2011.07.119
Taboada-González P, Aguilar-Virgen Q, Ojeda-Benítez S, Armijo C (2011) Waste characterization and waste management perception in rural communities in Mexico: a case study. Environ Eng Manag J 10:1751–1759. https://doi.org/10.30638/eemj.2011.238
Tan L, Liu Z, Liu T, Wang F (2019) Efficient fractionation of corn stover by bisulfite pretreatment for the production of bioethanol and high value products. BioResources 14:6501–6515
Tapia-Rodríguez A, Ibarra-Faz E, Razo-Flores E (2019) Hydrogen and methane production potential of agave bagasse enzymatic hydrolysates and comparative technoeconomic feasibility implications. Int J Hydrog Energy 44:17792–17801. https://doi.org/10.1016/j.ijhydene.2019.05.087
Varanasi JL, Kumari S, Das D (2018) Improvement of energy recovery from water hyacinth by using integrated system. Int J Hydrog Energy 43:1303–1318. https://doi.org/10.1016/j.ijhydene.2017.11.110
Wang J, Wan W (2009) Kinetic models for fermentative hydrogen production: a review. Int J Hydrog Energy 34:3313–3323. https://doi.org/10.1016/j.ijhydene.2009.02.031
Wang W, Xie L, Chen J, Luo G, Zhou Q (2011) Biohydrogen and methane production by co-digestion of cassava stillage and excess sludge under thermophilic condition. Bioresour Technol 102:3833–3839. https://doi.org/10.1016/j.biortech.2010.12.012
Wong YM, Wu TY, Ching-Juan J (2014) A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew Sust Energ Rev 34:471–482. https://doi.org/10.1016/j.rser.2014.03.008
Xia A, Cheng J, Lin R, Lu H, Zhou J, Cen K (2013) Comparison in dark hydrogen fermentation followed by photo hydrogen fermentation and methanogenesis between protein and carbohydrate compositions in Nannochloropsis oceanica biomass. Bioresour Technol 138:204–213. https://doi.org/10.1016/j.biortech.2013.03.171
Yan L, Zhang H, Chen J, Lin Z, Jin Q, Jia H, Huang H (2009) Dilute sulfuric acid cycle spray flow-through pretreatment of corn stover for enhancement of sugar recovery. Bioresour Technol 100:1803–1808. https://doi.org/10.1016/j.biortech.2008.10.001
Zhang K, Ren N, Wang A (2015) Fermentative hydrogen production from corn stover hydrolyzate by two typical seed sludges: effect of temperature. Int J Hydrog Energy 40:3838–3848. https://doi.org/10.1016/j.ijhydene.2015.01.120
Zhang W, Wei Q, Wu S, Qi D, Li W, Zuo Z, Dong R (2014) Batch anaerobic co-digestion of pig manure with dewatered sewage sludge under mesophilic conditions. Appl Energy 128:175–183. https://doi.org/10.1016/j.apenergy.2014.04.071
Zhu J, Wan C, Li Y (2010) Enhanced solid-state anaerobic digestion of corn stover by alkaline pretreatment. Bioresour Technol 101:7523–7528. https://doi.org/10.1016/j.biortech.2010.04.060
Funding
The authors express their gratitude to the Directive Board of the Chemical Sciences Faculty of the Universidad Autónoma de Nuevo León for supporting the project (02-106534-PST-15/123). Santiago Rodríguez-Valderrama thanks Consejo Nacional de Ciencia y Tecnología (Conacyt) for the Scholarship No. 714579.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodríguez-Valderrama, S., Escamilla-Alvarado, C., Rivas-García, P. et al. Biorefinery concept comprising acid hydrolysis, dark fermentation, and anaerobic digestion for co-processing of fruit and vegetable wastes and corn stover. Environ Sci Pollut Res 27, 28585–28596 (2020). https://doi.org/10.1007/s11356-020-08580-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08580-z