Abstract
Dark fermentation, also known as acidogenesis, involves the transformation of a wide range of organic substrates into a mixture of products, e.g. acetic acid, butyric acid and hydrogen. This bioprocess occurs in the absence of oxygen and light. The ability to synthesize hydrogen, by dark fermentation, has raised its scientific attention. Hydrogen is a non-polluting energy carrier molecule. However, for energy generation, there is a variety of other sustainable alternatives to hydrogen energy, e.g. solar, wind, tide, hydroelectric, biomass incineration, or nuclear fission. Nevertheless, dark fermentation appears as an important sustainable process in another area: the synthesis of valuable chemicals, i.e. an alternative to petrochemical refinery. Currently, acetic acid, butyric acid and hydrogen are mostly produced by petrochemical reforming, and they serve as precursors of ubiquitous petrochemical derived products. Hence, the future of dark fermentation relies as a core bioprocess in the biorefinery concept. The present article aims to present and discuss the current and future status of dark fermentation in the biorefinery concept. The first half of the article presents the metabolic pathways, product yields and its technological importance, microorganisms responsible for mixed dark fermentation, and operational parameters, e.g. substrates, pH, temperature and head-space composition, which affect dark fermentation. The minimal selling price of dark fermentation products is also presented in this section. The second half discusses the perspectives and future of dark fermentation as a core bioprocess. The relationship of dark fermentation with other (bio)processes, e.g. liquid fuels and fine chemicals, algae cultivation, biomethane–biohythane–biosyngas production, and syngas fermentation, is then explored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The preservation and management of the diverse natural resources is essential for a sustainable development in the present and future. An economy based on sustainable processes requires safe and sustainable resources for industrial production, investment and finance system, ecological and health safety, and sustainability (Reddy et al. 1997; Tanaka et al. 2010). Fossil resources are not sustainable and their availability is more than questionable in the long-term. Therefore, it is essential to establish solutions that will reduce the rapid consumption of fossil resources, i.e. petroleum, natural gas, coal, minerals. An approach is the stepwise conversion of large parts of the global economy into a sustainable biobased economy with bioenergy, biofuels, and biobased products as its main foundations (Kamm et al. 2006).
For energy generation, there is a variety of alternative renewable processes that can be established, e.g. solar, wind, tide, hydroelectric, biomass incineration, nuclear fission, etc. However, products derived from petrochemical processes are ubiquitous in our present day society, e.g. transport fuels, adhesives, plastics, among others. Research has to be addressed towards new technologies capable of replacing both petrochemical technology and products in order to transform our society into a sustainable one (Kamm et al. 2006). Therefore, the chemical-fuel industry and the industrial biotechnology will depend on the conversion of sustainable material, i.e. will be based on biomass technologies.
Biomass technologies are a vast and interdisciplinary renewable concept in which the main role is played by solar energy recovery and CO2 transformation into biomass, i.e. photosynthesis. Under any sustainable scenario of biomass based bioprocesses, organic residues will be produced. In this context, dark fermentation, also known as acidogenic mixed culture fermentation, appears as an attractive solution that will reduce those residues, and increase the global efficiency of biomass based production of energy and valuable chemicals, interlinked in a biorefinery concept.
As stated above, the dark fermentation of those organic residues yields energy carriers and fuels, such as hydrogen and ethanol, and chemicals like acetic acid, butyric acid, propionic acid, lactic acid, among others. In present days, these compounds are mostly produced by petrochemical reforming, except for ethanol and lactic acid that are mainly produced by bioprocesses. The production of those compounds by dark fermentation makes it attractive as a petrochemical refinery alternative.
In recent years, dark fermentation has been extensively studied and reviewed for its capacity of producing hydrogen (Hawkes et al. 2002; Liu et al. 2002; Lin and Lay 2004; Karlsson et al. 2008; Hallenbeck 2009; Guo et al. 2010; Rittmann and Herwig 2012). Nevertheless, as is shown in Sect. 2.2, in the best case scenario, i.e. assuming a maximum hydrogen yield of 4 \({\text{mol}}_{{{\text{H}}_{2} }}\)/molglucose, the produced hydrogen represents only a 4 % of the total substrate mass consumed, while 67 % corresponds to products in the liquid phase, e.g. acetic acid. Hence this review also explores other options besides hydrogen production from dark fermentation. It aims to present and discuss the current and future status of dark fermentation in the biorefinery concept.
The first half of the review presents dark fermentation metabolic pathways, product yields and the technological importance in the present industry, microorganisms present and responsible for mixed dark fermentation, operational parameters affecting dark fermentation, e.g. substrates, pH, temperature and head-space composition. This section also presents the process costs of conventional dark fermentation. The second half presents and discusses the perspectives and future of dark fermentation as a core bioprocess. Links with other (bio)processes are also explored, e.g. acetone–butanol–ethanol (ABE) fermentation, syngas fermentation, biohythane, bioelectrochemical systems, and catalytic hydrocarbon synthesis.
2 Dark fermentation—current status
This section presents the dark fermentation biochemistry and metabolism, product consideration as product yields, prices, and technological importance, and the operational factors that affect dark fermentation, e.g. microbial population structure, inocula sources, substrates and nutrients, pH, temperature, gas composition, and bioreactor configuration.
2.1 The biochemistry of dark fermentation
Dark fermentation involves the transformation of organic compounds to various inorganic and organic products. During this process, a portion of an organic compound may be oxidized while another portion is reduced. It is from this oxidation–reduction of organic compounds that dark fermentative microorganisms obtain their energy, producing numerous simplistic and soluble organic compounds (Gerardi 2003).
The dark fermentation process begins with bacterial hydrolysis of the fed organic materials in order to break down insoluble polymers, making them available for microorganism consumption. Acidogenic bacteria then convert the products of hydrolysis into carbon dioxide, hydrogen, ammonia, alcohols and organic acids. Hydrolysis and acidogenesis are detailed here below.
2.1.1 Hydrolysis
Hydrolysis is the first step required for anaerobic microbial utilization of complex polymers (Aceves-Lara et al. 2008c). Hydrolytic fermentative bacteria facilitate the extracellular enzymatic hydrolysis of the initial complex organic matter formed by polymers such as polysaccharides, proteins, and fats. The hydrolases (enzymes) that catalyzes these reactions are cellulase, amylase, protease, and lipase, among others. Hydrolases may be secreted to the extracellular environment or be bound to the cell surface (Pavlostathis and Gossett 1988; Mitchell and Gu 2010).
Polysaccharides are generally converted into simple monomeric or dimeric sugars. Hydrolysis of starch and cellulose yields glucose as monomeric sugar, while hemicellulose is degraded to galactose, arabinose, xylose, mannose and glucose. Proteins are broken down into amino acids, small peptide, ammonia, and carbon dioxide by proteases. Lipids are hydrolyzed into long and short chain fatty acids and glycerol by lipases (Gerardi 2003; Insam et al. 2010; Mitchell and Gu 2010). The hydrolysis products then become substrates for the fermentation processes that follow.
2.1.2 Acidogenesis
Monosaccharides and amino acids, released after the hydrolysis of their respective polymers, serve as substrates to the acidogenic fermentation (Insam et al. 2010). Groups of facultative and anaerobic fermentative or anaerobic oxidizing organisms utilize these substrates yielding compounds such as ethanol, acetate, propionate, H2 and CO2 as intermediary products.
Acidogenesis is a process in which intracellular reduced co-factors such as NADH are being oxidized. The regeneration of co-factors is vital to the process as a whole, as these co-factors serve as intermediary electron acceptors in the catabolic reactions that proceed continuously within the system. The regeneration of these co-factors is done by the production of the alcohols and organic acids yielded in this process (Gujer and Zehnder 1983; Zehnder and Svensson 1986; Gerardi 2003).
2.1.3 Acidogenesis fermentation metabolism
In a mixed culture acidogenic fermentation, where many microbial species are present, most of the fermentative conversions explained below are possible. Further detailed dark fermentation metabolic models can be found in the literature (Bastidas-Oyanedel et al. 2008; Gonzalez-Cabaleiro et al. 2015; Zhang et al. 2013c). The fermentation patterns observed are the result of the combined effect and interaction among the microbial consortium. Acidogenic bacteria are capable of performing a variety of oxidation–reduction reactions involving organic compounds, carbon dioxide, and molecular hydrogen. Acidogenic bacteria include facultative anaerobes, aerotolerant anaerobes, and strict anaerobes. In such an ecosystem, the microorganism consortia activity depends on environmental condition changes, e.g. gas phase composition or pH for instance. Environmental changes will conduct changes on the consortia metabolism and/or physiology, affecting the types and quantities of compounds that are produced through fermentation (Gerardi 2003).
Metabolism is the sum of all biochemical reactions performed by a living organism. The reactions have two main purposes, (1) to generate energy for the organism, and (2) to build new cell material. Reactions related to generating energy are called catabolism, and all reactions leading to formation of cell material are called anabolism. Metabolism is initiated by the consumption of biodegradable substrates. The most common substrate found in organic residues is glucose. The acidogenic fermentation of glucose extracts energy in the form of ATP by substrate-level phosphorylation during oxidative substrate breakdown (Thauer et al. 1977).
The resulting reducing equivalents, in the form of NADH and reduced ferredoxin are transferred into metabolic intermediates (Angenent et al. 2004; Zhang et al. 2013c), leading to the formation of a variety of reduced products such as H2, ethanol, and organic acids (lactic, propionic, formic, acetic, butyric acids), depending on the fermentation pathways utilized (Louis and Flint 2009). Acidogenic bacteria generally possess alternative pathways leading to the formation of these products. Hence the relative proportions of the different products formed depend on the environmental conditions.
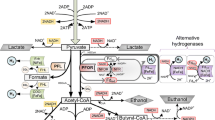
Figure 1 illustrates the main catabolic pathways in acidogenic glucose fermentation. Glycolysis (or Embden–Meyerhof–Parnas pathway) is thought to be the archetype of a universal metabolic pathway (Kurzynski 2006). It occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways known (Romano and Conway 1996). Glycolysis is a sequence of ten reactions. In Fig. 1, reaction (1), it is lumped into one reaction starting from glucose giving pyruvate. In this process, the consumption of 0.5 mol of glucose produces 1 mol of ATP, which is then used as energy source for biomass growth and maintenance processes.
The NADH resulting from glycolysis should be reconverted to NAD+ to allow glycolysis to continue. In anaerobic conditions, organisms are able to oxidase NADH back to NAD+ in several ways. One method is lactic acid fermentation, where pyruvate is converted into lactate, reaction (2). Propionic acid is formed also as a reduced product of glucose fermentation. Propionic acid can be produced through the so called acrylate pathway (Tholozan et al. 1992) or via the succinate pathway (Schink et al. 1987). The acrylate pathway, reaction (3), reconverts and extra NADH to NAD+. The succinate pathway, reactions (4) and (5), allows the reconversion of 2 NADH, this pathway also consumes CO2 for succinate synthesis. The production of ATP in the acrylate pathway and/or succinate pathway may not be possible (Seeliger et al. 2006; Zhang et al. 2013c).
Production of acetyl-coenzyme-A through pyruvate decarboxilation is a different way to regulate glucose catabolism. Two different pathways exist in anaerobic bacteria, the pyruvate formate lyase pathway and the pyruvate dehydrogenase pathway (Sparling et al. 2006; Gheshlaghi et al. 2009), reactions (6) and (7), respectively. Facultative anaerobes, e.g. Enterobacter, E. coli, Klebsiella, present the pyruvate formate lyase pathway (Waligorska 2012). Simultaneous presence and expression of genes encoding pyruvate formate lyase and pyruvate ferredoxin oxidoreductase can be found in members of the genus Clostridium (Sparling et al. 2006).
Formic acid, produced by reaction (6), is converted by the formate-hydrogen lyase, reaction (8) into H2 and CO2 (Waligorska 2012). The reduced ferredoxin, produced by reaction (7), transfers electrons to hydrogenase catalyzing the generation of H2, reaction (9) (Waligorska 2012). Reaction (10) consist on the NADH:ferredoxin oxidoreductase, which transfers electrons from reduced ferredoxine to NAD+ producing NADH (Hallenbeck 2009). This reaction does not seem to exist in many facultative anaerobes (Cai et al. 2011).
The synthesis of acetic acid proceeds from acetyl-CoA, reaction (11). This reaction produces ATP, via the phosphotransacetylase and the acetate kinase reactions. Both enzymes are present in all anaerobic bacteria that form acetyl-CoA in their energy metabolism and that use the acetyl-CoA to synthesise ATP (Thauer et al. 1977). The detection of acetate kinase activity in C. thermolacticum confirmed the presence of the acetate branch from acetyl-CoA and the supplementary ATP formation associated to this pathway (Collet et al. 2006). Another pathway for the generation of acetic acid is homoacetogenesis, reaction (14), which consumes H2 and CO2 (Zhao et al. 2010).
Butyric acid synthesis, reaction (12), takes place in a cycle starting with the condensation of molecules of acetyl-CoA. This cycle goes on until the formation of butyril-CoA, wich reacts with a molecule of acetic acid to yield one molecule of butyric acid and one of acetyl-CoA, which is the starting point for a new cycle. The simplification of this cycle starts with the condensation of acetyl-CoA and acetic acid to produce butyric acid. Butyrate fermentation has been proposed to not only regenerate NAD+ from NADH produced in the glycolysis, but that it might also lead to further energy gains. During the conversion of acetyl-CoA to butyric acid, one of the enzymatic complex, the butyryl-CoA dehydrogenase electron-transferring flavoprotein, may generate a proton motive force with a membrane-associated NADH:ferredoxin oxidoreductase (Louis and Flint 2009).
The formation of ethanol from acetyl-CoA, reaction (13), involves two enzyme, aldehyde dehydrogenase and ethanol dehydrogenase. The former catalyzes the reaction of acetyl-CoA to acetaldehyde (Thauer et al. 1977). The later catalyzes the dehydrogenation of acetaldehyde to ethanol (Collet et al. 2006). The synthesis of caproic acid by dark fermentation, reaction (15), is still under investigation. However, it is clear that acetyl-CoA is the precursor of caproic acid (Jeon et al. 2010).
2.2 Dark fermentation product considerations
This section analyzes the dark fermentation product yields. The analysis clearly shows that dark fermentation attention has not to focus only on hydrogen production, but also on organic acid production. Also, here the price and market size of organic acids and ethanol are presented. Furthermore, it is described the technological importance of dark fermentation products in the present industry.
2.2.1 Product yields and prices
Figure 2 shows the average dark fermentation product yields of acetic acid, butyric acid, propionic acid, caproic acid, lactic acid, ethanol, formic acid, H2, CO2 and biomass, expressed as mass percentage of substrate consumed. Glucose and organic solid waste were reviewed as substrates. For glucose, the data was obtained from mixed culture dark fermentation under continuous mode with suspended microorganisms, i.e. not immobilized (Aceves-Lara et al. 2008a, b; Temudo et al. 2008a, b, 2009; Bastidas-Oyanedel et al. 2012; Zhang et al. 2012). In the case of organic solid waste the data corresponds to the dark fermentation of organic fraction of municipal solid waste (Zahedi et al. 2013) and food waste (Jiang et al. 2013). Mass and electron balances (Bastidas-Oyanedel et al. 2008) have been applied to glucose and organic solid waste data. This was useful for the organic solid waste since it allowed estimating the CO2 and biomass average yield (data not available in the references).
Dark fermentation average product yields expressed as mass percentage of substrate consumed, i.e. glucose and organic solid waste. Glucose plot is based on experimental data of continuous mixed culture fermentation (Aceves-Lara et al. 2008a, 2008b; Temudo et al. 2008a, 2008b, 2009; Bastidas-Oyanedel et al. 2012; Zhang et al. 2012). Organic solid waste plot is based on Jiang et al. (2013) and Zahedi et al. (2013)
Both sorts of substrates have different scientific aims. Glucose has been used as a model substrate to study the metabolic changes in dark fermentation, while organic solid waste is a realistic substrate in order to apply dark fermentation in the biorefinery concept.
In both cases, organic acids and ethanol represent around 65 % w/w of substrate consumed, H2 does not represent more than 3 %, CO2 and biomass represents around 35 %, i.e. a large amount of the substrates, 65 %, is converted to valuable products different than H2. Also, the products conforming this 65 % are already traded in the international market, while H2 is difficult to be priced since it is mainly produced in the same industries that use it.
Table 1 shows the bulk prices for acetic acid, butyric acid, propionic acid, caproic acid, lactic acid, ethanol, and formic acid. As an end product, butyric acid is by far the compound that presents the highest price, 2500 USD/ton, followed by propionic acid, 1700 USD/ton. The lowest price is for acetic acid, 800 USD/ton. In this context mixed culture dark fermentation can be controlled to maximize the production of butyric acid over the other products. Average concentrations and productivities are presented in Table 2.
2.2.2 Technological importance of dark fermentation products
This section reviews the technological importance of acetic acid, butyric acid, propionic acid, caproic acid, lactic acid, ethanol, formic acid, H2, and CO2, in the current industry. Nowadays these compounds are produced by petroleum-based chemical synthesis, except for ethanol and lactic acid which are mainly produced by pure-culture bioprocess. As it was mentioned before, in a biobased economy, dark fermentation of organic residues supposed to increase the global efficiency of biomass based production of valuable chemicals. Hence dark fermentation appears as an alternative to petroleum-based synthesis.
Industrial acetic acid is mainly produced by petrochemical synthesis (Agreda and Zoeller 1993). Acetic acid is used in the synthesis of several derived molecules, among them, vinyl acetate represents the single largest use of acetic acid. Vinyl acetate derived polymers are ubiquitous in modern society, which are found as part of woodpanels, paper bags, cardboard boxes, labels adhesives, white glue, latex paints, highquality paper coating, textiles, and cement additive (Agreda and Zoeller 1993; Weissermel and Arpe 1997; Lindblad et al. 2002). Acetic acid also serves as precursor of cellulose acetate, alcohol acetates, halogenated acetic acid, acetic anhydride, citrate esters, diketene, methyl acetoacetate, acetoacetamides, acetoacetylated polymers, which serve as precursor molecules with vast applications on the production of pharmaceuticals (such as aspirin, vitamin E, beta-lactam and oxacillin antibiotics, antiepilectic drugs), agrochemicals (insecticides, fungicides), and dye, colorant and polymers. Furthermore, acetic acid chemistry still offers ample opportunity for providing new discoveries in the future of material science (Agreda and Zoeller 1993).
Butyric acid is produced at industrial scale via mainly a petroleum-based chemical synthesis (Zhang et al. 2009). The main industrial application of butyric acid is in the manufacture of cellulose acetate-butyrate plastics (Rogers et al. 2006; Dwidar et al. 2012). Another well-known polymer, based on butyric acid, is the poly-3-hydroxybutyrate (PHB). Butyric acid esters, e.g. methyl, ethyl and amyl butyrate, are used in beverages, foods and cosmetics industries as fragrant and flavoring agents (Rogers et al. 2006; Dwidar et al. 2012). Nowadays, butyric acid attention is focused on the synthesis of fuels, since it can be converted to butanol through biological or chemical transformation (Dwidar et al. 2012).
Butyric acid also has many applications in medicine, in the field of gastroenterology (Hamer et al. 2008; Vanhoutvin et al. 2009), treatment of haematological, metabolic and neurological pathologies (Sossai 2012). Various prodrugs (Rephaeli et al. 2000) that are derivatives of butyric acid were tried for their potential use in treatment of hypercholesterolemia (Canani et al. 2011), cancers (Canani et al. 2012) and hemoglobinopathies (Kim et al. 2009), including leukemia and sickle cell anemia, and also to protect hair follicles of radio- and chemotherapy-induced alopecia (Dwidar et al. 2012).
Propionic acid is used as antifungicide in the food industry to suppress mold growth in breads, meats, fruits, and on the surfaces of cheese and also as preservatives for grains, silage and tobacco during storage and transport (Rogers et al. 2006). As in the case of butyric acid, propionic acid is also used in the production of cellulose-based plastics, such as cellulose acetate propionate, which are used in textiles, filters, reverse osmosis membranes, sheeting, film products, lacquers, and molding plastics (Rogers et al. 2006). Propionic acid esters and other derivatives are used as plasticizers, e.g. phenyl propionate and glycerol tripropionate, and as specialized solvents. Propionic acid esters are also used as perfumes and flavours, e.g. citronellyl propionate and geranyl propionate (Rogers et al. 2006).
The commercial use of caproic acid, also known as hexanoic acid, is primarily as precursor for the synthesis of pharmaceuticals, flavors and other hexyl ester (Kenealy et al. 1995). Caproic acid fungicide properties have been reported as an alternative to synthetic fungicides, reducing environmental-human harm (Leyva et al. 2008). Caproic acid biosynthesis, by chain elongation of ethanol or acetic acid, is gaining interest since it can be separated with less energy input than ethanol and/or acetic acid (Vasudevan et al. 2014). In-situ extraction of caproic acid have been reported from fermentation broth by water-immiscible organic solvents (Choi et al. 2013; Jeon et al. 2013).
Industrial production of lactic acid is mainly based on bioprocesses. Lactic acid is used in the polymer industry, as two of its polymers, the polyester syndiotactic polylactide, and polylactic acid, are currently use in wide applications (www.purac.com). Other applications include its use in the food, textile, leather, dyeing, and the cosmetic industries (Kamm et al. 2006). Lactic acid is an example of a biobased molecule that is widely used in our society.
Ethanol is produced in large volumes by industrial fermentation. In the 2008 the total worldwide ethanol production accounted to 51 Mega tonnes, of wich USA and Brazil contributed 52 and 37 % respectively (Mussatto et al. 2010). Most of the ethanol in the United States is produced from corn while Brazilian ethanol is derived from sugar cane (Freudenberger 2009). Ethanol is also produced by petrochemical synthesis. In the chemical industry, ethanol is used as solvent, antifreeze and as fuel supplement (Mussatto et al. 2010). The major use of ethanol is as an intermediate feedstock in the synthesis of innumerable organic chemicals. Some of them include: (1) diethyl ether, a solvent, extractant, and anesthetic, (2) acetaldehyde, which is the raw material for production of a large number of organic chemicals such as butanol, acetic acid, acetic anhydride, chloral, crotonaldehyde, and ethylhexanol. These and other ethanol-derived chemicals are used in dyes, drugs, synthetic rubber, solvents, detergents, plasticisers, surface coatings, adhesives, mouldings, cosmetics, explosives, pesticides, and synthetic fibre resins (Roehr 2001).
Formic acid is used in the textile industry, in tanning, in rubber processing and in manufacturing of pharmaceuticals, and is also used as food preservative agent in livestock feed (Zacharof and Lovitt 2013). Recently, it has received more attention to be used as environmental storage and transportation medium for hydrogen (Joo 2008). Hydrogen can be generated by the catabolic decomposition of formic acid. Also some researchers have demonstrated that formic acid has the potential to direct power fuel cells for electricity generation and transportation (Rice et al. 2002; Uhm et al. 2008).
Hydrogen is industrially produced by steam reforming, in which natural gas is reacts with steam, releasing hydrogen. The production of hydrogen by water electrolysis, by running an electrical current through it, is used where electricity is cheap and where high purity is required (Hoffmann 2001). Hydrogen uses are in energy applications and the chemical industry. Hydrogen, in combination with oxygen, is used to fuel space shuttles (Boucher 2006). Hydrogen is a non-polluting fuel since the combustion of hydrogen with oxygen produces only water, unlike hydrocarbon internal-combustion engines that produce carbon monoxide, carbon dioxide, unburned hydrocarbons, stench, and smoke (Berry and Aceves 2005). Also, hydrogen can be used in fuel cells, producing electricity, in a flameless process which is 2.5 times more efficient than internal-combustion engines (Peighambardoust et al. 2010). Hydrogen has been thought to store renewable electricity when is less needed, e.g. during low consumption hours, through electrolysis of water (Boucher 2006). Hydrogen then can be converted back to electricity at peak hours. Hydrogen can be stored at high-pressure, as an integral component in certain alloys known as hydrides, on microscopic carbon fibers. Hydrogen can be converted to formic acid in order to be stored or been directly used in fuel cells (Rice et al. 2002; Joo 2008; Uhm et al. 2008). In addition its energy applications, hydrogen is widely used in chemical industries as a raw material for hydrogenation in the production of hydrocarbons, fertilizers, dyes, drugs, and plastics. Also, it is used in the food industry for the treatment of oils and fats (Hoffmann 2001).
The most common use of carbon dioxide in industry is as supercritical CO2 in materials manufacturing industry and in food industry. Carbon dioxide appears as a non-hazardous and environmental friendly supercritical fluid. It is used as solvent in microelectronic applications (Weibel and Ober 2003), in polymer foam applications (Tomasko et al. 2009), in the manufacture of fibres, microparticles and films (Davies et al. 2008). Supercritical CO2 applications in food industry include extraction, fractionation, refinement and deodorization of lipids or essential oils (Sahena et al. 2009). Another application in food industry is on dealcoholization process, i.e. reduction of ethanol content in alcoholic beverages (Ruiz-Rodriguez et al. 2010). As expose above, use of supercritical CO2 reduce the use of organic solvents, reducing health, environmental and safety hazards.
2.3 Operation factors affecting dark fermentation
As outlined above, dark fermentation produces a mixture of organic acids, solvents and gases. The proportion of these products depends upon the microorganisms, the substrates and operational conditions (Cai et al. 2011). These parameters are very important for the optimization and modeling of the dark fermentation process. Dark fermentation literature has been focused on optimization of hydrogen production, including extensive reviews on the topic. Changes on dark fermentation occur in two levels: (1) microbial population structure shifts, and (2) enzymatically/metabolic shifts. These two levels can be modified independently or simultaneously (Ye et al. 2007; Guo et al. 2010).
2.3.1 Dark fermentation microbial population
Mixed culture dark fermentation is an autocatalytic process mediated by specified microorganisms that are able to thrive under anaerobically conditions. Mixed culture dark fermentation has two advantages over pure cultures fermentation technology, there is no need for aseptic conditions and it can consume a vast range of substrates, which decreases the overall cost of the process (Ntaikou et al. 2010). On the other hand, pure cultures are characterized by high selectivity, yielding higher product efficiency, and well control by environmental parameters (Waligorska 2012).
Identified bacteria in mixed culture dark fermentation are strict anaerobes, e.g. Clostridia sp. (Quemeneur et al. 2011) and facultative anaerobes, e.g. E. coli, Enterobactericeae sp. (Aceves-Lara et al. 2008c), and Klebsiella sp. (Ntaikou et al. 2010). Other identified bacteria in thermophilic dark fermentation are Caldicellulosiruptor saccharolyticus and Thermoanaerobacter spp. (Zhang et al. 2014). One of the phenomena related to the microbial population shift in dark fermentation is sporulation. This phenomena appears with the microbes belonging to Clostridium sp. Sporulation is a protection system that is activated when the environmental conditions are not favorable, e.g. increase in temperature (over 90 °C), excess or limitation of nutrients, dissolved oxygen, or a significant decrease on pH (below 3.0) (Sauer et al. 1995). This produces both advantages and disadvantages for the selection of dark fermentative microorganisms and process stability, respectively, as presented in Sect. 2.3.2. Regarding the process stability, sporulation must be controlled and/or avoided. During process, if sporulation occurs, this will decrease the substrate consumption rates and the productivity of dark fermentation, and produce a wash out of the spores, then losing the autocatalytic capacity of the microbes (Hawkes et al. 2002), hence increasing the dominance of bacteria populations that do not have sporulate.
2.3.2 Inocula sources
Several studies of mixed culture dark fermentation have used inocula from sewage sludge (Chen and Lin 2001), organic waste treating sludges (Rajhi et al. 2013), animal dung (Guo et al. 2010), fluvial (Rajhi et al. 2013) and marine (Bastidas-Oyanedel et al. 2013) sediments. In literature, three methods have been utilized in order to select and/or enrich the dark fermentative bacteria, avoiding the presence of organisms that consume organic acids and H2, particularly methanogens (Hawkes et al. 2002). These three methods are thermal shock, pH shock, and short hydraulic retention time (HRT).
Thermal shock, consists of heating the inocula to 90 °C for a short time (Li and Fang 2007). Usually for reaction volumes ranging from 1 to 5 L, authors use a thermal shock time range from 10 to 20 min (Mu et al. 2007; Im et al. 2012). This treatment allows the selection of spore forming bacteria, e.g. Clostridium (Hawkes et al. 2002).
The pH shock method uses pH below 5 or above 10 (Chen et al. 2002). This method uses the principle of the wide pH range that acidogenic bacteria can stand (Temudo et al. 2007), while methane producing archea cannot tolerate these pH conditions, being inhibited and washout of the reactors.
The third method, short hydraulic retention time (HRT), uses HRT ranging from 6 to 20 h (Temudo et al. 2007; Aceves-Lara et al. 2008b; Bastidas-Oyanedel et al. 2012). This is based on the fact that acidogenic bacteria exhibit higher growth kinetics than methane producing archea. Methanogenic archea is highly present in wastewater anaerobic sludge, sewage sludge, cattle dung. These three methods can be applied independently or in a combination (Liu et al. 2002).
2.3.3 Substrates and nutrients
Mixed culture dark fermentation has a wide range of substrate utilization (Nielsen et al. 2001), from different biomasses and organic waste streams (Li and Fang 2007; Chong et al. 2009). They are able to consume the residues from biodiesel production (Nishio and Nakashimada 2007), food waste (Han and Shin 2004; Shin et al. 2004), biomass residues from agroindustries (Hussy et al. 2003; Kaparaju et al. 2009; Guo et al. 2010; Chu et al. 2012), lignocellulosic hydrolysates (Ueno et al. 1995; Ren et al. 2009), paper/wastepaper/cardboard (Lay 2001), and pretreated biomass (Prakasham et al. 2010). It can convert anaerobic sludge (Mu et al. 2006) and organic fraction of municipal solid waste (Lay et al. 1999; Tawfik and El-Qelish 2012; Zahedi et al. 2013). This wide range of non-sterile fermentable organic feedstock for mixed culture dark fermentation highlights its viability and flexibility as technology (Hawkes et al. 2002). Compared to other bioprocesses, e.g. bioethanol production, where yeasts use a narrow range of substrates and sterile conditions are needed to avoid microbial contamination.
As mention previously in Sect. 2.3, substrates have a significant effect on productivities. Lin and Lay (2004) have observed metabolic changes when decreasing C/N ratio, leading to a shift from acetic acid to ethanol production. Quemeneur et al. (2011) have studied the influence of monosaccharide (glucose and fructose), disaccharide (sucrose, maltose and cellobiose) and trisaccharide (maltotriose) on genetic diversity and product yields and in mixed culture dark fermentation.
Quemeneur et al. (2011) studied the effect of different types of carbohydrates on the microbial population structures. Glucose, fructose, sucrose and cellobiose cultures, displayed a dominant population related to Clostridium sporogenes. Sucrose culture showed a population related to C. acetobutylicum. While cellobiose showed the most diverse culture including populations of C. cellulolyticum, C. acetobutylicum, C. saccharobutylicum and C. kluyveri. In the case of maltose and maltotriose cultures the dominant population was related to C. acetobutylicum. They also found that the H2 yield decreased when increasing the chain length of carbohydrates, from 1.82 ± 0.1 to 1.38 ± 0.12 \(( {\text{mol}}_{{{\text{H}}_{2} }} /{\text{mol}}_{{{\text{hexose}}\_{\text{equivalent}}}} )\). They also observed changes in liquid product yields. While Acetic and butyric acids were the main products in all tested carbohydrates, yields were 290 ± 60 (molacetic/molhexose_equivalent) and 380 ± 90 (molbutyric/molhexose_equivalent), caproic acid only appeared when using di and trisaccharides, with yields ranging from 10 to 40 (molcaproic/molhexose_equivalent), and ethanol yield increased dramatically when using maltotriose (trisaccharide), from 40 ± 10 (for mono and disaccharides) to 117.5 (molethanol/molhexose_equivalent).
Minerals present on the fermentation media also affect dark fermentation. As said before, most of the literature on dark fermentation is focused on hydrogen production. In this context the Fe2+ ions media composition has shown to be important. These ions are essential constituents of the hydrogenase active site (Aceves-Lara et al. 2008c), and the concentration of Fe2+ that maximizes H2 production was found to be around 200 mg/L, at 35 °C (Zhang and Shen 2006). Other minerals that have been identified as important for the H2 production are magnesium, sodium and zinc (Lin and Lay 2005). In the case of phosphate, its limitation may favor solvent production over H2 and organic acids (Hawkes et al. 2002).
2.3.4 pH
Dark fermentation has a wide pH range, 3.0–11 (Chen et al. 2002; Temudo et al. 2007). The pH modifies the dark fermentation product yields. H2 production maximization occurs in the pH range of 5–7 (Aceves-Lara et al. 2008b; Bastidas-Oyanedel et al. 2012). Acetic acid production is favored at pH over 6.5, while butyric acid production is favored at pH below 6.0 (Fang and Liu 2002). The pH range between 4.5 and 6.0 maximizes the ethanol production, while pH between 5.0 and 6.0 the propionate production (Hwang et al. 2004). Production of butanol is favored at pH below 4.3 (Kim et al. 2004). The accumulation of organic acids at pH below 5 favors the production of solvents, e.g. ethanol, propanol or butanol (Sauer et al. 1995; Hawkes et al. 2002). Increase in the formic acid at pH below 5.5 induces the enzyme formate-hydrogen lyase, which catalyses its decomposition into H2 and CO2 (Temudo et al. 2007; Bastidas-Oyanedel et al. 2012; Waligorska 2012).
2.3.5 Temperature
Dark fermentation is a biological/biochemical mediated process. Increase on temperature reduces enzymes activation energy. Dark fermentation can take place in the presence of mesophilic bacteria (Clostridium, Enterobacter) with their range of temperature being around 35 °C, Thermophiles (Caldicellulosiruptor, Thermoanaerobacterium) temperature around 50 °C, or hyperthermophiles (Thermotoga) temperature approximately 70 °C (Waligorska 2012).
In mixed culture dark fermentation, changes in temperature may produce shifts in the dominant bacteria culture. Some authors have observed shifts from Clostridium at mesophilic conditions to Thermoanaerobacterium in thermophilic conditions (Karadag and Puhakka 2010). In the case of H2 production, their productivities are similar for mesophilic and thermophilic regions. Anyhow, H2 yields are lower for the mesophilic temperature range (Lin and Chang 2004; Li and Fang 2007), as well as the H2 gas fraction. This could be due to the fact that hyperthermophilic bacteria are less inhibited by the H2 partial pressure (Waligorska 2012). Temperatures below 35 °C decrease the kinetics of dark fermentation (Lin and Chang 2004). Increasing the temperature, in the range of 33 to 41 °C has demonstrated an increase in the substrate conversion, and a shift from ethanol to butyrate (Mu et al. 2006).
2.3.6 Head space partial pressure and dissolved gas concentration (gases composition)
H2 and CO2 produced during dark fermentation, generates supersaturation in the liquid phase, allowing them to be transferred to the gas phase (Pauss et al. 1990; Kraemer 2004). H2 liquid saturation and gas partial pressure affects negatively the reactions producing H2 and the conversion of NADH to H2 by hydrogenases (Tanisho et al. 1998; Mizuno et al. 2000; Hallenbeck 2005), see Sect. 2.1.3. These metabolic reactions are thermodynamically controlled (Bastidas-Oyanedel et al. 2012). The production of hydrogen using electrons from NADH is possible at very low partial pressure of hydrogen. Some authors reported H2 partial pressures below 0.09 bar (Bastidas-Oyanedel et al. 2012), while others propose the partial pressure should be less than 10−3 bar (Hallenbeck 2009). Other authors suggested that reduced ferredoxin is the suitable electron donor to produce H2 instead of NADH (Zhang et al. 2013c).
2.3.7 Bioreactor configuration
Bioreactor configuration affects the hydrodynamics influencing liquid–gas mass transfer phenomena, hence producing changes on the dominant microorganism population and enzymatically/metabolic shifts (Waligorska 2012). On the other hand, since the cost of the dark fermentation bioreactors must be low, their construction has to be based on low-cost technology, e.g. anaerobic digesters used in wastewater treatment plant. The most common bioreactor used for dark fermentation is the continuously stirred tank reactor (CSTR). In this type of reactor the stirring must be effective in order to homogenate the biomass suspended and the substrates. This provides a good contact between the substrate and the microorganisms, and enhances the mass transfer (Ntaikou et al. 2010; Show et al. 2011).
In CSTR the solid retention time (SRT) is the same as the hydraulic retention time (HRT). HRT of 6–12 h is favorable for the production of acetic and butyric acids, as well as hydrogen. Also it makes the SRT short enough to promote a dominance of the microbial population by dark fermentative bacteria, preventing the mixed culture from methanogenic microorganisms, which requires SRT in the order of days. The use of HRT below 6 h is risky since it could produce biomass concentration decay, followed by a biomass wash out from the reactor (Ntaikou et al. 2010; Show et al. 2011). In this context decoupling SRT from HRT prevents biomass wash out (Hafez et al. 2010). In the case of CSTR mode several types of bioreactors have been developed in this context, based on the capacity of the microorganisms to produce biofilms, or aided by membrane technologies. In both cases, the SRT is increased independently from the HRT.
In the presence of divalent cations and an increase in carbohydrate concentration in extracellular polymeric substances (EPS), bacteria can suddenly attach to surfaces, and/or each other, creating floccules and/or granules (Jung et al. 2011b). Zhang et al. (2007) have increased the biomass concentration over 30-fold compared to a CSTR without granulation. Hafez et al. (2010) demonstrated that decoupling SRT from HRT, by biofilm formation, increases H2 yield, and also allows to increasing the capacity of organic waste treatment.
Membrane bioreactors (MBR) possess many advantages that include higher biomass concentration in the bioreactor, reactor volume can be reduced according to a higher substrate consumption rate, reduced production of excess sludge due to biomass decay in the reactor, and a lack of microorganisms in the effluent due to their total retention by the used membrane (Oh et al. 2004; Jung et al. 2011b). The main disadvantage limiting the use of MBR is the membrane fouling produced by EPS accumulation (Lee et al. 2008, 2010; Zheng et al. 2010). Regular backpulsing is essential in order to remove membrane fouling, hence maintaining the permeate flux through the membrane (Oh et al. 2004). Anyhow, SRT can be controlled in order to prevent, or retard fouling.
Control of SRT also produces changes in the dark fermentation productivities. This has been evidenced by Lee et al. (2010), on a submerge membrane reactor, using glucose as substrate, with a HRT of 9 h. When the SRT increased from 2 to 12.5 days, the H2 productivity increased from 3.9 to 5.8 L/L/day. But using a SRT of 90 days caused a drop in H2 productivity. A similar trend was observed by (Oh et al. 2004), working on an external cross-flow membrane bioreactor, using glucose as substrate. When increasing the SRT from 3.3 to 12 h, the biomass concentration increased from 2.2 to 5.8 g/L, the glucose consumption increased from 90 to 98 % and the H2 productivity increased from 7.2 to 9.2 L/L/day. When increasing the SRT from 5 to 48 h, both biomass and glucose conversion increased, from 2.4 to 8.8 g/L and 99 to 99.5 %, respectively. But H2 productivity decreased from 9.2 to 4.5 L/L/day. The authors suggest that the decrease in hydrogen productivity must have been due to changes in the physiology or composition of the microbial community in the reactor, as the SRT was increased (Oh et al. 2004).
An alternative to CSTR are upflow bioreactors. The liquid influent containing the substrates enters the reactor at the bottom, and the liquid outlet, with minimum substrates, exits at the top. In the upflow bioreactors, biomass is immobilized either in granules or in biofilms or entrapped in packed support media. The support media includes sponge, granular activated carbon, expanded clay, polyethylene-octane elastomer, ceramic ball, alginate gel. The immobilized biomass can be packed or fluidized. Upflow packed bioreactors have a higher mass transfer resistance, compare to CSTR, resulting in a lower substrate conversion (Waligorska 2012). In a fluidised-bed reactor, gas or liquid passes through accumulated solid matter, causing its fluidization, enhancing the mixing and mass transfer.
Upflow anaerobic sludge blanket (UASB) reactors, used commonly for methane production, consist of a gas/liquid/solid separator on top, where microbial granules are formed. The granules sediment easily creating a thick biomass blanket zone at the bottom (Hawkes et al. 2007). The main disadvantage is a long start-up period of around 5 months (Wang et al. 2007). According to (Jung et al. 2011a), the granulation rate can be increased by means of a two-stage process, consisting on a CSTR which serves as seeding to the UASB. This strategy shortened the start-up time to 20 days.
In the case of solid substrates, the current developments liquefy the solids by different means. At laboratory-scale, the dark fermentation of municipal solid waste and slaughterhouse waste (Gomez et al. 2006), and household solid waste (Liu et al. 2006), have been studied in a two-stage mode, with the objective of produce biohythane (hydrogen and methane). In both experiments the solid waste have been grinded, and suspended in aqueous solution.
Full scale-technologies liquefy the solid substrates by hydrolysis, trying to avoid the size reduction of the solid waste. The Aikan® technology (Aikantechnology 2015; Bonk et al. 2015; Magid 2006; Zeeshan and Hinrich 2014), has been designed for biogas and compost production from the organic fraction of municipal solid waste and green waste. It uses the hydrolytic capabilities of a preexisting biogas reactor. It consists of percolation units and biogas reactors. In the percolation units an aqueous solution, from the biogas reactor, percolates through the waste, is collected and send back to the biogas reactors. In this technology, the waste is not suspended in the fermentation broth of the biogas reactors, reducing the necessary downstream purification cost to separate or use the organic acids from the dark fermentation effluent (Bonk et al. 2015). The use of Aikan® in dark fermentation have been explored in Bonk et al. (2015).
Another full-scale technology that could be eventually adapted to dark fermentation is the REnescience process (REnescience 2015). This process use unsorted and non-shredded municipal solid waste, which is wetted and mixed with hydrolytic enzymes. The mix is heated up for optimal enzymatic hydrolysis. The biodegradable mass is liquefied (bioliquid) by the enzymatic action. The non-degradable solids are then easily separated from the bioliquid. The bioliquid can be further processed by dark fermentation (Munster 2009).
2.4 Minimal selling price of dark fermentation products
There is scarce literature on the economic assessment of dark fermentation systems. Granda et al. (2009) analyze the economics of the MixAlco process for the production of liquid fuels from biomass. In this process biomass is converted via dark fermentation to volatile fatty acids (VFAs), i.e. acetic acid, propionic acids and butyric acid, forming carboxylate salts. The effluent is dewatered by vapor-compression evaporation and converted to short chain alcohols via esterification and subsequent hydrogenolysis. The MixAlco process minimum selling price of alcohols, with a 15 % return on investment, was calculated to be around $350/t_ethanol. At the time of the analysis, 2009, this was economically viable where the market price of ethanol was $750/t_ethanol.
The organic compounds produced by dark fermentation have the potential to be the intermediates to a vast range of molecules different to alcohols, e.g. ketones, aldehydes, olefins. They can be polymerized to produce plastics, liquid fuels, and molecules with biochemical activity. Anyhow, not all the chemical conversions of these organic compounds are economically feasible (Eggeman and Verser 2005).
A different approach could be followed. The VFAs do not need to be converted to alcohols but can be sold directly on the market if purified from the dark fermentation effluent. In a recent publication, Fasahati and Liu (2014) assess the techno-economic production and recovery of VFAs using membrane distillation followed by an methyl tert-butyl ether extraction step, and a final rectification column achieving a VFA stream free from water and extracting solvent. In their assessment it was considered the brown algae Laminaria japonica as the biomass converted to VFAs. The main result was the estimated minimum VFA selling price, $384/t_VFA, with a 10 % internal rate of return after 10 years of plant operation. Still the authors have not detailed the operation cost of the VFA separation/purification steps. Bonk et al. (2015) have estimated that the production of pure VFAs from dark fermentation would be cost efficient if the VFAs separation/purification costs do not exceed $15/m3_effluent. Yet, this estimation has to be validated. This is a possibility, considering the advancements in purification technologies.
In bioconversion of agricultural and food wastes to valuable chemicals, separation and purification of the products from the bulk liquid represents the highest percentage of the manufacturing cost (Angenent et al. 2004). Therefore, the economic feasibility of reusing wastes will strongly depend on the downstream processing efficiency, developing superior separation technologies (Agler et al. 2011).
In-situ (Online) product removal through diverse techniques including dialysis, distillation, adsorption, and extraction was tried for isolation of organic acids and other volatile products, and many of them can be applied for dark fermentation (Dwidar et al. 2012). In-situ removal of liquid dark fermentation products enhances the productivity by decreasing the concentration of these products in the culturing medium and therefore reducing its toxic effect on the cells. In the following section we detail the volatilisation of dark fermentation products by gas stripping/flushing.
The volatilisation of fermentation products by gas stripping is a known technology. In 1986, Ennis et al. (1986) used this principle on Clostridium acetobutylicum fermentations for separation and recovery of butanol. Further research, based on this principle, has been performed for the separation (from the fermentation broth) and recovery of acetone, butanol and ethanol (Groot et al. 1989; Maddox et al. 1995; Ezeji et al. 2003; Ezeji and Karcher 2005). This gas stripping fermentation product recovery has been reviewed by Vane (2005), and it was also applied to separate ethanol from winery wastewater (Colin et al. 2005). Though, product volatilisation by gas stripping, applied to acidogenic fermentation, is an attractive technology with an obvious application on biorefinery. This technology could be applied in both bioreactor and liquid outlet stream. As stated before, gas stripping directly applied in the bioreactor could increase the product yield of ethanol and organic acids, while stripping the outlet stream would allow recover the remaining volatile products. Organic acids are volatile in the undissociated form, thus their volatilisation depends on pH. The undissociated form of an organic acid increases with pH decrease, e.g. at pH 5.0 and 4.0, the undissociated form of both formic and lactic acids is 10 and 40 %, respectively, while for both acetic and butyric acids is 40 and 80 %. Thus low pH will improve the volatilisation and consequently, the product yield on acidogenic fermentation. The pH of a liquid solution decreases as a consequence of CO2 sparging. Then CO2 sparging is also attractive since it will allow organic acid volatilisation. However, literature shows that suspended biomass acidogenic fermentation is hardly accomplished at pH lowers than 4.5 (Temudo et al. 2007). Suspended biomass fermentation is a crucial system that allows the metabolic study of either pure or mixed cultures. Nevertheless, the acidogenic pH issue could be bypassed by the development of acidogenic biofilms in the reactor. Biofilms are aimed to be a technological system rather than an analysis system as suspended biomass fermentation.
3 Perspectives of dark fermentation in the biorefinery concept
As reviewed in Sect. 2, the role of dark fermentation in the bio-society is that of reduction of organic residues and increase the global efficiency of biomass based production of energy and valuable commodities. Hence the future of dark fermentation relies as a core bioprocess in the biorefinery concept. Here it is discussed the perspectives and future of dark fermentation. It is explored its role as a link with other (bio)process for the production of liquid fuels, biohythane (biosyngas), its role in photofermentative systems and fine chemicals production, syngas fermentation and bioelectrochemical systems.
Integration of dark fermentation with other (bio)processes has the aim of reducing residues and associated operational costs, and increasing revenues coming from high value chemicals and/or bioenergy. In this context, dark fermentation metabolic capabilities and/or products can be associate to other (bio)processes. Figure 3 depicts how dark fermentation can be used as the heart of the biorefinery concept. Dark fermentation can use residues produced by different biomass based activities, e.g. organic residues from agricultural and forestry activities (Ueno et al. 1995; Hussy et al. 2003; Kaparaju et al. 2009; Ren et al. 2009; Guo et al. 2010; Chu et al. 2012; Chaturvedi 2013; Uratani 2013), algae and microalgae activities (Sialve et al. 2009; Rashid 2013), food industry (Han and Shin 2004; Shin et al. 2004), municipal solid and liquid waste (Lay et al. 1999; Lay 2001; Li and Yu 2011; Mu et al. 2006; Nwobi 2013) and residues from other bio-industries (Nishio and Nakashimada 2007). These residues are transformed by dark fermentation into valuable chemicals that can be redirected for uses as bioenergy and biofuels, chemical and biochemical synthesis, bioplastics and new materials.
3.1 Liquid fuels production
The dark fermentation products contained in the liquid phase can be used for the production of liquid fuels by (bio)catalytic process. Ladygina et al. (2006) have reviewed the biosynthesis of hydrocarbons by both prokaryotes and eukaryote microorganisms. In this way, organic acids, e.g. acetic or butyric acids, can be converted into C10–C35 aliphatic hydrocarbons. Another alternative is the catalytic conversion of the organic acids into hydrocarbons. Leung et al. (1995) have demonstrate that pure saturated carboxylic acids, butyric acid among them, produces hydrocarbons when pyrolyzed at 450 °C and atmospheric pressure under the presence of activated alumina as catalyzer. In this same context, Wang et al. (2012) have obtained hydrocarbons and phenol derivatives from mixed and pure acetic acid and propionic acid by catalytic cracking using silicon/aluminum as catalyzers. Ketonization of caproic acid was demonstrated by Gaertner et al. (2009) using cerium/zirconium as catalyzer at temperatures from 178 to 350 K. As demonstrated by Leung et al. (1995), ketones can be further converted into hydrocarbons, by pyrolysis.
Butyric acid, acetic acid and ethanol produced by dark fermentation appear as carbon sources for the production of biobutanol. Biobutanol offers several advantages over ethanol as a transportation fuel, e.g. butanol provides more energy when burned, is less volatile, can replace gasoline in internal combustion engines without any mechanical modifications, does not attract water so it can be transported in existing pipelines, is not miscible with water, and is less sensitive to colder temperatures (Dwidar et al. 2012). Despite these benefits, the main issue of fermentative bioproduction of butanol is its toxicity to the microorganisms that produce it, e.g. Clostridia. This toxicity results in poor concentrations in the fermentation broth, and higher recovery costs. One feasible strategy to reduce the toxicity and improving the yield of butanol is to first convert biomass into organic acids by dark fermentation and then convert this downstream into butanol.
Biohydrogen, which is present in the gas phase product streams of dark fermentation, could be used in a biohydrogen based society, however this approach presents several drawbacks, e.g. infrastructure (Berry and Aceves 2005; Bossel 2006). In order to immediately decreasing human GHG emission, biohydrogen could instead be used in the refining and/or upgrading of liquid fuels. Hydrogen used for these purposes is today mainly produced from mineral oil. Biohydrogen produced via electrolysis is also possible but with the current technologies is expensive and GHG unfriendly if using fossil fuel energy (Tran et al. 2010).
3.2 Fine chemicals production
Fine chemicals, e.g. amino acids, polymers, and drugs, are known to have a higher market price than bulk chemicals, e.g. fuels, which are largely consumed (Willke and Vorlop 2004). Table 3 presents some organic molecules from both fine and bulk chemicals, as an example for comparison. Arginine, polylactic acid and salicylic acid are well known fine chemicals, and their market prices are in the range of 2.5–40 times higher than butanol, jet fuel or diesel. In the case of petroleum-based bulk chemicals, this is explained because the raw material is still inexpensive, compared to biomass, and the technologies and infrastructure are optimized and scaled for their respectively market size. As presented in Sect. 3.1, the production of liquid fuels from dark fermentation products seems economically feasible because of two factors, mixed culture dark fermentation seems to be a low-cost process, and the possibility of using organic residues. Furthermore, fermentation revenues could be increased if its products are directed to the production of fine chemicals.
As expressed above, dark fermentation could be used as an upstream process for the production of fine chemicals derived from biomass residues through (bio)catalytic processes. In the bioprocess area, dark fermentation could be linked to microbial systems, including pure cultures and engineered microbes, and/or enzymatic systems. Mixed culture systems have succeed in the production of biopolyesters, e.g. polyhydroxyalkanoates (PHA), by using acetate and propionate as carbon sources (Sudesh and Doi 2000; Hu et al. 2005). The effect in the PHA production by using the liquid effluent of dark fermentation must be studied.
Pure culturing systems could also use the organic compounds, in the liquid effluent of dark fermentation, as substrates for the production of valuable products such as drugs, industrial chemicals and biopolymers (Pirie et al. 2013). The case of engineered microbial pure cultures offers an expansion in number of products synthesized by microbial systems (Dhamankar and Prather 2011). The current trend is the use of biomass resources to be directly converted by engineered microbes into valuable chemicals. Pure cultures require sterile conditions and specific substrates. A direct usage of organic residues by engineered microbial systems is not advised. As exposed above, this could be done through dark fermentation in order to convert biomass residues into a mixture of organic acids and ethanol that can be therefore used as substrates for the engineered microbial systems. Alternative a fractionation of the organic residues is necessary before use as substrate for engineered microbial systems.
Enzymatic catalysis for synthesis of fine chemicals is already in used at industrial scale (Murzin and Leino 2008; Zhang 2014). Conventional enzymatic catalysis is carried out in organic solvents. Hence, the use of dark fermentation liquid effluent, as an upstream for enzymatic catalysis, must be treated in order to purify and extracts its organic compounds that are dissolved in an aqueous solution. Also it is highly recommended to develop new approaches to directly use organic compounds from dark fermentation by enzymatic catalysis.
Another option, the chemical catalysis, is being used for the conversion of biomass as a part of the so called green chemistry (Anastas et al. 2000, 2001; Gallezot 2007; Murzin and Simakova 2011; Bui et al. 2013). The biomass compounds that have been studied as platform molecules include carbohydrates, lignocellulose, triglycerides, glycerol, and organic acids as levulinic acid. Technically there are no limitations to use dark fermentation products, e.g. acetic acid, in chemical catalysis. Still is highly recommended to develop chemical catalysis based on this type of effluent. In this regard, as mention above, the direct use of dark fermentation effluent will entails the use of aqueous catalytic technologies (Cornils 1999; Ford 2001). These technologies have been reviewed for the conversion of carbohydrates (Queneau et al. 2011). Furthermore, conversion of glycerol into propylene glycol (Marinoiu et al. 2013), or polymerization of olefins (Mecking et al. 2002) have been achieved in aqueous solutions.
3.3 Algae, cyanobacteria and phototrophic bacteria cultivation
As it was detail in Sect. 3.1, residues from algae and micro algae cultivation and/or processing could be feed into dark fermentation. Also, products from dark fermentation can be feed to Algae, cyanobacteria (blue algae) and phototrophic bacteria, in order to optimize the production of valuable chemicals, recycle nutrients, and reduce CO2 emissions (Markou and Georgakakis 2011). Here it is explained how dark fermentation produced CO2, organic acids, and H2 can be used by these three types of photosynthetic systems.
The macroalgae world market size estimative in 2004 was USD $6 × 109/year, with 7.5 × 106 t/year biomass harvested, while microalgae world market was USD $1.25 × 109/year, 5000 t/year of biomass harvested (Pulz and Gross 2004). Valuable chemicals derived from algal products are economically attractive. Carotenoids pigments 2005 estimated marked was USD $935 × 106/year (Cardozo et al. 2007). Astaxanthin, a carotenoid used as pigmentation source and antioxidant, estimated 2006 market price was 2500 USD $/kg (Jeon et al. 2006), with a market estimation of USD $150 × 106/year (Pulz and Gross 2004). Another group of algal valuable chemicals are phycocolloids, where 2007 market for agar was valued at USD $200 × 106/year, with 10,000 t/year of traded agar. For the same year carrageenan market was valued at USD $200 × 106, and a market size of 25,000 t/year (Cardozo et al. 2007).
Other economically attractive molecules extractable from algae are alginate, used as phycocolloid, and biochemical active molecules with used in the cosmetic industry, e.g. mycosporine-like aminoacids for UV blocker sunscreen, or for pharmacological industry, e.g. lectins, halogenated products, poliketide, fatty acids, sterols, and harmful algal bloom (HAB) toxins (Cardozo et al. 2007). HBA toxins have a promising potential as antibacterial and antifungal molecules (Najdenski et al. 2013). If we compare the prices of astaxanthin with acetic acid, 2500 USD/kg and 800 USD/t respectively, see Table 1 for acetic acid details, it is clear that finding ways to convert the dark fermentation organic acids to algal valuable chemicals is economically attractive. Also the market sizes of astaxanthin and acetic acid, 60 and 3.5 × 106 t/year respectively, hints information regarding the saturation and/or expansion of their respectively markets.
Literature reports that organic acids can be used for microalgae growth in mixotrophic mode, i.e. the simultaneous use of light and organic compounds for energy and carbon requirements (Markou and Georgakakis 2011), and in aerobic heterotrophic conditions, i.e. use of organic compounds in absence of light. The algal acetate mixotrophic growth resulted in 56–91 % increase in chlorophyll content, relative to photoautotrophy, for the green algae Chlamydomonas globosa, Chlorella minutissima, and Scenedesmus bijuga (Bhatnagar et al. 2001). Chlorophyll content can be related to biomass production. The mixotrophic culture of Chlorella vulgaris in acetate increased the biomass productivity from 13 to 85 mg/L/day and the lipid productivity from 4 mg/L/day, at photoautotrophic conditions, to 28 mg/L/day (Liang et al. 2009). Another example of successful algal acetate mixotrophic growth was achieved by Kobayashi et al. (1993) and Jeon et al. (2006), where acetate enhanced both the productivity of Haematococcus pluvialis cell biomass, and its astaxanthin formation. Mixotrophy can also support harmful algal species (HAS) (Burkholder et al. 2008), from where valuable antimicrobial toxins can be extracted (Najdenski et al. 2013). Under aerobic heterotrophic conditions Chlorella sorokiniana, a single cell green algae, specific growth rate is improved with concentrations of acetate up to 6 g/L, while it does not consumes propionate (Ogbonna et al. 2000).
Organic acids produced from dark fermentation can also be used as substrates for the photofermentative H2 production. From the 4 groups of hydrogen producer microorganisms, i.e. green algae, cyanobacteria, phototrophic bacteria and dark acidogenic bacteria, only the latter two can produce hydrogen from organic compounds (Cai et al. 2012).
In literature is it reported that H2 producing phototrophic bacteria are capable of consuming the liquid products from dark fermentation, e.g. acetic or butyric, in presence of light (Shi and Yu 2005; Argun et al. 2009; Redwood et al. 2009). The resultant dual dark/light integrated system could reach a maximum theoretical H2 yield of 12 mol_H2/mol_hexose. Dark fermentation maximum theoretical yield is 4 mol_H2/mol_hexose. Experimental results using pure cultures or well-defined co-cultures of dark fermentative bacteria are promising. The integrated systems shows overall H2 yields ranging from 7 to 8 mol_H2/mol_hexose (Yokoi et al. 2001; Kim et al. 2006). Experimental results for mixed culture dark fermentation are still missing. Shi and Yu (2006) have showed a hydrogen overall yield of 4.56 mol_H2/mol_hexose using the effluent of an acidogenic reactor, but it is not defined if the acidogenic reactor consisted in mixed or pure culture.
Regarding the dark fermentation gas products, hydrogen can be used in a hydrogenation reaction to upgrade the algal oil/fat produced for biofuel purposes (Jang et al. 2005; Tran et al. 2010; Borowitzka and Moheimani 2013). Oil yields of microalgae, 58 to 137 m3/ha/year, are much higher than terrestrial crops dedicated for oil, e.g. oil palm 6 m3/ha/year (Chisti 2007; Amin 2009). Oil from microalgae can be transform into biodiesel by a conventional catalytic methanol transesterification reaction (Demirbas 2002). The challenge is still to extract the oil from the microalgae biomass due to the content of chlorophyll. The purification of lipids from algae requires additional treatments compared with vegetable oil (Petkov et al. 2012). Also, microalgal oils are rich in polyunsaturated fatty acids, which are susceptible to oxidation during storage and this reduces their acceptability for use in biodiesel (Chisti 2007). This issue is solved with hydrogenation converting the oils to the saturated form. Hydrogenation of algal biofuels using dark fermentative hydrogen appears as a sustainable process since hydrogenation process currently used petrochemical-based hydrogen (Tran et al. 2010).
The use of the CO2 produced by dark fermentation can be used for the phototrophic growth of macro and micro algae (Chung et al. 2011; Kumar et al. 2011; Singh and Ahluwalia 2013). The use of CO2 from alcoholic fermentation has been successfully attain by Bezerra et al. (2013) for the growth of Arthrospira platensis. In this context, the link of dark fermentation CO2 to algae cultivation would mitigate the CO2 emissions of this integrated bioprocess.
3.4 Biomethane, biohythane and biosyngas production
Dark fermentation liquid effluent can be converted to biomethane by anaerobic digestion. Anaerobic digestion is a conventional process used for the treatment of organic waste. This bioprocess requires a pH of around 7 in order to maintain the activity of the methanogenic archea, which are responsible of methane production. Fluctuations of pH could reduce, inhibit and/or inactivate the methanogenic activity, which is why dark ferementation is used as an upstream process for methane production, i.e. conferring stability to the global system (Cavinato et al. 2012; Willquist et al. 2012).
Furthermore, the hydrogen produced in the dark fermentation step can be blended to the methane, forming biohythane. Biohythane is a combustible gas, consisting in 10 % H2, 30 % CO2 and 60 % CH4 (Cavinato et al. 2011). The use of 10 % H2 in the biohythane blend, enhance combustion, thermal efficiency and power output compared to biomethane, together with reducing hydrocarbon emissions (Sierens and Rosseel 2000; Porpatham et al. 2007).
Biohythane, can be also considered as biosyngas. Syngas consist of a non-fixed mixture of CO, H2, CO2 and CH4, see Sect. 3.5. The conventional approach of production of biosyngas is the gasification of biomass (McKendry 2002b). This approach is useful for dried biomass stocks as lignocellulosic material. This approach is not advised for organic residues where the water content is above 30 %, e.g. food waste, organic fraction of municipal solid waste, and algae residues, since extra energy is needed to evaporate the contained water (McKendry 2002b). The tandem system dark fermentation-anaerobic digestion appears as a biogasification process of biomass (McKendry 2002a). The biosyngas produced can be used as chemical feedstock to produce liquid fuels (Wilhelm et al. 2001; Chuang 2012) and/or fine chemicals (Spivey et al. 2000; Hadipour and Sohrabi 2008).
3.5 Syngas dark-fermentation
Syngas fermentation by mixed culture dark-fermentation consists in the production of organic acids and solvents using synthesis gas (syngas). Homoacetogenesis (Sect. 2.1.3) is one example of a metabolic pathway involved in syngas dark-fermentation. Syngas dark-fermentation through homoacetogenesis (Zhao et al. 2010) consumes CO2 and H2, contained in the syngas, to produce acetic acid, whose technological importance was described in Sect. 2.2.2. Sustainable syngas, i.e. avoiding crude-oil resources, could be obtained, for example, from the gasification of non-biodegradable organic solids, e.g. lignocellulosic biomass and plastics, present in municipal solid waste (Arena 2012; Roddy 2013). Examples of co-gasification of lignocellulosic biomass and plastic waste are found in literature (Pinto et al. 2002; Ahmed et al. 2011). Syngas composition is not fixed (Williams et al. 2008). Pinto et al. (2002),working in co-gasification of pine-wood and polyethylene, have obtained syngas compositions in the following range, in v/v %: CO 25–45, H2 20–50, CO2 2–15, CH4 5–15, and hydrocarbons (CnHm) 0–15. The operational condition used by them were temperatures ranging the 720–900 °C, atmospheric pressure, and steam/waste ratio (0.45–0.9 w/w), and the polyethylene/pine-wood ratio (0–0.4 w/w).
Syngas fermentative microorganisms have been identified and studied. Most of the work has been done in pure culture fermentation (Heiskanen et al. 2007; Henstra et al. 2007; Mohammadi et al. 2011). Mixed culture syngas fermentation technology supposes low-cost process compared to pure culture syngas fermentation. Gas–liquid mass transfer presumes another issue to be solved. The low solubility of H2 in water (1.6 mg/L) in equilibrium with 1 atm of H2 at 298 K is the main challenge for hydrogen utilization (Zhang et al. 2013a). Various methods such as vigorous stirring and gas circulation were proposed (Henstra et al. 2007). Hollow-fiber membrane bioreactors, have been identified as a cheaper option to tackle this mass transfer issue (Henstra et al. 2007; Zhang et al. 2013a). In order to overcome these two pure cultures and mass transfer issues, Zhang et al. (2013a) have studied the syngas mixed culture dark-fermentation in a hollow-fiber membrane bioreactor. In this kind of bioreactor, syngas permeates through the lumen of hollow-fibers membranes through the liquid phase of the bioreactor, where is directly consumed, without loss of gas through bubble gas formation, by a naturally attached mixed culture dark-fermentative biofilm (enriched from an anaerobic digestion inoculum). In this work the authors have use a synthetic syngas composed by 60 % H2 and 40 % CO2 (% v/v). The bioreactor was operated at 35 °C and pH 4.5 Acetic acid represented 99 % of the liquid products, reaching concentrations of 12.7 g/L (HRT: 25 days) and 3.7 g/L (HRT 9 days) in batch and continuous modes respectively. For both bioreactor modes, their acetic acid productivities were very similar 0.02 g/L/h. Anyhow, the production of hexanoic acid and caprylic acid (medium chain fatty acids) by syngas fermentation could represent a lower operating cost and product separation when compared to acetic acid production (Agler et al. 2012; Zhang et al. 2013b).
Alternatives to syngas fermentation are the conventional catalytic syngas conversions, e.g. Fischer–Tropsch Tecnology (Mohammadi et al. 2011). This technology uses high temperatures ranging from 200 to 350 °C (Dry 2002) and high pressures 18–40 bar (Geerlings et al. 1999; Dry 2002), which is translated into very energy consuming process. Also it requires metallic catalysts, e.g. iron or cobalt based. Fast heat exchange is required to avoid catalyst deactivation. Catalysts are deactivated also by syngas impurities, e.g. H2S present in municipal waste syngas (Dry 2002). Fischer–Tropsch, depending in operational conditions, produces gasoline, olefins, and waxes (Geerlings et al. 1999; Dry 2002). Deviations in the syngas composition, mainly CO/H2 ratio, produce changes in the product selectivity. The process is fast, with retention times around 10 min (Geerlings et al. 1999).
In comparison, hollow-fiber membrane syngas dark-fermentation uses temperatures around 35 °C, and atmospheric pressure which results in substantial energy savings. The moderate temperature causes irreversibility of biological reactions, which results in high conversion efficiencies. This process does not require adding mineral catalysts since it is an autocatalytic process, using microorganism enzymatic systems. Hence high reaction specificity is achieved due to the enzymatic specificity. It is less sensitive to CO/H2 ratio (Mohammadi et al. 2011). A major drawback of syngas dark-fermentation is its high retention time, 9–25 days (Zhang et al. 2013a). Presently, syngas dark-fermentation is still at the research level. Further study is recommended with respect to the optimization of productivities, and scale-up of the process. Also long run experiments are required in hollow-fiber membrane bioreactor syngas mixed culture dark-fermentation.
4 Conclusions
This paper has reviewed both the present state and future potential of dark fermentation in the (bio)chemical industry. Dark fermentation is a viable and flexible technology since it consumes a vast range of substrates, most of which can be complex in composition, as residues streams from other bioprocess, or even municipal solid and liquid waste.
Dark fermentation contributes to the biobased-society reducing organic residues and increasing the global efficiency of biomass based production of energy and valuable commodities. The products of dark fermentation can be purified and/or use as platform chemicals in subsequent (bio)process for the production of fuels, fine chemicals, and biosyngas. These features make dark fermentation a core bioprocess in the biorefinery concept.
References
Aceves-Lara CA, Latrille E, Bernet N, Buffiere P, Steyer JP (2008a) A pseudo-stoichiometric dynamic model of anaerobic hydrogen production from molasses. Water Res 42:2539–2550
Aceves-Lara CA, Latrille E, Buffiere P, Bernet N, Steyer JP (2008b) Experimental determination by principal component analysis of a reaction pathway of biohydrogen production by anaerobic fermentation. Chem Eng Process Process Intensif 47:1968–1975
Aceves-Lara CA, Trably E, Bastidas-Oyanedel JR, Ramirez I, Latrille E, Steyer JP (2008c) Bioenergy production from waste: examples of biomethane and biohydrogen. J Soc Biol 202:177–189
Agler MT, Wrenn BA, Zinder SH, Angenent LT (2011) Waste to bioproducts conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol 29:70–78
Agler MT, Spirito CM, Usack JG, Werner JJ, Angenent LT (2012) Chain elongation with reactor microbiomes: upgrading dilute ethanol to medium-chain carboxylates. Energy Environ Sci 5:8189–8192
Agreda VH, Zoeller JR (1993) Acetic acid and its derivatives. Marcel Dekker, Inc., New York
Ahmed I, Nipattummakul N, Gupta A (2011) Characteristics of syngas from co-gasification of polyethylene and woodchips. Appl Energy 88:165–174
Aikantechnology (2015) Aikan technology, how it works, batch processing. http://www.aikantechnology.com/how-it-works/batch-processing.html. Accessed 12 June 2015
Alibaba (2014) Chinese e-commerce. Organic chemical bulk prices. www.alibaba.com. Accessed 24 Nov 2014
Amin S (2009) Review on biofuel oil and gas production processes from microalgae. Energy Convers Manag 50:1834–1840
Anastas P, Bartlett L, Kirchhoff M, Williamson T (2000) The role of catalysis in the design, development, and implementation of green chemistry. Catal Today 55:11–22
Anastas P, Kirchhoff M, Williamson T (2001) Catalysis as a foundational pillar of green chemistry. Appl Catal A-Gen 221:3–13
Angenent LT, Karim K, Al-Dahhan MH, Wrenn BA, Dominguez-Espinosa R (2004) Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 22:477–485
Arena U (2012) Process and technological aspects of municipal solid waste gasification: a review. Waste Manage 32:625–639
Argun H, Kargi F, Kapdan I (2009) Effects of the substrate and cell concentration on bio-hydrogen production from ground wheat by combined dark and photofermentation. Int J Hydrogen Energy 34:6181–6188
Bastidas-Oyanedel JR, Aceves-Lara CA, Ruiz-Filippi G, Steyer JP (2008) Thermodynamic analysis of energy transfer in acidogenic cultures. Eng Life Sci 8:487–498
Bastidas-Oyanedel JR, Mohd-Zaki Z, Zeng RJ, Bernet N, Pratt S, Steyer JP et al (2012) Gas controlled hydrogen fermentation. Bioresour Technol 110:503–509
Bastidas-Oyanedel J, Kumaraswany R, Rodriguez J (2013) Stability and structure of different microbial communities during dark fermentation of glucose. In: Presented at the 13th World Congress on Anaerobic Digestion, Santiago de Compostela, Spain: IWA Publishing
Berry GD, Aceves SM (2005) The case of hydrogen in a carbon constrained world. J Energ Resour ASME 127:189–194
Bezerra R, Matsudo M, Sato S, Converti A, de Carvalho J (2013) Fed-Batch Cultivation of Arthrospira platensis using carbon dioxide from alcoholic fermentation and urea as carbon and nitrogen sources. Bioenergy Res 6:1118–1125
Bhatnagar A, Chinnasamy S, Singh M, Das K (2001) Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl Energy 88:3425–3431
Bonk F, Bastidas-Oyanedel JR, Schmidt JE (2015) Converting the organic fraction of solid waste from the city of Abu Dhabi to valuable products via dark fermentation—economic and energy assessment. Waste Manage 40:82–91
Borowitzka M, Moheimani N (2013) Sustainable biofuels from algae. Mitig Adapt Strateg Glob Change 18:13–25
Bossel U (2006) Does a hydrogen economy make sense? Proc IEEE 94:1826–1837
Boucher S (2006) La revolution de l’hydrogene, vers une energie propre et performante? Paris. Editions du Felin, France
Bui L, Luo H, Gunther W, Roman-Leshkov Y (2013) Domino reaction catalyzed by zeolites with Bronsted and Lewis acid sites for the production of gamma-valerolactone from furfural. Angew Chem Int Ed 52:8022–8025
Burkholder J, Glibert P, Skelton H (2008) Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8:77–93
Cai G, Jin B, Monis P, Saint C (2011) Metabolic flux network and analysis of fermentative hydrogen production. Biotech Adv 29:375–387
Cai J, Wang G, Pan G (2012) Hydrogen production form butyrate by a marine mixed phototrophic bacterial consort. Int J Hydrogen Energy 37:4057–4067
Canani R, Di Constanzo M, Leone L, Pedata M, Meli R, Calignano A (2011) Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 17:1519–1528
Canani R, Di Constanzo M, Leone L (2012) The epigenic effects of butyrate: potential therapeutic implications for clinical practice. Clin Epigenetics 4:4
Cardozo K, Guaratini T, Barros M, Falcao V, Tonon A, Lopes N et al (2007) Metabolites from algae with economical impact. Comp Biochem Phys C 146:60–78
Cavinato C, Bolzonella D, Fatone F, Cecchi F, Pavan P (2011) Optimization of two-phase thermophilic anaerobic digestion of biowaste for hydrogen and methane production through reject water recirculation. Bioresour Technol 102:8605–8611
Cavinato C, Giuliano A, Bolzonella D, Pavan P, Cecchi F (2012) Bio-hythane production from food waste by dark fermentation coupled with anaerobic digestion process: a long-term pilot scale experience. Int J Hydrogen Energy 37:11548–11555
ccminternational (2012) China’s l-arginine industry enjoys rapid development. https://ccminternational.wordpress.com/2012/04/13/chinas-l-arginine-industry-enjoys-rapid-development/. Accessed 25 Nov 2014
Chaturvedi T (2013) Evaluation of bioenergy production from lignocellulosic biomass of Salicornia Bigelovii. MSc, Chemical Engineering, Masdar Institute of Science and Technology, Abu Dhabi, UAE
Chen CC, Lin CY (2001) Start-up of anaerobic hydrogen producing reactors seeded with sewage sludge. Acta Biotechnol 21:371–379
Chen CC, Lin CY, Lin M (2002) Acid-base enrichment enhances anaerobic hydrogen production process. Appl Microbiol Biotechnol 58:224–228
Cheze B, Gastineau P, Chevallier J (2011) Foreasting world and regional aviation jet fuel demands to the mid-term (2025). Energ Policy 39:5147–5158
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Choi K, Jeon B, Kim B, Oh M, Um Y, Sang B (2013) In situ biphasic extractive fermentation for hexanoic acid production from sucrose by megasphaera elsdenii NCIMB 702410. Appl Biochem Biotechnol 171:1094–1107
Chong ML, Sabaratnam V, Shirai Y, Hassan MA (2009) Biohydrogen production from biomass and industrial wastes by dark fermentation. Int J Hydrogen Energy 34:3277–3287
Chu CY, Sen B, Lay CH, Lin YC, Lin CY (2012) Direct Fermentation of sweet potato to produce maximal hydrogen and ethanol. Appl Energy 100:10–18
Chuang S (2012) Conversion of syngas to fuels. Handbook of climate change mitigation. Springer, Berlin
Chung I, Beardall J, Mehta S, Sahoo D, Stojkovic S (2011) Using marine macroalgae for carbon sequestration: a critical appraisal. J Appl Phycol 23:877–886
Colin T, Bories A, Sire Y, Perrin R (2005) Treatment and valorisation of winery wastewater by a new biophysical process (ECCF®). Water Sci Technol 51:99–106
Collet C, Girbal L, Peringer P, Schwitzguebel JP, Soucaille P (2006) Metabolism of lactose by Clostridium thermolacticum growing in continuous culture. Arch Microbiol 185:331–339
Cornils B (1999) Bulk and fine chemicals via aqueous biphasic catalysis. J Mol Catal A Chem 143:1–10
Davies O, Lewis A, Whitaker M, Tai H, Shakesheff K, Howdle S (2008) Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv Drug Deliver Rev 60:373–387
Demain A (2007) The business of biotechnology. Ind Biotechnol 3:269–283
Demirbas A (2002) Biodiesel from vegetable oils via transesterification in supercritical methanol. Energy Convers Manag 43:2349–2356
Dhamankar H, Prather K (2011) Microbial chemical factories: recent advances in pathway engineering for synthesis of value added chemicals. Curr Opin Struct Biol 21:488–494
Dry M (2002) The Fischer-Tropsch process: 1950–2000. Catal Today 71:227–241
Dwidar M, Park JY, Mitchell RJ, Sang BI (2012) The future of butyric acid in industry. Scientific World J. doi:10.1100/2012/471417
Eggeman T, Verser D (2005) Recovery of organic acids from fermentation broths. Appl Biochem Biotechnol 121–124:605–618
Ennis BM, Marshall CT, Maddox IS, Paterson AHJ (1986) Continuous product recovery by in situ gas stripping/condensation during solvent production from whey permeate using Clostridium acetobutylicum. Biotechnol Lett 8:725–730
Ezeji TC, Karcher PM (2005) Improving performance of a gas stripping-based recovery system to remove butanol from Clostridium beijerinckii fermentation. Bioprocess Biosyst Eng 27:207–214
Ezeji TC, Qureshi N, Blaschek HP (2003) Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J Microbiol Biotechnol 19:595–603
Fang HHP, Liu H (2002) Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour Technol 82:87–93
Fasahati P, Liu J (2014) Techno-economic analysis of production and recovery of volatile fatty acids from brown algae using membrane distillation. Comput Aided Chem Eng 34:303–308
Ford W (2001) Catalysis by colloidal polymers in aqueous media. React Funct Polym 48:3–13
Freudenberger R (2009) A guide to small-scale ethanol, alcohol fuel, making and using ethanol as a renewable fuel. New Society Publishers, Gabriola Island
Gaertner C, Serrano-Ruiz J, Braden D, Dumesic J (2009) Catalytic coupling of carboxylic acids by ketnonization as a processing step in biomass conversion. J Catal 266:71–78
Gallezot P (2007) Catalytic routes from renewables to fine chemicals. Catal Today 121:76–91
Geerlings J, Wilson J, Kramer G, Kuipers H, Hoek A, Huisman H (1999) Fischer-Tropsch technology—from active site to commercial process. Appl Catal A Gen 186:27–40
Gerardi MH (2003) The microbiology of anaerobic digesters. Wiley, Hoboken
Gheshlaghi R, Scharer JM, Moo-Young M, Chou CP (2009) Metabolic pathways of clostridia for producing butanol. Biotechnol Adv 27:764–781
Gomez X, Moran A, Cuetos MJ, Sanchez ME (2006) The production of hydrogen by dark fermentation of municipal solid wastes and slaughterhouse waste: a two-phase process. J Power Sources 157:727–732
Gonzalez-Cabaleiro R, Lema JM, Rodriguez J (2015) Metabolic energy-based modeling explains product yielding in anaerobic mixed culture fermentations. PLoS One. doi:10.1371/journal.pone.0126739
Grand View Research (2014) Global lactic acids and polylactic acid market. www.grandviewresearch.com/press-release/global-lactic-acid-poly-lactic-acid-market. Accessed 24 Nov 2014
Granda C, Holtzapple M, Luce G, Searcy K, Mamrosh D (2009) Carboxylate platform: the MixAlco process part 2: process economics. Appl Biochem Biotechnol 156:107–124
Groot WJ, van der Lans RGJM, Luyben KCAM (1989) Batch and continuous butanol fermentations with free cells: integration with product recovery by gas-stripping. Appl Microbiol Biotechnol 32:305–308
Gujer W, Zehnder AJB (1983) Conversion processes in anaerobic digestion. Water Sci Technol 15:127–167
Guo XM, Trably E, Latrille E, Carrere H, Steyer JP (2010) Hydrogen production from agricultural waste by dark fermentation: a review. Int J Hydrogen Energy 35:10660–10673
Hadipour A, Sohrabi M (2008) Synthesis of some bifunctional catalysts and determination of kinetic parameters for direct conversion of syngas to dimethyl ether. Chem Eng J 137:294–301
Hafez H, Naggar MHE, Nakhla G (2010) Steady-state and dynamic modeling of biohydrogen production in an integrated biohydrogen reactor clarifier system. Int J Hydrogen Energy 35:6634–6645
Hallenbeck PC (2005) Fundamentals of the fermentative production of hydrogen. Water Sci Technol 52:21–29
Hallenbeck P (2009) Fermentative hydrogen production: principles, progress and prognosis. Int J Hydrogen Energy 34:7379–7389
Hamer H, Jonkers D, Venema K, Vanhoutvin S, Troost F, Brummer R (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27:104–119
Han SK, Shin HS (2004) Biohydrogen production by anaerobic fermentation of food waste. Int J Hydrogen Energy 29:567–577
Hawkes FR, Dinsdale R, Hawkes DL, Hussy I (2002) Sustainable fermentative hydrogen production: challenges for process optimisation. Int J Hydrogen Energy 27:1339–1347
Hawkes FR, Hussy I, Kyazze G, Dinsdale R, Hawkes DL (2007) Continuous dark fermentative hydrogen production by mesophilic microflora: principles and progress. Int J Hydrogen Energy 32:172–184
Heiskanen H, Virkajarvi I, Viikari L (2007) The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzyme Microb Technol 41:362–367
Henstra A, Sipma J, Rinzema A, Stams A (2007) Microbiology of synthesis gas fermentation for biofuel production. Curr Opin Biotechnol 18:200–206
Hoffmann P (2001) Tomorrow’s energy, hydrogen, fuel cells, and the prospects for a cleaner planet. The MIT Press, Cambridge
Hu W, Sin S, Chua H, Yu P (2005) Synthesis of polyhydroxyalkanoate (PHA) from excess activated sludge under various oxidation-reduction potentials (ORP) by using acetate and propionate as carbon sources. Appl Biochem Biotechnol 121–124:289–301
Hussy I, Hawkes FR, Dinsdale R, Hawkes DL (2003) Continuous fermentative hydrogen production from a wheat starch co-product by mixed microflora. Biotechnol Bioeng 84:619–626
Hwang MH, Jang NJ, Hyun SH, Kim IS (2004) Anaerobic bio-hydrogen producion from ethanol fermentation: the role of pH. J Biotechnol 111:297–309
Im WT, Kim DH, Kim KH, Kim MS (2012) Bacterial community analyses by pyrosequencing in dark fermentative H2-producing reactor using organic wastes as a feedstock. Int J Hydrogen Energy 37:8330–8337
Indexmundi (2014) International prices of commodities. www.indexmundi.com. Accessed 24 Nov 2014
Insam H, Franke-Whittle I, Goberna M (2010) Microbes at work, from wastes to resources. Springer, Berlin Heidelberg
Jang E, Jung M, Min D (2005) Hydrogenation of low trans and high conjugated fatty acids. Compr Rev Food Sci Food Saf 1:22–30
Jeon Y, Cho C, Yun Y (2006) Combined effects of light intensity and acetate concentration on the growth of unicellular microalga Haematococcus pluvialis. Enzyme Microb Technol 39:490–495
Jeon B, Kim B, Um Y, Sang B (2010) Production of hexanoic acid from d-galactitol by a newly isolated Clostridium sp. BS-1. Appl Microbiol Biotechnol 88:1161–1167
Jeon B, Moon C, Kim B, Kim H, Um Y, Sang B (2013) In situ extractive fermentation for the production of hexanoic acid from galactitol by Clostridium sp. BS-1. Enzyme Microb Technol 53:143–151
Jiang J, Zhang Y, Li K, Wang Q, Gong C, Li M (2013) Volatile fatty acids production from food waste: effects of pH, temperature, and organic loading rate. Bioresour Technol 143:525–530
Joo F (2008) Breakthroughs in hydrogen storage—formic acid as a sustainable storage material for hydrogen. Chem Sus Chem 1:805–808
Jung K, Kim D, Shin H (2011a) A simple method to reduce the start-up in a H2-producing UASB reactor. Int J Hydrogen Energy 36:1466–1473
Jung K, Kim D, Shin H (2011b) Bioreactor design for continuous dark fermentative hydrogen production. Bioresour Technol 102:8612–8620
Kamm B, Gruber PR, Kamm M (2006) Biorefineries—industrial processes and products. Wiley-VCH, Weinheim
Kaparaju P, Serrano M, Thomsen AB, Kongjan P, Angelidaki I (2009) Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour Technol 100:2562–2568
Karadag D, Puhakka J (2010) Effect of changing temperature on anaerobic hydrogen production and microbial community composition in an open-mixed culture bioreactor. Int J Hydrogen Energy 35:10954–10959
Karlsson A, Vallin L, Ejlertsson J (2008) Effects of temperature, hydraulic retention time and hydrogen extraction rate on hydrogen production from the fermentation of food industry residues and manure. Int J Hydrogen Energy 33:953–962
Kenealy W, Cao Y, Weimer P (1995) Production of caproic acid by cocultures of ruminal cellulolytic bacteria and Clostridium kluyveri grown on cellulose and ethanol. Appl Microbiol Biotechnol 44:507–513
Khardenavis A, Wang J, Ng W, Purohit H (2013) Management of various organic fractions of municipal solid waste via recourse to VFA and biogas generation. Environ Technol 34:2085–2097
Kim I, Hwang MH, Jang NJ, Hyun SH, Lee S (2004) Effect of low pH on the activity of hydrogen utilizing methanogen in biohydrogen process. Int J Hydrogen Energy 29:1133–1140
Kim M, Baek J, Yun Y, Sim S, Park S, Kim S (2006) Hydrogen production from Chlamydomonas reinhardtii biomass using a two-step conversion process: anaerobic conversion and photosynthetic fermentation. Int J Hydrogen Energy 31:812–816
Kim H, Leeds P, Chuang D (2009) The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem 110:1226–1240
Kobayashi M, Kakizono T, Nagai S (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol 59:867–873
Kraemer JT (2004) Effects of methanogenic effluent recycle on fermentative hydrogen production PhD Thesis, University of Toronto, Toronto, Canada
Kumar K, Dasgupta C, Nayak B, Lindblad P, Das D (2011) Development of suitable photobioreactors for CO2 sequestration addressing global warming using green algae and cyanobacteria. Bioresour Technol 102:4945–4953
Kurzynski M (2006) The thermodynamic machinery of life. Springer, Leipzig
Ladygina N, Dedyukhina E, Vainshtein M (2006) A review on microbial synthesis of hydrocarbons. Process Biochem 41:1001–1004
Lay JJ (2001) Biohydrogen generation by mesophilic anaerobic fermentation of microcrystalline cellulose. Biotechnol Bioeng 74:280–287
Lay JJ, Lee YJ, Noike T (1999) Feasibility of biological hydrogen production from organic fraction of municipal solid waste. Water Res 33:2579–2586
Lee D, Li Y, Noike T, Cha G (2008) Behaviour of extracellular polymers and bio-fouling during hydrogen fermentation with a membrane bioreactor. J Memb Sci 322:13–18
Lee D, Li Y, Noike T (2010) Influence of solids retention time on continuous H2 production using membrane bioreactor. Int J Hydrogen Energy 35:52–60
Leung A, Boocock D, Konar S (1995) Pathway for the catalytic conversion of carboxylic acids to hydrocarbons over activated alumina. Energy Fuel 9:913–920
Leyva M, Vicedo B, Finiti I, Flors V, Del Amo G, Real M et al (2008) Preventive and post-infection control of Botrytis cinerea in tomato plants by hexanoic acid. Plant Pathol 57:1038–1046
Li C, Fang HHP (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit Rev Environ Sci Technol 37:1–39
Li W, Yu H (2011) From wastewater to bioenergy and biochemicals via two-stage bioconversion processes: a future paradigm. Biotechnol Adv 29:972–982
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Lin CY, Chang RC (2004) Fermentative hydrogen production at ambient temperature. Int J Hydrogen Energy 29:715–720
Lin CY, Lay CH (2004) Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int J Hydrogen Energy 29:41–45
Lin CY, Lay CH (2005) A nutrient formulation for fermentative hydrogen production using anaerobic sewage sludge microflora. Int J Hydrogen Energy 30:285–292
Lindblad MS, Liu Y, Albertsson AC, Ranucci E, Karlsson S (2002) Polymers from renewable resources. In: Albertsson AC (ed) Degradable aliphatic polyesters. Springer, Berlin, pp 139–161
Liu WT, Chan OC, Fang HHP (2002) Microbial community dynamics during start-up of acidogenic anaerobic reactors. Water Res 36:3203–3210
Liu D, Liu D, Zeng RJ, Angelidaki I (2006) Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res 40:2230–2236
Louis P, Flint HJ (2009) Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294:1–8
Maddox IS, Qureshi N, Roberts-Thomson K (1995) Production of acetone-butanol-ethanol from concentrated susbtrates using Clostridium acetobutylicum in an integrated fermentation-product removal process. Process Biochem 30:209–215
Magid J (2006) Short-Circuit short circuiting the carbon and nutrient cycles between urban and rural districts by establishing three new systems for source separation, collection and composting of organic waste in the greater Copenhagen area. Final report presented to the EU-life program. http://orgprints.org/9230/. Accessed 21 June 2015
Marinoiu A, Cobzaru C, Carcadea E, Capris C, Tanislav V, Raceanu M (2013) Hydrogenolysis of glycerol to propylene glycol using heterogeneous catalysis in basic aqueous solutions. React Kinet Mech Catal 110:63–73
Market Publishers (2012) Global n-butanol market to reach 4 Mt by 2020. http://marketpublishers.com/lists/13495/news.html. Accessed 24 Nov 2014
Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue-green algae) in agro-industrial wastes and wastewaters: a review. Appl Energy 88:3389–3401
McKendry P (2002a) Energy production from biomass (part 2): conversion technologies. Bioresour Technol 83:47–54
McKendry P (2002b) Energy production from biomass (part 3): gasification technologies. Bioresour Technol 83:55–63
Mecking S, Held A, Bauers F (2002) Aqueous catalytic polymerization of olefins. Angew Chem Int Ed 41:544–561
Mitchell R, Gu JD (2010) Environmental microbiology, 2nd edn. Wiley-Blackwell, Hoboken
Mizuno O, Dinsdale R, Hawkes FR, Hawkes DL, Noike T (2000) Enhancement of hydrogen production from glucose by nitrogen gas sparging. Bioresour Technol 73:59–65
Mohammadi M, Najafpour G, Younesi H, Lahijani P, Uzir M, Mohamed A (2011) Bioconversion of synthesis gas to second generation biofuels: a review. Renew Sustain Energy Rev 15:4255–4273
Mu Y, Zheng XJ, Yu HQ, Zhu R (2006) Biological hydrogen production by anaerobic sludge at various temperatures. Int J Hydrogen Energy 31:780–785
Mu Y, Yu HQ, Wang G (2007) A kinetic approach to anaerobic hydrogen-producing process. Water Res 41:1152–1160
Munster M (2009) Energy systems analysis of waste to energy technologies by use of energyPLAN. Riso report, Danish Technical University. http://orbit.dtu.dk/fedora/objects/orbit:81741/datastreams/file_4069900/content. Accessed 12 June 2015
Murzin D, Leino R (2008) Sustainable chemical technology through catalytic multistep reactions. Chem Eng Res Des 86:1002–1010
Murzin D, Simakova I (2011) Catalysis in biomass processing. Catal Ind 3:218–249
Mussatto SL, Dragone G, Guimaraes PMR, Silva JPA, Carneiro LM, Roberto IC et al (2010) Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv 28:817–830
Najdenski H, Gigova L, Illiev I, Pilarski P, Lukavsky J, Tsvetkova I et al (2013) Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int J Food Sci Technol 48:1533–1540
Nielsen AT, Amandusson H, Bjorklund R, Dannentun H, Ejlertsson J, Ekedahl LG et al (2001) Hydrogen production from organic waste. Int J Hydrogen Energy 26:547–550
Nishio N, Nakashimada Y (2007) Recent development of anaerobic digestion processes for energy recovery from wastes. J Biosci Bioeng 103:105–112
Ntaikou I, Antonopoulou G, Lyberatos G (2010) Biohydrogen production from biomass and wastes via drak fermentation: a review. Waste Biomass Valoriz 1:21–39
Nwobi A (2013) Techno-economic evaluation of biofuel production from organic fraction of municipal solid waste (OFMSW) generated in Abu Dhabi. MSc thesis, Masdar Institute of Science and Technology, Abu Dhabi, UAE
Ogbonna J, Yoshizawa H, Tanaka H (2000) Treatment of high strength organic wastewater by a mixed culture of photosynthetic microorganisms. J Appl Phycol 12:277–284
Oh S, Iyer P, Bruns M, Logan B (2004) Biological hydrogen production using a membrane bioreactor. Biotechnol Bioeng 87:119–127
OPEC (2014) 2014 world oil outlook. Organization of the Petroleum Exporting Countries, Vienna
Pauss A, Andre G, Perrier M, Guiot S (1990) Liquid-to-mass transfer in anaerobic processes: inevitable transfer limitations of methane and hydrogen in the biomethanation process. Appl Environ Microb 56:1636–1644
Pavlostathis SG, Gossett JM (1988) Preliminary conversion mechanisms in anaerobic digestion of biological sludges. J Environ Eng 114:575–592
Peighambardoust SJ, Rowshanzamir S, Amjadi M (2010) Review of the proton exchange membranes for fuel cell applications. Int J Hydrogen Energy 35:9349–9384
Petkov G, Ivanova A, Iliev I, Vaseva I (2012) A critical look at the microalgae biodiesel. Eur J Lipid Sci Technol 114:103–111
Pinto F, Franco C, Andre R, Miranda M, Gulyurtlu I, Cabrita I (2002) Co-gasification study of biomass mixed with plastic wastes. Fuel 81:291–297
Pirie C, De Mey M, Prather K, Ajikumar P (2013) Integrating the protein and metabolic engineering tollkits for next-generation chemical biosynthesis. ACS Chem Biol 8:662–672
Porpatham E, Ramesh A, Nagalingam B (2007) Effect of hydrogen addition on the performance of a biogas fuelled spark ignition engine. Int J Hydrogen Energy 32:2057–2065
Prakasham RS, Sathish T, Brahmaiah P (2010) Biohydrogen production process optimization using anaerobic mixed consortia: a prelude study for use of agro-industrial material hydrolysate as substrate. Bioresour Technol 101:5708–5711
PRnewswire (2013) Global salicylic acid market is expected to reach USD 5212 million in 2019. http://www.prnewswire.com/news-releases/global-salicylic-acid-market-is-expected-to-reach-usd-5212-million-in-2019-transparency-market-research-225332251.html. Accessed 24 Nov 2014
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Quemeneur M, Hamelin J, Guidici-Orticoni T, Latrille E, Steyer JP, Trably E (2011) Changes in hydrogenase genetic diversity and proteomic patterns in mixed-cultured dark fermentation of mono, di- and tri-saccharides. Int J Hydrogen Energy 36:11654–11665
Queneau Y, Pinel C, Scherrmann M (2011) Some chemical transformations of carbohydrates in aqueous medium. C R Chim 14:688–699
Rajhi H, Conthe M, Puyol D, Diaz E, Sanz J (2013) Dark fermentation: isolation and characterization of hydrogen-producing strains from sludges. Int Microbiol 16:53–62
Rashid K (2013) Prospects for algal biofuels in UAE: technology and economics. MSc Thesis Masdar Institute of Science and Technology, Abu Dhabi, UAE
Reddy AKN, Williams RH, Johansson T (1997) Energy after Rio: prospects and challenges. United Nations Development Programme (UNDP), New York
Redwood M, Paterson-Beedle M, Macaskie L (2009) Integrating dark and light bio-hydrogen production strategies: towards the hydrogen economy. Rev Environ Sci Biotechnol 8:149–185
Ren N, Cao G, Xu J, Gao L (2009) Bioconversion of lignocellulosic biomass to hydrogen: potential and challenges. Biotechnol Adv 27:1051–1060
REnescience (2015) Technology and services. http://www.renescience.com/en/technology-and-services/technology. Accessed 12 Jun 2015
Rephaeli A, Zhuk R, Nudelman A (2000) Prodrugs of butyric acid from bench to bedside: synthetic design, mechanisms of action, and clinical applications. Drug Devlop Res 50:379–391
Rice C, Ha S, Masel RI, Waszczuk P, Wieckowski A, Barnard T (2002) Direct formic acid fuel cells. J Power Sources 111:83–89
Rittmann S, Herwig C (2012) A comprehensive and quantitative review of dark fermentative biohydrogen production. Microb Cell Fact 11:115
Roddy D (2013) A syngas network for reducing industrial carbon footprint and energy use. Appl Therm Eng 53:299–304
Roehr M (2001) The biotechnology of ethanol, classical and future applications. Wiley-VCH verlag GmbH, Weinheim
Rogers P, Chen J, Zidwick M (2006) Organic acid and solvent production. The Prokaryotes, 3rd edn. Springer, Berlin Heidelberg, pp 511–755
Romano AH, Conway T (1996) Evolution of carbohydrate metabolic pathways. Res Microbiol 147:448–455
Ruiz-Rodriguez A, Fornan T, Hernandez E, Senorans F, Reglero G (2010) Thermodynamic modeling of dealcoholization of beverages using supercritical CO2: application to wine samples. J Supercrit Fluid 52:183–188
Sahena F, Zaidul I, Jinap S, Karim A, Abbas K, Norulaini N et al (2009) Application of supercritical CO2 in lipid extraction—a review. J Food Eng 95:240–253
Sauer U, Santangelo J, Treuner A, Buchholz M, Durre P (1995) Sigma factor and sporulation genes in Clostridium. FEMS Microbiol Rev 17:331–340
Schink B, Kremer DR, Hansen TA (1987) Pathway of propionate formation from ethanol in Pelobacter propionicus. Arch Microbiol 147:321–327
Seeliger S, Janssen PH, Schink B (2006) Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol Lett 211:65–70
Shi X, Yu H (2005) Optimization of volatile fatty acid compositions for hydrogen production by Rhodopseudomonas capsulata. J Chem Technol Biot 80:1198–1203
Shi X, Yu H (2006) Continuous production of hydrogen from mixed volatile fatty acids with Rhodopseudomonas capsulata. Int J Hydrogen Energy 31:1641–1647
Shin HS, Younb JH, Kim SH (2004) Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int J Hydrogen Energy 29:1355–1363
Show K, Lee D, Chang J (2011) Bioreactor and process design for biohydrogen production. Bioresour Technol 102:8524–8533
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechol Adv 27:409–416
Sierens R, Rosseel E (2000) Variable composition hydrogen/natural gas mixtures for increased engine efficiency and decreased emissions. J Eng Gas Turb Power 122:135–140
Singh U, Ahluwalia A (2013) Microalgae: a promising tool for carbon sequestration. Mitig Adapt Strateg Glob Change 18:73–95
Sossai P (2012) Butyric acid: what is the future for this old substance? Swiss Med Wkly 142:w13596
Sparling R, Islam R, Cicek N, Carere C, Chow H, Levin DB (2006) Formate synthesis by Clostridium thermocellum during anaerobic fermentation. Can J Microbiol 52:681–688
Spivey J, Gangwal S, Zoeller JR, Winslow J, Srivastava RD (2000) Syngas conversion to fuels and chemicals. Catal Today 58:231
Sudesh K, Doi A (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Tanaka N, Kjorven O, Yumkella K (2010) Energy poverty, how to make modern energy access universal?. International Energy Agency (IEA), United Nations Development Programme (UNDP), and United Nations Industrial Development Organization (UNIDO), Paris
Tanisho S, Kuromoto M, Kadokura N (1998) Effect of CO2 removal on hydrogen production by fermentation. Int J Hydrogen Energy 23:559–563
Tawfik A, El-Qelish M (2012) Continuous hydrogen production from co-digestion of municipal food waste and kitchen wastewater in mesophilic anaerobic baffled reactor. Bioresour Technol 114:270–274
Temudo MF, Kleerebezem R, van Loosdrecht MCM (2007) Influence of the pH on (open) mixed culture fermentation of glucose: a chemostat study. Biotechnol Bioeng 98:69–79
Temudo MF, Muyzer G, Kleerebezem R, van Loosdrecht MCM (2008a) Diversity of microbial communities in open mixed culture fermentations: impact of the pH and carbon source. Appl Microbiol Biotechnol 80:1121–1130
Temudo MF, Poldermans R, Kleerebezem R, van Loosdrecht MCM (2008b) Glycerol fermentation by (open) mixed cultures: a chemostat study. Biotechnol Bioeng 100:1088–1098
Temudo MF, Mato T, Kleerebezem R, van Loosdrecht MCM (2009) Xylose anaerobic conversion by open-mixed cultures. Appl Microbiol Biotechnol 82:231–239
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Tholozan JL, Touzel JP, Samain E, Grivet JP, Prensier G, Albagnac G (1992) Clostridium neopropionicum sp-nov a strict anaerobic bacterium fermenting ethanol to propionate through acrylate pathway. Arch Microbiol 157:249–257
Tomasko D, Burley A, Feng L, Yeh S, Miyazono K, Nirmal-Kumar S et al (2009) Development of CO2 for polymer foam applications. J Supercrit Fluid 47:493–499
Tran N, Barlett J, Kannangara G, Milev A, Volk H, Wilson M (2010) Catalytic upgrading of biorefinery oil from micro-algae. Fuel 89:265–274
Ueno Y, Kawai T, Sato S, Otsuka S, Morimoto M (1995) Biological production of hydrogen from cellulose by natural anaerobic microflora. J Ferment Bioeng 79:395–397
Uhm S, Chung ST, Lee J (2008) Characterization of direct formic acid fuel cells by impedance studies: in comparison of direct methanol fuel cells. J Power Sources 178:34–43
Uratani J (2013) Anerobic digestion of halophytic plant residues for biogas production and nutrient recovery. MSc Thesis, Masdar Institute of Science and Technology, Abu Dhabi, UAE
Vane LM (2005) A review of pervaporation for product recovery from biomass fermentation processes. J Chem Technol Biotechnol 80:603–629
Vanhoutvin S, Troost F, Kilkens T, Lindsey P, Hamer H, Jonkers D et al (2009) The effects of butyrate enemas on viceral perception in healthy volunteers. Neurogastroenterol Motil 21:952–e76
Vasudevan D, Richter H, Angenent L (2014) Upgrading dilute ethanol from syngas fermentation to n-caproate with reactor microbiomes. Bioresour Technol 151:378–382
Waligorska M (2012) Fermentative hydrogen production—process design and bioreactors. Chem Process Eng 33:585–594
Wang S, Guo Z, Cai Q, Guo L (2012) Catalytic conversion of carboxylic acids in bio-oil for liquid hydrocarbons production. Biomass Bioenergy 45:138–143
Wang Y, Mu Y, Yu HQ (2007) Comparative performance of two upflow anaerobic biohydrogen-producing reactors seeded with different sludges. Int J Hydrogen Energy 32:1086–1094
Weibel G, Ober C (2003) An overview of supercritical CO2 applications in microelectronics processing. Microelectron Eng 65:145–152
Weissermel K, Arpe HJ (1997) Industrial organic chemistry. VCH Publishers, New York
Wilhelm D, Simbeck D, Karp A, Dickenson R (2001) Syngas production for gas-to-liquids applications: technologies, issues and outlook. Fuel Process Technol 71:139–148
Williams T, Shaddix C, Schefer R (2008) Effect of syngas composition and CO2-diluted oxygen on performance of a premixed swirl-stabilized combustor. Combust Sci Technol 180:64–88
Willke T, Vorlop K (2004) Industrial bioconversion of renewable resources as an alternative to conventional chemistry. Appl Microbiol Biotechnol 66:131–142
Willquist K, Nkemba VN, Svensson H, Pawar S, Ljunggren M, Karlsson H et al (2012) Design of a novel biohythane process with high H2 and CH4 production rates. Int J Hydrogen Energy 37:17749–17762
Ye N, Lu F, Shao L, Godon J, He P (2007) Bacterial community dynamics and product distribution during pH-adjusted fermentation of vegetable wastes. J Appl Microbiol 103:1055–1065
Yokoi H, Saitsu A, Uchida H, Hirose J, Hayashi S, Takasaki Y (2001) Microbial hydrogen production from sweet potato starch residue. J Biosci Bioeng 91:58–63
Zacharof M, Lovitt R (2013) Complex effluent streams as a potential source of volatile acids. Waste Biomass Valor 4:557–581
Zahedi S, Sales D, Romero L, Solera R (2013) Hydrogen production from the organic fraction of municipal solid waste: in anaerobic thermophilic acidogenesis: influence of organic loading rate and microbial content of the solid waste. Bioresour Technol 129:85–91
Zeeshan N, Hinrich U (2014) Enhancing the hydrolysis process of a two-stage biogas technology for the organic fraction of municipal solid waste. In: Presented at the international conference on anaerobic digestion “BiogasScience 2014”, Vienna, Austria
Zehnder A, Svensson B (1986) Life without oxygen: what can and what cannot? Experentia 42:1197–1205
Zhang Y (2014) Production of biofuels and biochemicals by in vitro synthetic biosystems: opportunities and challenges. Biotechnol Adv. doi:10.1016/j.biotechadv.2014.10.009
Zhang Y, Shen J (2006) Effect of temperature and iron concentration on the growth and hydrogen production of mixed bacteria. Int J Hydrogen Energy 31:441–446
Zhang Z, Show K, Tay J, Liang D, Lee D, Jiang W (2007) Rapid formation of hydrogen producing granules in an anaerobic continuous stirred tank reactor induced by acid incubation. Biotechnol Bioeng 96:1040–1050
Zhang C, Yang H, Yang F, Ma Y (2009) Current progress on butyric acid production by fermentation. Curr Microbiol 59:656–663
Zhang F, Zhang Y, Chen M, Zeng RJ (2012) Hydrogen supersaturation in thermophilic mixed culture fermentation. Int J Hydrogen Energy 37:17809–17816
Zhang F, Ding J, Shen N, Zhang Y, Ding Z, Dai K et al (2013a) In situ hydrogen utilization for high fraction acetate production in mixed culture hollow-fiber membrane biofilm reactor. Appl Microbiol Biotechnol 97:10233–10240
Zhang F, Ding J, Zhang Y, Chen M, Ding ZW, van Loosdrecht MCM, Zeng RJ (2013b) Fatty acids production from hydrogen and carbon dioxide by mixed culture in the membrane biofilm reactor. Water Res 47:6122–6129
Zhang F, Zhang Y, Chen M, van Loosdrecht MCM, Zeng RJ (2013c) A modified metabolic model for mixed culture fermentation with energy conserving electron bifurcation reaction and metabolite transport energy. Biotechnol Bioeng 110:1884–1894
Zhang F, Zhang Y, Ding Y, Dai K, van Loosdrecht MCM, Zeng RJ (2014) Stable acetate production in extreme-thermophilic (70 °C) mixed culture fermentation by selective enrichment of hydrogenotrophic methanogens. Sci Rep. doi:10.1038/srep05268
Zhao Y, Chen Y, Zhang D, Zhu X (2010) Waste activated sludge fermentation for hydrogen production enhanced by anaerobic process improvement and acetobacteria inhibition: the role of fermentation pH. Environ Sci Technol 44:3317–3323
Zheng H, O’Sullivan C, Mereddy R, Zeng R, Duke M, Clarke W (2010) Experimental and theoretical investigation of diffusion process in membrane anaerobic reactor for bio-hydrogen production. Int J Hydrogen Energy 35:5301–5311
Acknowledgments
The authors would like to acknowledge the financial support from Masdar Institute, to help fulfill the vision of the late President Sheikh Zayed Bin Sultan Al Nahyan for sustainable development and empowerment of the United Arab Emirates and humankind, funding project 2GBIONRG (12KAMA4). The authors would like to acknowledge Kevin Garvey, Academic Writing Lecturer at Masdar Institute for the manuscript language review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bastidas-Oyanedel, JR., Bonk, F., Thomsen, M.H. et al. Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev Environ Sci Biotechnol 14, 473–498 (2015). https://doi.org/10.1007/s11157-015-9369-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-015-9369-3