Abstract
The phytohormone auxin is an essential mediator in many aspects of plant development. Its dynamic and differential distribution within the plant is regulated by a variety of environmental cues including heavy metal stimuli. In the present study, we first evaluated the toxic effects of seven heavy metals including Pb2+, Cd2+, Hg2+, Ni2+. Zn2+, Co2+ and Cu2+ in their excess on the model plant, Arabidopsis thaliana. Various morphological defects including loss in fresh weight and leaf area, decrease of the primary root length and stimulation of the lateral root density occurred to a different extent among seven heavy metals. Next, using an indicative DR5:GUS reporter line of Arabidopsis, the auxin accumulation and distribution within plant seedlings were found to be dramatically and differentially affected by these heavy metals. We further analyzed the transcriptional changes of 27 selected auxin homeostasis-related genes by qRT-PCR technique and found that upon various heavy metals, the expressions of the candidate genes were distinctly altered in shoots and roots. Our data indicated that when confronted with excessive heavy metals, plants could dynamically and differentially regulate the transcription of auxin-related genes to adjust the location and effective accumulation of auxin within the plant for better adaptation and survival under the adverse environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a class of plant hormones, auxin is essential for plant development, especially for patterning and organogenic processes. The metabolism and distribution pattern of auxin within the plant is dynamic and environment responsive, which in turn can modify the plant growth and morphology (Benkova et al. 2003; Friml 2003; Vanneste and Friml 2009). For example, in unilateral light, auxin accumulates at the shaded side of shoots and ultimately leads to organ bending and shade avoidance (Whippo and Hangarter 2006). The cellular concentration of auxin can be controlled at multiple levels including biosynthesis, conjugation, deconjugation, degradation, and intercellular transport, which undoubtedly constitute a complex and interacting network to regulate auxin activity at different developmental stages and in response to a variety of environmental signals (Vanneste and Friml 2009).
In modern society, extensive mining, industrial activities and improper agricultural practice have released a great amount of heavy metals to the environment. Some heavy metals such as zinc, copper, and iron are essential for the growth of plants, but they become toxic in excess. Heavy metals such as cadmium and mercury are generally considered as nonessential for plants and are potentially highly toxic due to their reactivity with S and N atoms in amino acid side chains (Chiang et al. 2006; Clemens 2001). It has been reported that the uptake and accumulation of heavy metals in plants lead to a series of physiological disturbances, including decreased growth, enhanced senescence and abnormal photosynthetic apparatus (Maksymiec 1997), which are known to be tightly linked to hormonal homeostasis.
Considering the important roles played by auxin in the coordination of plant growth and defense, it is highly desirable to investigate its homeostatic alteration in heavy metal excesses. Meanwhile, plants have evolved highly effective mechanisms to cope with heavy metal toxicity and modulating the auxin signaling cascade constitutes one key step. In this study, following a detailed description of the morphological changes of Arabidopsis seedlings subjected to different heavy metal treatments, the auxin level and distribution as well as the transcriptional changes of some important auxin-related genes was analyzed in shoots and roots, respectively. By focusing mainly on the physiological and transcriptional responses related to auxin signaling pathway, this study aims to provide new insights into the common and differential responses of Arabidopsis thaliana to a range of heavy metals.
Materials and methods
Plant growth and heavy metal treatments
Seeds of A. thaliana (L.) Heyhn ecotype Columbia (Col-0) and the DR5:GUS reporter line in a Col-0 background (Ulmasov et al. 1997) were used on this study. Arabidopsis seeds were surface sterilized, placed in the dark at 4 °C for 2 d, and then sown on one-half Murashige and Skoog medium (Murashige and Skoog 1962) containing 1 % sucrose and 0.8 % agar with pH adjusted to 5.7–5.8. The concentrations of each heavy metal used in this study were first determined by preliminary experiments and then compared to the concentrations previously reported in literature (Andres-Colas et al. 2006; Eren and Arguello, 2004; Kim et al. 2006; Lee et al. 2003; Paulose et al. 2013). For treatments, the growth medium were supplemented with one of the following metals (µM): CdCl2 (20, 40); HgCl2 (3, 5); CuSO4 (40, 60); CoCl2 (50, 70); Pb(NO3)2 (1,100, 1,200); ZnSO4 (150, 350) and NiCl2 (50, 75). Plates were kept vertically oriented for 8 or 12 days before morphological analyses were carried out. Photographs were taken from at least 50 plants per condition and the root length was measured using the ImageJ software (http://rsb.info.nih.gov/ij/). All experiments were performed at least three times.
Histochemical staining
β-glucuronidase (GUS) activity in DR5:GUS marker lines (Sabatini et al. 1999; Ulmasov et al. 1997) was visualized by incubating tissues for 18 h at 37 °C in a buffer containing 0.5 mg/mL X-gluc (5-bromo-4-chloro-3-indolyl-beta-d-glucuronic acid, cyclohexylammonium salt) dissolved in N-dimethyl-formamide, 0.05 M phosphate buffer (pH 7), 0.5 mM K3Fe(CN)6 and 0.1 % (v/v) Triton X-100. Samples were washed with 70 % (v/v) ethanol, prepared on microscopic slides and observed with a Nikon microscope 50i equipped with a Nikon DS-Fi1C camera.
RNA extraction and gene transcriptional level analyzed by qRT-PCR
After seedlings were washed with ddH2O twice to exchange surface-bound mental elements, total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) from 8-day-old Arabidopsis seedlings (roots and shoots separately) grown on 1/2 MS agar plates containing various metals. cDNA was synthesized with SuperScript III reverse transcriptase (Invitrogen) using oligo(dT)18 as primer and qPCR amplification was performed using SYBR Green detection method at universal cycling conditions (95 °C for 3 min, followed by 45 cycles of 95 °C for 20 s and 58 °C for 20 s, 72 °C for 30 s) in a total reaction volume of 10 μL (2 μL cDNA, 5 μL 2 × Fast SYBR® Green Master Mix, forward and reverse primers 0.3 μL each and 2.4 μL RNase free H2O). Relative expression compared to untreated plants was calculated as 2−ΔΔCt (Livak and Schmittgen 2001) and was normalized to the expression of reference gene translation elongation factor EF1α. The list of primers used for qPCR is given in Supplementary Table S1.
Statistical analysis
Statistical analyses were performed with SPSS 13.0 (SPSS Inc, Chicago, IL, USA). Data were analyzed using one-way ANOVA and means were compared using LSD (least significant difference) at the 0.05 and 0.01 probability levels. The results were based on at least three replicates from three independent experiments.
Results and discussion
Heavy metals severely and differentially affected the growth of A. thaliana seedlings
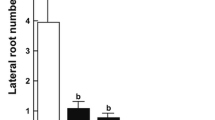
In a previous study investigating the impact of Cu2+ on the root system architecture of Arabidopsis seedlings, it was found that when seeds were directly germinated on medium supplemented with Cu2+, the effect on root growth was similar to the one measured in transfer experiments (Lequeux et al. 2010). Therefore, in this work, in order to determine the toxic effects of heavy metals on Arabidopsis seed germination, the sterilized seeds were directly sown on 1/2 MS medium. Without statistically significant difference in seed germination and radicle emergence, an 8-day or 12-day exposure of Arabidopsis seedlings to most tested heavy metals resulted in prominent phenotypic abnormalities when compared to the control plants (Fig. 1). For a same heavy metal, plants appeared to be more resistant to the lower concentration and the phenotypes became more pronounced at the higher concentration (Fig. 1). Most tested heavy metals affected Arabidopsis seedlings by reducing their biomass and slowing down their growth rate (Fig. 2). After 12 days, the fresh weight of seedlings grown on heavy metal-containing media varied from 100 % (Pb1100 and Hg3) to 38 % (Cu60 and Zn350) compared to the untreated controls (Fig. 2). In terms of hypocotyl elongation, Cu60 and Zn350 turned out to be the most detrimental and decreased the length of hypocotyls by nearly 50 % (Fig. 2). Similarly, 50 µm CuSO4 has been previously reported to reduce the hypocotyl length by about 45 % (Lequeux et al. 2010).
Growth comparison of A. thaliana seedlings exposed to various heavy metal concentrations for 8 or 12 days. Sterilized A. thaliana seeds were germinated and grown on vertical plates with half strength MS containing CdCl2 (20 and 40 μM), HgCl2 (3 and 5 μM), CuSO4 (40 and 60 μM), CoCl2 (50 and 70 μM), Pb(NO3)2 (1,100 and 1,200 μM), ZnSO4 (150 and 350 μM) and NiCl2 (50 and 75 μM), respectively. A representative result of three independent experiments that gave similar results is shown
Heavy metals also reduced the root growth prominently. Both Ni75 and Co70 caused a 70 % reduction in the primary root (PR) length (Fig. 2). Copper has been reported to block the division of root apical meristem cells, hence inhibit the elongation of primary root (Peto et al. 2011). In this work, Cu60 was found to inhibit the growth of main root by 67 % after 12 days. Since the fresh weight and leaf area of the treated seedlings were significantly correlated to their primary root length, root growth appeared to be the main factor affecting the overall performance of plants when exposed to heavy metals. Previously, exposure to cadmium, copper, zinc and lead has been reported to influence the root system architecture (RSA) characterized by inhibiting the primary root growth and simultaneously stimulating the lateral root (LR) formation (Lopez-Bucio et al. 2003; Pasternak et al. 2005; Potters et al. 2009, 2007). In this work, except for Zn2+, all other tested heavy metals induced an increased density of lateral root at day 8 at least at their higher concentrations (Fig. 2). This augmentation of secondary root numbers could be easily taken as an adaptation mechanism of plants to deal with the adverse environment. However, at day 12, this promoting effect on lateral root formation became less significant in all the treated plants (Fig. 2). Auxin is a major player in LR formation and the elevated level of free auxin has been shown to be able to inhibit the growth of main root while induce the formation of lateral root (Aloni et al. 2006; Dubrovsky et al. 2008; Nibau et al. 2008). In a previous work, a 35 % increase in the total LR density was recorded at 10 mM Cu2+ without affecting the PR growth and the authors proposed the involvement of auxin in the enhancement of lateral root initiation/emergence (Lequeux et al. 2010).
Auxin location and accumulation were differentially regulated in Arabidopsis shoots and roots by various heavy metals
Auxin is distributed differentially within plant tissues and modification of its metabolism and transport is important for the developmental adaptations of plants (Potters et al. 2009). Within the plant, auxin is mainly synthesized in young apical tissues and then transported downward via the phloem towards the root apical meristem (RAM) by a polar transport system. In Arabidopsis seedlings, auxin accumulates mainly at the root tip (Benkova et al. 2003), especially in the quiescent center (QC) and young columella cells (Friml et al. 2002; Sabatini et al. 1999), which pattern is necessary for maintaining a functional root meristem (Blilou et al. 2005). Therefore, the differential distribution of auxin within plant tissues and organs constitutes the basis of its action (Vanneste and Friml 2009). In this work, using an auxin-activity reporting system, the DR5:GUS line, we indirectly tracked the interference of various heavy metal stresses in the level and location of auxin across Arabidopsis seedlings.
After a 8-day exposure, seven types of heavy metals induced different changes in the GUS staining patterns in shoots and roots of DR5:GUS lines. Treatment with Cd2+ has previously been reported to reduce the free auxin content in the roots (Xu et al. 2010). However, in this work, Cd2+ caused an obviously stronger GUS staining in the root tips than the untreated control plants accompanied by a diffused GUS staining in the areas just above the apical meristems of both PR and LR (Fig. 3b). Neither Co2+ nor Hg2+ significantly altered the intensity or the location of GUS staining at RAMs (Fig. 3b). By comparison, four heavy metals including Pb2+, Zn2+, Ni2+ and Cu2+ weakened the GUS staining intensity dramatically in the tips of PR and LR. A close look at their respective responses revealed that in the case of Zn2+ and Cu2+, the auxin activity still resided in the apical meristem of root tips and consistent with the results of Lequeux et al. (2010), the activity of the DR5 promoter in Cu2+-treated seedlings got more evident in root regions above the meristem (Lequeux et al. 2010; Peto et al. 2011). However, exposure to Pb2+ and Ni2+ totally eliminated the auxin activity from the meristematic zone of root tips (Fig. 3b). Since the proper localization and perception of auxin maximum are important for organizing the root apical meristem (RAM) (Sabatini et al. 1999), these two heavy metals may disrupt the organization of cell types in the root apex.
In cotyledons of the untreated Arabidopsis seedlings, GUS staining is mainly observed in hydathodes and its neighboring leaf margins as well as in the leaf blades as scattered dots each of which represents a few cells with varied shapes from round and oval to elongated (Koizumi et al. 2005) (Fig. 3a). When seedlings were exposed to Co2+ or Hg2+, an intriguingly strong GUS expression appeared in the primary and first loops of the 2nd order procambial strands of cotyledons (Fig. 3a). This vascular-specific induction of GUS staining was reminiscent of the key role played by auxin in both initiation and formation of vascular tissue during leaf venation (Scarpella et al. 2010). Without appropriate polar auxin transport, Arabidopsis leaves could lose vascular continuity and the primary vein could not develop (Sieburth 1999). No significant change of auxin activity was observed in the Cu2+-treated cotyledons and for the remaining four types of heavy metals including Cd2+, Pb2+, Ni2+ and Zn2+, the number of GUS-expressing spots in leaf blades was significantly reduced and the histochemical staining in the cotyledons was visible only in the hydathodes (Fig. 3a).
Heavy metals affect the transcriptional expression levels of selected auxin-related genes
To test the hypothesis that plants could adapt to excessive heavy metals by modifying the transcriptional profile of genes involved in local auxin biosynthesis, accumulation, intercellular transport and degradation to precisely influence the auxin-mediated developmental events such as the RAM meristem maintenance and lateral root initiation and development (Bhalerao et al. 2002; Jones and Ljung 2012), we investigated the transcriptional changes of 27 selected auxin homeostasis-related genes in Arabidopsis seedlings after heavy metal exposure. Among genes involved in auxin biosynthetic pathways, PAT1 gene encodes the tryptophan (Trp) biosynthetic enzyme, phosphoribosylanthranilate transferase (Rose et al. 1992). In the indole-3-pyruvic acid (IPyA) pathway, the tryptophan aminotransferase encoded by TAA1 convert l-Trp to IPyA (Stepanova et al. 2008) and the YUCCA (YUC) enzymes subquently synthesize IAA from IPyA (Mashiguchi et al. 2011; Stepanova et al. 2008; Won et al. 2011). Two Arabidopsis cytochrome P450 monooxygenases CYP79B2 and CYP79B3 convert l-Trp to indo-3-acetaldoxime (IAOx), the common precursor for IAA (Zhao et al. 2002). Indole-3-acetonitrile (IAN) is a putative precursor that is synthesized from IAOx (Sugawara et al. 2009) and nitrilases (NITs) have been suggested to convert IAN to IAA in plants (Hillebrand et al. 1998; Vorwerk et al. 2001). In addition to de novo synthesis, conjugation and degradation are also important homeostatic mechanisms operating in plant cells to regulate the level of auxin (Normanly, 2010; Rosquete et al. 2012). The Gretchen Hagen (GH3) genes (GH3.4/3.3/GH3.1) encode IAA-amido synthases that conjugate Asp and other amino acids to auxin in vitro and thus are thought to be important in controlling free IAA levels (Staswick et al. 2005). An F box protein, called transport inhibitor response 1 (TIR1), is an auxin receptor and its auxin-dependent activity leads to ubiquitination-based proteolysis of Aux/IAA transcriptional repressors and plays an important role in conveying positional information during developmental processes (Dharmasiri et al. 2005; Gray et al. 2001; Maraschin Fdos et al. 2009). Auxin polar transportation (PAT) is essential in setting up the auxin gradients which has been attributed to a variety of important growth and developmental processes in plants (Tanaka et al. 2006). Two families of IAA efflux carrier proteins have so far been identified in Arabidopsis: the PIN family and the ABCB family (Blakeslee et al. 2007; Mravec et al. 2008). There are also specific IAA influx carrier proteins, such as the AUX1/LAX family, which are responsible for importing IAA into specific cell types. Two auxin response factors (ARFs) (ARF2 and ARF7) and one AP2/EREBP family member, PLETHORA 2 (PLT2), are included for analyses since that they are auxin-dependent transcriptional regulators essential for auxin signaling and a wide variety of early/primary auxin response in Arabidopsis seedlings (Harper et al. 2000; Okushima et al. 2005; Wang et al. 2005; Wilmoth et al. 2005; Aida et al. 2004; Galinha et al. 2007). Clearly, these selected genes only represent a small fraction of the complex auxin-mediated regulatory networks and our understanding of auxin modulation based on their similar or antagonistic effects on auxin abundance can only be fragmented and speculative.
By the means of qRT-PCR, the transcriptional regulations of the above-mentioned genes together with the GUS gene driven by the DR5 promoter were analyzed and the results were presented in the form of heat-map for an easy and direct view (Fig. 4). As expected, Arabidopsis seedlings responded to various heavy metals differentially, and shoots and roots also displayed dramatically dissimilar results. In particular, when confronted with Pb2+, Zn2+ and Ni2+, a contradictory gene modulation pattern was observed for shoots and roots. For example, upon Ni2+ application, 25 out of 27 tested genes got induced in shoots accompanied by a decreased expression level of GUS gene and a moderately weakened GUS staining. In contrast, 24 genes got repressed in roots with a concomitant absence of the QC-specific localization of auxin (Fig. 4). These results highlighted the tissue-specificity of dynamic expression regulation of auxin-related genes and the flexibility of the plant in mobilizing and metabolizing this important phytohormone to adjust itself to the heavy-metal contaminated environment.
Differential responsiveness of auxin homeostasis-related genes towards various heavy metal treatments in the shoots (s) and roots (r) of 8-day-old Arabidopsis seedlings. Transcriptional level of genes was determined by quantitative real-time PCR and the results were normalized, interpreted as the fold change relative to the unexposed plants and presented in the form of a heat-map
We then analyzed the gene expression data by hierarchical clustering in order to observe the potential common trends in these responses and two major groups were identified. In the first group, Ni2+-treated shoots was highlighted with a large-scale gene induction. Hg2+-treated shoots and Pb2+ or Zn2+-treated roots were clustered into the same group by sharing some of the up-regulated gene (Fig. 4). In the second group, Arabidopsis roots treated by Ni2+ or Co2+ exhibited a massive repression of almost all investigated genes despite that they influenced the auxin accumulation in root tips very differentially, from having a minor effect (Co2+) to almost totally dismissing the presence of auxin from the QC (Ni2+). Within the same group, Zn2+ or Cu2+-treated shoots were identified with a similar large-scale gene down-regulation and they also induced very distinct auxin activity patterns in cotyledons. Therefore, at least in this work, we did not find the correlation between the gene expression patterns and the local auxin accumulation upon various heavy metal stresses. Instead,it is plausible that the responsiveness and developmental plasticity of plants to heavy metals with regard to auxin activity are the outcome of multiple regulatory pathways, the dynamics of which are difficult to be predicted by the behaviors of individual genes. Considering that auxin network is intertwined with and affected by other signaling pathways, secondary messengers and some yet unidentified substances, further research is needed to elucidate the intrinsic reason for the responses of plants under heavy metal stresses.
Taken together, our data suggest that plants have different solutions to the problem of survival in heavy metal-contaminated environments and this developmental flexibility involves complex transcriptional reprogramming of auxin-related genes to modify the timing and localization of auxin accumulation which in turn to reshape and optimize the developmental programs of plants. The great challenge for the future will be to identify the key molecular players and integrators that link auxin signaling machinery to specific aspects of plant development and organogenesis under heavy metal stress conditions.
Conclusions
In this work, we first compared the effects of seven heavy metals on the phenotypic characters of Arabidopsis seedlings and investigated their respective disturbance on the distribution and accumulation of auxin within the plant using the DR5:GUS marker line. The pattern and intensity of GUS staining differed dramatically not only among different treatments but also between shoots and roots upon a same kind of exposure. Further real-time PCR analysis demonstrated that through a dynamic and tissue-specific transcription regulation of genes involved in de novo synthesis, conjugating attenuation and degradation of auxin, plants could respond to various heavy metal exposures with a fine and differential tunings of auxin level and distribution, which could help the plant to better acclimate and survive the adverse environment.
References
Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119:109–120
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 97:883–893
Andres-Colas N, Sancenon V, Rodriguez-Navarro S, Mayo S, Thiele DJ, Ecker JR, Puig S, Penarrubia L (2006) The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J 45:225–236
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29:325–332
Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J et al (2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19:131–147
Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433:39–44
Chiang HC, Lo JC, Yeh KC (2006) Genes associated with heavy metal tolerance and accumulation in Zn/Cd hyperaccumulator Arabidopsis halleri: a genomic survey with cDNA microarray. Environ Sci Technol 40:6792–6798
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Dharmasiri N, Dharmasiri S, Estelle M (2005) The F-box protein TIR1 is an auxin receptor. Nature 435:441–445
Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benkova E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci U S A 105:8790–8794
Eren E, Arguello JM (2004) Arabidopsis HMA2, a divalent heavy metal-transporting P(IB)-type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiol 136:3712–3723
Friml J (2003) Auxin transport—shaping the plant. Curr Opin Plant Biol 6:7–12
Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jurgens G et al (2002) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108:661–673
Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449:1053–1057
Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414:271–276
Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12:757–770
Hillebrand H, Bartling D, Weiler EW (1998) Structural analysis of the nit2/nit1/nit3 gene cluster encoding nitrilases, enzymes catalyzing the terminal activation step in indole-acetic acid biosynthesis in Arabidopsis thaliana. Plant Mol Biol 36:89–99
Jones B, Ljung K (2012) Subterranean space exploration: the development of root system architecture. Curr Opin Plant Biol 15:97–102
Kim DY, Bovet L, Kushnir S, Noh EW, Martinoia E, Lee Y (2006) AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiol 140:922–932
Koizumi K, Naramoto S, Sawa S, Yahara N, Ueda T, Nakano A, Sugiyama M, Fukuda H (2005) VAN3 ARF–GAP-mediated vesicle transport is involved in leaf vascular network formation. Development 132:1699–1711
Lee J, Bae H, Jeong J, Lee JY, Yang YY, Hwang I, Martinoia E, Lee Y (2003) Functional expression of a bacterial heavy metal transporter in Arabidopsis enhances resistance to and decreases uptake of heavy metals. Plant Physiol 133:589–596
Lequeux H, Hermans C, Lutts S, Verbruggen N (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Lopez-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
Maksymiec W (1997) Effect of copper on cellular processes in higher plants. Photosynthetica 34:321–342
Maraschin Fdos S, Memelink J, Offringa R (2009) Auxin-induced, SCF(TIR1)-mediated poly-ubiquitination marks AUX/IAA proteins for degradation. Plant J 59:100–109
Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H et al (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A 108:18512–18517
Mravec J, Kubes M, Bielach A, Gaykova V, Petrasek J, Skupa P, Chand S, Benkova E, Zazimalova E, Friml J (2008) Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135:3345–3354
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nibau C, Gibbs DJ, Coates JC (2008) Branching out in new directions: the control of root architecture by lateral root formation. New Phytol 179:595–614
Normanly J (2010) Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harb Perspect Biol 2:a001594
Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D et al (2005) Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17:444–463
Pasternak T, Rudas V, Potters G, Jansen MAK (2005) Morphogenic effects of abiotic stress: reorientation of growth in Arabidopsis thaliana seedlings. Environ Exp Bot 53:299–314
Paulose B, Chhikara S, Coomey J, Jung HI, Vatamaniuk O, Dhankher OP (2013) A gamma-glutamyl cyclotransferase protects Arabidopsis plants from heavy metal toxicity by recycling glutamate to maintain glutathione homeostasis. Plant Cell 25:4580–4595
Peto A, Lehotai N, Lozano-Juste J, Leon J, Tari I, Erdei L, Kolbert Z (2011) Involvement of nitric oxide and auxin in signal transduction of copper-induced morphological responses in Arabidopsis seedlings. Ann Bot 108:449–457
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105
Potters G, Pasternak TP, Guisez Y, Jansen MA (2009) Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ 32:158–169
Rose AB, Casselman AL, Last RL (1992) A phosphoribosylanthranilate transferase gene is defective in blue fluorescent Arabidopsis thaliana tryptophan mutants. Plant Physiol 100:582–592
Rosquete MR, Barbez E, Kleine-Vehn J (2012) Cellular auxin homeostasis: gatekeeping is housekeeping. Mol Plant 5:772–786
Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P et al (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99:463–472
Scarpella E, Barkoulas M, Tsiantis M (2010) Control of leaf and vein development by auxin. Cold Spring Harb Perspect Biol 2:a001511
Sieburth LE (1999) Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol 121:1179–1190
Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17:616–627
Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191
Sugawara S, Hishiyama S, Jikumaru Y, Hanada A, Nishimura T, Koshiba T, Zhao Y, Kamiya Y, Kasahara H (2009) Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc Natl Acad Sci U S A 106:5430–5435
Tanaka H, Dhonukshe P, Brewer PB, Friml J (2006) Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci 63:2738–2754
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136:1005–1016
Vorwerk S, Biernacki S, Hillebrand H, Janzik I, Muller A, Weiler EW, Piotrowski M (2001) Enzymatic characterization of the recombinant Arabidopsis thaliana nitrilase subfamily encoded by the NIT2/NIT1/NIT3-gene cluster. Planta 212:508–516
Wang S, Tiwari SB, Hagen G, Guilfoyle TJ (2005) AUXIN RESPONSE FACTOR7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts. Plant Cell 17:1979–1993
Whippo CW, Hangarter RP (2006) Phototropism: bending towards enlightenment. Plant Cell 18:1110–1119
Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW (2005) NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 43:118–130
Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci U S A 108:18518–18523
Xu J, Yin H, Liu X, Li X (2010) Salt affects plant Cd-stress responses by modulating growth and Cd accumulation. Planta 231:449–459
Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL (2002) Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev 16:3100–3112
Acknowledgments
We thank Professor ShuZhen Men (Nankai University, China) for kindly donating DR5:GUS Arabidopsis seeds. This study was funded by Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2012BAC07B02), National Transgenic Major Project (2014ZX08004-004B) and Tianjin Research Program of Application Foundation and Advanced Technology (13JCQNJC14500).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, R., Wang, J., Zhao, L. et al. Impact of heavy metal stresses on the growth and auxin homeostasis of Arabidopsis seedlings. Biometals 28, 123–132 (2015). https://doi.org/10.1007/s10534-014-9808-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-014-9808-6