Abstract
Cadmium (Cd) is one of the dangerous factors that have negative impacts on plants and human health. Recently, many researchers have been looking for biostimulants to use as bioprotectants that can help or ameliorate plants’ tolerance against abiotic stress, including Cd. To test the dangerousness of Cd accumulated in the soil, 200 µM of the latter was applied to sorghum seeds at germination and maturation stages. At the same time, Atriplex halimus water extract (0.1%, 0.25%, 0.5%) was applied to test its efficacy on Cd alleviation in sorghum plants. The obtained results showed that the tested concentrations enhanced the tolerance of sorghum to Cd by enhancing the germination indexes parameters such as germination percentage (GP), seedling vigor index (SVI), and reducing the mean germination time (MGT) of sorghum seeds grown under cadmium stress. On the other hand, the morphological parameters (height and weight) as well as the physiological parameters (chlorophyll and carotenoid) were stimulated in treated maturated sorghum plants under Cd stress. In addition, 0.5% and 0.25% of Atriplex halimus extract (AHE) stimulated the antioxidant enzymes, including superoxide dismutase, catalase, glutathione peroxidase, glutathione-s-transferase, and glutathione reductase. In the same time, an increase in carbon–nitrogen enzymes was recorded in the case of AHE treatment; phosphoenol pyruvate carboxylase, glutamine synthase, glutamate dehydrogenase, and amino acid transferase were all upregulated. These results suggest that using AHE as a biostimulant could be a better strategy to enhance the tolerance of sorghum plants to Cd stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world’s economic activities increased, leading to increased pollution. In this manner, heavy metals are considered major elements that contaminate soil (Li et al. 2012). Cadmium (Cd) is the most toxic heavy metal that can accumulate since it is highly mobile in the soil (Muratova et al. 2015). The latter is widely used in industrial production (nuclear power, electroplating, battery manufacture, etc.) (Muratova et al. 2015). It’s known that Cd, and other heavy metals, present a high negative impact on human health throughout the food chain (Piechalak et al. 2002). It is also harmful toplant’s health since it reduces nutrition absorption ability and alters many physiological and biochemical parameters in plants, causing oxidative stress (Anjum et al. 2015).
Cliquez ou appuyez ici pour entrer du texte.Sorghum is considered one of the most important cereals (Ben Mrid et al. 2019). It is cultivated for many purposes including food, forage, fiber, and fuel (Bouargalne et al. 2022). It occupies the fifth class in produced cereal grain in the world (Ben Mrid et al. 2019), making it an essential element in the economic world activity (de Morais Cardoso et al. 2017). Sorghum bicolor is considered one of the important plants in phytoremediation thanks to its capacity to remove heavy metals from contaminated soil (Shafiei Darabi et al. 2016). Additionally, this crop is an excellent source of nutrients and bioactive compounds essential for human health and diet, and it is a C4 plant capable of tolerating multiple environmental stressors, making it an ideal crop to grow in many arid regions (Roussi et al. 2022a). However, the development and growth of this plant are still negatively affected (Sharmila et al. 2017). To increase the tolerance of sorghum to cadmium and other heavy metals, researchers are working to find natural biostimulants like microorganisms, molecules, seaweed extracts, and plant extracts (Ben Mrid et al. 2021).
During this study, the tested plant-based extract was obtained from Atriplex halimus, a shrub that is up to 3 m in height and starts to branch from the base. Its leaves measures 10 to 30 mm long and 5 to 20 mm wide (Franclet and Le Houérou 1971). This plant is found in semi-arid and arid areas and is widely distributed in Morocco, especially in the southern area where we collected these species’ leaves. Atriplex halimus is rich in protein (Walker et al. 2014), and used to feed cattle and sheep (Walker et al. 2014). The used extract contain many secondary metabolites that play a crucial role in plant tolerance against abiotic stress. It was mentioned in the literature that Atriplex halimus extract is rich in flavonoids responsible for antioxidant activities, and other compounds like peptides, organic acids, and proline, which exist in strong concentrations in halophytes plants. These molecules are generally responsible for enhancing the tolerance of plants to abiotic stress (Benhammou et al. 2009).
In order to valorize Atriplex-halimus which is wildly spread in southern Morocco as a natural and cost-effective biostimulant for cadmium-stressed sorghum plants, we opt to test the water extract of this wild shrub on the germination and maturation of sorghum bicolor grown under 200 µM cadmium stress.
To achieve this objectif, we tested the effect of Atriplex-halimus extract using three concentrations, 0.1%, 0.25%, and 0.5% on sorghum germination, then on the morphological, physiological, and biochemical parameters. Based on the obtained results, we selected 0.25% and 0.5% for treating matured sorghum plants suffering from Cd stress. The morphological and physiological parameters in addition to the antioxidant and carbon nitrogen enzymes activities were determined for the harvested plants.
Material and methods
Material collection and extract preparation
In this study, Atriplex halimus plan was collected from the south of Morocco region called Akka (29° 23′ 27″ north, 8° 15′ 23″ west) near Tata city. The leaves of this plant were handpicked and transferred to the biochemistry and genetics molecular laboratory in Tangier. The plant material was dried at 37 °C for 7 days. The dried leaves were crushed and the obtained powder was used for extraction.
One hundred g of powder was homogenized with 1L of distilled water; the mixte was boiled at 100 °C for 2 h. After filtration, the supernatant obtained was considered to be at a concentration of 100%, 0.1%, 0.25%, and 0.5% were prepared by adding distilled water.
Germination assay of sorghum seeds under Cd stress
After being sterilized with 5% of sodium hypochlorite for 10 min, sorghum seeds that were obtained from the national research institute for agriculture (INRA) were rinsed with distillate water. For germination assay, 25 seeds were placed in Whatman No. 1 filter paper in 90 mm diameter sterilized Petri plates. Each condition was repeated four times:
-
First condition (C): soaked with water
-
Second condition (C+): soaked with 200 µM of cadmium
-
Third condition (T0.1%): Soaked with a solution containing 200 µM Cd and 0.1% Atriplex halimus extract (AHE)
-
Fourth condition (T0.25%): Soaked with a solution containing 200 µM Cd and 0.25% AHE
-
Fifth condition (T0.5%): Soaked with a solution containing 200 µM Cd and 0.5% AHE.
The Petries plates were incubated in the dark for 3 days until the seeds started germination, and then placed in a 16/8 h (light/dark) photoperiod in an average temperature of 27 °C. The number of germinated seeds was noted every day. After 8 days, the length and weight of seedlings were measured.
To study the effect of cadmium on sorghum bicolor seeds germination, and the effect of AHE on seeds germination under cadmium stress, many parameters were calculated such as germination percentage (GP), rate germination index (RGI), seedlings vigor index (SVI), mean germination time (MGT). The formulas used to calculate those indexes are mentioned in the following table.
Parameters | Formula | Reference |
|---|---|---|
Germination pourcentage (GP) | GP = number of germinated seeds/Total number of seeds | Ait Elallem et al. (2022) |
Rate germination index (RGI) | GI = (ixN1) + ((i − 1) x N2) + … + (1xNi) N1, N2…Ni: number of seeds germinated every day, and the multipliers are weights given to the days of the germination | Ranal et al. (2009) |
Seedling vigor index (SVI) | SVI = G% × SL G%: germination percentage SL: seedling length (cm) | Ait Elallem et al. (2022) |
Mean germination Time (MGT) | MGT = ΣNt.T/ΣN Nt: number of seeds newly germinated at time t T: number of days from the start of the germination test until time t ΣN: number of seeds that germinated on the final germination day | Ait Elallem et al. (2022) |
Growth assay of sorghum seeds under Cd stress
After selecting the best concentrations (0.25%, 0.5%) that have a high positive impact on sorghum germination under cadmium stress, another test was made, this time on maturate sorghum plants cultivated in vermiculite.
Eight sterilized seeds of sorghum were cultivated in medium plastic pots (5.5 inches in inner diameter; 5 inches in height) full with vermiculite (3 pots were used as repetition) (Fig. 1), after the apparition of the third leaf (10 days), the treatments started as 4 conditions were made (treatments were applied every 4 days):
-
First condition (C): irrigated with culture medium
-
Second condition (C+): irrigated with culture medium containing 200 µM Cd
-
Third condition (T0.25%): irrigated with culture medium containing 200 µM Cd and 0.25% AHE
-
Fourth condition (T0.5%): irrigated with culture medium containing 200 µM Cd and 0.5% AHE
The elements of the medium with nutrients were; 0.5 mM KNO3, 0.375 mM KH2PO4, 0.125 mM K2HPO4, 0.375 mM MgSO4, 1.25 mM CaSO4, 10 mg/L Fe-ethylene-diamine tetraacetate (EDTA) and micronutrients (Ben Mrid 2016).
Growth and biochemical parameters
After 50 days from starting the treatment, the plants were harvested, the growth parameters of each condition were noted (weight and height), and plant leaves of each condition were conserved at − 80 °C for enzyme activities’ determination.
Preparation for enzymes’ extraction
0.2 g of fresh leaves ( conserved at − 80 °C) was extract in medium that contained: 0.1 M HEPES–KOH, 0.02 mM of flavin adenine dinucleotide (FAD), 0.01 M of Magnesium chloride (MgCl2), 0.001 M phenylmethylsulfonyl fluoride (PMSF), and 14 mM β-Mercaptoethanol. The homogenate was centrifuged (20,000 g) for 20 min at 4 °C. The supernatant was used to determine the activities of enzymes.
Antioxidant and carbon–nitrogen enzyme activities
The antioxidant enzymes’ system was presented by five enzymes; superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-s-transferase (GST), and glutathione reductase (GR).
The SOD activity was determined based on the protocol used by Ben Mrid et al. (2022), 5 ul of enzyme extract was mixed with a reaction medium that contained 50 mM phosphate buffer (pH 7.5), 0.01 M methionine, 0.1 uM EDTA, 0.002 Mm riboflavin, 0.075 mM of nitro blue tetrazolium (NBT). The activity was determined at 560 nm. One unit of SOD activity was defined as the quantity of SOD required to obtain a 50% inhibition of the reduction of NBT. The activity was expressed as units per mg of protein content.
The CAT activitywas done using the protocol ofEl Omari et al. (2016), the reaction mixture contained 0.05 M phosphate buffer (pH 7), 10 mM of H2O2 was added to the enzyme extract, the activity was followed at 240 nm for 5 min.
GPx activity was made according to Bouchmaa et al. (2018), the reaction mixture contained 0.05 M of potassium phosphate buffer (pH 7.4), 0.001 M sodium azide, 0.001 M EDTA, 4 ug/mL of glutathione reductase, 0.001 M of reduced glutathione (GSH), 0.2 mM NADPH, and 0. 25 mM of H2O2 was added to the enzyme extract, the oxidation of NADPH was followed at 340 nm.
The protocol used for GR activity was mentioned by Roussi et al. (2022b). A reaction mixture that contained 0.1 M potassium phosphate buffer (pH 7.8), 0.001 M of oxidized glutathione (GSSG), and 0.2 mM of NADPH was added to the enzyme extract, and the oxidation of NADPH was followed at 340 nm.
The GST activity, was determined according to the protocol of Latique et al. (2021); The assay mixture contained the enzyme extract, 5 mM GSH, 2.5 mM 1-chloro-2, 4-dinitrobenzene (CDNB), and 0.1 M phosphate buffer (pH 5.5). The reaction was monitored spectrophotometrically at 340 nm at 30 °C, and the product concentrations were calculated using a molar extinction coefficient of 9.6 mM−1 cm−1.
The phosphoenolpyruvate carboxylase (PEPC), glutamate dehydrogenase (GDH), glutamine synthase (GS), and aspartate amino transferase (AAT) represented the carbon–nitrogen enzyme system.
The PEPC activity was determined according to the protocol used by Kchikich et al. (2021a). The activity was assayed by coupling to NAD-malic dehydrogenase (MDH), and monitoring NADH oxidation at 340 nm spectrophotometrically in a 1 mL assay mixture containing 100 mM Hepes–KOH (pH 7.3), 5 mM MgCl2, 0.2 mM NADH, 5 U of MDH, 2.5 mM PEP, 5 mM NaHCO3 and the enzyme extract. One unit of PEPC is the amount of the enzyme extract, which catalyzes the transformation of 1 μmol substrate per minute at 30 °C.
The GDH, and GS enzyme activities were measured in the aminating direction, as described by Kchikich et al. (2021b). The first activity was made at 30 °C by adding a reaction mixture that contained 0.1 M Tris–Hcl (pH 8), 0.001 M CaCl2, 0.013 M α-ketoglutarate, 0.05 M (NH4)2SO4, and 0.25 mM NADH. The kinetic activity was determined at 340 nm. While the second one was measured using the transferase method according to Kchikich et al. (2021a),the assay mixture contained 90 mM imidazole–HCl (pH 7.0), 0.12 M L-glutamine, 0.003 M MnCl2, 0.4 mM ADP, 0.02 M sodium arsenate, 0.06 M NH2OH and the enzyme extract. The L-glutamine was omitted in the blank test of each condition. After incubation of the mixture at 37 °C for 20 min, the reaction was stopped by adding a mixture (1:1:1) of 10% FeCl3•6H2O (in 0.2 N HCl), 24% TCA, and 5% HCl after 15 min. The appearance of γ-glutamyl hydroxamate was measured at 540 nm.
The AAT activity was measured according to Ben Mrid et al. (2017), the activity was determined by following the oxidation of NADH at 340 nm, the assay mixture contained: Tris–HCl 50 mM, pH 7.8, L-aspartate 50 mM, 2-oxoglutarate 10 mM, NADH 0.1 mM, 2U of MDH and 20 µl of the enzyme extract. The reaction was initiated by adding 2-oxoglutarate.
Chlorophyll content determination
The chlorophyll content was measured according to the protocol used by Naboulsi et al. (2022a) with some modifications. The fresh leaf samples were extracted in a mixture of 80% acetone, the solution was incubated at 4 °C for 72 h. The optic density of the supernatant was measured at 645 nm and 663 nm, and chlorophyll a, chlorophyll b, and total chlorophyll contents were estimated using the following formula:
MDA content determination
The lipid peroxidation was measured by detecting malondialdehyde (MDA) content in each sample. The MDA content was determined according to a previous study done by Ben Mrid et al. (2019). In brief, cell homogenate, in different conditions, was mixed with trichloroacetic acid (20%) and TBA (0.67%). The mixture was heated at 95 °C for 1 h. After cooling, 1 mL n-butanol was added to the mixture followed by centrifugation at 12,000 g for 10 min. The organic supernatant was collected to measure the absorbance at 532 nm and 600 nm.
H2O2 content determination
The peroxide (H2O2) content was determined according to (Ennoury et al. 2022), 0.25 mL of cell homogenate was mixed with 1 mL of TCA 0.1%, and the mixture was centrifuged (12,000) for 15 min. The obtained supernatant was mixed with 1 ml of 10 mM phosphate buffer ph 7, and 2 mL of 1 M iodide potassium (KI). After incubation in the dark for 1 h, the O.D was read at 390 nm. H2O2 concentrations were calculated using a standard curve.
O2 − content determination
The superoxide content was estimated using the protocol mentioned by Kubiś (2008) with slight modification; 0.1 g of leaves were cut into small paces and immersed in a mix containing 10 mM (K) phosphate buffer, pH 7.8, 0.05% NBT, and 10 mM NaN3; then, the mixture was incubated for 1 h at room temperature. 2 ml of immersed solution was heated at 85 °C for 15 min. After cooling at room temperature, the optical density was measured colorimetrically at 580 nm, and the O2− content was expressed in A580 g−1 FW.
Statistical analysis
SPSS 25 for Windows software, version 10.0.1 was used for all statistical analyses. A one-way ANOVA, followed by the Student Newman-Keuls post hoc test, was used to compare the differences in means (p < 0.05). Different letters indicate significant differences.
Results
Atriplex halimus extract composition
The Atriplex halimus extract composition was identified, and the tested extract presents a diverse chemical compound with different concentrations. For instant, the secondary metabolites flavonoid and polyphenols present 27.65 mg.g−1 DW and 5.726 mg.g−1 DW, respectively. Additionally, an amount of 0.224 mg.g−1 DW, 3.104 mg.g−1 DW, and 0.303 mg.g−1 DW for indole acetic acid (IAA), soluble sugar, and the free amino acid was detected in the 100% of tested extract (Table 1).
Effect of Atriplex halimus extract on sorghum bicolor growth parameters in germination, seedlings, and plant stage
Exposure of sorghum bicolor seeds to cadmium stress (200 µM) resulted in a significant decrease in germination parameters; the germination percentage (GP) index showed a decrease of 48%, 45.5%, 43.75%, and 38.7% in 24 h, 48 h, 72 h, and 288 h, respectively compared to untreated seeds(irrigated only with water). In addition, the rate germination index (RGI) and seeds vigor index (SVI) of sorghum bicolor seeds under cadmium stress were reduced by 46.06%, and 61.22% respectively, while an increase of 8.51% in mean germination time (MGT) was recorded compared to the untreated group. However, sorghum bicolor seedlings parameters; germinated seedlings fresh weight (GSFW), germinated seedlings shoot length (GSSL), and germinated seedlings root length (GSRL) showed a reduction of 42.9%,37.3%, and 53.3%, respectively compared to control (untreated seeds) (Table2).
The Atriplex halimus extract application reversed the harm caused by cadmium, the treatment with 0.5% of the tested extract showed important results; it increased GP by 73.1%, 63.4%, 55.9%, and 37.1% after 24 h, 48 h, 72 h, and 288 h. Respectively, it also increased the rate germination and the seedlings’ vigor index; both of those parameters are showing an increase of 64.9% and 82.2% respectively. Additionally, treatment with 0.5% of AHE decreased the mean germination time by 10% compared to stressed plants (irrigated with cadmium only) (Table 2).
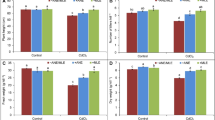
The AHE application has also stimulated the morphological parameters; results showed an increase in GSFW, GSSL, and GSRL in the case of treatment, especially the case of treatment by 0.5% of AHE that showed the highest values of the three parameters (94.74 g, 5.1 cm, 9.8 cm, respectively). However, Table 2 showed that the treatment with 0.1% of AHE showed a non-significant difference in germination and morphological parameters compared to those in the cadmium condition. Based on the obtained findings, 0.25% and 0.5% of the tested extract were selected to carry on our study on sorghum bicolor plants at the maturated stage using vermiculite. The results obtained showed that Cd reduced the morphological parameters; a decrease of 44.3%, 68.6%, 40.7%, and 88.8% were observed in maturated plants’ shoot lengths (MPSL), plant shoot weight (MPSW), plant root lengths (MPRL) and plant root weight (MPRW). The application of AHE with 0.25% and 0.5% has increased those parameters in stressed plants; the most efficient concentration was 0.5%, and it increased MPSL, MPSW, MPRL, and MPRW by 64%, 144.5%, 63.15%, and 1493.5%, respectively, compared to stressed plants (Table 2).
Effect of Atriplex halimus extract on chlorophyll and carotenoid pigments in sorghum bicolor plants under cadmium stress
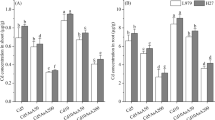
The effect of Cd stress on chlorophyllous pigments content in sorghum leaves’ cells’ results are shown in Fig. 2A. According to the findings, chlorophyll a, chlorophyll b, and total chlorophyll content decreased considerably in the presence of 200 µM Cd compared to the untreated condition. The total chlorophyll content of sorghum leaves showed a decrease of 28.82% under Cd exposure. However, this impairment under Cd stress was less pronounced with the AHE treatment when applied repeatedly at very low doses, showing a positive effect on this pigment content. The chlochlorophyll content in plants exposed at 200 µM Cd and treated with 0.25% AHE was twice the one found in the control, while plants treated with 0.5% had an increase of 35%. In the case of chlorophyll b content, the stressed plants subjected to 0.25% AHE treatment showed an increase of 14.43%. This pigment content of plants treated with 0.5% AHE was unaffected. The treatment with 0.25% AHE resulted in the greatest levels of total chlorophyll (Fig. 2A).
Figure 2B has reported the content of carotenoid determined in sorghum leaves treated or non-treated with AHE. Under no use of AHE, plants subjected to Cd stress decreased this chlorophyllous pigment by 87% compared to non-stressed plants. In the current experiment, we observed that treatment with doses of AHE, 0.25%, and 0.5%, reduced the adverse effects of Cd exposure by displaying significant increases in the carotenoid content in stressed sorghum leaves with 200 µM Cadmium. Plants treated with 0.25% AHE had a higher content, and this treatment was the most effective.
Effect of Atriplex halimus extract on H2O2, O2 −, MDA, and free thiol content in Sorghum bicolor plants under cadmium stress
The Cd stress has increased O2−, H2O2, and MDA contents in the sorghum bicolor plants. The three toxic molecules showed an increase in plants exposed to cadmium by 18%, 190%, and 16.9%, respectively. In addition, the non-protein thiol (NPT) concentration also decreased by 7.8% compared to non-stressed plants (Fig. 3).
Treatment by 0.25% and 0.5% of AHE has a positive impact on stressed plants, both concentrations have suppressed the accumulation of stress markers (O2−, H2O2, and MDA) and increased non-protein thiol content, it was shown that 0.5% of AHE has significantly decreased O2−, H2O2, and MDA by 19%, 60%, and 19%, respectively. While it increased significantly NPT content by 15.99% compared to cadmium-stressed plants.
Effect of Atriplex halimus extract on antioxidant enzymes system in Sorghum bicolor plants under cadmium stress
Sorghum bicolor plants’ exposure to cadmium has shown an alteration in antioxidant enzymes’ activities, superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-s-transferase (GST), and glutathione reductase (GR) showed an increase under cadmium toxicity. In contrast, catalase activity showed a decrease in the case of toxicity by cadmium. SOD, GPx, GST, and GR activities showed an augmentation of 33.6%, 19.7%, 32.2%, and 3%, respectively compared to non-stressed conditions (Fig. 4A, C–E). However, CAT activity was shown to be 50% less active under stressful situations (Fig. 4B).
The plant-based extract treatment has improved the antioxidant enzyme activities considerably. The five activities have shown an increase in treated plants, especially in those treated by 0.5% of AHE, an increase of 39.7%, 8.35%, 32.9%, 83.5%, and 75.8% was recorded in SOD, GPx, GST, GR, and CAT activities, respectively, compared to those under cadmium stress.
Effect of Atriplex halimus extract on carbon–nitrogen enzymes system in Sorghum bicolor plants under cadmium stress
Cadmium toxicity has altered the activity of the carbon–nitrogen enzymes in sorghum bicolor plants; phosphoenol pyruvate carboxylase (PEPC), Aspartate-amino transferase (AAT), and Glutamine synthase (GS) showed a decrease of 17.36%, 10.29%, and 80% under cadmium stress compared to untreated conditions (Fig. 5A, B, D). While glutamate dehydrogenase (GDH) showed an increase of 3% compared to non-stress plants (Fig. 5C).
AHE application has improved the four enzymes; 0.5% of AHE has raised PEPc, GS, and GDH activities by 20.64%, 395%, and 33.44%, respectively. While AAT activity increased by 8.7% when 0.25% of AHE was applied compared to stressed plants.
Correlation analysis
Table 3 presents the correlation of morphological parameters (MP); it was observed that the MP of both germinated and maturated stages were positively correlated. The GSFW, GSSL, GSRL, and the MPSL, MPSW, and MPRL were positively correlated.
During this study, we also made a correlation between the physiological and biochemical parameters in matured plants. The results showed that chlorophyll and carotenoid contents are positively correlated between them and correlated between the antioxidant enzymes, as it was also observed that MDA has a positive relation with both H2O2 and O2− (Table 4).
Discussion
Heavy metals, especially cadmium (Cd) are considered very dangerous to cereals and other crops (An 2004). Additionally, they are harmful to humans health (Ben Mrid et al. 2021). Cd appears to have no beneficial role in plant and human metabolisms (Doganlar and Yurekli 2009). As shown in the obtained results, Cd negatively affects sorghum bicolor seeds germination. It reduces GP, GRI, and SVI and increases MGT. The difference observed in these parameters reflects the negative effect of Cd on the germination of seeds (Ahmad et al. 2012). The reason behind this change can be related to the accelerated breakdown of food reserved in the seed’s embryo (Raziuddin et al. 2011). It has also been reported that Cd toxicity leads to nutrition loss (Sfaxi-Bousbih et al. 2010). In addition, stress by Cd imposed an inhibitory effect on initial germination enzymes such as alpha-amylase and invertases (Tumanyan et al. 2020). Cd had drastically affected roots and shoots lengths, in addition to seedlings weight. The reduction in seedlings’ morphological parameters is considered a good indicator of metal toxicity (Da Rosa Corrêa et al. 2006). Previous studies showed that heavy metals favor the accumulation of ROS, accelerate the process of lipid peroxidation, and affect the membrane cells’ permeability (Tumanyan et al. 2020).
The Cd toxicity also affected the growth parameters of the Sorghum bicolor plants at the maturated stage. It’s known that Cd negatively affects plant growth, causing an imbalance in nutrients and inhibiting the absorption of many elements that play a vital role for plants (Ben Mrid et al. 2021). However, the loss in biomass and morphological parameters can be a consequence of alteration in phytochemical processes. It was reported that Cd uptake inhibits leaf photosynthesis by favoring chlorophyll degradation and photosynthetic activity damage (Ben Mrid et al. 2021). In our case, chlorophyll a and b decreased significantly when Cd was applied. The outcomes of this experiment also indicate that Cd stress had a negative effect on carotenoid content. This could be attributed to cadmium’s deleterious effects on photosynthesis, which include a blockage of the photosynthetic electron transport pathway as well as severe photo-inhibition of photosystems I and II (El Rasafi et al. 2022). These findings are in accordance with previous studies in which they demonstrated that the photosynthetic apparatus is one of the most important sites of Cd inhibition (Rahman et al. 2021).
It has been previously revealed that Cd stress enhances the production of reactive oxygen species (ROS) such as O2− and H2O2. This enhancement of ROS not simply shatters cell membranes by lipid peroxidation but also reduces the biosynthesis of essential metabolites needed for plant life, like nucleic acids, proteins, carbohydrates (Jung et al. 2020). Throughout this study, O2− and H2O2 contents increased in sorghum bicolor plants under Cd stress. In addition, malondialdehyde (MDA) content has significantly increased in plants under Cd stress, which could be related to the increase in ROS caused by Cd toxicity. This molecule is a major indicator of oxidative stress, thus its accumulation is related to lipid peroxidation of cell membranes (Ben Mrid et al. 2021). On the other hand, the NPT content was also altered; the reduction of these molecules is considered one of the oxidative stress markers. It is known that Cd has an affinity with thiol, which reduces this molecule in the presence of oxidative stress (Ben Mrid et al. 2022).
To reduce the damage caused by oxidative stress, plants developed an antioxidant system through many enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-s-transferase (GST), and glutathione reductase (GR). SOD is the first enzyme that initiates the antioxidant process (Naboulsi et al. 2022a). It has been reported that this enzyme removes superoxide radicals in cells (Ennoury et al. 2022). SOD have a key role in controlling ROS levels in stressed plants by catalyzing the dismutation of superoxide to molecular oxygen and hydrogen peroxide (H2O2). Under normal conditions, catalase, peroxidase, and GST enzymes effectively scavenge the resulting H2O2 (Scott et al. 2010). Glutathione is a substrate for GPx when converting H2O2 into a non-toxic form or water. Besides, GST also uses GSH to convert toxic xenobiotics to non/less toxic molecules (Hasanuzzaman et al. 2017). Both GPx and GST use glutathione as a substrat to neutralize ROS (Ben Mrid et al. 2022). GR enzyme feeds the chain with glutathione to maintain an adequate presence of this substance in the cell. As it’s known, GR regulates the GSH/GSSG ratio and supplies GSH for several enzymes, such as dehydro ascorbate reductase (DHAR), GPX, and GST (Bhuyan et al. 2020).
Cd stress also caused an alteration in sorghum bicolor plant's carbon–nitrogen enzymes activity. phosphoenol pyruvate carboxylase (PEPC) is one of carbon–nitrogen enzymes affected by this heavy metal. The decrease in this enzyme was also reported in a study done by Roussi et al. (2022a). Wang et al. (2008) also mentioned that stress by Cd causes a decrease in PEPC. The latter is involved in the carboxylation of phosphoenolpyruvate to synthesize oxaloacetate (Bouargalne et al. 2018).At the same time, our study showed that glutamine synthase (GS) activity decreased while glutamate dehydrogenase (GDH) increased under Cd stress; these findings were also reported by Kchikich et al. (2021a). As mentioned in previous studies, GDH replaces GS activity to assure ammonium incorporation (Zhou et al. 2004). While amino acid transferase (AAT) plays an important role in carbon and nitrogen metabolism by the trapping and liberation of key oxo-acids that help co-ordinate N metabolism and amino acid synthesis with the availability of C skeletons from the Krebs cycle (Kchikich et al. 2021b); this enzyme was affected by the presence of Cd.
Atriplex halimus extract treatment has repaired the damage caused by cadmium and fixed the altered germination and maturation stages parameters. The improvement in growth parameters can be due to the presence of IAA and other metabolites in the used extract. IAA is one of the phytohormones that can stimulate the growth of plants (Ennoury et al. 2022). Our findings are in accordance with numerous studies conducted to find a way for plants to survive the negative impact caused by Cd stress. Ahmad et al. (2017) proved that jasmonic acid helps faba bean plants to tolerate Cd stress. Roussi et al. (2022a) have found that Cistus Salviifolius water extract supplementation enhanced sorghum plants’ tolerance to Cd toxicity. Also, Megha et al. (2020) mentioned that the supplementation of potassium alleviates oxidative stress in sorghum plants, while Ben Mrid et al. (2021), demonstrated that treatment with secondary metabolites increased the growth parameters of plants under Cd stress. The tested extract also helped stressed plants tolerate this metal’s drastic impact by suppressing chlorophyll and carotenoid degradation. The increased total chlorophyll content detected in AHE-treated sorghum leaves is most probably attributable to the effects of ROS deterioration and photosynthesis augmentation, which positively affects the growth of plants (Azizi et al. 2021).We note that treatment with both concentrations of AHE, 0.25% and 0.5%, have a beneficial impact on sorghum growth by stimulating photosynthetic metabolism. In addition, the ROS content was reduced in the case of treatment by AHE compared to non-treated stressed plants. This reduction can be explained by the presence of polyphenols and flavonoids in this plant-based extract, known for their antioxidant activity against ROS formation and for suppressing the enzymes involved in ROS formation (Stagos 2020). Sorghum plants treated with AHE showed a significant reduction in MDA content, especially at the concentration of 0.5%. This decrease could be explained by the presence of soluble sugars in AHE and also the presence of plant hormones that have the capacity to increase antioxidants levels and reduce ROS (H2O2 and O2−) and consequently reducing lipid peroxidation to induce healthy growth of plants (Alzahrani and Rady 2019). Our results are in the same line with those obtained by Rayees et al. (2022) who reported that the use of Tagetes erecta L. extract reduced the MDA content in wheat plants. Additionally, the reduction of ROS can be linked to the enhancement in oxidative stress enzymes’ activities (Ennoury et al 2022). It was found in multiple studies Cliquez ou appuyez ici pour entrer du texte that algal and plant extracts as biostimulants enhance the antioxidant enzymes' activities (Latique et al. 2021; Naboulsi et al. 2022b; Roussi et al. 2022b).
The AHE also stimulates the antioxidant enzymes SOD, GPx, GR, GST, and CAT. This stimulation increased the tolerance against the applied stress by reducing the amount of ROS in cells, as well as maintaining homeostasis station of plants by stabilizing GSSG/GSH ration in cells (Hasanuzzaman et al. 2017). On the other hand, the tested extract helped sorghum bicolor grow by repairing the carbon–nitrogen incorporation system throughout carbon enzymes like PEPC that could be essential for the supply of carbon compounds required for the synthesis of amino acids and, thus, proteins (Ben Mrid et al. 2017). The latteralso necessities nitrogen that GS and GDH incorporate. AAT enzyme was reported to have a direct role in the furniture of key organic acids for ammonium assimilation. The upregulation of the carbon–nitrogen enzymes has a positive impact through the accumulation of carbohydrates, proteins, amino acids, and other metabolites (Ben Mrid 2016).
These results proved that the use of AHE extract had a significant positive impact on sorghum plants’ growth and tolerance to Cd stress in both germination and maturation stages. Therefore, it can be said that this plant-based extract could be successfully used as a biostimulant in order to face the negative effects caused by Cd stress in sorghum bicolor plants and enhance their defense system and tolerance to this heavy metal.
Conclusion
In conclusion, the outcome of this study showed that Cd stress could negatively affect the germination and growth of sorghum. Many changes were observed in seeds germinated under Cd stress (200 µM); a reduction in germination percentage, rate germinated index, and seedling vigor index as well as the increase in mean germination time reflected the harmful impact of Cd. Moreover, this negative influence of Cd also affected plants at the maturation stage, which was apparent by the reduction of morphological parameters as well as the physiological parameters, including chlorophyll and carotenoid. In addition, Cd promotes the accumulation of ROS and MDA known as major oxidative stress markers. As a consequence, the antioxidant and carbon–nitrogen enzymes were highly affected. The use of Atriplex halimus extract showed optimistic results regarding the reduction of damage caused by Cd on sorghum at both the germination and maturation stages. The tested extract increased the germination percentage, rate germination index, and seedlings vigor index, and reduced the mean germination time. On the other hand, our results showed that treatment with Atriplex halimus extract reduced ROS accumulation and increased the antioxidant and carbon–nitrogen enzyme activities, especially with the two concentrations of 0.5% and 0.25%. It can be concluded that Atriplex halimus extract may represent an effective biostimulant to improve the germination and growth of plants in Cd contaminated soils, as well as boost their tolerance against it.
Data availability
The data used during this study is included in the article.
References
Ahmad I, Akhtar MJ, Zahir ZA, Jamil A (2012) Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak J Bot 44:1569–1574
Ahmad P, Alyemeni MN, Wijaya L et al (2017) Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch Agron Soil Sci 63:1889–1899. https://doi.org/10.1080/03650340.2017.1313406
Ait Elallem K, Ben Bakrim W, Ennoury A et al (2022) Germination parameters and responses of antioxidant enzymatic activities of two medicinal plants (Peganum harmala L. and Origanum majorana L.) under heavy metal stress. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00943-4
Alzahrani Y, Rady MM (2019) Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2019.109378
An YJ (2004) Soil ecotoxicity assessment using cadmium sensitive plants. Environ Pollut 127:21–26. https://doi.org/10.1016/S0269-7491(03)00263-X
Anjum SA, Tanveer M, Hussain S et al (2015) Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ Sci Pollut Res 22:17022–17030. https://doi.org/10.1007/s11356-015-4882-z
Azizi I, Esmaielpour B, Fatemi H (2021) Exogenous nitric oxide on morphological, biochemical and antioxidant enzyme activity on savory (Satureja hortensis L.) plants under cadmium stress. J Saudi Soc Agric Sci 20:417–423. https://doi.org/10.1016/j.jssas.2021.05.003
Ben MR, Bouchmaa N, Bouargalne Y et al (2019) Phytochemical characterization, antioxidant and in vitro cytotoxic activity evaluation of Juniperus oxycedrus Subsp Oxycedrus needles and berries. Molecules. https://doi.org/10.3390/molecules24030502
Ben Mrid R (2016) Effect of nitrogen source and concentration on growth and activity of nitrogen assimilation enzymes in roots of a Moroccan sorghum ecotype. Plant 4:71. https://doi.org/10.11648/j.plant.20160406.14
Ben Mrid R, El Omari R, Bouargalne Y et al (2017) Activities of carbon and nitrogen metabolism enzymes during germinating sorghum seeds and early seedlings growth. Cereal Res Commun 45:587–597. https://doi.org/10.1556/0806.45.2017.044
Ben Mrid R, Benmrid B, Hafsa J et al (2021) Secondary metabolites as biostimulant and bioprotectant agents: a review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.146204
Ben Mrid R, Bouchmaa N, Ouedrhiri W et al (2022) Synergistic antioxidant effects of natural compounds on H2O2-induced cytotoxicity of human monocytes. Front Pharmacol. https://doi.org/10.3389/fphar.2022.830323
Benhammou N, Bekkara FA, Kadifkova Panovska T (2009) Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplex halimus. C R Chim 12:1259–1266. https://doi.org/10.1016/j.crci.2009.02.004
Bhuyan MHMB, Parvin K, Mohsin SM et al (2020) Modulation of cadmium tolerance in rice: insight into vanillic acid-induced upregulation of antioxidant defense and glyoxalase systems. Plants. https://doi.org/10.3390/plants9020188
Bouargalne Y, Ben MR, El Omari R, Nhiri M (2018) Phosphoenolpyruvate carboxylase during maturation and germination sorghum seeds: enzyme activity and regulation. Russ J Plant Physiol 65:824–832. https://doi.org/10.1134/S1021443718060031
Bouargalne Y, Ben Mrid R, Bouchmaa N et al (2022) Genetic diversity for agromorphological traits, phytochemical profile, and antioxidant activity in Moroccan sorghum ecotypes. Sci Rep. https://doi.org/10.1038/s41598-022-09810-9
Bouchmaa N, Ben Mrid R, Boukharsa Y et al (2018) Cytotoxicity of new pyridazin-3(2H)-one derivatives orchestrating oxidative stress in human triple-negative breast cancer (MDA-MB-468). Arch Pharm (weinheim). https://doi.org/10.1002/ardp.201800128
Da Rosa Corrêa AX, Rörig LR, Verdinelli MA et al (2006) Cadmium phytotoxicity: quantitative sensitivity relationships between classical endpoints and antioxidative enzyme biomarkers. Sci Total Environ 357:120–127. https://doi.org/10.1016/j.scitotenv.2005.05.002
de Morais CL, Pinheiro SS, Martino HSD, Pinheiro-Sant’Ana HM (2017) Sorghum (Sorghum bicolor L.): nutrients, bioactive compounds, and potential impact on human health. Crit Rev Food Sci Nutr 57:372–390. https://doi.org/10.1080/10408398.2014.887057
Doganlar ZBP, Yurekli F (2009) Interactions between cadmium and phytochelatin accumulation in two different sunflower cultivars. Fresenius Environ Bull 18:304–310
El Omari R, Ben Mrid R, Chibi F, Nhiri M (2016) Involvement of phosphoenolpyruvate carboxylase and antioxydants enzymes in nitrogen nutrition tolerance in Sorghum bicolor plants. Russ J Plant Physiol 63:719–726. https://doi.org/10.1134/S102144371606008X
El Rasafi T, Oukarroum A, Haddioui A et al (2022) Cadmium stress in plants: a critical review of the effects, mechanisms, and tolerance strategies. Crit Rev Environ Sci Technol 52:675–726. https://doi.org/10.1080/10643389.2020.1835435
Ennoury A, BenMrid R, Nhhala N et al (2022) River’s Ulva intestinalis extract protects common bean plants (Phaseolus vulgaris L.) against salt stress. S Afr J Bot 150:334–341. https://doi.org/10.1016/j.sajb.2022.07.035
Franclet A, Le Houérou HN (1971) Les Atriplex en Tunisie et en Afrique du Nord. FAO, Rome
Hasanuzzaman M, Nahar K, Anee TI, Fujita M (2017) Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants 23:249–268. https://doi.org/10.1007/s12298-017-0422-2
Jung HI, Lee BR, Chae MJ et al (2020) Ascorbate-mediated modulation of cadmium stress responses: reactive oxygen species and redox status in Brassica napus. Front Plant Sci. https://doi.org/10.3389/fpls.2020.586547
Kchikich A, El Omari R, Kabach I et al (2021a) Effect of arbuscular mycorrhizal fungus and the γ-aminobutyric acid treatment in nitrate assimilation under nitrogen deficiency in sorghum plant. Russ J Plant Physiol 68:901–908. https://doi.org/10.1134/S102144372105006X
Kchikich A, Ben MR, Kabach I et al (2021b) Arbuscular mycorrhizal fungi enhance sorghum plant growth under nitrogen-deficient conditions through activation of nitrogen and carbon metabolism enzymes. Int J Agric Biol 26:201–208. https://doi.org/10.17957/IJAB/15.1825
Kubiś J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406. https://doi.org/10.1016/j.jplph.2007.02.005
Latique S, Ben MR, Kabach I et al (2021) Foliar application of ulva rigida water extracts improves salinity tolerance in wheat (Triticum durum L.). Agronomy. https://doi.org/10.3390/agronomy11020265
Li X, Bu N, Li Y et al (2012) Growth, photosynthesis and antioxidant responses of endophyte infected and non-infected rice under lead stress conditions. J Hazard Mater 213–214:55–61. https://doi.org/10.1016/j.jhazmat.2012.01.052
Megha T, Mir RA, Jatav KS, Agarwal RM (2020) Supplementation of potassium alleviates water stress induced changes in Sorghum bicolor L. Physiol Plant. https://doi.org/10.1111/ppl.13306
Muratova A, Lyubun Y, German K, Turkovskaya O (2015) Effect of cadmium stress and inoculation with a heavy-metal-resistant bacterium on the growth and enzyme activity of Sorghum bicolor. Environ Sci Pollut Res 22:16098–16109. https://doi.org/10.1007/s11356-015-4798-7
Naboulsi I, Ben MR, Ennoury A et al (2022a) Crataegus oxyacantha extract as a biostimulant to enhance tolerance to salinity in tomato plants. Plants. https://doi.org/10.3390/plants11101283
Piechalak A, Tomaszewska B, Baralkiewicz D, Malecka A (2002) Accumulation and detoxification of lead ions in legumes. Phytochemistry 60:153–162. https://doi.org/10.1016/S0031-9422(02)00067-5
Rahman SU, Xuebin Q, Riaz L et al (2021) The interactive effect of pH variation and cadmium stress on wheat (Triticum aestivum L.) growth, physiological and biochemical parameters. PLoS ONE. https://doi.org/10.1371/journal.pone.0253798
Ranal MA, de Santana DG, Ferreira WR, Mendes-Rodrigues C (2009) Calculando medidas de germinação e organizando planilhas eletrônicas. Rev Brasileira Botanica 32:849–855. https://doi.org/10.1590/S0100-84042009000400022
Rayees Ahmad M, Argal S, Ahanger MA, Jatav KS, Agarwal RM (2022) Differential activity of wheat antioxidant defence system and alterations in the accumulation of osmolytes at different developmental stages as influenced by marigold (Tagetes erecta L.) leachates. Front Plant Sci. https://doi.org/10.3389/fpls.2022.1001394
Raziuddin F, Hassan G et al (2011) Effects of cadmium and salinity on growth and photosynthesis parameters of Brassica species. Pak J Bot 43:333–340
Roussi Z, Ben Mrid R, Ennoury A et al (2022a) Insight into Cistus salviifolius extract for potential biostimulant effects in modulating cadmium-induced stress in sorghum plant. Physiol Mol Biol Plants 28:1323–1334. https://doi.org/10.1007/s12298-022-01202-7
Roussi Z, Kchikich A, Nhhala N, Krid A, Ennoury A, Asri SE, Zouaoui Z, Nhiri N, Nhiri M (2022b) Cistus monspeliensis extract as a prospective biostimulant in enhancing tolerance to cadmium in sorghum plant. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-03542-6
Scott JL, Gabrielides C, Davidson RK et al (2010) Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis 69:1502–1510. https://doi.org/10.1136/ard.2009.119966
Sfaxi-Bousbih A, Chaoui A, El Ferjani E (2010) Cadmium impairs mineral and carbohydrate mobilization during the germination of bean seeds. Ecotoxicol Environ Saf 73:1123–1129. https://doi.org/10.1016/j.ecoenv.2010.01.005
Shafiei Darabi S, Almodares A, Ebrahimi M (2016) Phytoremediation efficiency of sorghum bicolor (L) Moench in removing cadmium, lead and arsenic. Open J Environ Biol 1:001–006. https://doi.org/10.17352/ojeb.000001
Sharmila P, Kumari PK, Singh K et al (2017) Cadmium toxicity-induced proline accumulation is coupled to iron depletion. Protoplasma 254:763–770. https://doi.org/10.1007/s00709-016-0988-5
Stagos D (2020) Antioxidant activity of polyphenolic plant extracts. Antioxidants. https://doi.org/10.3390/antiox9010019
Tumanyan AF, Seliverstova AP, Zaitseva NA (2020) Effect of heavy metals on ecosystems. Chem Technol Fuels Oils 56:390–394. https://doi.org/10.1007/s10553-020-01149-z
Walker DJ, Lutts S, Sánchez-García M, Correal E (2014) Atriplex halimus L.: its biology and uses. J Arid Environ 100–101:111–121. https://doi.org/10.1016/j.jaridenv.2013.09.004
Wang L, Zhou Q, Ding L, Sun Y (2008) Effect of cadmium toxicity on nitrogen metabolism in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. J Hazard Mater 154:818–825. https://doi.org/10.1016/j.jhazmat.2007.10.097
Zhou W, Sun QJ, Zhang CF et al (2004) Effect of salt stress on ammonium assimilation enzymes of the roots of rice (Oryza sativa) cultivars differing in salinity resistance. Acta Bot Sin 46:921–927
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: AE; Methodology: AE, NN, AK, ZR, SEA, ZZ; Formal analysis and investigation: AE, NN, SEA; Writing—original draft preparation: AE, NN, SEA; Writing—review and editing: AE, MN; Supervision: MN.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ennoury, A., Nhhala, N., Kchikich, A. et al. Saltbuch extract: a bio-solutionfor cadmium stress sorghum plants in germination and maturation. Biometals 36, 997–1012 (2023). https://doi.org/10.1007/s10534-023-00499-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-023-00499-5