Abstract
Cadmium (Cd) is a serious threat for environmental sustainability as it can be taken up quickly by plants and transported to the food chain of living organisms. It alters plants’ metabolic and physiological activities and causes yield loss, thereby, enhancing plant tolerance to Cd stress is of utmost essential. Therefore, an experiment was executed to investigate the potential role of Ascophyllum nodosum extract (ANE) and moringa (Moringa oleifera) leaf extract (MLE) to confer Cd tolerance in rice (Oryza sativa cv. BRRI dhan89). Thirty-five-day-old seedling was subjected to Cd stress (50 mg kg−1 CdCl2) alone and in a combination of ANE (0.25%) or MLE (0.5%) in a semi-controlled net house. Exposure to Cd resulted in accelerated production of reactive oxygen species, enhanced lipid peroxidation, and disrupted antioxidant defense and glyoxalase system, thus retarded plant growth, biomass production, and yield attributes of rice. On the contrary, the supplementation of ANE or MLE enhanced the contents of ascorbate and glutathione, and the activities of antioxidant enzymes such as ascorbate peroxidase, dehydroascorbate reductase, monodehydroascorbate reductase, glutathione reductase, glutathione peroxidase, and catalase. Moreover, supplementation of ANE and MLE enhanced the activities of glyoxalase I and glyoxalase II which prevented the overgeneration of methylglyoxal in Cd stressed rice plants. Thus, because of ANE and MLE addition Cd-induced rice plants showed a noticeable declination in membrane lipid peroxidation, hydrogen peroxide generation, and electrolyte leakage, whereas improved water balance. Furthermore, the growth and yield attributes of Cd-affected rice plants were improved with the supplementation of ANE and MLE. All the studied parameters indicates the potential role of ANE and MLE in mitigating Cd stress in rice plants through improving the physiological attributes, modulating antioxidant defense and glyoxalase system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal/metalloids are toxic environmental pollutants that seriously threaten the sustainability of agroecosystems (Vardhan et al. 2019). Both natural and anthropogenic activities accelerate the contamination of heavy metals in agricultural lands, thereby negatively affecting the existing fauna and flora (Seifikalhor et al. 2020). Cadmium (Cd) is one of the toxic minerals, and its contamination has increased rapidly in the last few decades (Xue et al. 2009). Cadmium, even at a lower concentration, can alter the physiological activities of plants. Cadmium is highly mobile in plant-soil systems and becomes phytotoxic beyond the concentration of 5–10 mg g−1 dry weight of leaf of plants (Gill et al. 2013). Upon accumulation of Cd, biosynthesis of photosynthetic pigments is inhibited, thus hampering photosynthesis (Li et al. 2015). Besides, it also restricts the efficacy of proteins associated with the photosystem-II and declines the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) enzyme, which is engaged in the trapping of carbon dioxide and photoassimilate production of plants (Silva et al. 2017). Overaccumulated Cd diminishes the essential mineral uptake, transport rate, stomatal conductance, and water balance in plants (Karalija and Selović 2018).
Cadmium, a redox inactive metal, cannot produce reactive oxygen species (ROS) directly through plants’ Haber–Weiss and Fenton reactions. However, Cd participates in other metabolic processes, i.e., cationic replacement of enzymes, activation of NADPH oxidase, reduction in GSH pool, thus generating ROS (Mahmud et al. 2019). Excessive generation of ROS such as singlet oxygen (1O2), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and superoxide anion (O2•−), have damaging effects on the proteins, nucleic acids, photoinhibition, and membrane structure (Alyemeni et al. 2018). Moreover, exposure to Cd also accelerates oxidative stress in plants due to the altered antioxidant defense mechanism (Mahmud et al. 2019).
Plants are equipped with some avoidance mechanisms to cope with the adversity of metal toxicity which includes excretion, metal chelation, and compartmentalization. Still, unfortunately, these mechanisms are not enough to compensate the damaging of toxic metal stress (Gratao et al. 2008). Plants have antioxidant defense system consisting of non-enzymatic (ascorbate, AsA; glutathione, GSH, tocopherols, phenolic compounds, etc.) and enzymatic (ascorbate peroxidase, APX; monodehydroascorbate reductase, MDHAR; dehydroascorbate reductase, DHAR; glutathione reductase, GR; glutathione peroxidase, GPX; catalase, CAT, etc.) components which are working coordinately to detoxify ROS and retain the redox balance (Hasanuzzaman et al. 2019). Another reactive carbonyl species, methylglyoxal (MG), is generated through various cellular processes under adverse environmental conditions. It has the role of transducing signals to metabolic pathways when produced at an optimum level, but at a higher level becomes cytotoxic and produces glycation end products (Garai et al. 2021). Therefore, to reduce the MG-induced damage, enzymes of the glyoxalase system, i.e., glyoxalase I (Gly I) and glyoxalase II (Gly II), are engaged in the detoxification system and thus, protect the cellular and subcellular organelles (Hasanuzzaman et al. 2019).
To retain the growth and development of plants under abiotic stresses, the use of different types of natural and synthetic biostimulants is now gaining attention due to their potential role in inducing stress tolerance. These biostimulating compounds include humic and fulvic substances, extract from plants and algae, protein hydrolysates, phytohormones, chelating agents, nonessential but beneficial trace elements, fungi, plant growth-promoting bacteria, etc. (Hasanuzzaman et al. 2021a). Seaweeds are perennial, free-floating, and autotrophic marine algae that are comprising around 10,000 species of green, brown, and red categories (Battacharyya et al. 2015). They are eco-friendly, nontoxic, biodegradable, and bioindicators of the water quality status of coastal areas (Craigie 2011). Among the species, brown seaweeds extracts, such as Ascophyllum nodosum, Durvillea potatorum, Ecklonia maxima, Macrocystis pyrifera, and Sargassum cymosum are the most widely used commercial product of agriculture (Khan et al. 2009). A. nodosum extracts (ANE) contain a diverse range of inorganic (i.e., nitrogen, phosphorous, potassium, magnesium, calcium, iron, zinc, and sulfur), organic (i.e., betaines), polysaccharides (i.e., alginates, fucoidans, laminarans), vitamins, phenolic compounds, and other bioactive secondary metabolites (Rayorath et al. 2009; MacKinnon et al. 2010; Battacharyya et al. 2015). These compounds have interactive effect on the activation of signaling cascades and enhancing the metabolic processes of plants to endure stressed conditions as well as it also improves root microbe interactions and nutrient availability (Frioni et al. 2018; Di Stasio et al. 2018). Previous reports on the ANE also indicated a synergistic effect on improving the growth, development, yield, and quality of crops through alleviating abiotic stress of many plants (Fan et al. 2011; Di Stasio et al. 2018; Carrasco-Gil et al. 2018).
Moringa (Moringa oleifera) is a fast-growing and medicinal plant belonging to the family of Moringaceae, commonly found in the tropical climates of Africa and Asia (Sreelatha et al. 2011). Moringa leaf extract (MLE) contains many bioactive substances, including proteins, vitamins, carotene, calcium, potassium, and natural antioxidants (Moyo et al. 2012). Besides this, MLE is also considered a natural growth regulator due to the presence of zeatin (a derivative of cytokinin) and other phenolic compounds that boost the growth of plants by preserving water status, membrane integrity, and antioxidant activities (El-Hack et al. 2018). Thus, the use of MLE has gained immense attention from researchers to attenuate the abiotically stressed crops (Khan et al. 2022; Ramzan et al. 2022; Al-Taisan et al. 2022). Therefore, both ANE and MLE can increase nutrient availability, enhance phytohormone synthesis, maintain osmotic balance, and reduce overaccumulation of ROS by improving the antioxidant defense system of plants (Trivedi et al. 2018; Habiba et al. 2019; Hafeez et al. 2022; Auesukaree et al. 2022).
Rice (Oryza sativa L.) is the second most important cereal crop after wheat due to its highest calorie consumption (IRRI 2022). Heavy metals/metalloids toxicity adversely affects the growth, development, and yields of crops, including Cd, and thereby enters into the food chain of humans upon consumption (Aziz et al. 2015). However, scientists have adopted several mitigation strategies to alleviate the Cd contamination from the field, but the ANE and MLE have rarely been used. Thus, the present experiment was set with a hypothesis that the application of ANE and MLE on the Cd-affected rice will alleviate the Cd-induced oxidative stress through enhancing antioxidant defense and ROS metabolism.
Materials and methods
Plant materials, growth condition and stress treatments
Rice seeds (O. sativa cv. BRRI dhan89) were collected from the Bangladesh Rice Research Institute (BRRI), Bangladesh. Healthy, vigorous, uniform, and disease free seedlings of 35-day-old were transplanted in 14-L plastic pot supplemented with the recommended dose of fertilizers (BRRI 2020). The texture of the soil used for the experiment was clay loam which consist of sand (30%), silt (40%), and clay (30%), organic carbon (0.78%), and organic matter (1.35%) (Hasanuzzaman et al. 2021b). After seedling establishment, at 25 days after transplanting (DAT), plants were treated with cadmium chloride (50 mg kg−1 soil) through irrigation. Soil incorporation of 0.25% ANE (Jollive, Chiba, Japan) and 0.5% MLE (NatureVit, Jodhpur, India) was done once on the same day of stress exposure. The ANE contains carbohydrate (44.7 ± 2.1%), ash (18.6 ± 0.9%), protein (5.2 ± 0.2%), lipids (3.0 ± 0.1%), phenolics (1.4 ± 0.2%), and other compounds (13.6%) like sodium polymannuronate, starch, and glutamate. On the other hand, MLE consists of different types of bioactive compounds such as vitamin A, vitamin C, ascorbic acid, tocopherol, carotenoids, total phenols, phenolic acids, flavonoids, and some essential minerals. The plants without Cd and ANE or MLE supplemented were considered as control. Different attributes of growth, physiology and biochemical parameters were estimated at 55 DAT, whereas the yield components were measured after the completion of the life cycle of the plants at 125 DAT. The experiment followed a completely randomized design (CRD) with three replications.
Determination of plant growth parameters
The plant height was determined by measuring the height of four hills of each treatment from the base to the uppermost leaf tip. Then the values were averaged and expressed as cm. The number of secondary tillers was counted manually from four hills of each treatment, and the average value was expressed as the number of tillers hill−1.
Three hills from each treatment were uprooted and thoroughly washed with distilled water (dH2O) to remove the adhering mud. Then the fresh weight (FW) of the hills was measured with a digital balance. To determine the dry weight (DW), these hills were air-dried for a couple of days to reduce the excess moisture and then oven-dried for 72 h at 80 °C in an electric oven. The weight of the hills was further divided by three to express as g hill−1.
Determination of physiological attributes
Leaf relative water content
Three leaf laminas were plucked from each treatment and weighed for the FW. These laminas were soaked in dH2O for 24 h covered with filter paper, and kept in a dark place. After 24 h, the turgid weight (TW) of the leaves was measured by wiping the excess dH2O with blotter paper. The DW of the leaves was taken after drying in an electric oven at 80 °C for 48 h. The relative water content (RWC) of the leaf was calculated following this equation: RWC (%) = [(FW − DW)/(TW − DW)] × 100 (Barrs and Weatherley 1962).
Proline content
For estimating the proline (Pro) content, fresh leaf (0.5 g) was homogenized with aqueous sulfosalicylic acid (3%) in an ice-cooled mortal pestle. A clear aliquot was obtained by centrifuging the homogenate at 12,000 × g for 15 min. For incubating in a water bath at 100 °C for 1 h, acid ninhydrin solution (dissolved in 6 M phosphoric acid), glacial acetic acid, and the supernatant were incorporated at a ratio of 1:1:1. The incubated mixture was then cooled in an ice bath, and 4 ml of toluene was added to separate the free Pro. The optical density of the colored chromophore was observed at 520 nm in a spectrophotometer, and the calculation was done by plotting the value against a standard concentration of Pro (Bates et al. 1973).
Quantification of oxidative stress indicators
Malondialdehyde content
Half of a gram of fresh leaf sample was macerated with 5% trichloroacetic acid (TCA) in an ice-chilled mortar pestle to determine the amount of lipid peroxidation as MDA content. After centrifugation of the homogenate at 12,000 × g for 12 min, a clear aliquot was obtained. Then 1 ml of this aliquot was fused with 4 ml of thiobarbituric (TBA) acid reagent (0.5% TBA and 20% TCA) following the protocol of Heath and Packer (1968) and the mixture was heated in a water bath at 95 °C for 30 min. After cooling in an ice bath, the absorbance of the colored chromophore was spectrophotometrically detected at 532 nm, and for a non-specific value, it was observed at 600 nm. The final quantification of the amount of MDA content was estimated by subtracting the value of non-specific absorbance using 155 mM−1 cm−1 as an extinction coefficient.
Hydrogen peroxide content
A fresh leaf of 0.5 g was ground with 5% TCA solution, and the homogenate was centrifuged at 12,000 × g for 12 min. A clear supernatant was found after the centrifugation, and 1 ml of this extracted solution was mixed with 1 ml of potassium-phosphate (K-P) buffer (10 mM, pH 7.0) and 1 ml of potassium iodide (KI). Then after mixing properly with a vortex machine, it was incubated for 1 h in a dark place. The optical density of the mixture was taken at 390 nm in a spectrophotometer, and the amount of H2O2 content was calculated using 0.28 μM–1 cm–1 as an extinction coefficient (Yu et al. 2003).
Electrolyte leakage
For estimating the EL, two different electrical conductivities viz. EC1 and EC2 were needed for the calculation. For EC1, 0.5 g of a leaf was plucked and chopped into small pieces. The leaves were put into a Falcon tube, and after adding 15 ml of dH2O, it was incubated for 1 h at 40 °C in a water bath. Then the EC1 was measured with an electrical conductivity meter (HI-993310, Hanna, USA) after cooling at room temperature. The same sample was further heated in an autoclave at 121 °C for 40 min for the measurement of EC2. Following the formula, the EL of the leaf was calculated: EL (%) = EC1/EC2 × 100 (Dionisio-Sese and Tobita 1998).
Estimation of ascorbate and glutathione content
Fresh leaf (0.5 g) was homogenized in an ice-cooled mortar pestle using metaphosphoric acid (5%) and ethylenediaminetetraacetic acid (EDTA; 1 mM). The homogenate was centrifuged (12,000 × g, 15 min) to collect the supernatant. The content of AsA and GSH were estimated using the collected supernatant following the protocol of Huang et al. (2005) and Hasanuzzaman et al. (2018), respectively. The supernatant was neutralized with 0.5 M, pH 7.0 K-P buffer and dH2O for the estimation of reduced AsA, whereas neutralization was done with 0.1 M dithiothreitol (DTT) for total AsA. The optical density was then assayed with 100 mM, pH 6.5 K-P buffer, and ascorbate oxidase (AO; 0.5 U) at 265 nm spectrophotometrically. The final calculation was done by plotting the value of AsA and total AsA against a standard curve, and the dehydroascorbate (DHA) content was estimated following the formula, DHA = total AsA-reduced AsA (Huang et al. 2005).
For estimating the GSH and GSSG, the supernatant was neutralized with dH2O and 2–vinylpyridine, respectively, along with 0.5 M pH 7.0 K-P buffer. The neutralized solution was further incorporated with 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB), nicotinamide adenine dinucleotide phosphate (NADPH), and glutathione reductase (GR). Then the optical density of the mixture was spectrophotometrically observed at 412 nm, and the content was estimated by plotting the values against a standard curve. The GSH content was estimated following the formula, GSH = total GSH – GSSG (Hasanuzzaman et al. 2018).
Enzyme extraction, soluble protein quantification, and antioxidant enzymes assay
The enzyme was extracted using an extraction buffer prepared with K-P buffer (100 mM, pH 7.0), l-ascorbic acid (AsA; 1 mM), KCl (100 mM), β-mercaptoethanol (5 mM), and glycerol (10%). For extracting the enzyme, 0.5 g of the fresh leaf was homogenized with the buffer solution (1 ml) and centrifuged for 12 min at 12,000 × g. The aliquot was further preserved to determine the free protein and antioxidant enzyme activity (Hasanuzzaman et al. 2018).
The free protein was quantified using a standard of bovine serum albumin (BSA) following the procedure of Bradford (1976). The optical density was measured after incorporating the extracted supernatant with the Bradford reagent (containing coomassie brilliant blue (CBB G-250), 100% ethanol, 85% ortho-phosphoric acid, and dH2O) at 595 nm in a spectrophotometer. The final concentration of protein was determined by plotting the values against a standard curve of BSA.
The CAT (EC: 1.11.1.6) activity was determined using a reaction buffer prepared with H2O2 (15 mM) and K-P buffer (50 mM, pH 7.0). The enzymatic activity was accelerated with the addition of H2O2. The final CAT activity was quantified using an extinction coefficient of 39.4 M−1 cm−1 (Hasanuzzaman et al. 2018).
The GPX (EC: 1.11.1.9) activity was estimated following the methods of Hasanuzzaman et al. (2018) and Elia et al. (2003). A reaction buffer consists of NADPH (0.12 mM), GR (1 U), GSH (2 mM), GR (1 unit), sodium azide (NaN3; 1 mM), K-P buffer (100 mM, pH 7.0), and EDTA (1 mM), which was required for observing the activity. The H2O2 was added lastly to initiate the enzymatic reaction, and an extinction coefficient of 6.62 mM−1 cm−1 was used to quantify the activity.
The APX (EC: 1.11.1.11) activity was assayed with a reaction buffer containing Asc (0.5 mM), EDTA (0.1 mM), K-P buffer (15 mM, pH 7.0), and H2O2 (0.1 mM). The inclusion of H2O2 should be done for the activation of enzymatic reactions. The final activity was computed using 2.8 mM−1 cm−1 as an extinction coefficient (Nakano and Asada 1981).
The activity of MDHAR (EC: 1.6.5.4) was determined using an assay buffer containing Asc (2.5 mM), NADPH (0.2 mM), Tris–HCl (50 mM, pH 7.5), and AO (1 U). Here, the inclusion of AO initiated the reaction of the enzymes, and the final quantification was done with an extinction coefficient of 6.2 mM−1 cm−1 (Hossain et al. 1984).
The DHAR (EC: 1.8.5.1) activity was determined according to the procedure of Nakano and Asada (1981). An assay buffer containing GSH (2.5 mM), DHA (0.1 mM), EDTA (0.1 mM), and K-P buffer (50 mM, pH 7.0) was needed to observe the enzymatic activity. The DHA should be added last to initiate the reaction, and the calculation was done with an extinction coefficient of 14 mM−1 cm−1.
The activity of GR (EC: 1.6.4.2) was estimated following the method of Hasanuzzaman et al. (2018). For observing the activity, an assay buffer containing GSSG (1 mM), NADPH (0.2 mM), EDTA (1 mM), and K-P buffer (0.1 M, pH 7.8) was required where the GSSG acts as a precursor of the reaction. The activity was quantified using 6.2 mM−1 cm−1 as an extinction coefficient.
The Gly I (EC: 4.4.1.5) activity was assayed with a reaction buffer prepared with GSH (100 mM), magnesium phosphate (MgSO4; 16 mM), methylglyoxal (MG; 35 mM), and sodium-phosphate (Na-P) buffer, in which MG should be added lastly to commence the enzymatic reactions. The estimation of the final activity was calculated using an extinction coefficient of 3.37 mM−1 cm−1 (Hasanuzzaman et al. 2018).
The Gly II (EC: 3.1.2.6) activity was quantified with an assay buffer consisting of DTNB (0.2 mM), S-D-lactoylglutathione (SLG; 1 mM), and Tris–HCl buffer (100 mM, pH 7.2). Here, the addition of SLG commenced the enzymatic activity, and the final computation of the activity was done with 13.6 mM−1 cm−1 as an extinction coefficient (Principato et al. 1987).
Determination of yield and yield attributes
The panicle length was measured from the base to the apex of each panicle from each treatment. The average value from 10 randomly selected panicles was expressed as cm.
The fully developed kernel was considered as filled grain, whereas the partially developed or unfilled kernel was regarded as unfilled grain. The filled and unfilled grains were separated manually from 10 panicles, and the number was counted with an automatic seed counter. The average value of 10 panicles was expressed as the number of filled or unfilled grains panicle−1.
Primary branches from 10 panicles were counted for the estimation of the rachis number from each treatment. The average value was expressed as the number of rachis panicle−1.
One thousand filled and dried grains were counted with an automatic seed counter and weighed for the estimation of 1000-grain weight. At the same time, the total grain of a hill was considered as the grain yield hill−1. The 1000-grain weight and grain yield hill−1 were expressed as g.
Statistical analysis
A computer-based software CoStat v.6.400 (CoHort Software, Monterey, CA, USA) was used for the statistical analysis. The average values were obtained from three replications, and Tukey’s HSD test was used to compare the treatments at p ≤ 0.05 (CoStat 2008). The correlation analysis among the different parameters was done using Origin Pro 2022 software (OriginLab, USA).
Results
Effects of Cd and ANE or MLE supplementation on plant growth and biomass accumulation of rice
Upon exposure to Cd stress, the plant height was decreased by 15%, while the number of tillers hill−1 was declined by 21% compared to the controls. However, the supplementation of ANE improved the plant height by 7%, whereas a 16% increase in the height was found with MLE compared to the corresponding Cd stressed alone. Similarly, the number of tillers hill−1 was also enhanced with the ANE and MLE under Cd stress by 21 and 33%, respectively (Fig. 1A, B).
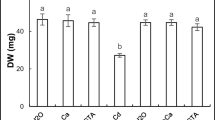
Effect of cadmium stress and supplementation of ANE or MLE on the plant height (A), number of tillers hill−1 (B), fresh weight (C), and dry weight (D) of rice. Mean value (± SD) was calculated from three replications and the letters on the respective bars indicated significant differences among the treatments after Tukey’s HSD test
The reduction of FW and DW in the Cd stressed plants was 36 and 19%, respectively, compared to the controls. However, the deleterious effect of Cd was reverted with ANE supplementation, and it improved the FW and DW by 26 and 19%, respectively, compared to the Cd affected alone. Conversely, the MLE improved the FW and DW by 48 and 22%, respectively in the Cd stressed plants (Fig. 1C, D).
Effects of ANE or MLE on relative water content and proline accumulation of rice under Cd stress
The leaf RWC was declined by 18% when exposed to Cd compared to the controls. However, the RWC of the Cd stressed plants were improved when supplemented with ANE or MLE (Fig. 2A). A notable increase of Pro was seen under Cd by 99% compared to the controls. Moreover, supplementation of ANE and MLE was found to be effective in diminishing the Pro accumulation under Cd stress (Fig. 2B).
Effect of cadmium stress and supplementation of ANE or MLE on the relative water content (A) and proline accumulation (B) of rice. Mean value (± SD) was calculated from three replications and the letters on the respective bars indicated significant differences among the treatments after Tukey’s HSD test
Effects of ANE or MLE on reducing oxidative stress indicators of rice under Cd toxicity
When exposed to Cd, a remarkable increase of MDA by 26% was observed compared to the controls. Supplementation of ANE decreased MDA by 15%, while a 34% reduction was found with MLE compared to the Cd treated only (Fig. 3A). Similarly, the H2O2 was elevated by 36% under Cd compared to the controls. However, the ANE reduced it by 26%, whereas the declination was 30% with MLE compared to the Cd affected alone (Fig. 3B). Further, increment of EL was found by 8% due to Cd stress, which was reduced with ANE and MLE supplementation (Fig. 3C).
Effect of cadmium stress and supplementation of ANE or MLE on the MDA content (A), H2O2 content (B), and electrolyte leakage (C) of rice. Mean value (± SD) was calculated from three replications and the letters on the respective bars indicated significant differences among the treatments after Tukey’s HSD test
Effects of Cd and ANE or MLE on the AsA-GSH pool of rice
A notable declination of the AsA content was found by 35% under Cd, while the DHA content was increased by 43% compared to the controls. Thus, the ratio of AsA/DHA also declined by 55% in the Cd stressed plants. However, MLE supplementation was found to be more beneficial rather than ANE. The MLE increased the AsA content by 53% and AsA/DHA ratio by 100%, while it reduced the DHA content by 24% compared to the Cd only (Fig. 4A–C).
Effect of cadmium stress and supplementation of ANE or MLE on the AsA (A), DHA (B), AsA/DHA ratio (C), GSH (D), GSSG (E), and GSH/GSSG (F) ratio of rice. Mean value (± SD) was calculated from three replications and the letters on the respective bars indicated significant differences among the treatments after Tukey’s HSD test
Under Cd stress, the GSH and GSSG contents were increased by 26 and 88%, respectively, while the GSH/GSSG reduced by 33% compared to the controls. Nevertheless, enhancement of GSH was further observed with MLE supplementation by 34%, whereas it declined the GSSG by 24% compared to the Cd affected only. Consequently, the ratio of GSH/GSSG was also increased by 77% with MLE under Cd (Fig. 4D–F).
Effects of ANE or MLE on the activities of antioxidant enzymes under Cd stress
The APX activity was increased by 44% upon exposure to Cd compared to the controls. Further improvement of APX was seen with the MLE incorporated plants by 19%. When exposed to Cd, the MDHAR activity was upgraded by 34%, whereas the DHAR activity was degraded by 31% compared to the controls. The MLE incorporation enhanced the activities of both MDHAR and DHAR by 32 and 38%, respectively, in the Cd-affected plants. Similarly, the activity of GR also accelerated under Cd by 64%, which was further augmented with the MLE supplementation by 34% (Fig. 5A–D). Compared to the controls, the CAT activity was enhanced by 46%, while the GPX activity was declined by 21% under Cd. Moreover, the activity of CAT was accelerated by 41% with the supplementation of MLE compared to the Cd affected alone. Nevertheless, the GPX activity was also upgraded with the ANE (27%) and MLE (67%) supplementation compared to the Cd only (Fig. 5E, F).
Effect of cadmium stress and supplementation of ANE or MLE on the activities of APX (A), MDHAR (B), DHAR (C), GR (D), CAT (E), and GPX (F) of rice. Mean value (± SD) was calculated from three replications and the letters on the respective bars indicated significant differences among the treatments after Tukey’s HSD test
Effects of ANE or MLE on the activities of glyoxalase system enzyme under Cd stress
Compared to the controls, the activities of Gly I and Gly II were declined by 59 and 80%, respectively under Cd stress. Moreover, the supplementation of the ANE uplifted the Gly I activity by 38% and increased the Gly II by 182% compared to the Cd stressed alone. Similarly, the MLE application also upregulated the activities of Gly I and Gly II by 59 and 437%, respectively compared to the Cd only (Fig. 6A, B).
Effect of Cd and ANE or MLE on the yield and yield attributes of rice
The imposition of Cd adversely affected the yield contributing parameters of rice. The panicle length was reduced by 27% under Cd compared to the controls. However, the MLE supplemented plants increased the panicle length by 19% compared to the Cd alone. When exposed to Cd, the number of filled grains panicle−1 was declined by 45%, whereas the unfilled grains panicle−1 was increased by 32%, compared to the controls. Moreover, the MLE incorporation only enhanced the number of filled grains panicle−1 by 46% compared to the Cd only. Nevertheless, the supplemental ANE decreased the number of unfilled grains panicle−1 by 10%, in contrast, a 15% reduction was found with the MLE incorporated plants compared to the Cd affected alone. A notable reduction of 1000-grain weight was also observed by 24% when exposed to Cd stress, and the weight was increased with the MLE supplementation in Cd treated plants by 13%. The grain yield hill−1 was also declined by 25% upon exposure to Cd, compared to the controls. However, the ANE and MLE supplementation further improved the grain yield hill−1 by 14 and 24%, respectively, compared to the Cd stressed alone (Fig. 7A–E).
Effect of cadmium stress and supplementation of ANE or MLE on the panicle length (A), number of filled grains panicle−1 (B), number of unfilled grains panicle−1 (C), 1000-grain weight (D), and grain yield hill−1 (E) of rice. Mean value (± SD) was calculated from three replications and the letters on the respective bars indicated significant differences among the treatments after Tukey’s HSD test
Correlation analysis among the different parameters as affected by Cd stress
From the correlations among the variables, it is clear that oxidative stress (MDA, H2O2, and EL) have a negative correlation with RWC, whereas the Pro content is positively correlated. The AsA, AsA/DHA, and GSH/GSSG are negatively associated with the MDA, H2O2, and EL but have positive relationships with the DHA and GSSG. The GSH, MDHAR, and CAT are negatively correlated with MDA, but they positively correlate with H2O2 and EL. Furthermore, the APX and GR have positive relationships with the MDA, H2O2, and EL; in contrast, the DHAR, GPX, Gly I, and Gly II are negatively related. Thus, the growth (plant height, tiller, FW, and DW) and yield components (panicle length, filled grain, 1000-grain weight, grain yield) are adversely affected and negatively correlated with the oxidative stress indicators (Fig. 8).
Correlation analysis among the parameters of Cd-affected rice. Here, PHT- plant height; FW—fresh weight; DW—dry weight; RWC—relative water content; Pro—proline; MDA—malondialdehyde; H2O2—hydrogen peroxide; EL—electrolyte leakage; AsA—ascorbate; DHA—dehydroascorbate; GSH—reduced glutathione; GSSG—oxidized glutathione or glutathione disulfide; APX—ascorbate peroxidase; MDHAR—monodehydroascorbate reductase; DHAR—dehydroascorbate reductase; GR—glutathione reductase; CAT—catalase; GPX—glutathione peroxidase; Gly I—glyoxalase I; Gly II—glyoxalase II; TSW—1000-grain weight
Discussion
Developing plant tolerance to Cd stress is becoming one of the most significant challenges for plant biologists due to its toxic effects on plants. Use of plant biostimulants is one of the efficient and prospective approaches for improving plant defense against Cd toxicity. Seaweeds and moringa are both natural creatures. Seaweed is an alga of coastal areas enriched with different nutrients, minerals, vitamins, and other secondary metabolites (Battacharyya et al. 2015). The polysaccharide (e.g., alginate) content and osmoregulators (mannitol) of seaweed can take part in regulation of lipid peroxidation, ROS contents and osmotic balance (Li et al. 2018; Habiba et al. 2019). Moringa has stimulating effect on germination, plant height, photosynthesis, reproductive features, crop yield, and quality. The micronutrients of MLE have been found to be beneficial in various plant growth (Auesukaree et al. 2022; Karthiga et al. 2022). Moreover, moringa contains zeatin and ascorbic acid which have the potentiality to decrease ROS-induced damages and to improve antioxidant defense system during stressful condition (Zhang et al. 2019; Azzam et al. 2022). It is evident that plant biostimulants, including seaweed and moringa extracts, provide protection against oxidative stress in several crops (Hasanuzzaman et al. 2021a).
Plant exposed to Cd exhibits cellular disintegration and inhibition of cell expansion and cell division which results in growth inhibition (Chen et al. 2019). Cadmium could inhibit cellular differentiation, plant cell division, and accumulation of photosynthates, and these phenomena are in line with our result that showed Cd treatment caused a reduction in plant height, FW, and DW as a consequence of the retardation of normal cellular growth. However, supplementation with ANE and MLE showed improvement in Cd-treated plants. Previous reports provided the notion that SWE and MLE can synthesize growth regulators, which directly contribute to better growth even under stress condition (Bonomelli et al. 2018 and Al-Taisan et al. 2022). The minerals and secondary metabolites of seaweed extract (SWE), particularly calcium being an antagonistic ion of Cd has the potency to impair Cd entry and absorption to plant root. Thus, it helps in maintaining cell functions and enhancing plant metabolism for supporting plant growth even under Cd toxicity (Ye et al. 2020). Moreover, some of the promising effects of MLE in terms of growth improvement are also reported in T. aestivum (Basu et al. 2022) and Mentha piperita (Al-Taisan et al. 2022). It is known that moringa leaf has a high content of plant growth-promoting hormones like auxin (Moyo et al. 2012), which has a pivotal role in maintaining plant growth even under stress (Santner and Estelle 2009). So, MLE application in our experiment accelerated rice growth parameters by mitigating the Cd stress. Furthermore, Hafeez et al. (2022) explained the improved plant morphological character as a consequence of increased minerals, photosynthesis, and enzyme activity after MLE treatment, which is in congruence with our findings.
Inhibition of water uptake is one of the primary responses to metal toxicity. Due to Cd exposure, RWC was decreased in our experiment. The negative impact of Cd on the permeability of plasma membrane can be considered as the reason behind the reduced water content (Ferńandez et al. 2013). Under stress condition, plants synthesize different types of compatible solutes (i.e., Pro) which act as osmoprotectants to protect cells from damaging and induce stress tolerance in plants (Forlani et al. 2019). A similar pattern was observed in our experiment, where the highest Pro content was recorded in Cd-induced rice plants. Ozfidan-Konakci et al. (2018) stated that to mitigate Cd-induced damages, rice accumulated more Pro that takes part in scavenging ROS which conferred Cd stress tolerance and their findings are in congruence with our finding. However, both ANE and MLE evolved in mitigating the stress through increasing the RWC of leaves and reducing Pro content. It has been observed that SWE can maintain leaf water potential, water use efficiency, and osmo-protection as it has a fair amount of osmotically active molecules (Di Stasio et al. 2020). Moreover, seaweed contains some osmoregulators like mannitols and minerals like potassium which is vital for the regulation of osmotic balance in plants (Oddo et al. 2011; Habiba et al. 2019). Therefore, ANE-induced osmoprotection was evident in our crop. Besides, Basu et al. (2022) reported the positive effects of moringa, where it has been explained that the accumulation of micronutrients (B, Zn, Si) in moringa-treated T. aestivum improved osmoprotectants (total soluble solid, Pro) and flag leaf RWC as well conferring MLE-induced tolerance to the unfavorable condition that supports our results.
An increasing trend of MDA and H2O2 contents was exhibited when rice was treated with Cd. It is known that stressful condition is responsible for oxidative burst that overgenerates ROS (indicated by H2O2 content in the present study), exceeding the scavenging capacity and ultimately leading to increased lipid peroxidation (MDA content), and as a result, increased EL was also noticeable. Reduction of oxidative stress markers along with a lower abscisic acid (ABA) under stress condition was evident by Campobenedetto et al. (2021). This might be happened due to the role of the alginate content of seaweed in regulating lipid peroxidation, ROS contents, and ABA signaling genes that ultimately resulted in enhanced resistance capacity (Li et al. 2018). As MLE during stressful condition can recover tissue membrane maintaining the integrity and give protection against stress-induced damages and subsequently decreases oxidative stress markers (El-Mageed et al. 2017), this may explain the better Cd detoxification capacity of MLE. Moreover, the efficiency of MLE in generating zeatin content (a form of cytokinin) that has better potency to reduce free radical–induced damages (Azzam et al. 2022) can also be illustrated as the reason for arresting overproduced ROS in stress condition.
When plants are exposed to stressful condition, the non-enzymatic antioxidants, particularly the AsA-GSH pool efficiently act in sequestering stress-induced overgenerated ROS so that they can protect the cell from oxidative damage (Zhao et al. 2020). Moreover, the scavenging potency of APX to detoxify ROS-induced H2O2 is dependent on AsA (electron donor substrate). The scavenging reaction also evolves DHA later, which is also a component of the AsA-GSH pool (Sofo et al. 2015). The reduction of AsA with an increment of DHA and, subsequently the decreasing trend of AsA/DHA indicates Cd imposed oxidative stress in rice. Additionally, during detoxifying the ROS generated by Cd toxicity, GSH changes into GSSG, so an increased GSSG content with decreased GSH/GSSG ratio at the same time might be considered as an indicator of the oxidative stress under Cd stress. This kind of changing the behavior of the AsA-GSH pool as a response to Cd exposure was also observed by Han et al. (2018), and it is also to be noted that GSH being a precursor of phytochelatin can bind and transport metal and therefore effectively works in chelating Cd (Zagorchev et al. 2013). It was observed that in MLE treated plants both the contents of AsA and GSH were increased further with decreased DHA and GSSG contents, which in turn increased AsA/DHA and GSH/GSSG ratios. This conforms better tolerance to Cd-induced oxidative stress. Moringa-treated plant with better AsA-GSH regulation was also exhibited in Phaseolus vulgaris (Rady et al. 2013), highlighting the availability of minerals, amino acids, and antioxidants in MLE and its attribution towards antioxidant defense. Additionally, MLE is rich in ascorbic acid, an integral part of antioxidant defense. Ascorbic acid is a multifunctional metabolite that can strongly negate ROS-induced oxidative stress and induce antioxidative defense cooperating with GSH (Zhang et al. 2019). Therefore, MLE can attenuate strong antioxidant defense with better osmoprotection.
Activities of APX, GR, and CAT were upregulated due to Cd exposure. This indicates plants self-defense mechanism to protect from oxidative stress-induced damage and the similar stimulated enzyme activities were observed in Cd-induced Capsicum annuum (Kaya et al. 2020). On the contrary, DHAR and GPX activities were reduced upon Cd treatment in the present experiment. The tendency of Cd to combine with cysteine residues (thiol group) make barrier to the enzyme activities, which are account for ROS scavenging and redox homeostasis maintaining (DalCorso et al. 2013). Therefore, reductions of these enzymes with an increment of MDA and H2O2 contents indicate Cd induced toxicity to rice. Seaweed extract increased antioxidant enzyme activities to some extent, which agrees with Trivedi et al. (2018) in G. max. Moreover, MLE attenuated Cd-induced damages in rice, elevating the activities of all measured antioxidant enzymes along with reducing ROS. A similar trend of moringa in stress adaptation was recorded in P. vulgaris L. (Rady et al. 2013) and Carthamus tinctorius (Hafeez et al. 2022).
The Gly I and Gly II are enzymes that build up glyoxalase system to reduce overproduced MG toxicity upon stress exposure (Shivani et al. 2022). Therefore, decreased Gly I and Gly II activities indicate plant stress-induced damage. Cadmium-induced downregulation of glyoxalase system was reported in Brassica campestris (Raihan et al. 2022), which agrees with our present study. Salicylic acid is an important polyphenol of seaweed that can activate glyoxalase activities to detoxify the methylglyoxal-mediated toxicity under metal stress (Zaid et al. 2019), which supports our results. As a consequence of MLE application, the glyoxalase activities (Gly I and Gly II) were increased noticeably, which indicates that rice, along with MLE was able to detoxify MG-induced toxicity caused by Cd in the present experiment.
The yield of rice was significantly affected by Cd stress and reflected as reduced tiller number, panicle length, no. of filled grain, grain weight as well as grain yield. As Cd started the damaging effect from the very initial stage through affecting growth attributes and impairing normal physiological and biochemical functions of rice, the yield contributing characteristics was recorded to decrease markedly. Li et al. (2020) demonstrated that Cd causes a reduction of effective tiller number, grain number, 1000-grain weight, and increment of Cd absorption in rice, which resulted in poor yield. The findings are in consistent with our experiment. As a result of seaweed addition, rice yield and quality were improved along with higher nutrient use efficiency as reported in previous research (Layek et al. 2018). This corroborates with our findings where the positive effect of ANE addition was noticed in grain yield even under Cd stress. Yield and yield attributes were increased due to MLE addition because of improved growth, physiology, and biochemical profile of MLE-treated rice even under Cd toxicity. El-Mageed et al. (2017) explained the capability of MLE in improving leaf anatomy as well as photosynthesis under the stressed condition as a precursor of compensated yield, which is in line with our findings.
Conclusions
In a nutshell, it can be said that both ANE and MLE have the capacity to improve Cd tolerance to rice but in depth, the data of the present study proved that ANE and MLE improved rice growth and physiology, enhanced antioxidant defense, reduced oxidative stress, and ultimately recovered yield which indicate improved Cd toxicity tolerance. ANE showed better performance in uplifting plant growth and biomass, RWC, AsA content, GPX activity, glyoxalase system, and grain yield. Besides, it also played a role in scavenging ROS, reducing, EL, and boosting osmoprotection. Furthermore, MLE was proved as an excellent stimulator combating Cd-induced damages in almost all measured parameters. Since moringa contains antioxidants that facilitated better defense by activating the AsA-GSH pool, accelerating APX, MDHAR, DHAR, GR, CAT, and GPX activities under Cd exposure. Additionally, the improved antioxidant defense showed better detoxification with reduced oxidative stress markers, Pro content, DHA, and GSSG contents. The improved Gly I and Gly II activities further confirmed MG toxicity tolerance in MLE added Cd treatment (Fig. 9). Therefore, the application of ANE/MLE can be suggested as an efficient eco-friendly tool to mitigate oxidative stress and to improve rice production.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Al-Taisan WA, Alabdallah NM, Almuqadam L (2022) Moringa leaf extract and green algae improve the growth and physiological attributes of Mentha species under salt stress. Sci Rep 12:14205. https://doi.org/10.1038/s41598-022-18481-5
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 255:459–469
Auesukaree C, Bussarakum J, Sirirakphaisarn S, Saengwilai PJ (2022) Effects of aqueous Moringa oleifera leaf extract on growth performance and accumulation of cadmium in a Thai jasmine rice—Khao Dawk Mali 105 variety. Environ Sci Pollut Res 29:46968–46976
Aziz R, Rafiq MT, Li T, Liu D, He Z, Stoffella PJ, Sun K, Xiaoe Y (2015) Uptake of cadmium by rice grown on contaminated soils and its bioavailability/toxicity in human cell lines (Caco-2/HL-7702). J Agric Food Chem 63:3599–3608
Azzam CR, S-nS Z, Bamagoos AA, Rady MM, Alharby HF (2022) Soaking maize seeds in zeatin-type cytokinin biostimulators improves salt tolerance by enhancing the antioxidant system and photosynthetic efficiency. Plants 11:1004. https://doi.org/10.3390/plants11081004
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Basu S, Prabhakar AA, Kumari S, Aabha, Kumar RR, Shekhar S, Prakash K, Singh JP, Singh GP, Prasad R, Kumar G (2022) Micronutrient and redox homeostasis contribute to Moringa oleifera-regulated drought tolerance in wheat. Plant Growth Regul 2022. https://doi.org/10.1007/s10725-022-00795-z
Bates LS, Waldren RP, Teari D (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic 196:39–48
Bonomelli C, Celis V, Lombardi G, Mártiz J (2018) Salt stress effects on avocado (Persea Americana mill.) plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy 8:64. https://doi.org/10.3390/agronomy8050064
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
BRRI (Bangladesh Rice Research Institute) (2020) Adhunik Dhaner Chash (in Bengali). Joydebpur, Dhaka- 1701. p.106
Campobenedetto C, Agliassa C, Mannino G, Vigliante I, Contartese V, Secchi F, Bertea CM (2021) A biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 11:557. https://doi.org/10.3390/agriculture11060557
Carrasco-Gil S, Hernandez-Apaolaza L, Lucena JJ (2018) Effect of several commercial seaweed extracts in the mitigation of iron chlorosis of tomato plants (Solanum lycopersicum L.). Plant Growth Regul 86:401–411
Chen D, Chen D, Xue R, Long J, Lin X, Lin Y, Jia L, Zeng R, Song Y (2019) Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J Hazard Mater 367:447–455
CoStat (2008) CoStat- Statistics Software Version 6.400. CoHort Software, 798 Lighthouse Ave. PMB 320. Monterey, CA, 93940, USA
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393
DalCorso G, Manara A, Furini A (2013) An overview of heavy metal challenge in plants: from roots to shoots. Metallomics 5:1117–1132
Di Stasio E, Van Oosten MJ, Silletti S, Raimondi G, Carillo P, Maggio A (2018) Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J Appl Phycol 30:2675–2686
Di Stasio E, Cirillo V, Raimondi G, Giordano M, Esposito M, Maggio A (2020) Osmo-priming with seaweed extracts enhances yield of salt-stressed tomato plants. Agronomy 10:1559. https://doi.org/10.3390/agronomy10101559
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. https://doi.org/10.1016/s0168-9452(98)00025-9
El-Hack ME, Alagawany M, Elrys AS, Desoky E-SM, Tolba HMN, Elnahal ASM, Elnesr SS, Swelum AA (2018) Effect of forage Moringa oleifera L. on animal health and nutrition and its beneficial applications in soil, plants and water purification. Agriculture 8:145. https://doi.org/10.3390/agriculture8090145
Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
El-Mageed TAA, Semida WM, Rady MM (2017) Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agril Water Manag 193:46–54
Fan D, Hodges DM, Zhang J, Kirby CW, Ji X, Locke SJ, Critchley AT, Prithiviraj B (2011) Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem 124:195–202
Ferńandez R, Bertrand A, Reis R, Mourato MP, Martins LL, Gonźalez A (2013) Growth and physiological responses to cadmium stress of two populations of Dittrichia viscosa (L.) Greuter. J Hazard Mater 244–245:555–562
Forlani G, Bertazzini M, Cagnano G (2019) Stress-driven increase in proline levels, and not proline levels themselves, correlates with the ability to withstand excess salt in a group of 17 Italian rice genotypes. Plant Biol 21:336–342
Frioni T, Sabbatini P, Tombesi S, Norrie J, Poni S, Gatti M, Palliotti A (2018) Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci Hortic 232:97–106
Garai S, Bhowal B, Kaur C, Singla-Pareek SL, Sopory SK (2021) What signals the glyoxalase pathway in plants? Physiol Mol Biol Plants 27:2407–2420
Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trived DK, Ahmad I, Pereira E, Tuteja N (2013) Glutathione reductase and glutathione: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem 70:204–212
Gratao PL, Pompeu GB, Capaldi FR, Vitorello VA, Lea PJ, Azevedo RA (2008) Antioxidant response of Nicotiana tabacum cv. Bright Yellow 2 cells to cadmium and nickel stress. Plant Cell Tiss Organ Cult 94:73. https://doi.org/10.1007/s11240-008-9389-6
Habiba U, Ali S, Rizwan M, Ibrahim M, Hussain A, Shahid MR, Alamri SA, Alyemeni MN, Ahmad P (2019) Alleviative role of exogenously applied mannitol in maize cultivars differing in chromium stress tolerance. Environ Sci Pollut Res 26:5111–5121
Hafeez A, Tipu MI, Saleem MH, Al-Ashkar I, Saneoka H, El Sabagh A (2022) Foliar application of moringa leaf extract (MLE) enhanced antioxidant system, growth, and biomass related attributes in safflower plants. S Afr J Bot 150:1087–1095
Han Y, Wu M, Hao L, Yi H (2018) Sulfur dioxide derivatives alleviate cadmium toxicity by enhancing antioxidant defence and reducing Cd2+ uptake and translocation in foxtail millet seedlings. Ecotoxicol Environ Saf 157:207–215
Hasanuzzaman M, Nahar K, Anee TI, Khan MIR, Fujita M (2018) Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S Afr J Bot 115:50–57
Hasanuzzaman M, Bhuyan MHMB, Anee TI, Parvin K, Nahar K, Mahmud JA, Fujita M (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8:384. https://doi.org/10.3390/antiox8090384
Hasanuzzaman M, Parvin K, Bardhan K, Nahar K, Anee TI, Masud AAC, Fotopoulos V (2021a) Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 10:2537. https://doi.org/10.3390/cells10102537
Hasanuzzaman M, Raihan MRH, Khojah E, Samra BN, Fujita M, Nahar K (2021b) Biochar and chitosan regulate antioxidant defense and methylglyoxal detoxification systems and enhance salt tolerance in jute (Corchorus olitorius L.). Antioxidants 10:2017. https://doi.org/10.3390/antiox10122017
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049
IRRI (International Rice Research Institute) (2022) http://www.knowledgebank.irri.org. accessed on October 6, 2022
Karalija E, Selović A (2018) The effect of hydro and proline seed priming on growth, proline and sugar content, and antioxidant activity of maize under cadmium stress. Environ Sci Pollut Res 25:33370–33380
Karthiga D, Chozhavendhan S, Gandhiraj V, Aniskumar M (2022) The effects of Moringa oleifera leaf extract as an organic biostimulants for the growth of various plants: review. Biocatal Agric Biotechnol 43:102446
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) The role of nitrate reductase in brassinosteroid induced endogenous nitric oxide generation to improve cadmium stress tolerance of pepper plants by upregulating the ascorbate-glutathione cycle. Ecotoxicol Environ Saf 196:110483
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Khan S, Ibrar D, Bashir S, Rashid N, Hasnain Z, Nawaz M, Al-Ghamdi AA, Elshikh MS, Dvořáčková H, Dvořáček J (2022) Application of moringa leaf extract as a seed priming agent enhances growth and physiological attributes of rice seedlings cultivated under water deficit regime. Plants 11:261. https://doi.org/10.3390/plants11030261
Layek J, Das A, Idapuganti RG, Sarkar D, Ghosh A, Zodape ST, Lal R, Yadav GS, Panwar AS, Ngachan S, Meena RS (2018) Seaweed extract as organic bio-stimulant improves productivity and quality of rice in eastern Himalayas. J Appl Phycol 30:547–558
Li S, Yang W, Yang T, Chen Y, Ni W (2015) Effects of cadmium stress on leaf chlorophyll fluorescence and photosynthesis of Elsholtzia argyi—a cadmium accumulating plant. Int J Phytoremediat 17:85–92
Li J, Wang X, Lin X, Yan G, Liu L, Zheng H, Zhao B, Tang J, Guo Y-D (2018) Alginate-derived oligosaccharides promote water stress tolerance in cucumber (Cucumis sativus L.). Plant Physiol Biochem 130:80–88
Li N, Feng A, Liu N, Jiang Z, Wei S (2020) Silicon application improved the yield and nutritional quality while reduced cadmium concentration in rice. Environ Sci Pollut Res 27:20370–20379
MacKinnon SA, Craft CA, Hiltz D, Ugarte R (2010) Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J Appl Phycol 22:489–494
Mahmud JA, Bhuyan MHMB, Anee TI, Nahar K, Fujita M, Hasanuzzaman M (2019) Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In: Hasanuzzaman M, Hakeem KR, Nahar K, Alharby H (eds) Plant abiotic stress tolerance. Springer, Cham, pp 221–257
Moyo B, Oyedemi S, Masika PJ, Muchenje V (2012) Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci 91:441–447
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Oddo E, Inzerillo S, La Bella F, Grisafi F, Salleo S, Nardini A (2011) Short-term effects of potassium fertilization on the hydraulic conductance of Laurus nobilis L. Tree Physiol 31:131–138
Ozfidan-Konakci C, Yildiztugay E, Bahtiyar M, Kucukoduk M (2018) The humic acid-induced changes in the water status, chlorophyll fluorescence and antioxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol Environ Saf 155:66–75
Principato GB, Rosi G, Talesa V, Govannini E, Uolila L (1987) Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochim Biophys Acta 911:349–355
Rady MM, Varma CB, Howladar SM (2013) Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci Hortic 162:63–70
Raihan MRH, Rahman M, Mahmud NU, Adak MK, Islam T, Fujita M, Hasanuzzaman M (2022) Application of rhizobacteria, Paraburkholderia fungorum and Delftia sp. confer cadmium tolerance in rapeseed (Brassica campestris) through modulating antioxidant defense and glyoxalase systems. Plants 11:2738. https://doi.org/10.3390/plants11202738
Ramzan M, Ayub F, Shah AA, Naz G, Shah AN, Malik A, Sardar R, Telesiński A, Kalaji HM, Dessoky ES, Elgawad HA (2022) Synergistic effect of zinc oxide nanoparticles and Moringa oleifera leaf extract alleviates cadmium toxicity in Linum usitatissimum: antioxidants and physiochemical studies. Front Plant Sci 13:900347. https://doi.org/10.3389/fpls.2022.900347
Rayorath P, Benkel B, Hodges DM, Allan-Wojtas P, MacKinnon S, Critchley AT, Prithiviraj B (2009) Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta 230:135–147
Santner A, Estelle M (2009) Recent advances and emerging trends in plant hormone signaling. Nature 459:1071–1078
Seifikalhor M, Hassani SB, Aliniaeifard S (2020) Seed priming by cyanobacteria (Spirulina platensis) and salep gum enhances tolerance of maize plant against cadmium toxicity. J Plant Growth Regul 39:1009–1021
Shivani GSK, Gill RK, Virk HK, Bhardwaj RD (2022) Methylglyoxal detoxification pathway-explored first time for imazethapyr tolerance in lentil (Lens culinaris L.). Plant Physiol Biochem 177:10–22
Silva AJ, Nascimento CWA, Gouveia-Neto AS (2017) Assessment of cadmium phytotoxicity alleviation by silicon using chlorophyll a fluorescence. Photosynthetica 55:648–654
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578
Sreelatha S, Jeyachitra A, Padma PR (2011) Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol 49:1270–1275
Trivedi K, Anand KGV, Vaghela P, Ghosh A (2018) Differential growth, yield and biochemical responses of maize to the exogenous application of Kappaphycus alvarezii seaweed extract, at grain-filling stage under normal and drought conditions. Algal Res 35:236–244
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 290:111197. https://doi.org/10.1016/j.molliq.2019.111197
Xue D, Chen M, Zhang G (2009) Mapping of QTLs associated with cadmium tolerance and accumulation during seedling stage in rice (Oryza sativa L.). Euphytica 165:587–596
Ye W, Wu F, Zhang G, Fang Q, Lu H, Hu H (2020) Calcium decreases cadmium concentration in root but facilitates cadmium translocation from root to shoot in rice. J Plant Growth Regul 39:422–429
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955–963
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432
Zaid A, Mohammad F, Wani SH, Siddique KM (2019) Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol Environ Saf 180:575–587
Zhang K, Wang G, Bao M, Wang L, Xie X (2019) Exogenous application of ascorbic acid mitigates cadmium toxicity and uptake in Maize (Zea mays L.). Environ Sci Pollut Res Int 26:19261–19271
Zhao C, Zhang H, Song C, Zhu JK, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. Innovation 1:100017
Acknowledgements
We cordially acknowledge Md. Mahabub Alam for his generous help during the biochemical analysis. We are thankful to Khussboo Rahman, Mira Rahman, Naznin Ahmed, and Khadeja Sultana Sathi for their assistance during the fieldwork. We also thankful to Ayesha Siddika for her critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
M.H. and K.N. conceived and designed the experiments. M.R.H.R., F.N., and K.N. conducted the experiments. M.H. provided the methodologies and supervised the experiments. M.H. analyzed the data and prepared the illustration. M.H., M.R.H.R, and F.N. wrote the manuscript draft. M.H. and K.N. revised, edited, and formatted the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasanuzzaman, M., Raihan, M.R.H., Nowroz, F. et al. Insight into the physiological and biochemical mechanisms of biostimulating effect of Ascophyllum nodosum and Moringa oleifera extracts to minimize cadmium-induced oxidative stress in rice. Environ Sci Pollut Res 30, 55298–55313 (2023). https://doi.org/10.1007/s11356-023-26251-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26251-7