Abstract
We evaluated the toxic effects of cadmium (Cd) on reproduction, the mitotic cycle, and on the root and leaf anatomy of Pistia stratiotes. Reductions in reproductive structures, symptoms of toxicity, and abnormalities in the cell cycle were noted in plants submitted to the highest Cd concentrations. The effects of the highest concentrations of cadmium could also be observed in the thickening of the epidermis, exoderm, and endoderm of the roots and by reductions in the number of crystalline idioblasts. Furthermore, decreases in the percentages of aerenchyma in the leaf blades and increases in the Carlquist vulnerability indexes of plants exposed to the highest concentrations of Cd were observed. The phytotoxicity of cadmium was quite evident in P. stratiotes, and it could thus be used as a bioindicator species as it was also tolerant of that pollutant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals, also known as trace metals or trace elements, occur naturally in the earth’s crust at very low concentrations (Tan 2000) as a result of geological weathering. Starting with the Industrial Revolution, however, human activities began to significantly increase the concentrations of these metals in the environment, principally due to the indiscriminant use of pesticides and fertilizers, the dumping of untreated industrial wastes, and the extraction and processing of minerals (including electroplating and the fabrication of metallic alloys, batteries, and radiators) (Kenskinkan et al. 2003; Zhou et al. 2008; Melo et al. 2009).
Heavy metals have adverse effects on essentially all living organisms and can make land areas inappropriate for food production or biodiversity preservation as these pollutants accumulate throughout the trophic chain (Melo et al. 2009). Cadmium (Cd) is used in making pigments (cadmium-yellow), as a stabilizer for polyvinyl plastics, in cadmium–nickel batteries, electroplating, and it is a principal subproduct of phosphate fertilizer production (Malavolta 1994).
Cadmium is one of the most toxic heavy metal because it tends to bioaccumulate, has a half-life of 19–40 years in the human body and magnifies the effects of other mutagens (Rojas et al. 1999; Fatur et al. 2003). It has no known function in biological systems and is toxic to living organism even in trace quantities (Mirsha and Tripathi 2008). Among the known effects in humans are liver and kidney problems, hypertension, emphysema, low reproductive function, deformation in the bone structure due to the deleterious effect on the absorption of calcium and, in more severe cases, cancer (Kabata-Pendias and Mukherjee 2007; Arroyo et al. 2012; Kah et al. 2012). In plants, exposure to Cd often causes changes in photosynthetic metabolism and absorption of nutrients, affecting their growth and development (Mirsha and Tripathi 2008).
One of the ways to predict the effects of this element on living organisms is through environmental biomonitoring, which can furnish information about pollution levels in different ecosystems.
Aquatic macrophytes have been widely used as bioindicators and phytoremediators of environmental pollution (Zimmels et al. 2006; Skinner et al. 2007; Wolff et al. 2009; Lu et al. 2010) as they show readily measurable damage to their vital functions when exposed to different contaminants and/or have the capacity to accumulate significant quantities of certain pollutants in their tissues without overt negative effects on their growth and development (Pilon-Smits 2005; Zhou et al. 2008). However, considering that each species will demonstrate specific responses in terms of its accumulation capacity, has different mechanisms of pollutant removal, and each pollutant has a distinct mode of action in these organisms (Zhou et al. 2008), specific bioassays must be performed to confirm the characteristics of each plant as a bioindicator or phytoremediator with each specific chemical contaminant. One aquatic plant with significant potential for use in phytoremediation/bioindicator programs is Pistia stratiotes (Araceae), also known as water lettuce. This species displays useful characteristics such as a high potential for absorbing and accumulating different pollutants, natural abundance, wide distribution, high propagation rates under laboratory conditions, it is easy to grow, and its cells and tissues can be easily analyzed microscopically—all of which facilitate the evaluation of the damages caused by pollutants (Zhou et al. 2008).
The present study evaluated the effects of Cd on the clonal growth and inflorescence production of P. stratiotes, its mitotic cycle, and anatomy to further our understanding of how Cd acts in living organisms and to contribute to biomonitoring programs in contaminated aquatic environments.
2 Materials and Methods
Samples of P. stratiotes were obtained in a lake that was not suspected of being contaminated by Cd, near the community of Pedra Negra in the municipality of Ijaci, Minas Gerais State, Brazil (44°55′ W; 21°10′ S). The test plants were selected from among eighth-generation clones (to assure the elimination of possible heavy metal residues or other contaminants); their reproductive structures were removed to evaluate clonal growth when exposed to Cd.
The bioassays were composed of seven treatments with five repetitions, using three rosettes per repetition. The plants were selected for size uniformity and placed in trays containing 4 L of Hoagland and Arnon nutrient solution (1950) modified to ¼ of its original concentration. Cd was added to the nutrient solutions in the form of Cd(NO3)2.4H2O in increasing concentrations, based on the maximum permitted value of total Cd for effluent disposal into freshwater bodies (0.2 mg L−1 of Cd) (Brasil 2011). The concentrations used were 0.0, 0.4, 0.8, 1.6, 3.2, 6.4, and 12.8 mg L−1 of Cd. The experimental exposure period was 15 days, during which time, the plants were maintained in a green house; the solutions were renewed on the eighth day of treatment. Clonal growth was determined by counting the numbers of daughter rosettes at the end of the experimental period. Visual symptoms of Cd were toxicity recorded starting at the seventh day of exposition.
Young roots were collected for cytogenetic analyses, fixed in Carnoy’s solution (ethyl alcohol and glacial acetic acid; 3:1 v/v), and stored at −4 °C. Slide preparation used the technique of squashing the root tips in 2 % acetic orcein. The slides were examined using a clear field microscope (Leica DMLS), and images were captured using a micro-camera (Nikon Digital Sight DS-Fil), both with × 1,000 magnifying power. Counts were made of approximately 5,000 cells at each Cd concentration tested to obtain the mitotic indexes. Cell cycle abnormalities were also quantified.
Anatomical analyses were performed on roots and young leaves (totally expanded) that were collected, washed in running water, fixed in FAA70% (Johansen 1940) for 72 h, and conserved in 70° L.G. ethanol (Jensen 1962). Slides were prepared from samples of the root proliferation zones and the median regions of leaves by dehydration in an ethyl alcohol series (for 2 h at each concentration), pre-infiltration in resin base and ethanol 95 % (1:1), and subsequent infiltration in the resin base for 48 h. After infiltration, the material was polymerized in resin base/hardener (15:1) in polyethylene molds. The histomolds were cured for 24 h, and sections were cut using a rotary microtome calibrated for 8-μm sections; the sections were extended on slides placed on a warming plate (40 °C), and subsequently stained with 0.05 % toluidine blue (in pH = 6.8 potassium phosphate buffer) for 30 min. The slides were then washed in running water, air-dried, and mounted in Permount. Anatomical analyses were made using photomicrographs obtained with a digital camera (Cannon PowerShot A620, 8.0 megapixels) coupled to a microscope (KEN-A-VISION TT18). Measurements were made using the Image Tool® program (Wilcox et al. 2002) and calibrated using a micrometric ruler that was micro-photographed at the same magnification. The percentages of aerenchyma in the leaf blades were determined by calculating the areas of the leaf blades and the sums of the individual areas of the aerenchyma present in the photomicrograph.
The data obtained in each of the analyses were submitted to tests of normality of errors and homogeneity of variances. Those values not showing normal distributions and homogeneous variances were transformed before being submitted to analysis of variance and Scott-Knott means grouping tests at a 5 % probability level.
3 Results and Discussion
The evaluations undertaken at the end of the experimental period (15th day) showed large numbers of daughter rosettes growing from plants in the control group, with statistically significant decreases in their numbers (p < 0.05) as Cd concentrations increased. Larger numbers of inflorescences were detected in plants exposed to concentrations of 1.6 mg L−1 of Cd (p < 0.05), while significant reductions were observed at higher concentrations that were proportional to increases in Cd concentrations; the lowest numbers of inflorescences were observed (p < 0.05) in the maximum Cd concentration tested (Fig. 1). Our results were consistent with observations made by Coelho et al. (2005) and Oliveira (2010), who noted that although reproduction in P. stratiotes is predominantly vegetative, these plants tend to reproduce sexually under stress conditions to maximize their chances of perpetuation. The decreases seen in the numbers of daughter rosettes and of inflorescences at high Cd concentrations (above 3.2 mg L−1) appear to be direct responses to heavy metal toxicity and that these plants adopt strategies under these conditions directed towards allocating nutrients only for their own survival, reducing investments in reproduction.
Clonal growth and numbers of reproductive structures of P. stratiotes in relation to the concentrations of Cd in the nutrient solutions (mg L−1). Horizontal bars represent the standard errors. Capital letters compare the numbers of daughter rosettes; lowercase letters compare the numbers of inflorescences on the mother rosettes. Similar letters do not statistically differ by the Scott-Knott test at a 5 % probability level
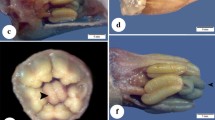
Symptoms of Cd contamination were detected in plants after the seventh day of heavy metal exposure in the leaves of plants exposed to the greatest metal concentrations (1.6; 3.2; 6.4, and 12.8 mg L−1) would then begin to show signs of chlorosis (Fig. 2). In addition to these symptoms, Monteiro et al. (2010) reported a darkening of the roots and leaf fall in Lactuca sativa when exposed to this metal. All of these symptoms were noted by Zhou et al. (2008) as characteristic of Cd toxicity in plants. Plant mortality was not observed under the conditions and concentrations of Cd tested here, suggesting that this species is tolerant to this metal at the doses used, although their development was affected.
Morpho-anatomical alterations in P. stratiotes plants exposed to Cd. a, c, and e 0.0 mg L−1 Cd; b, d, and f 12.8 mg L−1 Cd. a–b Visual symptoms observed after 7 days of exposure to the Cd treatments; c–d Transversal sections of the leaves and e–f roots of P. stratiotes after 15 days of exposure to the Cd treatments. Arq, aerenchyma; Ead, adaxial epidermis; Eab, abaxial epidermis; arrow, endoderm; star, druse
The appearance of chlorotic blotches on the leaves of plants treated with the highest Cd concentrations can be explained by decreasing zinc (Zn) and iron (Fe) absorption, elements chemically very similar to Cd (Hart et al. 1998; di Toppi and Gabrielli 1999). According to Hart (1998), Cd and Zn compete for the same cell membrane transport proteins, resulting in Zn deficiencies that are characterized by the appearance of internerval chlorosis and growth reductions (Taiz and Zeiger 2010). Chlorotic blotches are also symptoms of Fe deficiency since this element is directly involved in chlorophyll synthesis (Taiz and Zeiger 2010).
The analyses of the cell cycles of P. stratiotes plants exposed to Cd demonstrated that there were no significant reductions in the mitotic indexes of these plants as compared to control plants. This result demonstrates that Cd (at the concentrations studied) did not inhibit or increase cell proliferation. In terms of the normality of the mitotic cycle, we observed that Cd significantly increased the numbers of metaphase irregularities (p < 0.05). Plants submitted to the highest Cd concentration (12.8 mg L−1) demonstrated the greatest percentages of metaphase abnormalities, followed by the treatments 1.6, 3.2, and 6.4 mg L−1 of Cd (although these did not differ among themselves). The percentages of abnormalities at the other concentrations were not statistically different from controls.
The metaphase alterations most commonly encountered were sticky chromosomes, C-metaphases, and nonoriented chromosomes; multipolar anaphases, bridges, and fragments were also encountered, although at lower frequencies (Table 1). These events represent visible records of direct or indirect genotoxic effects of Cd in provoking many kinds of damage, including breaks in the DNA double helix, chromosomal alterations, the formation of micronuclei, as well as structural damage to the nucleolus and to RNA (Misra et al. 1998; Jonak et al. 2004; Ünyayar et al. 2006). The occurrence of C-metaphases and multipolar anaphases indicated that Cd can affect the assembly/disassembly of the microtubules forming the mitotic spindle—resulting in the complete or partial inactivation of the mitotic spindle and delays in (or the impediment of) centromere division (Fiskesjö 1985; Fiskesjö 1993). The interaction of Cd with the mitotic spindle also helps explain the occurrence of nonoriented chromosomes generated by syntelic and merotelic attachments of microtubules (with consequent problems of orientation and segregation). These lost chromosomes appear to be associated with stickiness. The formation of fragments and lost chromosomes indicates both clastogenic and aneugenic effects. The dose-dependent increases in metaphase stickiness frequencies are a strong indication of the toxic effects of Cd on chromatin. Stickiness occurs as a result of partial dissociation of nucleoproteins and alteration in the organizational patterns of the chromosomes (Evans 1962), or sometimes because of alterations in the nonhistone proteins that are necessary for successful segregation (Gaulden 1987).
The large numbers of condensed nuclei (47.46 % of the total number of abnormalities observed in this study) in the roots of the plants submitted to the highest Cd doses suggest the induction of programmed cell death. In their study of the roots of Allium cepa treated with Cd, Behboodi and Samadi (2004) concluded that this heavy metal caused programmed cell death and that its induction was dose-dependent, occurring at concentrations above 25–50 μM of Cd. Programmed cell death is a natural process and highly controlled, but can be induced by toxic agents, such as Cd, considered one of the major molecular responses and a biomarker of occurrence of the same (Chiarelli and Roccheri, 2012).
Cadmium exposure significantly affected the thicknesses of the root tissue (p < 0.05), and a thickening of the epidermis, exoderm, and the endoderm at the highest Cd concentrations was also observed. The numbers of crystalline idioblasts were significantly reduced in plants exposed to 3.2, 6.4, and 12.8 mg L−1 of Cd. There were no alterations in the percentages of aerenchyma or in the Carlquist vulnerability index—CVI (1977) with increasing Cd exposure (Table 2 and Fig. 2). Thickening of the epidermis and endoderm were reported by Marques et al. (2011) in Eucalyptus camaldulensis plants exposed to Cd. As the epidermis contains accumulation of negative charges, increases in its thickness could amplify its role as a biological filter of metallic irons. Endoderm and exoderm form apoplastic barriers with important roles in protection against environmental stress (Enstone et al. 2003; Lux et al. 2004; Marques et al. 2011). Thickening of these tissues due to exposure to high concentrations of Cd may also result in less translocation of this element to the aerial parts of the plant, as observed by Oliveira (2010), which reports the highest Cd accumulation in the roots of P. stratiotes, accounting for about 91 % of total Cd absorbed.
The analyses of the leaf tissues of P. stratiotes exposed to the different Cd concentrations used did not demonstrate alterations in epidermal thickness on the abaxial or adaxial faces, in leaf blade thicknesses, or in the thicknesses of the perivascular fibers. Aerenchyma tissues showed area reductions of 21.57 and 32.44 % at Cd concentrations of 6.4 and 12.8 mg L−1, respectively (as compared to controls). The CVI increased 64.28 and 57.65 % at Cd concentrations of 3.2 and 6.4 mg L−1, respectively (Table 3 and Fig. 2), indicating that the hydraulic systems of these plants had become more vulnerable, resulting in poor distributions of water and nutrients (Hacke and Sperry 2001).
The observed reductions in the numbers of crystalline idioblasts in the roots of P. stratiotes in response to cadmium exposure may be explained by decreased absorption of calcium in the presence of this pollutant (Soares et al. 2005). Similar observations were reported by Souza et al. (2009) in Alternanthera philoxeroides after treatment with Cd. The interference of Cd in the absorption and translocation of Ca to the aerial portions of the plants may also be responsible for the decreases in the percentages of aerenchyma in the leaf blades of plants exposed to the highest concentrations of Cd. He et al. (1996a and 1996b) reported that intercellular spaces develop where the greatest concentrations of cytosol Ca are found and that substances that reduce Ca concentrations will inhibit enzymes such as cellulase that act in forming aerenchyma. These authors also pointed out that high intracellular Ca concentrations stimulate ethylene synthesis that will, in turn, stimulate cellulase synthesis, resulting in increased formation of aerenchyma through the degradation and loosening of cell walls (Pereira et al. 2008). These effects reflect the toxicity of Cd at high concentrations that affect the plant’s capacity to float and to store oxygen, thus interfering with its normal development.
In light of the observed reproductive, cellular, and anatomical alterations observed in P. stratiotes due to cadmium exposure, it is clear that this element exerts cyto-genotoxic effects in this plant—and this species therefore demonstrates significant potential as a bioindicator of cadmium pollution in light of the fact that it is also largely tolerant of that element, since the observed effects are typical, though nonspecific, exposure to pollutants from plants, mainly heavy metals.
4 Conclusions
The obtained results suggest the following conclusions:
-
1.
The effects of the highest concentrations of cadmium could also be observed in the thickening of the epidermis, exoderm, and endoderm of the roots, decreases in the percentages of aerenchyma in the leaf blades, and by reductions in the number of crystalline idioblasts.
-
2.
The phytotoxicity of cadmium was quite evident in P. stratiotes, and it could thus be used as a bioindicator species as it was also tolerant of that pollutant.
References
Arroyo, V. S., Flores, K. M., Ortiz, L. B., Gómez-Quiroz, L. E., & Gutiérrez-Ruiz, M. C. (2012). Liver and cadmium toxicity. Journal of Drug Metabolism & Toxicology. doi:10.4172/2157-7609.S5-001.
Behboodi, B. S., & Samadi, L. (2004). Detection of apoptotic bodies and oligonucleosomal DNA fragments in cadmium-treated root apical cells of Allium cepa Linnaeus. Plant Science, 167(3), 411–416.
Brasil. Congresso, Senado, Resolução nº 430, 13 de maio de 2011. Dispõe sobre as condições e padrões de lançamento de efluentes, complementa e altera a Resolução nº 357, de 17 de março de 2005, do Conselho Nacional do Meio Ambiente-CONAMA. Coleção de Leis da República Federativa do Brasil, Brasília.
Carlquist, S. (1977). Ecological factors in wood evolution: a floristic approach. American Journal of Botany, 64(7), 887–896.
Chiarelli, R., & Roccheri, M. C. (2012). Heavy metals and metalloids as autophagy inducing agents: focus on cadmium and arsenic. Cells, 1, 597–616.
Coelho, F. F., Deboni, L., & Lopes, F. S. (2005). Density-dependent reproductive and vegetative allocation in the aquatic plant Pistia stratiotes (Araceae). Revista de Biologia Tropical, 53(3–4), 369–376.
Enstone, D. E., Peterson, C. A., & Ma, F. S. (2003). Root endodermis and exodermis: structure, function, and responses to the environment. Journal of Plant Growth Regulation, 21(4), 335–351.
Evans, T. H. J. (1962). Chromosome aberrations induced by ionizing radiations. International Review of Cytology, 13, 221–321.
Fatur, T., Lah, T. T., & Filipic, M. (2003). Cadmium inhibits repair of UV-, methyl methanesulfonate- and N-methyl-N-nitrosourea-induced DNA damage in Chinese hamster ovary cells. Mutation Research, 529(1–2), 109–116.
Fiskesjö, G. (1985). The Allium test as a standard in environmental monitoring. Hereditas, 102(1), 99–112.
Fiskesjö, G. (1993). Technical methods section, Allium test I: A 2-3 day plant test for toxicity assessment by measuring the mean root growth of onions (Allium cepa L,). Environmental Toxicology and Water, 8(4), 461–470.
Gaulden, M. E. (1987). Hypothesis: some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosome stickiness, which causes chromosome aberrations. Mutagenesis, 2(5), 357–365.
Hacke, U. G., & Sperry, J. S. (2001). Functional and ecological xylem anatomy. Perspectives in Plant Ecology, 4(2), 97–115.
Hart, J. J., Welch, R. M., Norvell, W. A., Sullivan, L. A., & Kochian, L. V. (1998). Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiology, 116(44), 1413–1420.
He, C. J., Finlayson, S. A., Drew, M. C., Jordan, W. R., & Morgan, P. W. (1996). Ethylene biosynthesis during aerenchyma formation in roots of Zea mays subjected to mechanical impedance and hypoxia. Plant Physiology, 112(4), 1679–1685.
He, C. J., Morgan, P. W., & Drew, M. C. (1996). Transduction of an ethylene signal required for cell death and lysis in the root cortex of maize during aerenchyma formation during hypoxia. Plant Physiology, 112(2), 463–472.
Hoagland, D. R., & Arnon, D. I. (1950). The water-culture method for growing plants without soil. California: California Agricultural Experimental Station.
Jensen, W. A. (1962). Botanical histochemistry: principle and practice. San Francisco: W H Freeman.
Jonak, C., Nakagami, H., & Hirt, H. (2004). Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiology, 136(19), 3276–3283.
Johansen, D. A. (1940). Plant microtechnique. New York: McGraw- Hill Book Company.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Berlin: Springer.
Kah, M., Levy, L., & Brown, C. (2012). Potential for effects of land contamination on human health. 1. The Case of Cadmium. Journal of Toxicology and Environmental Health, 15(5), 348–363.
Kenskinkan, O., Goksu, M. Z. L., Yuceer, A., Basibuyuk, M., & Foster, C. F. (2003). Heavy metal adsorption characteristics of a submerged aquatic plant (Myriophyllum spicatum). Process Biochemistry, 39(2), 179–183.
Lu, Q., He, Z. L., Graetz, D. A., Stofella, P. J., & Yang, X. (2010). Phytoremediation to remove nutrients and improve eutrophic stormwaters using water lettuce (Pistia stratiotes L.). Environmental Science & Pollution Research, 17(1), 84–96.
Lux, A., Sottníková, A., Opatrná, J., & Greger, M. (2004). Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiologia Plantarum, 120(4), 537–545.
Malavolta, E. (1994). Fertilizantes e seu impacto ambiental: micronutrientes e metais pesados, mitos, mistificações e fatos. São Paulo: ProduQuímica.
Marques, T. C. L. L. S. M., Soares, A. M., Gomes, M. P., & Martins, G. (2011). Respostas fisiológicas e anatômicas de plantas jovens de eucalipto expostas ao cádmio. Revista Árvore, 29(5), 997–1006.
Mirsha, V. K., & Tripathi, B. D. (2008). Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Bioresource Technology, 99(15), 7091–7097.
Misra, R. R., Smith, G. T., & Waalkes, M. P. (1998). Evaluation or the direct genotoxic potential of cadmium in four different rodent cell lines. Toxicology, 126(2), 103–114.
Melo, E. E. C., Costa, E. T. C., Guilherme, L. R. G., Faquin, V., & Nascimento, C. W. A. (2009). Accumulation of arsenic and nutrients by castor bean plants grown on an As-enriched nutrient solution. Journal of Hazardous Materials, 168(1), 479–483.
Monteiro, M. S., Rodriguez, E., Loureiro, J., Mann, R. M., Soares, A. M. V. M., & Santos, C. (2010). Flow cytometric assessment of Cd genotoxicity in three plants with different metal accumulation and detoxification capacities. Ecotoxicology and Environmental Safety, 73(6), 1231–1237.
Pereira, F. J., Castro, E. M., Souza, T. C., & Magalhães, P. C. (2008). Evolução da anatomia radicular do milho ‘Saracura’ em ciclos de seleção sucessivos. Pesquisa Agropecuária Brasileira, 43(12), 1649–1656.
Pilon-Smits, E. (2005). Phytoremediation. Annual Review of Plant Biology, 56(1), 15–39.
Oliveira, C. (2010). Características morfoanatômicas e fisiológicas na avaliação do potencial bioindicador e fitorremediador de Pistia stratiotes L., na presença de cádmio, chumbo e arsênio. Dissertação (Mestrado em Agronomia/ Fisiologia Vegetal). Lavras: Universidade Federal de Lavras.
Rojas, E., Herrera, L. A., Poirier, L. A., & Ostrosky-Wegman, P. (1999). Are metals dietary carcinogens? Mutation Research, 443(1–2), 157–181.
Skinner, K., Wright, N., & Porter-Goff, E. (2007). Mercury uptake and accumulation by four species of aquatic plants. Environmental Pollution, 145(1), 234–237.
Soares, C. R. F. S., Siqueira, J. O., Carvalho, J. G., & Moreira, F. M. S. (2005). Fitotoxidez de cádmio para Eucalyptus maculata e E. urophylla em solução nutritiva. Revista Árvore, 29(2), 175–183.
Souza, V. L., Silva, D. C., Santana, K. B., Mielke, M. S., Almeida, A. F., Mangabeira, P. A. O., et al. (2009). Efeitos do cádmio na anatomia e na fotossíntese de duas macrófitas aquáticas. Acta Botanica Brasilica, 23(2), 343–354.
Taiz, L., & Zeiger, E. (2010). Plant Physiology. Sunderland: Sinauer Associates.
Tan, K. H. (2000). Enviromental soil science. New York: Marcel Dekker.
di Toppi, L. S., & Gabrielli, R. (1999). Response to cadmium in higher plants. Environmental and Experimental Botany, 41(2), 105–130.
Ünyayar, S., Çelik, A., Özlem, Ç. F., & Gözel, A. (2006). Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis, 21(1), 77–81.
Wilcox, D., Dove, B., McDavid, D., & Greer, D. (2002). Software Image tool Version 3.0. Texas: The University of Texas, Health Science Center in San Antonio.
Wolff, G. A., Assis, L. R., Pereira, G. C., Carvalho, J. G., & Castro, E. M. (2009). Efeitos da toxicidade do zinco em folhas de Salvinia auriculata cultivadas em solução nutritiva. Planta Daninha, 27(1), 33–137.
Zimmels, Y., Kirzhner, F., & Malkovskaja, A. (2006). Application of Eichhornia crassipes and Pistia stratiotes for treatment of urban sewage in Israel. Journal of Environmental Management, 81(4), 420–428.
Zhou, Q. A., Zhang, J., Fu, J., Shi, J., & Jiang, G. (2008). Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Analytica Chimica Acta, 606(2), 135–150.
Acknowledgments
The authors would like to thank Fundação de Apoio à Pesquisa de Minas Gerais for sponsoring this project and Dr. Fernando Lisboa Guedes for helping with the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, S.A., Techio, V.H., de Castro, E.M. et al. Reproductive, Cellular, and Anatomical Alterations in Pistia stratiotes L. Plants Exposed to Cadmium. Water Air Soil Pollut 224, 1454 (2013). https://doi.org/10.1007/s11270-013-1454-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1454-z