Abstract
Sorghum bicolor (sorghum) is a species known for accumulating high quantities of cadmium (Cd), which can damage physiological and metabolic functions, impede growth, and reduce yield. Maintaining sorghum’s production, therefore, requires enhancing its tolerance to the toxic effects of Cd. In this study, we investigate the effects of Cistus monspeliensis extract (CME) on Cd stress tolerance in sorghum. Sorghum plants exposed to Cd (200 μM) showed a decrease in their growth, biomass, and chlorophyll content compared to unstressed ones. However, CME supplementation (5 mg/l, 20 mg/l, and 60 mg/l) to the stressed plants reversed the detrimental effect of Cd and elevated biomass and pigment content. CME also reduced superoxide ions (O2−) accumulation and boosted the activities of antioxidant system-related enzymes: superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferase (GST). Moreover, through examining several carbon–nitrogen enzyme activities (phosphoenolpyruvate carboxylase (PEPC), malate dehydrogenase (NAD-MDH), glutamine synthase (GS), glutamate dehydrogenase (GDH), and aspartate aminotransferase (AAT)), we discovered that CME supplementation modulated the perturbations of carbon and nitrogen metabolism in sorghum plants under Cd stress. CME, therefore, appears to improve Cd stress tolerance by upregulating antioxidant defense enzymes, decreasing ROS production, and improving carbon metabolism and nitrogen assimilation, thus leading to a better growth rate. CME’s Cd stress alleviation effect was generally more prominent at 5 mg/L and 20 mg/L.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cadmium (Cd) is a major environmental contaminant that causes a considerable threat to nature and human health alike. Cd pollution is predominantly caused by anthropogenic activities such as mining, chemical, and metallurgical industries [1, 2]. It is a redox-inactive and poisonous heavy metal that exists at low levels in nature and becomes extremely toxic when accumulated in soil and water [3]. Cd is quickly uptaken by plants, translocated, and accumulated in the edible parts consumed by humans [4, 5]. The ingested Cd can be accumulated in the human body and cause various diseases like liver damage, kidney stones, renal tubular damage, bone diseases, and calcium metabolism disorder [6, 7]. Cd exposure can also disrupt the antioxidant defense system in plants and increase the generation of reactive oxygen species (ROS), which eventually damages physiological and metabolic functions [8]. Photosynthesis, nutrient acquisition, mineral uptake, nitrogen assimilation, gas exchange, and carbon fixation are all retarded during Cd stress, causing reduced yield, growth, and development in the affected plants [9]. Due to its high mobility, Cd can be found in any part of the plant and cause multiple anatomical abnormalities in the leaves, stems, and roots [10]. Previous reports have linked Cd exposure to the reduction of aerenchyma proportion in the leaves, reduction in leaf thickness, stomatal closure, narrowing in the xylem and phloem vessels, and decrease in root tissues [11,12,13,14]. These alterations damage the ultrastructure of the affected plants, resulting in a reduction in biomass and yield, in which the severity depends on Cd levels, plants specie, and exposure period [15].
Sorghum (Sorghum bicolor (L.)) is a C4 grass that is cultivated for food, feed, fiber, and fuel [16, 17]. It ranks fifth among the world's economically important crops and is a source of nutrients and bioactive substances in the human diet [18, 19]. This crop has the ability to accumulate high quantities of heavy metals, including Cd, and nonetheless endure the generated stress [20]. However, even though sorghum species can survive and accumulate high Cd levels, their growth and development can be severely hampered, hence, the need for suitable remediation [21, 22]. Natural bio-stimulants, such as plant materials, are nowadays considered a promising and sustainable approach for addressing yield losses induced by abiotic stressors [23]. Biostimulants contain a wide range of mineral and organic compounds that plants can use as metabolites, growth regulators, and nutrients in order to increase their growth and output and combat stress [24]. Plant-derived biostimulants can improve growth patterns and yield of several crops cultivated under environmental stresses by improving various physiological, biochemical, and molecular mechanisms. Several studies have previously described the effect of numerous plant extracts like licorice root extract (LRE), lemongrass, garlic extract, and moringa leaves extract (MLE) as biostimulants in different stressful conditions [25, 26]. Foliar application of MLE to bean plants grown under Cd and salt stress significantly increased photosynthetic pigments and proline content and boosted the activities of antioxidant enzymes (superoxide dismutase, catalase, peroxidase, and glutathione reductase) [27]. Treatments of maize with silymarin-enriched maize grain extract under Cd stress increased photosynthetic capacity and reduced ROSs by enhancing the antioxidant machinery [28]. LRE application to common bean plants grown under salt stress restored their mineral nutrients, maintained their water status, and protected their leaf anatomy [29]. LRE also effectively improved the plant's enzymatic and non-enzymatic antioxidants, resulting in improved growth and a better yield even under stress [29]. Likewise, pear tree leaves treated with LRE exhibited an improvement in nutrient and hormonal contents in pear tree leaves [30]. In addition, aqueous garlic extract improved the growth of salt stressed eggplants through enhancing plant morphology, biomass, photosynthesis, and antioxidant enzymes (superoxide dismutase and peroxidase) [31].
However, to the best of our knowledge, this is the first work reporting the biostimulant effect of Cistus monspeliensis (CM). CM is a shrub that belongs to the Cistaceae family and grows mostly in the Mediterranean semi-arid ecosystems [32]. It is known for its medicinal attributes and has antimicrobial, antifungal, anti-inflammatory, and antiproliferative properties [33,34,35]. Phytochemical studies of CM revealed the presence of many phenolic compounds, notably flavonoids, which have been linked to oxidative stress prevention mechanisms [36, 37]. CM is also known to have a high terpenoid content, especially diterpenes, that plays essential roles in plant growth, defense, and adaptation to biotic and abiotic stress [38, 39]. This multitude of biologically active compounds could be helpful in mitigating Cd-induced toxicity and ensuring better plant growth even in stressful conditions. Therefore, we aimed to investigate the effect of Cistus monspeliensis extract (CME) on sorghum plants grown under Cd stress contributing to its alleviation. For this purpose, we focused on evaluating some physiological and biochemical parameters, such as plant growth parameters, antioxidant defensive enzymes (SOD, GPX, GST, GR), carbon–nitrogen activities (PEPC, GS, MDH, GDH, AAT), malondialdehyde (MDA) levels, chlorophyll, O2− content, and some osmolytes studied following the different treatments of CME: 5 mg/l, 20 mg/l, and 60 mg/l combined with Cd stress (200 µM).
2 Materials and methods
2.1 Cistus monspeliensis extract preparation
The Cistus monspeliensis (CM) plant was acquired from the “Jbel Lahbib” mountain in northern Morocco (exact geographical coordinates, latitude, and longitude: 35.4664997, − 5.7971542). CM aerial parts (leaves and stems) were dried at room temperature and then crushed to obtain a fine powder. One hundred grams of this powder was mixed with 1 L of distilled water, boiled for 2 h at 100 °C, poured into Petri dishes, and dried at 37 °C in an incubator until the water had evaporated entirely. The dried extract was then used to prepare three concentrations: 5 mg/l, 20 mg/l, and 60 mg/l. The selected concentrations were based on our preliminary study.
2.2 Cistus monspeliensis extract chemical composition analysis
The chemical composition of Cistus monspeliensis extract was evaluated; the polyphenol content was determined according to Ben Mrid et al. [19], and the flavonoid content was estimated following the protocol of Bouargalne et al. [17]. While IAA, free amino acid, and protein contents protocols are mentioned in details in following sections of materials and methods.
2.3 Plant materials, experimental treatments, and growth conditions
Sorghum bicolor (L.) plants were cultivated in the plant nursery at Tangier’s Faculty of Sciences and Technologies. Sorghum seeds were surface sterilized with 5% sodium hypochlorite for 3 min, washed with distilled water, and left to germinate for 48 h. Six pre-germinated seeds were planted in each plastic pot (26 cm deep and 30 cm in diameter) using Cd-free soil. The composition of the used soil was as follows (%): Ca, 1.68 ± 0.113; Mg, 0.902 ± 0.006; Cl, non-detected; S, 0.048 ± 0.001; Fe, 5.007 ± 0.024; I, 0.024 ± 0.001; Mn, 0.097 ± 0.002; Zn, 0.012 ± 0.001; Na, 0.403 ± 0.006; Al, 9.949 ± 0.097; OM, 2.592 ± 0.011; CaCO3, 17.059 ± 0.002; Cd, non-detected, with a pH of 8. The experiment was laid out in October 2021. The weather during this month was warm, humid, sunny, and partially cloudy, with daytime maximum temperatures averaging about 25 °C, nighttime temperatures around 18 °C, and an average of 10.4 h of sunlight each day. Seven days after planting, the pots were separated into five groups comprising plants irrigated with water only (C), plants irrigated with 200 μM of Cd (C +), plants irrigated with 5 mg/l of CME + 200 μM of Cd (CM5), plants with 20 mg/l of CME + 200 μM of Cd (CM20), and plants treated with 60 mg/l of CME + 200 μM of Cd (CM60). Each treatment was performed in 4 replicates (pots).
2.4 Agronomic traits measurements
Sorghum plants were harvested after 35 days from planting to measure plants’ height and weight. Shoots were weighted to register their fresh weight and then placed in an incubator at 37 °C until completely dry to record the dry weight.
2.5 Estimation of MDA and O2 − content
MDA content was quantified using Bouchmaa et al. method with a few adjustments [40]. Plant cell homogenate was mixed with trichloroacetic acid (20%) and tetrabutylammonium hydroxide (TBA) (0.67%). The mix was heated at 95 °C for 1 h. After cooling, 1 mL n-butanol was added, and the mixture was centrifuged at 12,000 for 12 min. The supernatant was gathered to determine the absorbance at 532 and 600 nm.
Superoxide ions (O2−) content was determined using the method of Kubiś [41]. One hundred milligrams of fresh sorghum leaves were cut into 1 mm fragments and immersed for 1 h at room temperature in a mixture containing: 10 mM K-phosphate buffer (pH 7.8), 0.05% nitro blue tetrazolium (NBT), and 10 mM sodium azide. Next, 2 ml of the immersed solution was heated at 85 °C for 15 min, then cooled rapidly. Optical density was measured colorimetrically at 580 nm, and O2− content was expressed as an increase of absorbance/1 g of fresh weight (A580 g−1 FW).
2.6 Determination of chlorophyll content
To determine the chlorophyll content, 200 µl of the plant’s soluble pellets homogenized in water was added to 800 µL of 80% acetone. After 72 h of incubation at 4 °C, the chlorophyll content was determined in three independent replicates. The method of Armon [42] was used to estimate chlorophyll a, chlorophyll b, and total chlorophyll contents as follows:
where OD645nm and OD663nm are the optical densities at 645 and 663 nm, respectively.
2.7 Preparation of plant extracts and estimation of indole acetic acid and amino acid contents
Harvested sorghum leaves were placed in an incubator to dry (37 °C) for 48 h. 200 mg of the dried material was crushed using a mortar at 4 °C in the presence of ethanol (80%). The homogenate was centrifuged (5000 g) for 15 min at 4 °C, and the supernatant was used to determine the content of indole acetic acid and amino acid.
Indole acetic acid (IAA) content in sorghum leaves was estimated using Salkowski’s reagent method and commercial IAA as standard [43]. About 1.5 ml of the supernatant was mixed with 500 ml of Salkowski’s reagent (FeCl3 in 36% perchloric acid) and incubated at 25 °C for 30 min. The optical density at 530 was then measured against a blank containing 1.5 ml of distilled water and 500 µl of Salkowski’s reagent.
To quantify the amino acid content, 200 µl of the supernatant was mixed with 1.8 ml of 2% ninhydrin reactive solution (solubilized in 0.2 M citrate buffer, pH 5, and ethylene glycol). The mixture was vortexed and then heated in a water bath for 15 min. After cooling at room temperature, the optical density at 570 nm was measured, and the amino acid content was calculated using a calibration curve established by varied glycine concentrations.
2.8 Extraction and assay of SOD, GPx, GST, GR, and GDH
A solution comprising 100 mM HEPES–KOH, 20 µM FAD, 10 mM MgCl2, 1 mM PMSF, and 14 mM β-mercaptoethanol was used to ground 200 mg of fresh sorghum leaves in a chilled mortar. The mixture was centrifuged (20,000 g) at 4 °C for 20 min. SOD, GPx, GST, GR, and GDH enzyme activities were determined using the supernatant. The total protein content was estimated using the method of Bradford with Bovine Serum Albumin (BSA) as standard [44].
SOD activity was determined by measuring its capacity to limit the photochemical reduction of nitroblue tetrazolium chloride (NBT), as established by Beauchamp et Fridovich [45]. The mixture used for the reaction contained 50 mM phosphate buffer (7.8), 2 mM methionine, 75 µM NBT, 1 µM EDTA, 2 µM riboflavin, and the enzyme extract. Absorbance at 560 nm was measured after 30 min under a flat light panel. Controls without the enzyme developed the maximum color, and 1 unit of the activity was defined as the amount of enzyme that caused a 50% decrease in absorbance compared to the control.
The activity of GPx was carried out according to the method described by Bouchmaa et al. with some modifications [46]. The reaction mixture contained 50 mM potassium phosphate, pH 7.4, 1 mM EDTA, 1 mM sodium azide, 1 mM GSH, GR (4 μg/mL), 0.2 mM NADPH, 0.25 mM of H2O2, and enzyme extract. The rate of NADPH oxidation was monitored at 340 nm.
GST activity was calculated using a slightly modified Habig, Pabst, and Jakoby technique [47]. The assay mixture contained the supernatant, 5 mM GSH, 2.5 mM 1-chloro-2,4-dinitrobenzene (CDNB), and 0.1 M phosphate buffer (pH 5.5). The reaction was spectrophotometrically monitored at 340 nm at 30 °C, and the product concentrations were calculated using a molar extinction coefficient of 9.6 mM−1 cm−1.
The oxidation of NADPH at 340 nm was used to assess GR activity, as described by Latique et al. [48]. Some modifications were applied. The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.8), 0.2 mM NADPH, 1 mM GSSG, and the required amount of enzyme extract. The reaction was started by adding NADPH at 30 °C.
GDH activity was measured as described by Ben Mrid et al. [49]. The reaction mixture contained 100 mM Tris–HCl (pH 8), 1 mM CaCl2, 13 mM α-ketoglutarate, 50 mM (NH4)2SO4, 0.25 mM NADH, and the required amount of enzyme extract. The activity was monitored spectrophotometrically for 30 min at 340 nm.
2.9 Extraction and assay of PEPC, GS, AAT, and NADH-MDH
Sorghum leaves were crushed in a cooled mortar using Tris–HCl buffer (100 mM, pH 8) that contains 10 mM MgCl2, 1.4 mM glycerol, 1.4 mM β–mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM EDTA, 1 mM EGTA, 9.4 µM leupeptin, and 16.5 µM chymostatin. The homogenate was centrifuged at 12,000 g for 15 min at 4 °C. After that, the supernatant was saturated (60%) with solid ammonium sulfate for 30 min. Under the same settings, the saturated supernatant was centrifuged anew, and the pellet was resuspended in the extraction buffer and utilized for enzyme activities. The total protein content was determined following the method of Bradford (1976) with BSA as a protein standard [44].
PEPC activity was measured as described by El Omari et al. following the oxidation of NADH at 340 nm [50]. The assay mixture contained 100 mM Hepes–KOH (pH 7.3), 5 mM MgCl2, 5 mM NaHCO3, 2.5 mM PEP, 0.25 mM NADH, 3 units of MDH, and the enzyme extract.
GS activity was estimated as described by Ben Mrid, Omari, and Nhiri [51]. The reaction mixture contained 90 mM imidazole–HCl (pH 7.0), 120 mM L-glutamine, 3 mM MnCl2, 0.4 mM ADP, 20 mM potassium arsenate, 60 mM hydroxylamine, and the enzyme extract. L-glutamine and ADP were not included in the blank test. The mixture was incubated at 37 °C for 20 min, then stopped by adding a solution of (1:1:1) of 10% FeCl3·6H2O (in 0.2 N HCl), 24% TCA, and 5% HCl. The appearance of γ-glutamyl hydroxamate was measured at 540 nm.
AAT activity was measured using the method described by Ben Mrid et al. [52]. The assay mixture containing Tris–HCl 50 mM, pH 7.8, L-aspartate 50 mM, 2-oxoglutarate 10 mM, NADH 0.1 mM, and 2 U of MDH was added to the extracts and the reaction was initiated by adding 2-oxoglutarate. The activity was monitored spectrophotometrically at 340 nm for 30 min.
NADH-MDH activity was assayed as described by Setién et al. [53]. Briefly, a reaction buffer made up of 100 mM Hepes–KOH (pH 7.5), 5 mM MgCl2, 2 mM oxaloacetate, and 0.2 mM NADH was added to the extracts, and the activity was monitored spectrophotometrically at 340 nm for 30 min. MDH activity was determined by surveying the oxidation of NADH and the reduction kinetics of NAD + .
2.10 Statistical analysis
IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp was used for all statistical analyses. ANOVA one factor, followed by the Student–Newman–Keuls post hoc test, was used to compare differences in the means (p < 0.05). Different letters indicate significant differences. The used data are mean values ± S.D.
3 Results
3.1 Cistus monspeliensis extract composition
Cistus monspeliensis plant extract analysis (Table 1) revealed the presence of several metabolites in 1 mg/ml sample concentration. We note the presence of 594.01 ± 4.09 mg g−1 DW of amino acid content, 4.97 ± 0.03 mg g−1 DW of indole acetic acid content, 84.15 ± 0.00 mg g−1 DW of flavonoids content, 375.66 ± 0.00 mg g−1 DW of polyphenols, and 107.33 ± 8.51 mg g−1 DW of protein content.
3.2 Effect of Cd and CME supplementation on plant growth and biomass of sorghum
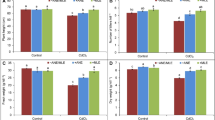
Cd exposure posed significant stress to sorghum plants during this study. We recorded a reduction of 14.74% in plant length due to Cd treatment compared to the control treated with water only (Table 2). Cd also reduced sorghum leaves’ fresh and dry weight by 38.84% and 42.85%, respectively, compared to the control (Table 2). However, supplementations with CME in the presence of Cd increased plants’ length and weight significantly. Five, 20, and 60 mg/l of CME were able to increase the length of sorghum plants by 23.34%, 26.02%, and 17.72%; fresh weight by 110.76%, 137.27%, and 63.25; and dry weight by 228.75%, 108.75%, and 66.25%, respectively, compared to Cd-control (Table 2).
3.3 Effect of CME on Cd‑induced oxidative stress in sorghum plants
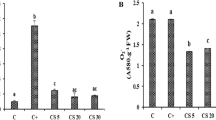
Cd-stressed plants showed an accumulation of superoxide radicals (218.97%) (Fig. 1A). Sorghum plants exposed to 200 µM Cd and then treated with 5, 20, and 60 mg/l of CME displayed significant reductions in O2− production of 64.08%, 30.43%, and 67.93%, respectively when compared to Cd control (Fig. 1A).
O2 − (A) and MDA (B) contents in sorghum plants treated with different concentrations of CME under Cd stress. C, treated only with water; C + , treated with 200 µM of Cd; CM5, treated with 5 mg/l of CME + 200 μM Cd; CM20, treated with 20 mg/l of CME + 200 μM Cd and CM60: treated with 60 mg/l of CME + 200 μM Cd. Each value represents the mean of four independent observations ± SD. Means with the same letter are not significantly different at the 5% probability
The results of the present investigation showed that 200 µM of Cd caused an increase of 46.65% in MDA content compared to the control (Fig. 1B). Moreover, MDA levels in Cd-contaminated plants treated with CME further increased, especially at 20 and 60 mg/l, by 20.72% and 31.55%, respectively, compared to the Cd-control (Fig. 1B).
3.4 Effect of Cd and CME on photosynthetic pigment and osmolytes of sorghum plants
Our findings revealed that Cd exposure and photosynthetic pigment content are inversely related. Lower contents of chlorophyll a, chlorophyll b, and total chlorophyll were detected in sorghum plants exposed to 200 µM of Cd compared to non-stressed plants (Fig. 2). However, the treatments with 5 and 20 mg/l of CME exhibited a significant increase of 38.16% and 54.64% in chlorophyll a content, 39.66% and 58.81% in chlorophyll b content, as well as 38.5% and 55.65% in total chlorophyll content, respectively, compared to Cd-control (Fig. 2).
Chlorophyll a (A), b (B), and total (C) contents in sorghum plants treated by different concentrations of CME under Cd stress. C, treated only with water; C + , treated with 200 µM of Cd; CM5, treated with 5 mg/l of CME + 200 μM Cd; CM20, treated with 20 mg/l of CME + 200 μM Cd; and CM60, treated with 60 mg/l of CME + 200 μM Cd. Each value represents the mean of four independent observations ± SD. Means with the same letter are not significantly different at the 5% probability
IAA is an auxin that hugely influences plants’ development and growth through various cellular mechanisms, such as altering cell orientation, organ development, fertility, and cell elongation. In this study, IAA content was slightly increased due to Cd toxicity (18.53%) (Fig. 3A). CME application decreased this hormone’s content in sorghum plants by 14.34%, 26.21%, and 30.19% under 5, 20, and 60 mg/l, respectively, compared to Cd-control (Fig. 3A).
Indole acetic acid (IAA) (A) and amino acid (B) contents in sorghum plants treated by different concentrations of CME under Cd stress. C, treated only with water; C + , treated with 200 µM of Cd; CM5, treated with 5 mg/l of CME + 200 μM Cd; CM20, treated with 20 mg/l of CME + 200 μM Cd; and CM60: treated with 60 mg/l of CME + 200 μM Cd. Each value represents the mean of four independent observations ± SD. Means with the same letter are not significantly different at the 5% probability
The amino acids and their derivatives have various remarkable functions in plants, such as protein synthesis, growth and development, nutrition, and stress responses. The amino acid content in sorghum leaf extract is illustrated in Fig. 3B. Cd stress significantly decreased amino acid content compared to the control (6.89%). In comparison, treatments with CME under Cd stress significantly improved the content of amino acids in sorghum leaves by 12.6%, 10.5%, and 12.99% using 5, 20, and 60 mg/l of our extract, respectively, compared to Cd-control (Fig. 3B).
3.5 Effect of Cd and CME on antioxidative enzyme activities of sorghum plants
The effect of Cd and CME treatments on oxidative stress was also evaluated by determining the enzyme activities of the antioxidant system. Cd toxicity significantly increased the activity of the antioxidant enzyme SOD by 34.83% compared to the control plants (Fig. 4A). Supplementation with 5 mg/l of CME in the presence of Cd further increased SOD activity by 20.33% compared to Cd-control (Fig. 4A). In addition, GR activity significantly increased under Cd stress compared to the control (7.10%) (Fig. 4B). Moreover, a further increase was observed under 5, 20, and 60 mg/l of CME combined with Cd by 20.10%, 12.41%, and 12.77%, respectively, compared to Cd-control (Fig. 4B).
Activities of superoxide dismutase (SOD) (A), glutathione reductase (GR) (B), glutathione-peroxidase (GPx) (C), and glutathione-s-transferase (GST) (D) in sorghum plants treated by different concentrations of CME under Cd stress. C, treated only with water; C + , treated with 200 µM of Cd; CM5, treated with 5 mg/l of CME + 200 μM Cd; CM20, treated with 20 mg/l of CME + 200 μM Cd; and CM60, treated with 60 mg/l of CME + 200 μM Cd. Each value represents the mean of four independent observations ± SD. Means with the same letter are not significantly different at the 5% probability
In the present study, there was no significant difference in GPx activity between Cd-stressed plants and the control plants treated with water only (Fig. 4C). However, under Cd stress, CME seems to stimulate this activity. GPx activity showed an increase of 36.63%, 4.86%, and 21.52% upon treatments with 5, 20, and 60 mg/l of CME, respectively, compared to the Cd control (Fig. 4C). Additionally, Cd toxicity increased GST activity significantly compared to the control (14.86%) (Fig. 4D). The addition of 5, 20, and 60 mg/l of CME reduced this activity by 21.51%, 30.29%, and 23.17%, respectively, compared to Cd control (Fig. 4D).
3.6 Effect of Cd and CME on carbon–nitrogen enzymes-system of sorghum plants
We observed the effect of Cd and CME supplementation on enzyme activity during nitrogen assimilation and carbon metabolism. Cd toxicity decreased the activities of GS and AAT enzymes by 60.94% and 67.71%, respectively, compared to control plants (Fig. 5). CME supplementation in the presence of Cd significantly increased GS and AAT activities, especially at 5 and 20 mg/l of our extract, where we noted an increase of 131.96% and 102.64% for GS activity (Fig. 5A) and an increase of 154.96% and 249.59% for AAT activity (Fig. 5B), respectively, compared to their respective stress control.
Activities of glutamine synthase (GS) (A), aspartate aminotransferase (AAT) (B), glutamate dehydrogenase (GDH) (C), phosphoenol pyruvate carboxylase (PEPC) (D), and malate dehydrogenase (MDH) (E) in sorghum plants treated by different concentrations of CME under Cd stress. C, treated only with water; C + , treated with 200 µM of Cd; CM5, treated with 5 mg/l of CME + 200 μM Cd; CM20, treated with 20 mg/l of CME + 200 μM Cd; and CM60, treated with 60 mg/l of CME + 200 μM Cd. Each value represents the mean of four independent observations ± SD. Means with the same letter are not significantly different at the 5% probability
GDH activity of sorghum plants’ leaves extract subjected to Cd toxicity is shown in Fig. 5C. This enzyme’s activity has increased by 76.62% following treatment with Cd compared to the control (Fig. 5C). However, supplementation with 5, 20, and 60 mg/l of CME reduced GDH activity by 8.27%, 56.24%, and 27.58%, respectively, compared to Cd-control (Fig. 5C).
In the present work, PEPC and MDH activities were reduced by 30.96% and 62.64%, respectively, due to Cd stress compared to the control (Fig. 5). Treatments with CME greatly increased the two activities in sorghum plants subjected to Cd stress, particularly at 20 mg/l. An enhancement of 88.10% was recorded for PEPC activity (Fig. 5D) and 116.50% for MDH activity (Fig. 5E) compared to their Cd control.
4 Discussion
Plant length, dry weight, and fresh weight are indicators that can represent the impact of stress on growth. This study found that the parameters mentioned decreased in Cd-stressed sorghum plants. It has been firmly established that Cd toxicity inhibits plant growth; it can cause a reduction in biomass and yield and induce chlorosis [10, 54]. The Cd-based declines were significantly reversed with the addition of CME. Similarly, previous studies have demonstrated the effectiveness of various remediations in mitigating Cd toxicity. Desoky et al. [55] studied the effect of foliar application of maize grain and propolis extract on faba bean plants exposed to drought, salinity, or cadmium. The two extracts displayed a notable improvement in morphological, physiological, biochemical, and productivity characteristics under stress, mainly when the two extracts were used combined [55]. Another study by Howladar [27] reported that Moringa oleifera leaf extract (MLE) was able to mitigate the stress effects of salinity and cadmium in bean plants. Growth traits, yield, and photosynthetic pigment levels reduced upon exposure to stress were detoxified after MLE application [27]. MLE was also effective in improving growth characteristics and mitigating Cd-induced phytotoxicity up to 500 μM Cd concentration in wheat plants [56].
The effects of abiotic stress are characterized by the enhanced formation of ROS, leading to various biochemical and metabolic processes, including photosynthesis [57]. It is generally known that heavy metals, particularly Cd, act primarily through the photosynthetic apparatus and the pigment it produces [58]. Cd-induced oxidative disorders interrupt the photosystems and photosynthetic electron transport, causing denaturation of chlorophyllous pigments and reducing the efficiency of photosynthetic activity [59]. In this work, chlorophyll content in sorghum plants decreased in response to Cd stress. Our findings align with previous studies indicating that Cd contamination can harm the photosynthesis of many other plants [60,61,62,63]. On the other hand, the application of CME significantly lowered the impact of Cd stress and had a favorable influence on the chlorophyll content, which can lead to an increased photosynthesis rate. The positive influence of CME on chlorophyll content could be attributable to the ability of this extract to alleviate oxidative damage, increase the tolerance to Cd toxicity and ultimately improve the growth of plants.
Meanwhile, MDA content in sorghum plants exposed to Cd and treated with CME sharply increased. As an aldehyde molecule, end-product, and indicator of lipid peroxidation, MDA has been demonstrated to provide cell protection under oxidative stress and play a positive effect in plant defense and development by activating regulatory genes, despite its potential toxicity [64]. Indeed, its role as a destroyer or protector relies on the enzymatic activity of aldehyde dehydrogenases, which are activated to control MDA levels by oxidizing them to their respective carboxylic acids, reestablishing low cellular levels so that they can operate as signals rather than cause cell damage [65]. In our investigation, the elevation in MDA levels under CME treatments may indicate that MDA acts as a preventative mechanism rather than a marker of damage, given that there were no signs of oxidative damage or devastating symptoms on sorghum growth and development.
The high levels of ROS due to Cd stress can cause degradation of proteins, amino acids, lipids, carbohydrates, DNA, and eventually, the death of plants [66]. In this study, the treatments with CME have proven to minimize these adverse effects of Cd as we observed a significant increase in amino acid content that had plummeted due to Cd exposure. On the other hand, IAA content has increased under Cd stress. Among the auxin class, IAA is the most common plant hormone with its regulating effect on various aspects of plant growth and development [67]. IAA can help mediate the morphological reaction of plants in response to abiotic stresses by promoting roots and sometimes shoots growth [68]. Under heavy metal stress, plants can dynamically adjust auxin accumulation and location depending on the environmental conditions for better adaptation [69]. The increase in auxin-responsive protein IAA content under Cd stress was previously registered by Sun et al. [70] in rice shoots. Meanwhile, CME supplementations have reduced IAA levels in sorghum shoots under Cd stress, while no significant outcomes were observed on plant biomass under any of the used concentrations.
Superoxide anion (O2−), a free radical and oxygen derivative, is the source of most intracellular ROS [71]. Cd exposure could disturb the balance between ROS production and scavenging, resulting in oxidative disorder [72]. In the present investigation, a noticeable increase in the level of O2− in sorghum plants exposed to Cd stress was noted, which could be related to the oxidative damage produced by Cd’s detrimental effects. However, the application of CME protected sorghum plants from Cd-induced damage resulting from O2− dismutation. To mitigate the overly produced ROS, plants have developed different defense mechanisms against abiotic stress, such as the antioxidant system. The activities of the antioxidant enzymes (SOD, GR, GPx, GST) have been linked to the modulations of plants’ growth under stress, including Cd toxicity. In this work, SOD activity was upregulated by Cd stress, and the application of 5 mg/l of CME in the presence of Cd has further increased this activity. SOD is an enzyme that catalyzes the dismutation of superoxide radicals (O2−) to oxygen (O2) and hydrogen peroxide (H2O2), thereby providing cellular protection against ROS [73]. The resulting H2O2 is then neutralized by its conversion to water and oxygen in a GPx-catalyzed reaction [74]. The increase in SOD activity in plants treated with CME under Cd stress was accompanied by a decrease in O2− content and a significant increase in GPx activity, suggesting that CME may help sorghum plants cope with the oxidative stress caused by Cd. Except for GPx activity under Cd stress, where we did not notice a significant difference compared to the control, our findings were similar to previous researchers; Bhuyan et al. [75] found that Cd toxicity elevated SOD and GPx activity in rice and that supplementation with vanillic acid was able to further increase both activities under Cd stress. Another study by Alzahrani and Rady [76] suggested using maize-grain-derived organic biostimulants to improve Cd tolerance in wheat plants. Likewise, they found that Cd toxicity significantly increased SOD and GPx activities, and treatments with these biostimulants upregulated both activities even more [76].
The current research shows that GR activity in sorghum leaves subjected to Cd stress has significantly increased. Moreover, this activity was upregulated in plants treated with CME, especially with 5 mg/l. GR is an enzyme that catalyzes the reduction of GSSG (oxidized glutathione) to GSH (reduced glutathione), which is a crucial molecule in resisting oxidative stress and sustaining the reducing environment in plant cells for the active functioning of proteins [77]. GR enzyme's high activity might indicate a greater demand for GSH, required in different metabolisms involved in plant growth and development and for detoxifying plant cells from H2O2 [78]. GR is also essential for maintaining a high GSSG/GSH ratio, especially when plants face stressful conditions [77]. In addition, the increase in GST activity alongside GSH in plants exposed to heavy metals has been documented in previous studies [75, 79], which aligns with our results. GST activity has decreased in CME-treated Cd-stressed sorghum plants, thus indicating the potentiality of this extract in mitigating Cd stress. The effectiveness of CME in decreasing the oxidative damage resulting from Cd exposure is likely related to the combination of secondary metabolites that are naturally present in CM. Studies conducted using CM revealed the presence of flavonoids such as catechins that have potent antioxidant properties and are considered ROS scavengers and metal ion chelators [36, 80]. Other compounds were also identified in CM, including gallic acid, ellagitannins, hydrocarbons, fatty acids, and carbonylic compounds, in addition to an abundance of terpenoids, particularly diterpenes [36, 38]. The latter serve various functions in plants, including acting as hormones and antioxidants, and they were reported to be essential elements of biotic and abiotic stress resistance in many crops like rice and maize [39, 81]. The richness of CM in these compounds is potentially the reason for the boosted antioxidant system that has led to reducing ROSs and restoring the oxidative balance in sorghum plants, resulting in better growth.
We also investigated the role of CME in regulating carbon and nitrogen metabolism in Cd-stressed sorghum plants. For plants, nitrogen metabolism is an important physiological process that can affect growth and determine the yield and quality of plants [82]. The inhibition of plant growth due to Cd stress has been previously linked to the modulation of nitrogen assimilation enzymes’ activities under Cd toxicity [83]. The activities of two key enzymes (GS and GDH) that play a crucial role in nitrogen assimilation were studied. GS catalyzes the condensation of glutamate and ammonia to generate glutamine [84], whereas GDH catalyzes a reversible enzymatic reaction involving ammonium assimilation into glutamate and glutamate deamination into 2-oxoglutarate and ammonium [85]. Under Cd stress, sorghum plants showed a significant reduction in GS activity and a significant increase in GDH activity. Similar results have been previously described in tomato, bean, and maize plants [86,87,88]. The presence of Cd in the soil was found to decrease the activity of the GS/GOGAT cycle due to the oxidative damage caused by the increase in ROS generation [89]. The inhibition of GS and GOGAT enzymes causes ammonium accumulation, which GDH then assimilates to reduce its excess content, thus explaining the increase in GDH activity under Cd stress [90]. On the other hand, we remarked that CME promoted the activity of GS and decreased the activity of GDH, which marks the capability of this extract in restoring the regular order of ammonium assimilation in plants under Cd stress.
In addition, Cd-induced decline detected in AAT activity, an enzyme that catalyzes the reversible transamination between glutamate and oxaloacetate, resulting in aspartate and 2-oxoglutarate [91], was reversed with CME application (5 and 20 mg/l). The elevated AAT activity can be linked to its role in maintaining the relative stability of glutamate concentration in plants, which serve as a nitrogen donor for producing other amino acids [92, 93].
Cd was previously reported to cause a significant reduction in carbon metabolism [94]. In the current work, Cd stress has decreased the activities of PEPC and MDH; two enzymes were shown to fulfill an essential role in replenishing the tricarboxylic acid cycle (TCA) utilized for energy and biosynthetic metabolism [95]. PEPC also catalyzes the carboxylation of phosphoenolpyruvate to oxaloacetate, which is then transformed to malate by MDH [96]. Similar to our results, a reduction in PEPC activity was described in Cd-stressed maize and wheat plants [97, 98], and a significant inhibition in MDH activity was reported in Miscanthus treated with 200 µM of Cd [99]. In our study, CME has upregulated the activities of both PEPC and MDH, especially at 5 and 20 mg/l treatments. This increase could be valuable in supplying TCA with carbon compounds needed to synthesize amino acids, therefore, proteins [52]. Furthermore, the malate accumulation in C4 plants due to the upregulation of MDH activity can boost the Calvin cycle and grant higher tolerance to Cd stress [99]. These findings imply that CME can effectively regulate the perturbations of carbon and nitrogen metabolism, allowing for better growth of sorghum plants in Cd-polluted soil.
5 Conclusion
Cadmium toxicity strongly reduced the growth of sorghum plants and damaged the photosynthetic pigment. Cd exposure also promoted ROS generation and altered the antioxidant system and carbon and nitrogen metabolism. CME supplementation in the presence of Cd significantly reduced the loss of photosynthetic pigment, suppressed ROS generations, and improved antioxidant defense-related enzymes and carbon and nitrogen metabolism enzymes which led to better growth and development of sorghum plants under Cd stress. Interestingly, CME treatments elevated MDA and reduced IAA contents under Cd stress with no signs of damage on the enzymatic and morphological levels. We note that the enhanced tolerance to Cd stress was more significant at 5 mg/l and 20 mg/l of CME treatments.
Our findings can serve as insight to guide further studies of CME effect in enhancing the resistance to Cd and other heavy metals in sorghum plants and other crops. While CME can be a sustainable solution for elevating Cd stress in sorghum plants and improving their yield, extensive studies are needed to investigate the mechanisms and mode of action of this extract in plants.

Photograph of sorghum plants at the end of treatments with CME. C: treated only with water; C+: treated with 200 µM of Cd; CM5: treated with 5 mg/l of CME + 200 μM Cd; CM20: treated with 20 mg/l of CME + 200 μM Cd and CM60: treated with 60 mg/l of CME + 200 μM Cd.
Data availability
This declaration is not applicable.
References
Geng N et al (2019) Bioaccumulation of potentially toxic elements by submerged plants and biofilms: a critical review. Environ Int 131:105015. https://doi.org/10.1016/j.envint.2019.105015
Palansooriya KN, Shaheen SM, Chen SS, Tsang DC, Hashimoto Y, Hou D, Bolan NS, Rinklebe J, Ok YS (2020) Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int 134:105046. https://doi.org/10.1016/j.envint.2019.105046
Atabayeva SD, Minocha R, Minocha SC, Rakhymgozhina A, Nabieva AM, Nurmahanova AC, Кenzhebayeva SS, Alybayeva RA, Asrandina SS (2020) Response of plants to cadmium stress. Int J Biol Che vol. 13, no 1, p. 109‑117. https://doi.org/10.26577/ijbch.2020.v13.i1.11.
Song W, Chen S, Liu J, Chen L, Song N, Li N, Liu B (2015) Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J Integr Agric 14(9):1845–1854. https://doi.org/10.1016/S2095-3119(14)60926-6
Adil MF, Sehar S, Chen G, Chen ZH, Jilani G, Chaudhry AN, Shamsi IH (2020) Cadmium-zinc cross-talk delineates toxicity tolerance in rice via differential genes expression and physiological/ultrastructural adjustments. Ecotoxicol Environ Saf 190:110076. https://doi.org/10.1016/j.ecoenv.2019.110076
Pan J, Plant JA, Voulvoulis N, Oates CJ, Ihlenfeld C (2010) Cadmium levels in Europe: implications for human health. Environ Geochem Health 32(1):1–12. https://doi.org/10.1007/s10653-009-9273-2
Mahajan P, Kaushal J (2018) Role of phytoremediation in reducing cadmium toxicity in soil and water. J. Toxicol., vol. 2018. https://doi.org/10.1155/2018/4864365
Imran M et al (2020) Molybdenum supply alleviates the cadmium toxicity in fragrant rice by modulating oxidative stress and antioxidant gene expression. Biomolecules 10(11):1582. https://doi.org/10.3390/biom10111582
Sebastian A, Prasad MNV (2019) Mitigation of cadmium stress in cereals: molecular signaling and agronomic aspects. In Cadmium Tolerance in Plants, Elsevier, p. 401‑422. https://doi.org/10.1016/B978-0-12-815794-7.00015-1
Zulfiqar U et al (2022) Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils; a comprehensive review. Front Plant Sci 13:773815–773815. https://doi.org/10.3389/fpls.2022.773815
Techio VH, de Castro EM, de Faria MR, Palmieri MJ (2013) Reproductive, cellular, and anatomical alterations in Pistia stratiotes L plants exposed to cadmium. Water Air Soil Pollut 224:31–8. https://doi.org/10.1007/s11270-013-1454-z
Li J, Yu J, Du D, Liu J, Lu H, Yan C (2019) Analysis of anatomical changes and cadmium distribution in Aegiceras corniculatum (L) Blanco roots under cadmium stress. Mar. Pollut. Bull. 149:110536. https://doi.org/10.1016/j.marpolbul.2019.110536
Liza SJ, Shethi KJ, Rashid P (2020) Effects of cadmium on the anatomical structures of vegetative organs of chickpea (Cicer arientinum L). Dhaka Univ. J. Biol. Sci. 29(1):45–52. https://doi.org/10.3329/dujbs.v29i1.46530
Bora MS, Sarma KP (2021) Anatomical and ultrastructural alterations in Ceratopteris pteridoides under cadmium stress: a mechanism of cadmium tolerance. Ecotoxicol Environ Saf 218:112285. https://doi.org/10.1016/j.ecoenv.2021.112285
Shanmugaraj BM, Malla A, Ramalingam S (2019) Cadmium stress and toxicity in plants: an overview. Cadmium Toxic. Toler. Plants, p. 1‑17. https://doi.org/10.1016/B978-0-12-814864-8.00001-2
Paterson AH et al. (2009) The Sorghum bicolor genome and the diversification of grasses. nature, vol. 457, no 7229, p. 551‑556. https://doi.org/10.1038/nature07723
Bouargalne Y, Ben Mrid R, Bouchmaa N, Zouaoui Z, Benmrid B, Kchikich A, El Omari R, Kabach I, Mohamed N (2022) Genetic diversity for agromorphological traits, phytochemical profile, and antioxidant activity in Moroccan sorghum ecotypes. Sci Rep 12(1):1–13. https://doi.org/10.1038/s41598-022-09810-9
de Morais Cardoso L, Pinheiro SS, Martino HSD, Pinheiro-Sant’Ana HM, (2017) Sorghum (Sorghum bicolor L): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 57(2):372–390. https://doi.org/10.1080/10408398.2014.887057
Ben Mrid R et al. (2019) Phytochemical characterization, antioxidant and in vitro cytotoxic activity evaluation of Juniperus oxycedrus subsp. oxycedrus needles and berries. Molecules, vol. 24, no 3, p. 502. https://doi.org/10.3390/molecules24030502
Soudek P, Petrová Š, Vaňková R, Song J, Vaněk T (2014) Accumulation of heavy metals using Sorghum sp. Chemosphere 104:15–24. https://doi.org/10.1016/j.chemosphere.2013.09.079
Da-lin L, Kai-qi H, Jing-jing M, Wei-wei Q, Xiu-ping W, Shu-pan Z (2011) Effects of cadmium on the growth and physiological characteristics of sorghum plants. Afr J Biotechnol 10(70):15770–15776. https://doi.org/10.5897/AJB11.848
Jawad Hassan M et al (2020) Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants 9(11):1575. https://doi.org/10.3390/plants9111575
Ben Mrid R, Benmrid B, Hafsa J, Boukcim H, Sobeh M, Yasri A (2021) Secondary metabolites as biostimulant and bioprotectant agents: a review. Sci. Total Environ., p. 146204. https://doi.org/10.1016/j.scitotenv.2021.146204
Para\djiković N, Teklić T, Zeljković S, Lisjak M, Špoljarević M (2019) Biostimulants research in some horticultural plant species—A review. Food Energy Secur., vol. 8, no 2, p. e00162. https://doi.org/10.1002/fes3.162
Ali Q et al. (2020) Plant-based biostimulants and plant stress responses. In Plant ecophysiology and adaptation under climate change: mechanisms and perspectives I, Springer, p. 625‑661. https://doi.org/10.1007/978-981-15-2156-0_22
Zulfiqar F, Casadesús A, Brockman H, Munné-Bosch S (2020) An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci 295:110194. https://doi.org/10.1016/j.plantsci.2019.110194
Howladar SM (2014) A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol Environ Saf 100:69–75. https://doi.org/10.1016/j.ecoenv.2013.11.022
Alharby HF, Al-Zahrani HS, Hakeem KR, Alsamadany H, Desoky ESM, Rady MM (2021) Silymarin-enriched biostimulant foliar application minimizes the toxicity of cadmium in maize by suppressing oxidative stress and elevating antioxidant gene expression. Biomolecules 11(3):465. https://doi.org/10.3390/biom11030465
Rady MM, Desoky ES, Elrys AS, Boghdady MS (2019) Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? South Afr. J Bot 121:294–305. https://doi.org/10.1016/j.sajb.2018.11.019
Thanaa SM, Nabila EK, Abou Rayya MS, Eisa RA (2016) Response of nonpareil seedlings almond to foliar application of licorice root extract and bread yeast suspend under south Sinai conditions. J Innov Pharm Biol Sci 3:123–132
Ali M, Cheng Z, Hayat S, Ahmad H, Ghani MI, Tao LIU (2019) Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L). J. Integr. Agric. 18(5):1001–1013. https://doi.org/10.1016/S2095-3119(18)62129-X
Mechbal N, Bouhrim M, Bnouham M, Hammouti B, Karzazi Y, Kaya S, Serdaroğlu G (2021) Anticorrosive and antioxidant effect of the aqueous extract of the leaves, flowers, and stems of Cistus monspeliensis L: experimental and computational study. J Mol Liq 331:115771. https://doi.org/10.1016/j.molliq.2021.115771
Bouamama H, Noel T, Villard J, Jana Benharref A, M, (2006) Antimicrobial activities of the leaf extracts of two Moroccan Cistus L species. J. Ethnopharmacol. 104(1–2):104–107. https://doi.org/10.1016/j.jep.2005.08.062
Vitali F, Pennisi G, Attaguile G, Savoca F, Tita B (2011) Antiproliferative and cytotoxic activity of extracts from Cistus incanus L and Cistus monspeliensis L on human prostate cell lines. Nat. Prod. Res. 25(3):188–202. https://doi.org/10.1080/14786410802583148
Rebaya A, Belghith SI, Hammrouni S, Maaroufi A, Ayadi MT, Chérif JK (2016) Antibacterial and antifungal activities of ethanol extracts of Halimium halimifolium, Cistus salviifolius and Cistus monspeliensis. Int J Pharm Clin Res 8(4):243–247
Barrajón-Catalán E, Fernández-Arroyo S, Roldán C, Guillén E, Saura D, Segura-Carretero A, Micol V (2011) A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: evolutionary relationship. Phytochem Anal 22(4):303–312. https://doi.org/10.1002/pca.1281
Zalegh I, Akssira M, Bourhia M, Mellouki F, Rhallabi N, Salamatullah AM, Alkatham MS, Alyahya HK, Mhand RA (2021) A review on cistus sp.: Phytochemical and antimicrobial activities. Plants, vol. 10, no 6, p. 1214. https://doi.org/10.3390/plants10061214
Papaefthimiou D, Papanikolaou A, Falara V, Givanoudi S, Kostas S, Kanellis AK (2014) Genus Cistus: a model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front Chem 2:35. https://doi.org/10.3389/fchem.2014.00035
Pelot KA, Chen R, Hagelthorn DM, Young CA, Addison JB, Muchlinski A, Tholl D, Zerbe P (2018) Functional diversity of diterpene synthases in the biofuel crop switchgrass. Plant Physiol 178(1):54–71. https://doi.org/10.1104/pp.18.00590
Bouchmaa N, Mrid RB, Boukharsa Y, Bouargalne Y, Nhiri M, Idir A, Taoufik J, Ansar M, Zyad A (2019) Reactive oxygen species-mediated apoptosis and cytotoxicity of newly synthesized pyridazin-3-ones in P815 (murin mastocytoma) cell line. Drug Res 69(10):528–536. https://doi.org/10.1055/a-0762-3775
Kubiś J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165(4):397–406. https://doi.org/10.1016/j.jplph.2007.02.005
Arnon D (1949) Copper enzymes in isolated chloroplast. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Ehmann A (1977) The Van Urk-Salkowski reagent—a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr A 132(2):267–276. https://doi.org/10.1016/S0021-9673(00)89300-0
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bouchmaa N, Ben Mrid R, Boukharsa Y, Nhiri M, Ait Mouse H, Taoufik J, Ansar M, Zyad A (2018) Cytotoxicity of new pyridazin-3 (2H)-one derivatives orchestrating oxidative stress in human triple-negative breast cancer (MDA-MB-468). Arch Pharm (Weinheim) 351(12):1800128. https://doi.org/10.1002/ardp.201800128
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139. https://doi.org/10.1016/S0021-9258(19)42083-8
Latique S et al (2021) Foliar application of ulva rigida water extracts improves salinity tolerance in wheat (Triticum durum L). Agronomy 11(2):265. https://doi.org/10.3390/agronomy11020265
Ben Mrid R, El Omari R, Bouargalne Y, El Mourabit N, Nhiri M (2017) Activities of carbon and nitrogen metabolism enzymes during germinating sorghum seeds and early seedlings growth. Cereal Res Commun 45(4):587–597. https://doi.org/10.1556/0806.45.2017.044
El Omari R, Ben Mrid R, Amakran A, Nhiri M (2018) Effect of fungicide (Maneb) on antioxidant system and carbon assimilation in leaves of sorghum plants. Russ J Plant Physiol 65(2):237–243. https://doi.org/10.1134/S1021443718020103
Ben Mrid R, El Omari R, Nhiri M (2016) Effect of nitrogen source and concentration on growth and activity of nitrogen assimilation enzymes in roots of a Moroccan sorghum ecotype. Plant 4(6):71. https://doi.org/10.11648/j.plant.20160406.14
Ben Mrid R, El Omari R, El Mourabit N, Bouargalne Y, Nhiri M (2018) Changes in the antioxidant and glyoxalase enzyme activities in leaves of two Moroccan sorghum ecotypes with differential tolerance to nitrogen stress. Aust J Crop Sci 12(8):1280–1287
Setién I, Vega-Mas I, Celestino N, Calleja-Cervantes ME, González-Murua C, Estavillo JM, González-Moro MB (2014) Root phosphoenolpyruvate carboxylase and NAD-malic enzymes activity increase the ammonium-assimilating capacity in tomato. J Plant Physiol 171(5):49–63. https://doi.org/10.1016/j.jplph.2013.10.021
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46. https://doi.org/10.1016/j.envexpbot.2012.04.006
Desoky ESM, Elrys AS, Mansour E, Eid RS, Selem E, Rady MM, Ali EF, Mersal GAM, Semida WM (2021) Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci Hortic 288:110340. https://doi.org/10.1016/j.scienta.2021.110340
Farhat F et al (2022) The impact of bio-stimulants on Cd-stressed wheat (Triticum aestivum L.): insights into growth, chlorophyll fluorescence, Cd accumulation, and osmolyte regulation. Front Plant Sci 13:850567–850567. https://doi.org/10.3389/fpls.2022.850567
Sharma A et al (2020) Photosynthetic response of plants under different abiotic stresses: a review. J Plant Growth Regul 39(2):509–531. https://doi.org/10.1007/s00344-019-10018-x
Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M (2021) Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf 211:111887. https://doi.org/10.1016/j.ecoenv.2020.111887
Faraz A, Faizan M, Sami F, Siddiqui H, Hayat S (2020) Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J Plant Growth Regul 39(2):641–655. https://doi.org/10.1007/s00344-019-10007-0
An M, Wang H, Fan H, Ippolito JA, Meng C, Li Y, Wang K, Wei C (2019) Effects of modifiers on the growth, photosynthesis, and antioxidant enzymes of cotton under cadmium toxicity. J Plant Growth Regul 38(4):1196–1205. https://doi.org/10.1007/s00344-019-09924-x
Kaya C, Okant M, Ugurlar F, Alyemeni MN, Ashraf M, Ahmad P (2019) Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 225:627–638. https://doi.org/10.1016/j.chemosphere.2019.03.026
Shah AA, Khan WU, Yasin NA, Akram W, Ahmad A, Abbas M, Ali A, Safdar MN (2020) Butanolide alleviated cadmium stress by improving plant growth, photosynthetic parameters and antioxidant defense system of Brassica oleracea. Chemosphere 261:127728. https://doi.org/10.1016/j.chemosphere.2020.127728
Zhao H, Guan J, Liang Q, Zhang X, Hu H, Zhang J (2021) Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci Rep 11(1):1–11. https://doi.org/10.1038/s41598-021-89322-0
Morales M, Munné-Bosch S (2019) Malondialdehyde: facts and artifacts. Plant Physiol 180(3):1246–1250. https://doi.org/10.1104/pp.19.00405
Tagnon MD, Simeon KO (2017) Aldehyde dehydrogenases may modulate signaling by lipid peroxidation-derived bioactive aldehydes. Plant Signal Behav 12(11):e1387707. https://doi.org/10.1080/15592324.2017.1387707
Asgher M, Khan MIR, Anjum NA, Khan NA (2015) Minimising toxicity of cadmium in plants–role of plant growth regulators. Protoplasma 252(2):399–413. https://doi.org/10.1007/s00709-014-0710-4
Fu SF, Wei JY, Chen HW, Liu YY, Lu HY, Chou JY (2015) Indole-3-acetic acid: a widespread physiological code in interactions of fungi with other organisms. Plant Signal Behav 10(8):e1048052. https://doi.org/10.1080/15592324.2015.1048052
Fässler E, Evangelou MW, Robinson BH, Schulin R (2010) Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere 80(8):901–907. https://doi.org/10.1016/j.chemosphere.2010.04.077
Wang R, Wang J, Zhao L, Yang S, Song Y (2015) Impact of heavy metal stresses on the growth and auxin homeostasis of Arabidopsis seedlings. Biometals 28(1):123–132. https://doi.org/10.1007/s10534-014-9808-6
Sun L, Wang J, Song K, Sun Y, Qin Q, Xue Y (2019) Transcriptome analysis of rice (Oryza sativa L) shoots responsive to cadmium stress. Sci. Rep., vol. 9, no 1, Art. no 1. https://doi.org/10.1038/s41598-019-46684-w.
Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY (2019) Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev 2019:5080843. https://doi.org/10.1155/2019/5080843
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782. https://doi.org/10.3390/ijerph17113782
Wang Y, Branicky R, Noë A, Hekimi S (2018) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 217(6):1915–1928. https://doi.org/10.1083/jcb.201708007
Zhang L, Wu M, Teng Y, Jia S, Yu D, Wei T, Chen C, Song W (2019) Overexpression of the glutathione peroxidase 5 (RcGPX5) gene from rhodiola crenulata increases drought tolerance in Salvia miltiorrhiza. Front Plant Sci 9:1950. https://doi.org/10.3389/fpls.2018.01950
Bhuyan MB, Parvin K, Mohsin SM, Mahmud JA, Hasanuzzaman M, Fujita M (2020) Modulation of cadmium tolerance in rice: insight into vanillic acid-induced upregulation of antioxidant defense and glyoxalase systems. Plants 9(2):188. https://doi.org/10.3390/plants9020188
Alzahrani Y, Rady MM (2019) Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol Environ Saf 182:109378. https://doi.org/10.1016/j.ecoenv.2019.109378
Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I, Pereira E, Tuteja N (2013) Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem 70:204–212. https://doi.org/10.1016/j.plaphy.2013.05.032
Latique S, Mrid RB, Kabach I, Yasri A, Kchikich A, Nhiri M, El Kaoua M, Douira A, Selmaoui K (2021) The effect of foliar application of Ulva rigida extract on the growth and biochemical parameters of wheat plants. In E3S Web of Conferences, vol. 234, p. 00103. https://doi.org/10.1051/e3sconf/202123400103
Jan S, Noman A, Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) 24-Epibrassinolide alleviates the injurious effects of Cr (VI) toxicity in tomato plants: Insights into growth, physio-biochemical attributes, antioxidant activity and regulation of Ascorbate–glutathione and Glyoxalase cycles. J Plant Growth Regul 39(4):1587–1604. https://doi.org/10.1007/s00344-020-10169-2
Bernatoniene J, Kopustinskiene DM (2018) The role of catechins in cellular responses to oxidative stress. Molecules 23(4):965. https://doi.org/10.3390/molecules23040965
Valares Masa C, Sosa Díaz T, AlíasGallego JC, Chaves Lobón N (2016) Quantitative variation of flavonoids and diterpenes in leaves and stems of Cistus ladanifer L at different ages. Molecules 21(3):275. https://doi.org/10.3390/molecules21030275
Yang S, Zu Y, Li B, Bi Y, Jia L, He Y, Li Y (2019) Response and intraspecific differences in nitrogen metabolism of alfalfa (Medicago sativa L.) under cadmium stress. Chemosphere 220:69–76. https://doi.org/10.1016/j.chemosphere.2018.12.101
Mobin M (2013) Effects of cadmium-induced oxidative stress on growth and nitrogen assimilation in Blackgram [Vigna mungo (L) Hepper]. J. Agric. Sci. Belgrade 58(1):31–39. https://doi.org/10.2298/JAS1301031M
Tercé-Laforgue T, Bedu M, Dargel-Grafin C, Dubois F, Gibon Y, Restivo FM, Hirel B (2013) Resolving the role of plant glutamate dehydrogenase: II Physiological characterization of plants overexpressing the two enzyme subunits individually or simultaneously. Plant Cell Physiol. 54(10):1635–1647. https://doi.org/10.1093/pcp/pct108
Lan G, Jiao C, Wang G, Sun Y, Sun Y (2020) Effects of dopamine on growth, carbon metabolism, and nitrogen metabolism in cucumber under nitrate stress. Sci Hortic 260:108790. https://doi.org/10.1016/j.scienta.2019.108790
Gouia H, Suzuki A, Brulfert J, Ghorbal MH (2003) Effects of cadmium on the co-ordination of nitrogen and carbon metabolism in bean seedlings. J Plant Physiol 160(4):367–376. https://doi.org/10.1078/0176-1617-00785
Chaffei-Haouari C, Hajjaji-Nasraoui A, Carrayol E, Debouba M, Ghorbel MH, Gouia H (2011) Glutamate metabolism on Solanum lycopersicon grown under cadmium stress conditions. Acta Bot Gallica 158(2):147–159. https://doi.org/10.1080/12538078.2011.10516262
Erdal S, Turk H (2016) Cysteine-induced upregulation of nitrogen metabolism-related genes and enzyme activities enhance tolerance of maize seedlings to cadmium stress. Environ Exp Bot 132:92–99. https://doi.org/10.1016/j.envexpbot.2016.08.014
Wani AS, Tahir I, Ahmad SS, Dar RA, Nisar S (2017) Efficacy of 24-epibrassinolide in improving the nitrogen metabolism and antioxidant system in chickpea cultivars under cadmium and/or NaCl stress. Sci Hortic 225:48–55. https://doi.org/10.1016/j.scienta.2017.06.063
Paul S, Guha T, Dey S, Paul S, Kundu R (2022) Amelioration of cadmium toxicity by enhancing nitrogen assimilation and photosynthetic activity by two different nitrogen supplements in rice (Oryza sativa L) cv. Lalat. Plant Stress 4:100082. https://doi.org/10.1016/j.stress.2022.100082
de la Torre F, Cañas RA, Pascual MB, Avila C, Cánovas FM (2014) Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J Exp Bot 65(19):5527–5534. https://doi.org/10.1093/jxb/eru240
Cánovas FM, Avila C, Canton FR, Canas RA, de la Torre F (2007) Ammonium assimilation and amino acid metabolism in conifers. J Exp Bot 58(9):2307–2318. https://doi.org/10.1093/jxb/erm051
Gajewska E, Wielanek M, Bergier K, Skłodowska M (2009) Nickel-induced depression of nitrogen assimilation in wheat roots. Acta Physiol Plant 31(6):1291–1300. https://doi.org/10.1007/s11738-009-0370-8
Liu L, Li J, Yue F, Yan X, Wang F, Bloszies S, Wang Y (2018) Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 194:495–503. https://doi.org/10.1016/j.chemosphere.2017.12.025
Kchikich A, Ben Mrid R, Kabach I, Nhiri M, El Omari R (2021) Arbuscular mycorrhizal fungi enhance sorghum plant growth under nitrogen-deficient conditions through activation of nitrogen and carbon metabolism enzymes. Int J Agric Biol 26:201–208. https://doi.org/10.17957/IJAB/15.1825
Bouargalne Y, Ben Mrid R, El Omari R, Nhiri M (2018) Phosphoenolpyruvate carboxylase during maturation and germination sorghum seeds: enzyme activity and regulation. Russ J Plant Physiol 65(6):824–832. https://doi.org/10.1134/S1021443718060031
Wang H, Zhao SC, Liu RL, Zhou W, Jin JY (2009) Changes of photosynthetic activities of maize (Zea mays L) seedlings in response to cadmium stress. Photosynthetica 47(2):277–283. https://doi.org/10.1007/s11099-009-0043-2
Moussa HR, El-Gamal SM (2010) Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol Plant 54(2):315–320. https://doi.org/10.1007/s10535-010-0054-7
Guo H, Hong C, Chen X, Xu Y, Liu Y, Jiang D, Zheng B (2016) Different growth and physiological responses to cadmium of the three Miscanthus species. PLoS ONE 11(4):e0153475. https://doi.org/10.1371/journal.pone.0153475
Author information
Authors and Affiliations
Contributions
Conceptualization, M.N and Z.R; methodology, Z.R, N.N, S.E, A.E, Z.Z, A.K, and A.KR; writing—original draft preparation, Z.R.; writing—review and editing, A.KR and M.N; supervision, M.N and N.NHI. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roussi, Z., Kchikich, A., Nhhala, N. et al. Cistus monspeliensis extract as a prospective biostimulant in enhancing tolerance to cadmium in sorghum plant. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03542-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03542-6