Abstract
Biomolecules present in algae, such as carbohydrates, proteins, pigments, and lipids, have wide applications as ingredients in different cosmeceutical, pharmaceutical, and nutraceutical products. Research efforts have been made to establish efficient extraction processes of the high-value bioactive compounds from algae to overcome the limitations of traditional extraction processes. Supercritical fluid extraction (SFE) and subcritical water extraction (SWE) are identified as economically sustainable, promising green extraction technologies which have wide applicability in the extraction of valuable bioactive molecules from natural resources including micro- and macroalgae. This review presents a detailed discussion for the extraction of biomolecules from the algae using the SFE and SWE techniques. In addition, the improvement of these technologies has been discussed considering the extraction of different bioactive and valuable compounds from different algae strains. Optimized process conditions and choice of solvents in the SFE and SWE processes depend on the biomass composition. Research endeavors for the enhancement of extraction yield of the different biomolecules are addressed. The integrated extraction process by combining the SFE and SWE techniques appears to be an effective method for extracting different valuable bioactive molecules. This review also discussed the perspective and challenge for using SFE and SWE processes on algae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Algae are autotrophic organisms and large varieties of algae strains are found on the Earth’s surface. Both micro- and macroalgae are regarded as a potential source of bioactive molecules such as polysaccharides, proteins, pigments, unsaturated fatty acids, sterols, phytohormones, minerals, vitamins, and phlorotannins. Specifically, high-valued bioactive compounds (e.g., lutein, β-carotene, fucoxanthin, astaxanthin, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA)), have commercial interests. These biomolecules are widely used as ingredients to produce a wide range of products in pharmaceutical, nutraceutical, chemical, and cosmeceutical industries. Different biomolecules from micro- and macroalgae are generally extracted by using conventional organic solvents such as ethyl acetate, n-hexane, diethyl ether, dichloromethane, and chloroform. These solvents have limited applications due to their toxicity, low selectivity, and flammability [1]. These solvents also have carcinogenic and environmentally harmful effects due to their toxic properties. Generally, to mitigate these issues, one additional separation step is required in the solvent extraction process to remove the hazardous solvent from the extracted product, which is generally energy and time-consuming [1].
Selective extraction of biomolecules from algae with specific biological activity still remains as a challenge. Extraction of bioactive molecules from the cells depends on several factors such as solubilization or diffusion rate of the molecules from a solid matrix, type of the solvents used in the method, temperature, and extraction time [2]. Furthermore, the extraction process may cause changes in bioactivity or degradation of the molecules due to the cell handling, storage, and several processing steps involved. Nowadays, green extraction technology is ensuring a safe and high quality bioactive natural products by utilizing less energy and fewer or no organic solvents and reducing environmental and health hazards. Green extraction process is known as organic solvent free extraction process that satisfy the demand on the market for the chemically safe and high-quality natural products in modern civilizations [3].
At critical temperature and pressure, supercritical fluids (SCFs) have intermediate behavior between the liquid and gaseous state. For example, supercritical carbon dioxide (SCCO2) has gas-like viscosities, liquid-like densities [4]. The SFE has several advantages over traditional extraction processes like higher selectivity by changing temperature and pressure, lower viscosity,and higher diffusivity of the solutes. In this technique, low operating temperature is used which maintains the bioactivity of the products. In the SFE technique, carbon dioxide (CO2) is the most common choice as supercritical fluid due to the low critical temperature (31 °C) and pressure (73.8 bar), chemical inertness, low toxicity, no flammability, and non-corrosive property. Moreover, both European Food Safety Authority and American Food and Drug Administration approved CO2 as Generally Recognized as Safe (GRAS) chemical [5]. The critical temperature and pressure of the CO2 helps to preserve the bioactivity in the extracted compounds. In addition, the bioactive nature of the extracted compound is preserved without the presence of atmospheric oxygen, which may oxidize the molecules [6]. The elimination of CO2 from extract can be easily achieved to get a solvent-free bioactive compound due to the gaseous nature of CO2 at room temperature. Despite of several benefits, supercritical carbon dioxide (SCCO2) is limited in its ability to remove polar molecules due to its non-polar chemical nature. Therefore, co-solvents are used at low concentrations for the extraction of polar molecules (e.g., polar pigments such as chlorophyll a, chlorophyll b, anthocyanins, xanthophylls, and polar lipids such as phospholipids and sphingolipids) to improve the dissolving power of the fluid towards the target compounds. In the SFE process, the most commonly used co-solvents are methanol and ethanol [5]. Solubility parameters of the supercritical fluids are critical factors for the selection of appropriate solvent to achieve good selectivity during the extraction process [7]. Application of Hansen solubility parameters (HSP) is growing for selecting the appropriate solvent and optimizing the supercritical extraction process from the natural products [8, 9]. Sánchez -Camargo et al. had applied Hansen solubility approach to select the suitable solvent to extract phlorotannins from Cystoseira abies-marina seaweeds using different bio-based green solvents under subcritical (ethanol ethyl lactate and water) and supercritical (SCCO2 and SCCO2 with ethanol) conditions [10]. Theoretical calculation of the solubility parameters of the different solvents had identified ethanol as a suitable solvent at 25 ℃ temperature. However, the highest extraction selectivity of the phlorotannins was observed experimentally in case of extraction with subcritical pure ethanol (50 ℃ and 10.3 MPa pressure).
As reviewed by Michalak and Chojnaka (2014), several researches had shown the use of SFE process to extract pigments, lipids (omega-6 fatty acids, polyunsaturated fatty acids (PUFAs)), polyphenols, vitamins, etc. from different microalgae cells [11]. Santana et al. had used the SCCO2 in SFE process to extract lipids from green microalga Botryococcus braunii for biodiesel production at 50–80 ℃ and 200–250 bar pressure. Results had shown that polyunsaturated fatty acid (dicosahexaenoic acid (C22:6ω3)) content in the extracted lipids was 38.4% (w/w) while maintaining the operational temperature at 80 ℃ and 220 bar pressure. The essential fatty acid (myristic acid (C14:0)) was found 28.5% (w/w) in the extracted lipid while maintaining the operational temperature at 50 ℃ and 200 bar pressure [12]. Halim et al. had reported the extraction of fatty acids suitable for biodiesel synthesis from green microalgae Chlorococcum sp. at laboratory scale using SCCO2 as a solvent. Extraction yield of the fatty acid using SCCO2 was found more efficient than the conventional extraction process with the n-hexane [13]. In another study, Quitain et al. employed SCCO2 at 25–60 °C temperature and 200 to 400 bar pressure to extract fucoxanthin from the brown seaweed Undaria pinnatifida. This study reported that within 180 min of the operation ~ 80% of the fucoxanthin was extracted from the seaweed by maintaining operational temperature at 40 °C and pressure at 300 bar [14].
Pressurized hot water extraction (PHWE) which is one kind of subcritical water extraction (SWE) process is under research focus for green extraction process due to its high quality extracted product and environmental safety. Water is inexpensive, non-toxic, non-hazardous, and safe to work as a solvent in the SWE process [15]. In particular, water remains in the liquid state under pressurized conditions at the temperature between 100 and 374 °C. Studies had shown the physicochemical properties (relative dielectric constant, polarity) of the water decreases significantly with the rise of the temperature and at the subcritical condition water able to dissolve both polar and non-polar compounds [16, 17]. In subcritical water, the viscosity and surface tension tend to decrease with increasing temperature, which leads to increased extraction efficiency [18]. The diffusion coefficient of the solute molecules also increases in the subcritical water compared to the water at ambient temperature, resulting in higher mass transfer rate in the subcritical water [19]. In this process, the dissociation constant of subcritical water for hydrogen and hydroxyl ions is three orders of magnitude higher than ambient water. Consequently, subcritical water can act as an acid or an alkali which helps to extract and hydrolyze polysaccharides [18, 20]. In this method, water worked as both solvent and catalyst to transform biomass into high-value bioactive products. Additionally, subcritical water modifies the molecular structure of the biomolecules, which is advantageous for increasing the biological activities of the extracted molecules [21]. Generally, a short static extraction time (5–20 min) in SWE is employed to avoid degradation of thermally labile molecules and extract bioactive molecules efficiently from natural matrices. Thus, SWE process is able to contribute to better extraction, improving mass transfer efficiency of extract and maintaining its biological activities. SWE technique is currently used for extracting biomolecules from natural resources by restoring their biological activity and stability. Zakaria et al. had used the SWE process to extract the large amount of polar bioactive molecules such as proteins, polysaccharides, antioxidants, and polyphenols under high temperature (above the boiling point, 100 °C, but below the critical temperature, 374 °C) and high pressure (50–100 bar) [22]. In another study, the SWE process is used for the extraction of antimicrobial and antioxidant molecules from Haematococcus pluvialis at different temperatures (50 °C, 100 °C, 150 °C, and 200 °C) with a very short period (20 min) of extraction process. According to this study, the subcritical water extracts under 200 °C exhibited high antioxidant activity, due to the presence of vitamin E in the extract. In addition, the presence of short-chain fatty acids in water extracts, is responsible for antioxidant and antibacterial activity while tested on four different bacterial species (E. coli, S. aureus, C. albicans, A. niger) [23]. Researches had shown the extraction of antiviral compounds (against herpes simplex virus type 1) from different algae cells such as Chlorella vulgaris [24], Himanthalia elongata [25], Haematococcus pluvialis, and Dunaliella salina [26] by using SWE process. Results had shown that water extracts contain polysaccharide-rich fractions, which are responsible for the antiviral activity. SWE process also had been employed for efficient extraction of pigments such as chlorophylls and carotenoids from Haematococcus pluvialis [23] and Chlorella vulgaris [27]. Besides SWE extraction, subcritical CO2 extraction can be used to extract much smaller molecules (carotenoids) under low temperature and pressure. In a recent study, Mónica Bueno et al. improve the recovery (135%) and purity of carotenoids from D. salina at 250–400 bar pressure and 15–45 ℃ under subcritical and supercritical CO2 conditions [28].
Several cell disintegration techniques (physical or mechanical) considered as pretreatment methods are found most effective to enhance the extraction efficiency of SFE and SWE process. Examples of such techniques are freezing, alkaline, and organic solvents application, osmotic shocks, sonication, high-pressure homogenization, bead milling, ball-milling, etc. [11, 29]. Removal of excess water or drying as pretreatment method effectively enhances the yield in the SFE and SWE process. Crampon et al. had shown that the drying (air drying and freeze-drying) influences the extraction kinetics of neutral lipid from N. ovulata. In this process, faster extraction kinetics was observed due to the drying under air flow [30]. After drying the cell wall was crushed to break leading to more increment in the extraction kinetics and higher yield of the extraction process. Microwave or ultrasound-assisted cell disintegration techniques also facilitate the extraction of intracellular products increasing productivity and reducing processing time [29]. It has been found that the extraction yield of carotenoids from the homogenized microalgae Synechococcus sp. was increased than the uncrushed cell (91.8% recovery against 58.7%, respectively) significantly.
In this review, the emphasis has been given on the following: (i) the effects of different pretreatment methods for the extraction of biomolecules from algal biomass using SFE and SWE process; (ii) advances in the extraction of high-value products from algae by using SFE method including SCCO2; (iii) extraction of biomolecules from algae using SWE; (iv) combinatorial effect of SCCO2 and SWE techniques to extract lipid, pigments, protein, and carbohydrates from algal biomass; and (v) future perspective and challenges during the extraction of bioactive molecules from algae.
2 Effect of different pretreatments on algal biomass for SFE and SWE techniques
In SFE, different parameters such as pressure, temperature, and solvating power have influence on the extraction yield of bioactive molecules. If the temperature remains constant, extraction yield increases with the rising pressure [29, 31]. The effect of temperature in case of SFE process also depends on pressure. The relationship between temperature and pressure varies. At low pressure, solubility of the compound decreases with rising temperature. However, above the critical pressure, solvating power is enhanced with temperature. Solvating power is another factor in SFE process. By increasing the density of the supercritical fluid, the solvating power of the extracted compound increases. The density of the SCF can be adjusted by pressure, temperature and composition of the modifier. Extraction efficiency also depends on the concentration and molecular weight of the analytes, strength of bond to the matrix and their solubility in supercritical fluids [31]. Different types of co-solvents such as ethanol, methanol and water are used to extract polar compounds by SCCO2 extraction process. The solvating power of pure SCCO2 is similar to hexane. Thus, to extract non-polar molecules SCCO2 is commonly used. Addition of small amount of co-solvent causes enhancement of the solubility of polar molecules in the SCCO2.
In case of SWE process, temperature is the most important factor. The use of high temperature brings many beneficial effects to the SWE process, such as (i) improvement in mass transfer, which is favored by the disruption of intermolecular forces in the matrix sample; (ii) solubility increase of the extracted molecules; and (iii) decrease in surface tension of water, which causes better matrix penetration [32]. SWE at different temperatures causes Maillard and caramelization reactions in different glycation model systems. Maillard and caramelization reactions products show antioxidant activities [33]. Pressure also has an important effect in SWE process. A specific pressure (50–100 bar) is required to maintain the liquid state of water at high temperature [34]. A specific extraction time is required for full extraction of a particular molecule. An appropriate flow rate of the solvent is also required due the short contact time between the solvent and extracted molecule [32]. SWE process also depends on the physical state of the sample. The extraction efficiency increases with the enhancement of the surface area of contact with the solvent. Thus, the sample size should be considered and it should be appropriate for the maximization of the contact surface to avoid the formation of preferential path. In some cases, for the maximum extraction yields, some dispersants are applied with the sample molecules which cause uniform distribution of the solvents. Absorbents are also used to maximize the extraction yield [32].
Proper pretreatment is highly recommended for the maximum yield of extraction before applying supercritical fluid and subcritical water on the algal biomass. A schematic of the various types of pretreatment techniques is presented in Fig. 1. Cultivated biomass is harvested with sequential steps of centrifugation and filtration when scale of operation is small. However, in the case of bulk harvesting, pre-concentration is done by flocculation or flotation process. After that, the concentrated algal suspension is laid upon to the drying and crushing process which enhances mass transfer of biomolecules during the extraction process [35]. High moisture content in biomass can have a few drawbacks such as limiting the interaction of matrix-supercritical fluid and acidic hydrolysis of molecules owing to carbonic acid production caused by supercritical CO2 application [36]. Therefore, excess water should be removed during sample pretreatment. Two methods are mentioned for the drying step: freeze-drying or drying at low temperature. In the freeze-drying process, samples are first cleaned and then freeze-dried at − 20 °C for few days to avoid degradation of thermo-labile molecules [29]. The concentrated dried alga is milled into small pieces to improve the mass transfer. Most microalgae have a strong cell wall that makes it harder to recover different biomolecules and decreases the yield of extraction. To overcome this problem, algae are crushed to break the cell wall by the mechanical and non-mechanical processes [29]. Different pretreatment techniques are summarized in Table 1 with details of process conditions of the pretreatment methods.
Mercer et al. had reviewed the different technologies used for the extraction of oil from microalgae and shown that ultrasounds and microwaves assisted extraction processes are able to facilitate extraction quality and reduce process time [37]. Several numbers of other cell disruption techniques such as freezing, alkaline freezing, sonication, organic solvents, osmotic shocks, homogenization at high pressure, crushing, bead milling, and ball-milling are also used to increase the efficiency of the SFE method as mentioned in several reports (Fig. 1) [11, 38]. Safi et al. had shown the use of bead milling as a pretreatment process of SFE to enhanced the total yield of extraction by 16% with enhanced chlorophyll and carotenoids yield of extraction by 61% and 52%, respectively, from Chlorella vulgaris [39]. Halim et al. had shown the grinding of oven dried microalgal powder in a ring mill and centrifugation of wet algae in bench top centrifuge before SFE process leads toward increasing lipid yield from the wet algae biomass (0.071 g lipid/g of dried microalgae) [13]. In another study, Fujii et al. had extracted 2.02 ± 0.20 mg of astaxanthin and 29.4 ± 1.70 mg of chlorophyll from 1 g freeze-dried microalgae biomass by using SCCO2 fluid with ethanol as co-solvent at 60 °C temperature and 200 bar pressure [40]. Men’shova et al. had investigated the effect of different pretreatment conditions for the extraction of fucoidan from brown algae strains (F. evanescens, S. japonica, S. oligocystum). In the study three pretreatment conditions such as extraction by using 70% ethanol, SCCO2 with 5% ethanol and pure SCCO2 were applied. Results of the study had shown that, for each pretreatment condition, the yield of extraction and the structural characteristics of fucoidan were similar for each species. However, highly sulfated fucoidan along with homogenous monosaccharides were obtained when SCCO2 fluid was used with 5% ethanol as a co-solvent [41]. For lipid extraction, mechanical pretreatment is carried out on algal biomass before applying supercritical fluid, which improves the lipid extraction and decreases the extract selectivity. Patil et al. used microwave pre-treatment method on Nannochloropsis biomass and applied an azeotropic mixture (hexane/ethanol) as co-solvent to improve the affinity of SCCO2 to neutral lipids, offering a low selective extraction process [42]. Cheng et al. had used freeze-dried method for the disruption of Pavlov sp. prior to the SCCO2 extraction of neutral lipids. Cheng et al. had worked on marine microalgae Pavlova sp. and extracted lipid globules from the cells efficiently and effectively using the pressurized SCCO2 extraction process [43]. Dejoye et al. had compared microwave-assisted SCCO2 extraction with SCCO2 extraction alone while extracting fatty acids from freeze-dried Chlorella vulgaris. This study had shown microwave-assisted SCCO2 extraction process leads to higher extraction yield (4.73%) of lipid compared to the SCCO2 extraction alone (1.81%) [44]. Microwave irradiation in the process helped to break the rigid cell wall of microalgae. In the process, application of force implied by the microwave allows SCCO2 to solubilize the lipids, which leads to the higher extraction yield [44]. Balboa et al. reported that drying of algae in microwave before SCCO2 extraction process, enhances the extraction yield and purity of the extract (fucoxanthin). Two different microwave drying conditions was applied on the brown algae S. muticum: (i) 600 W for 5 min then 300 W for 5 min and (ii) 600 W for 5 min then 200 W for 10 min. Results confirmed that microwave drying before SCCO2 extraction (45 °C temperature, 350 bar pressure, and 25 g CO2 flow/min), under the first condition, showed the highest extraction yield (160 mg extract/g dry biomass) than the second condition (84 mg extract/g dry biomass) [45]. Rodríguez-Meizoso et al. had shown that pretreatment before SWE process increase the extraction yield (~ 30% of dry biomass) of different bioactive biomolecules from another microalgae strain H. pluvialis. Different bioactive biomolecules such as vitamin E, simple phenols and caramelized products were extracted by using the SWE process [23].
3 Application of supercritical fluids/SCCO2 techniques for the extraction of valuable compounds from algae
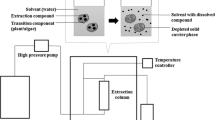
SFE process is preferred for the extraction of high-value compounds from algae for the production of safe and high-quality natural products. SCFs have low surface tension, low viscosity, high diffusivity, and compressibility. This complex nature of SCF causes effective penetration into the cells which leads to improved mass transfer between phases. It is worth to highlighting that the SFE process eliminates oxygen and uses a low processing temperature (depending on the type of fluid) to get volatile or labile components without deterioration [46]. A schematic diagram of SFE process has been shown in Fig. 2. SFE technique requires equipment that consists of a pump to pressurize the fluid, a mobile phase, an oven consisting of matrix-containing vessels, a restrictor to control the high pressure inside the system, and a trapping vessel. The extracts are collected during the decompression of the SCF-containing analyte into a vessel, through a solvent, or into a solid or liquid substance. SFE process has three different modes of operation namely dynamic mode, static mode, and combination mode. In dynamic mode, the fluid continuously flows through the sample inside the extraction vessel and out of the restrictor to the trapping vessel. In static mode, there is a flow of fluid in the loop inside the extraction vessel for specified time before releasing the trapping vessel via the restrictor. In combination mode, static extraction is performed for a certain time, then dynamic extraction is followed [47]. Dynamic mode is generally carried out for medium and large scale SFE extraction technique. In this case, the supercritical solvents flow through the solid material to extract the specific compounds until the substrate is exhausted. On the other side, continuous mode is generally used to extract liquid samples based on the extractor design. This technique can be improved by applying several extraction cycles in series with multiple extraction vessels. Extraction can be carried out offline at any moment by receiving the mobile phase containing extracted molecules. Then, the extracted molecules are directed to the collection vessel. After the completion of extraction for one vessel, CO2 flow is routed to another vessel which is already filled with the molecules that has to be extracted. This time first vessel either remains empty or can be filled with fresh sample. At the end of the extraction process, the CO2 is not released to the atmosphere, rather CO2 is reused by supplying into another vessel from one vessel. This cyclical depressurization process enables to collect different extracted molecules in separate collectors based on the solubility of the molecules under the different process parameters [47]. As a result, this modified method has higher processing and economic efficiency, as well as a reduced chance of adverse environmental impact.
In SFE technique different types of polar solvents (e.g., ammonia, nitrogen, water, ethanol, methanol, and acetone) and non-polar solvents (CO2, chlorotrifluoromethane, benzene, dimethyl ether, etc.) are used. A number of solvents have been prohibited due to their potentially toxic or dangerous effects on the environment [48]. These solvents are not used in the extraction process of bioactive compounds from algae. Patil et al. had used methanol as a solvent in the supercritical extraction procedure for the extraction of lipids. In this study, a single step process was adopted for direct transformation and liquefaction of wet algal biomass (Nannochloropsis sp.). This single-step procedure was carried out at 255 °C temperature and 82.74 bar pressure for 25 min with a 1:9 ratio of wet algae to methanol [42]. Among the different supercritical solvents, CO2 in its supercritical state (SCCO2) is the most suitable choice as a greenest solvent in the SFE process due to its non-hazardous, non-flammability property. However, SCCO2 cannot be used as a universal solvent due to its limited solvation capacity. Several types of co-solvents (e.g., methanol, ethanol, acetone) are mixed at low concentrations with SCCO2 to improve the dissolving power of the fluid towards the target component, for the extraction of polar compounds [5]. In general, SCCO2 is used to extract oil and oil derivative compounds from microalgae. SCCO2 is also deployed to extract carbohydrate-based compounds from macro- or microalgae [36]. Crampon et al. had reported the extraction of different biomolecules including lipids and pigments by using SCCO2 fluid at different operational conditions from algae cells [29]. Sequential steps for the extraction of different biomolecules including lipids and pigments are shown in Fig. 3. At the beginning of the process, homogenization is required to prepare a broken algal cell suspension. Cell fragments, cell wall, and other cell organelles are removed by centrifugation process to get a protein rich supernatant from the cell suspension. Protein rich supernatant is treated with RNase and Dnase at pH 4 for the removal of the nucleic acids. This protein-rich algal extract treated with SCCO2 fluid at 40 °C and 379 bar pressure to extract neutral lipids and polar lipid (extraction yield ~ 84%). Residual part of the algal extract is collected and treated with SCCO2 fluid in presence of ethanol at 40 °C and 379 bar pressure to separate polar lipids (14.7% of glycolipids and a trace amount of phospholipids) from the protein fraction. A significant amount of pigments (extraction yield ~ 97%) such as chlorophyll A and B and carotenoids (astaxanthin, canthaxanthin, fucoxanthin) is extracted in the next step of the process where SCCO2 fluid used with co-solvents (ethanol, acetone, and oil) and process operated at 40–50 °C and 200–550 bar pressure. This process leads toward the extraction of different type of lipids molecules such as neutral lipids (extraction yield ~ 67.89%), glycolipids (extraction yield ~ 22.52%), and phospholipids (extraction yield ~ 9.59%) by varying the operating conditions [29, 49]. In general, a narrow range of operating conditions (40–80 °C and 100–600 bar) are maintained in the SCCO2 extraction process to extract the high-value compounds from the microalgae [36].
Steps in the SCCO2 extraction process for the extraction of different biomolecules from algae [29]
The solubility of molecules in the SCCO2 fluid depends on the density of CO2, which is determined by the applied temperature and pressure in the process. The effect of operational pressures has a beneficial role on the yield of biomolecules extracted from macro- and microalgae [29]. The higher operating pressure resulted in higher CO2 density at a constant temperature, which enhanced yields and improved extraction kinetics. On the other hand, high operating pressures hinder the diffusion of SCCO2 into the matrices of macro- and microalgae, thereby reducing the yield of extraction. Therefore, optimum pressure can be determined for a specific extraction process through various approaches such as phase equilibrium strategy and design of experiment (DOE) with statistical modeling. The latter is currently the best option for optimization due to achieve a predictive knowledge of a complex, multi-variable process with the fewest acceptable trials [5]. The density of CO2 and the solubility of biomolecules decrease while operating temperature rises. However, in the SCCO2 extraction process, high dissolving capacity of solvent is typically targeted. Considering the low polarity of SCCO2, the application of this extraction technique is restricted for the low or medium polar molecules. For the extraction of a highly polar molecule (e.g., lutein, astaxanthin), the process is modified by adding co-solvents (polar modifiers) which enhance the dissolving power of the fluid. For the effective extraction of extremely polar compounds from macro- and microalgae matrices, multiple co-solvents are used as polarity modifiers such as acetone, methanol, ethanol, propanol, butanol, and vegetable oil. The use of co-solvents improves the extraction yield substantially while it reduces the selectivity of the molecules. Therefore, the use of a co-solvent should be considered carefully, which may threaten the purity of the targeted compound [50].
3.1 SFE extraction of biomolecules from micro- and macroalgae
3.1.1 Extraction of biomolecules from microalgae
Different bioactive molecules, such as carotenoids, β-carotene, zeaxanthin, astaxanthin, chlorophyll, fatty acids, lipids, γ-linolenic acids, and hydrocarbon, have been extracted from microalgae using SCCO2, as listed in Table 2. In microalgae, different carotenoid (non-polar in nature) molecules having wide biological activities are synthesized based on their metabolism. Examples of primary carotenoids are α-carotene, β-carotene, fucoxanthin, zeaxanthin, and neoxanthin, violaxanthin, and lutein. Secondary carotenoids include echinenone, astaxanthin, and canthaxanthin [51]. Both types of carotenoids are used as anticancer, antioxidant, antiobesity antidiabetic, and as a natural colorant in different pharmaceutical products [52]. For the SCCO2 extraction of carotenoid molecules, organic co-solvent is used as modifier to enhance the compound’s solubility by reducing the interaction of this compound with matrix. Liau et al. revealed that 7.61 mg/g of carotenoids can be extracted from Nannochloropsis oculata by using 16.7% ethanol as co-solvent at 50 ℃ temperature and 350 bar pressure [53]. Yen et al. had shown that extraction of lutein molecule from Scenedesmus sp., was achieved by using different co-solvents such as methanol, ethanol, propanol, butanol, and acetone with SCCO2 fluid. In the process, maximum extraction yield of the lutein molecule was found ~ 76% by operating the process using ethanol as a co-solvent (30%) at 400 bar pressure and 70 ℃ temperature [54]. Cardoso et al. had used 5% ethanol with the SCCO2 fluid for the extraction of β-carotene from Synechococcus sp. [55]. Yield of β-carotene was found 0.70 mg/g of dried biomass of algae, while operating the process at 40 °C and 400 bar pressure. Abrahamsson et al. extracted different carotenoids such as astaxanthin, β-carotene, canthxanthin, echinenone, lutein, neoxanthin, violaxnthin, and zeaxanthin from Scenedesmus sp. by using SFE with 10% ethanol at 60 °C and 300 bar pressure [56]. This process provide extraction yield of zeaxanthin ~ 0.09 mg/g, astaxanthin ~ 0.07 mg/g, lutein 0.44 mg/g of freeze dried algae biomass. Another investigation had shown, SCCO2 extraction process provide astaxanthin yield 2.02 ± 0.2 mg and chlorophyll yield 9.4 ± 1.7 mg from 1 g of dried biomass of Monoraphidium sp. by operating the process at 60 °C and 200 bar pressure [40].
The use of SCCO2 in the extraction process is being investigated to enhance the yield of extraction of lipids from microalgae [57]. Halim et al. had shown the extraction of lipid using SCCO2 fluid with a mixture of microalgae biomass in a tightly packed extraction vessel. SCCO2 fluid was flown on the surface of the packed microalgae mixture. Reports had shown that higher packing density inside the extraction vessels increases the lipid concentration in the fluid. Macro- or microalgae matrix inside the extraction vessel absorbs the SCCO2 solvent which leads to the expansion of the cellular structure. This step induces the flow of CO2 solvent through a decreased resistance in mass transfer process. The amount of lipids extracted in the process from microalgae biomass was described by the following first-order kinetics equation [57].
where Me is the amount of SCCO2 extracted lipid (lipid weight/weight of dried biomass) at time t, Ms,o is the original amount of lipid in the microalgae cells (g lipid/g dried biomass), k is the mass transfer coefficient of lipids from microalgae cells to eluted SCCO2 fluid (min−1), and t is the extraction time (min). In another study, it was reported that the use of co-solvent with SCCO2 and bead milling of Chlorella vulgaris cells before the extraction, effectively extract lipid along with chlorophyll and carotenoids [39]. The use of 5% ethanol as a co-solvent increased the total yield of extraction of lipids by 27%, whereas bead milling increased the total yield of extraction of lipids by 16%. The addition of 5% ethanol increases chlorophyll and carotenoids content by 81% and 65%, respectively. Bead milling also influences the extraction yield of chlorophyll and carotenoids by increasing 61% and 52%, respectively [39]. Different operating parameters such as pressure, temperature, modifier addition, and flow rate of fluid influence the performance of SCCO2 extraction of lipids from microalgae. The optimum operating conditions for efficient extraction of lipid using SCCO2 fluid from microalgae cells were pressure (200–600 bar), temperature (30–50 °C), and flow rate of CO2 (0.06–30 g/min) [58]. Research with different microalgae strains (Chlorella protothecoides, Scenedesmus obliquus, Nannochloropsis salina) had shown higher lipid yield (18.15 w%) in case of SCCO2 fluid extraction compared to the lipid yield (14.84 w%) through the conventional Soxhlet extraction process [59]. Solana et al. had compared the lipid extraction from different microalgae strains (Chlorella protothecoides, Scenedesmus obliquus, and Nannochloropsis salina) by SCCO2 fluid extraction and conventional Soxhlet extraction. The results had shown higher lipid yield (18.15 w%) in SCCO2 fluid extraction than through Soxhlet (14.84 w%). This study also showed the extraction of α-linolenic acid-rich oil from the three microalgae strains by using SCCO2 extraction process. The maximum extraction yield (73.6%) of free fatty acid was achieved from Scenedesmus obliquus when process parameters were kept at 60 °C and 300 bar pressure with a CO2 flow rate of 0.4 kg/h and 5% of ethanol as modifier [59]. Li et al. had tested many extraction processes and compared with the SCCO2 extraction process for the lipid extraction, particularly for the long-chain unsaturated fatty acids from Tetraselmis sp. The SCCO2 extraction process was found to be the most effective of all the tested approaches for this purpose due to the improved extraction efficiency of fatty acids and lipid molecules [60]. The use of SCCO2 plays a beneficial role in the extraction of only neutral lipids due to the non-polar nature of the solvent molecule [61]. Soh et al. had extracted algal lipid from Scenedesmus dimorphus for biodiesel production using SCCO2 at 414 bar pressure and 100 °C temperature. In this study, it has been shown that the SCCO2 flow rate throughout the extraction vessel influences the extraction kinetics of lipids [62].
3.1.2 Extraction of biomolecules from macroalgae
Most research on the development of supercritical extracts from macroalgae has focused on the relationship between the solvating capacity of SCFs and organic extractants. Different biomolecules such as pigments, lipids, and terpenes extracted from different macroalgae strains using SCCO2 are shown in Table 3. Carotenoid pigments such as fucoxanthin has wide biological activities (e.g., antitumor, antiobesity, anti-inflammatory) mainly found in marine micro- and macroalgae [63]. Kanda et al. and Quitain et al. used SCCO2 fluid for the fucoxanthin extraction from brown macroalgae Undaria pinnatifida [14, 64]. It has been shown that the maximum yield of fucoxanthin (~ 80%) was obtained by keeping the process parameters at 40 °C, 400 bar, and 3 h of extraction time [14]. Variation in the extraction time at different temperatures and the pressure showed a difference in the yield of extraction. The yield of extraction of fucoxanthin was achieved about 47 μg/g of biomass after 30 min at 400 bar pressure and 60 °C temperature. However, extraction yield was enhanced to 58 μg/g biomass after 150 min [64]. When ethanol is used in the SCCO2 extraction procedure, the dissolving power of SCCO2 was increased, which improved the extraction efficiency of various compounds [64]. Extraction of fucoxanthin from Phaeodactylum tricornutum was achieved by operating the process at 60 °C and 70 °C temperature with ethanol as co-solvent [65]. Balboa et al. performed SCCO2 extraction process at three different temperatures (40 °C, 50 °C, and 60 °C) on S. muticum to obtain fucoxanthin enriched extract. The concentration of fucoxanthin in the extract was highest (7 mg/g extract) when process was carried out at 40 °C [45]. Crampon et al. had reviewed the supercritical extraction of biomolecules including different pigments from macro- and microalgae. After reviewing thirty different studies related with supercritical extraction, it has been reported that biomolecules can be extracted from the micro- and macroalgae by varying operating parameters such as temperature (313.15–349.15 K), pressure (7.8–70 MPa) and CO2 algae mass ratio 6–500 [29]. Quitain et al., extracted highly sulfated cell wall polysaccharides fucoidan from Undaria pinnatifida by employing SCCO2 at 40 °C temperature and 400 bar pressure [66]. Fucoidan had been extracted from different macroalgae strains (Fucus evanescens, Saccharina japonica, Sargassum oligocystum) using SCCO2 as shown in other studies [41]. Marine red macroalgae Hypnea charoides were explored as a novel source of ω-3 fatty acids which extracted by using SCCO2 under mild operating conditions (40–50 °C and pressure 241–379 bar) [36]. Interestingly, it has been observed that the recovery of lipid and unsaturated fatty acids fraction increased at higher operating temperature and pressure. Moreover, the solubility of ω-3 fatty acids in SCCO2 mainly depends on the chain length of fatty acids [36]. Conde et al. had extracted fatty acids, fucoxanthin, and phenolic compounds from brown macroalgae Sargassum muticum by using SCCO2 with ethanol as co-solvent at 100 bar pressure and 40 ℃ temperature. It was reported that the use of 0.5 to 10% ethanol as co-solvent results in 3 times more total extraction yield and 90 times more fucoxanthin yield with 2.5 times higher free radical scavenging activity [67]. Pascale et al. extracted volatile oil from brown alga Dilophus ligulatus by using SCCO2 fluid and keeping operating parameters at 35–55 °C and 80–250 bar pressure [65]. Extraction of other valuable compounds (e.g., isoflavones, hydrocarbon, vitamin E) also had been shown by using the SCCO2 extraction process in different studies [29, 68].
4 Subcritical water extraction of different biomolecules from algae
Subcritical water extraction (SWE) is a sophisticated extraction technique that employs water at a temperature beyond its boiling point (100 °C) but below the critical temperature (374 °C). In the SWE process, high pressure is applied to maintain the liquid state during the whole extraction method. Water in the subcritical state increases the mass transfer and the polarity of water decreases significantly with rising temperature [69,70,71]. As a green extraction method, SWE is used for the extraction of different polar biomolecules from algae. SWE process is usually used to extract bio-oil from the matrix of microalgae. Research had shown the depolymerization of alginate produces antioxidants and other valuable compounds by applying the SWE process on Saccharina japonica cells [72]. At subcritical conditions, water possesses properties very different from liquid water at ambient temperature. At subcritical state, dissociation constant of the water increases with the increasing temperature, whereas dielectric constant of water decreases considerably with increasing the temperature. Low dielectric constant of subcritical water enables easy solubility of the less polar organic compounds. In the subcritical water, ionic reactions dominate due to the liquid-like properties of the water. This causes a large effects on the distribution of the products, such as gas, liquid, or solid, from hydrothermal conversion of biomass [70]. Thus, the extraction and fractionation of valuable compounds from micro- or macroalgae matrices can be performed by applying the SWE process. Table 4 shows the extracted valuable compounds from algae using the SWE process. Researches had shown the successful extraction of sulfated polysaccharides such as agar, alginate, and carrageenan from different algae by using the SWE process [73,74,75].
Although water is the preferred solvent in the SWE process for many applications, some other polar solvents are also considered to extract medium polar compounds. Pressurized liquid extraction (PLE) is another technique, based on the same principles of SWE process but uses other solvents to carry out the extraction [76]. Application of high pressure and temperatures in the PLE process ensures faster and higher extraction yield than the one achievable under normal ambient conditions[77].
In the conventional method, polysaccharides are extracted from microalgae by concentrating them at a lower temperature, followed by precipitation of molecules by using suitable organic solvents (e.g., acetone, acetic acids, acetonitrile, chloroform) [78]. The application of low temperatures cannot extract carbohydrates completely from algae due to the inherent extensible strength of the cell wall of microalgae. Therefore, complete extractions of carbohydrates from microalgae require high temperature along with high pressure. Among all the pressurized extraction techniques, the SWE approach is the most often used for the extraction of carbohydrate, making their recovery promising. In a review article, Gallego et al. explored the utilization of pressurized fluids-based extraction technologies such as PLE, gas-expanded liquids extraction (GXL), and SFE process to extract bioactive molecules (e.g., phlorotannins, phenolic compounds, carotenoids, carbohydrates, and proteins) from different natural sources including micro- and macroalgae [2]. Carbohydrates are more soluble in subcritical water at 100–150 °C temperature because the dielectric constant of water is reduced. It has been stated that the maximum yield of carbohydrate extraction was achieved near to 200 °C, and above this temperature, polysaccharides are degraded which resulted in the lower yield of polysaccharides. Most of the studies had shown that many factors such as pressure, temperature, biomass loading, extraction time, and particle size significantly influence the extraction of carbohydrates through PLE process [79]. Awaluddin et al. investigated the application of the SWE process for the high yield extraction of carbohydrate and protein molecules from C. vulgaris. The SWE process was optimized by varying process parameters such as temperature (180–374 °C), extraction time (1–20 min), loading of microalgae biomass (5–40 w%), and particulate size of biomass (38–250 μm) [79]. The study showed that 5% microalgae (C. vulgaris) biomass loading in 5 min of extraction period yields maximum carbohydrate (14.2 g/100 g biomass) and protein content (31.2 g/100 g biomass), at 277 °C. Statistical analysis revealed that among all parameters, temperature was the most critical factor for the extraction of carbohydrates and protein from microalgae [79, 80].
PLE method was employed by Santoyo et al. to extract the antiviral compounds from the seaweed Himanthalia elongata. This study reported that the extracted product obtained through the PLE technique with acetone, water, and ethanol had more antiviral activity than the original water extracts. Water and ethanol extract both can inhibit virus replication in HSV-1-infected Vero cells (in vitro). The antiviral activity of the ethanol extract of Himanthalia elongate cell was found at lower concentration (IC50 80.23 µg/ML) compared to the water extract (IC50 104.81 µg/ML) [25]. Analysis of the extracted carbohydrate from these two different organisms was done by using GC–MS. Results had shown that Dunaliella salina contains 94.34% glucose molecules as carbohydrates. Whereas, the major component of the extract from Haematococcus pluvialis was mannose along with the high quantity of galactose and glucose [26].
Subcritical hot solvents at elevated pressures are commonly used for the efficient extraction of valuable biomolecules from the living system. Bioactive molecules are wasted at high temperatures, and thus, supercritical extraction may not be the best way to extract bioactive molecules. On the contrary, SWE can be easily applied to the biological system, including macro- and microalgae, to extract bioactive polysaccharide molecules from the cells. Pressurized water is commonly used to extract polysaccharides from algae, both alone and in combination with acids or alkali [81]. Subcritical water has a threefold higher dissociation constant than ambient water. Thus subcritical water acts as acid or alkali which helps in the extraction of polysaccharide molecules [79]. Different parameters applied for the extraction of polysaccharides and other valuable molecules from different micro- and macroalgae species are presented in Table 4. Scientific study had shown the extraction of sulfated polysaccharides (rhamnan sulphate) from Ulva sp. and Monostroma latissimum using SWE process at 100–180 °C temperature [82]. Sulfated polysaccharide is used in the treatment and prevention of photo-aging by inhibiting the expression of UV-B-induced metalloproteinase-1 and the dose-dependent expression of type-1 pro-collagen mRNA and protein [82]. Alboofetileh et al. extracted another sulfated polysachharide (fucoidan) from the Nizamuddinia zanardinii strain by using the SWE method and showed that the yield of fucoidan at optimum condition was 25.98%, which was almost five times greater than the traditional solvent extraction method (5.2%) [83]. Carrageenan is a sulfated polysaccharide mainly obtained from red alga and used as an important ingredient in many products of the cosmeceutical industries [84]. Machmudah et al. had reported the efficient extraction of carrageenan molecules from Eucheuma cottonii and Gracilaria sp. by operating the SWE process at 120–200 °C and 10 to 100 bar pressure. The process was carried out at 120 °C temperature, 10 bar pressure for 150 min to pull off 86% of extraction yield of carrageenan from E. cottonii. However, only 73% of yield of carragenan was reported from Gracilaria sp. by operating the process at 200 °C temperature, 10 bar pressure with 150 min of extraction time [74]. Vázquez-Delfín et al. explored the extraction of carrageenan (extraction yield ~ 16.6%) from Hypnea musciformis by operating SWE process at 105ºC for 10 min [75].
Subcritical water hydrolysis is also considered as a useful process for alginate depolymerization. Meillisa et al. had reported the application of subcritical water hydrolysis on S. japonica at 180 to 260 °C by using 1% formic acid as a catalyst for effective depolymerization of the alginate and to maintain the molecule’s antioxidant properties [85]. Another high-value hydrocolloid molecule is agar, which is derived commercially from Gracilaria sp. and Gelidium sp. at a large scale [73, 86]. Agar is being used as an emulsifier and gelling agent in food processing to shape gel, food gum, and food additives. SFE, PLE, and microwave-assisted extraction processes are recently considered as eco-friendly alternative processes for the extraction of agar molecules [87].
The algal cell synthesizes phenolic compounds which protect themselves against abiotic and biotic stresses. Brown marine algae have a group of phenol-containing polymers known as phlorotannins, which include phlorethols, fucophlorethols, fucols, fuhalols, sulfated, and halogenated phlorotannin [88]. Polyphenol compounds were extracted by using water as a solvent [89, 90]. It seems from different research reports that at low temperature, water is unable to provide an effective outcome as those achieved by using co-solvents. An enzyme-assisted extraction, which comprises an enzymatic step before or simultaneously with the water extraction, is one way to improve the extraction efficiency of polyphenols with water [32]. Astaxanthin is carotenoid pigment that is produced by several algae strain. Astaxanthin was extracted from H. pluvialis cells through subcritical water extraction process [23]. In this study, other solvents, such as hexane and ethanol, were also used in subcritical conditions. The effect of the extraction temperatures (50 °C, 100 °C, 150 °C, and 200 °C) and the polarity of the solvent were estimated in terms of in vitro antioxidant activity. The antioxidant activity of subcritical water extracts from algae obtained at high temperatures can be partially attributed to the formation of Maillard reaction products during the extraction process. The extraction temperature exerted a positive influence on the antioxidant activity indicating a possible correlation between the antioxidant activity, vitamin E, simple phenols (gallate derivatives), caramelization products, and possible Maillard reaction compounds obtained during high temperatures extraction with subcritical water. Several researches had shown the efficient extraction of antioxidant molecules from different microalgae strains (Synechocystis sp., Himanthalia elongata, Haematococcus pluvialis, Chlorella vulgaris) using the SWE process [23, 27, 91]. The antiviral compounds were extracted by using different solvents (water, ethanol, and hexane or acetone) from different algae such as Himanthalia elongata [25], Chlorella vulgaris [24], Haematococcus pluvialis, and Dunaliella salina [26]. SWE process can be employed for commercial production of bio-fuels from algae at lower costs, as well as alternatives to develop byproducts. Reddy et al. extracted lipids from wet algal biomass of Nannochloropsis salina by using the SWE process and microwave-assisted SWE process [92]. This study reported that the extraction efficiency of lipid was achieved 70% in conventional SWE process and 100% in microwave-assisted SWE method. The optimized extraction conditions maintained in this process were 220 °C and 205 °C temperature, 25 min extraction time, and 7.5% and 25% of biomass loading for both in conventional SWE process and microwave-assisted SWE method [92]. Thiruvenkadam et al. had shown the extraction of oil from Chlorella pyrenoidosa using SWE process. It has been reported that the maximum oil yield was obtained at 12.89 wt.% keeping process parameters at 320 °C, 15 min extraction time, and 3% biomass loading [93]. Zainan et al. had extracted protein and carbohydrates from Chlorella pyrenoidosa by using SWE process at 170–370 °C. Highest extraction yield of protein (18.77%) and carbohydrates (2.40%) were achieved by operating SWE process at 270 °C and 170 °C for 10 min, respectively [94].
5 Combinatorial effect of SCCO2 and SWE technique to extract important biomolecules from algae
Integrated approaches with combination of high-pressure extraction (SFE and PLE) techniques should be taken into account in order to increase the extraction performance and to reduce the energy and resource consumption for the development of the algae-based biorefinery concept. High-pressure extraction is mainly used to extract non-polar (lipids, pigments) and polar molecules (carbohydrates and protein) from algal sources. It has been shown in different studies that SCCO2 extraction is mainly used to extract lipids from Chaetoceros muelleri [95], ω-3-fatty acids from Hypnea charoides [96], fucoxanthin from Undaria pinnatifida [60], β-carotene and lutein, and Scenedesmus almeriensis [97]. The use of a high concentration of co-solvent along with gas-expanded liquids (GXLs) acts as a favorable intermediate between PLE and SFE to extract high and medium polar molecules [98]. Gilbert-Lopez et al. extract phenol, proteins, sugar and carotenoids by an innovative sequential process. At first, the non-polar triglycerides were extracted by pure SCCO2. In the second step, mid polar compounds were extracted by gas expanded liquids (SCCO2 with 75% ethanol) and lastly polar molecules were extracted by PLE process using water as solvents. Authors reported 100% extraction of lipid and lutein which is around fourfold than conventional liquid extraction method [99]. Sánchez-Camargo et al. in another study had shown the similar approach. In this extraction process, high-pressure homogenization (HPH) was applied on Nannochloropsis gaditana to increase the extraction efficiency by breaking the cell wall of the cells. Then, pure SCCO2 extraction process was employed to extract pigments and non polar lipid molecules. Thereafter, PLE process was performed to extract antioxidant molecules. Pigment extraction was found twofold higher compared to liquid extraction using acetone [100]. Carbon dioxide expanded ethanol (CXE) was employed to extract astaxanthin from H. pluvialis [101]. PLE seems to have an interesting ability for the extraction of bioactive molecules from macro- and microalgae. This extraction approach helps to increase extraction yields in less time and less solvent consumption than traditional extraction techniques[101, 102]. It has been reported that carotenoids was extracted from Neochloris oleoabundans [32] and Chlorella ellipsoidea [103] by using ethanol as a solvent in the PLE process. Extraction of different biomolecules from microalgae (Isochrysis galbana) cells combining SCCO2 and SWE process is shown in Fig. 4. Gilbert-López et al. suggested integrated sequential extraction method to extract bioactive biomolecules from microalgae Isochrysis galbana by using compressed fluids with increasing polarity (SCCO2 < CXE < ethanol < water) [104]. The extraction process was carried out in four sequential steps by using SCCO2, SCCO2 plus ethanol (CXE), PLE plus pure ethanol, and PLE-SWE respectively. First SCCO2 extraction conditions should be optimized for establishing a relationship between operational parameters and extraction yield. Extraction was performed in variable pressure (200–300 bar) and temperature (40–50 °C) on Isochrysis galbana biomass to extract carotenoids, chlorophylls, and non-polar lipids from the cells. In the first step of the optimized process, 10 g of algal biomass was treated with SCCO2 at 300 bar pressure and 50 °C. Then, CXE was used to enhance the polarity of the extracted fraction on the residual biomass from the first step. In the second step, 70 bar pressure was applied to residual biomass and temperature maintained at 50 °C to extract carotenoids, chlorophyll, and polar lipids. Extraction was performed by using three different concentrations (15%, 45%, and 75%) of ethanol. In the third step, PLE was carried out by adding pure ethanol (100%) on the remaining algal biomass obtained in the second step. Here, operational pressure was maintained at 100 bar and temperature at 80 °C for 30 min to extract polar lipids, protein, and sugar molecules. In the final step, SWE or PLE was performed using water as a solvent and maintaining pressure at 100 bar and temperature at 80 °C for 30 min to extract protein and sugar molecules [104].
Combinatorial effect of SCCO2 and SWE of different biomolecules from biomass of Isochrysis galbana [104]
6 Effect of SFE and SWE on the environment
Life cycle assessment (LCA) process can be used to assess the environmental performance of SFE and PHWE processes at pilot scale by using a gate-to-gate approach to extract the products. According to ISO 14044, LCA is a standardized process which is used to assess the environmental impacts of SFE and PHWE process, as well as the effect of the extracted products on the environment [105, 106]. This process is also used for characterization and quantification of materials and energy [105]. LCA can be performed on two processes such as extraction process and drying process. A selected drying method maintains antioxidant property of the extracts such as freeze-drying for PHWE and vacuum-drying for SFE. LCA also used to assess the environmental disposal of solids and liquids that are produced during the processes. Environmental impact of PHWE is 28%, and in case of SFE process, the impact is around 37%. In SFE and PHWE, a small amount of green solvents are used (CO2 + ethanol for SFE and water for PHWE), which have no adverse effect on the environment. On the other hand, electricity production has highly significant impact on the environment. This factor causes 96.3% human toxicity and 99.7% terrestrial eco-toxicity in each extraction process. Electricity is applied for heating and pumping process and the electrical heating can be substituted by natural heating process (gas burning) to reduce the environment impact. The freeze drying process has great environmental effect that can to be substituted easily [105]. To determine the economic feasibility of the processes, an economic evaluation of compressed fluid extractions should be conducted. Software SuperPro Design is used to analyze capital and manufacturing cost. Cardenas-Toro et al. showed that the cost of manufacturing to extract carotenoid by PLE process is 29.2US$/kg extract while the selling price is greater than 667US$/kg extract. The higher selling price is due to the greater productivity and faster extraction time [107]. Process intensification is the process to improve economic status. Process intensification can be achieved by tuning the process parameters and integrating techniques (MW, ultrasound, and enzyme-assisted pressured fluid extraction), resulting in the improvement of the techno-economical system [8]. Nowadays, researchers have used the innovative concept of CO2 as a switchable solvent for the biorefinery valorization of algal biomass to improve the economic competitiveness of these processes [108].
7 Conclusions, future perspective, and challenges
The market of the natural extract is growing rapidly over the years as the consumer’s interests are shifting towards the natural ingredients used in the different nutraceutical and pharmaceutical industrial products. Public health, environmental impact, and safety concerns are some of the factors that must be considered while developing an effective extraction method. To satisfy this need, it is important to establish a green technology capable of producing high-quality extracts. SCCO2 extraction and SWE techniques are in the developmental stage as attractive and alternatives to substitute the conventional methods to extract valuable compounds from solid biomass matrices including macro- and microalgae. Operating cost of SCCO2 extraction, which is the most common application in supercritical fluid technologies, is comparable to the other high-pressure methods. A low operating temperature and low operating pressure are the main benefits of using SCCO2 for the extraction of biomolecules from natural sources macro- and microalgae. Considering the low polarity of SCCO2, the combination with another green solvent like ethanol as a modifier would increase the ability of SCCO2 to dissolve relatively higher polar compounds. Combination of pretreatments methods such as freezing, alkaline freezing, sonication, homogenization at high pressure, osmotic shocks, bead milling, ball-milling, etc., with SFE, increase the extraction yields of the biomolecules from algae cells and enhance the efficiency of the SFE method. A large number of algae-based molecules like carotenoids (e.g., astaxanthin, fucoxanthin), lipids (e.g., ω-3 fatty acids), and pigment (chlorophyll) are having health benefits and commercial values. SFE technology hold promises for extracting these molecules with high extraction yield and maintaining their biological activity. It has been seen in many reported work that extraction of wide varieties of biomolecules from different algae strains successfully done using SCCO2 as fluid, CO2 flow rate (0.06–30 g/min), maintaining the pressure (200–600 bar), and temperature (30–50 °C).
Sometimes it is difficult to maintain the original chemical characteristics of the extracted products. Therefore, integration of a purification or fractionation step is required. The most common technique is the integration of subcritical and supercritical extraction process and the fractionation applying supercritical antisolvent fractionation (SAF). By the process of SAF, the compounds can be precipitated based on their polarity and system’s polarity. The continuous contact between SCCO2 and liquid extracts (PLE with ethanol/water), dissolve the less polar molecules in the extracts and thus these molecules can be separated from the polar molecules. Another integrated approach is coupling of PLE with SAF to extract phenol rich compounds [109]. The current research focus is based on reducing component deterioration during extraction, purification, and storage. In this regard, integration of PLE or SFE with drying process is becoming famous. In this process, at first PLE or SFE process is optimized for the extraction of specific molecules and then extracted molecules are dried using supercritical antisolvent (SAS). In this process, an organic solution should be put with SCCO2 like SAF process. By supersaturating the solute, the SAS approach can also be applied to co-precipitate or to enclose target chemicals, resulting in sub-micrometric particles of regulated size. Some sequential extraction process is also used for the fractionation of different products from microalgae [2].
SWE process is also considered as a green technology, and still under developmental stage had shown many applications as environment-friendly integrated systems for the extraction of different biomolecules from algae cells. This process is able to perform different operations (e.g., extraction, fractionation, reaction) and is usually used for the extraction of bio-oil from microalgae. Several researches have shown the use of the SWE method for the extraction of valuable compounds such as polysaccharides from micro- and macroalgae cells with higher extraction yields. This extraction process allows the extraction of valuable compounds by reducing the extraction time and use of water at the subcritical state as the solvent. Since the heat of vaporization of water is relatively high, there are some difficulties in concentrating the valuable compounds in extract when subcritical water extraction was applied as an extraction media. The presence of water reduces the extract stability, freeze-drying is one way to remove water from the extract. However, freeze-drying is costly and time-consuming and promotes degradation of the molecules due to light and oxygen contact during the process. In general, this SWE technique is used for the extraction of carbohydrate molecules from different algal strains. SWE process is also used for the extraction of the protein molecules from the cells. In the SWE process, a number of factors that affect the extraction efficiency of biomolecules from the algae cells are temperature, extraction time, biomass loading, and particle size. SWE process also can be used together with acid or alkali which helps in the extraction of polysaccharide molecules from the microalgae. Many applications have shown efficient extraction of biomolecules by using another polar protic solvent, instead of subcritical water and the process termed pressurized liquid extraction. Some valuable compounds like sulfated polysaccharides (fucose) and agar are extracted from the algae cells by using an eco-friendly microwave-assisted SWE process. This process is also used for the depolymerization of alginate and efficient extraction of the molecules from the algae cells. Wide range of biomolecules which are present inside the algae cells can be extracted efficiently by the integration of the SCCO2 and SWE process. However, further research is required in this area to understand the benefit of this combined process completely. A future trend is also observed where the SWE process is combined with enzymatic catalysis. This enzymatic step will enable the enzymatic reaction to be converted into a subcritical enzymatic water reaction and extraction [110]. There are presently only a few examples provided in the literature using this approach. The production and application of this method will greatly increase the development of new methods for extraction, fractionation, and purification using thermo-stable molecules. New approaches are, therefore, expected in this area with the final goals of simplifying the technique, increasing the efficiency, and decreasing the risks for the environment and human health. Sustainability is the key to providing new answers to the challenges we are facing today. Finally, it could be said that SCCO2 and subcritical water are efficient methods and identified as future green technologies for the extraction of natural product from macro- and microalgae matrices.
References
Chemat F, et al. (2019) Review of alternative solvents for green extraction of food and natural products: panorama, principles, applications and prospects. Molecules 24(16)
Gallego R, Bueno M, Herrero M (2019) Sub-and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae–an update. TrAC, Trends Anal Chem 116:198–213
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. Int J Mol Sci 13(7):8615–8627
Budisa N, Schulze-Makuch D (2014) Supercritical carbon dioxide and its potential as a life-sustaining solvent in a planetary environment. Life 4(3):331–340
Herrero M et al (2010) Supercritical fluid extraction: recent advances and applications. J Chromatogr A 1217(16):2495–2511
Barbosa HM et al (2014) Optimization of the supercritical fluid coextraction of oil and diterpenes from spent coffee grounds using experimental design and response surface methodology. J Supercrit Fluid 85:165–172
Allada SR (1984) Solubility parameters of supercritical fluids. Ind Eng Chem Process Des Dev 23(2):344–348
del Pilar Sánchez-Camargo A et al (2017) Bioactives obtained from plants, seaweeds, microalgae and food by-products using pressurized liquid extraction and supercritical fluid extraction. Green Extraction Techniques: Principles, Advances and Applications 76:27
Li Z, Smith KH, Stevens GW (2016) The use of environmentally sustainable bio-derived solvents in solvent extraction applications—a review. Chin J Chem Eng 24(2):215–220
Sánchez-Camargo A et al (2016) Application of Hansen solubility approach for the subcritical and supercritical selective extraction of phlorotannins from Cystoseira abies-marina. RSC Adv 6(97):94884–94895
Michalak I, Chojnacka K (2014) Algal extracts: technology and advances. Eng Life Sci 14(6):581–591
Santana A, Jesus S, Larrayoz M (2012) Supercritical carbon dioxide extraction of algal lipids for the biodiesel production. Procedia Eng 42:1755–1761
Halim R et al (2011) Oil extraction from microalgae for biodiesel production. Biores Technol 102(1):178–185
Quitain AT et al (2013) Supercritical carbon dioxide extraction of fucoxanthin from Undaria pinnatifida. J Agric Food Chem 61(24):5792–5797
Abdelmoez W, Nakahasi T, Yoshida H (2007) Amino acid transformation and decomposition in saturated subcritical water conditions. Ind Eng Chem Res 46(16):5286–5294
Alghoul ZM, Ogden PB, Dorsey JG (2017) Characterization of the polarity of subcritical water. J Chromatogr A 1486:42–49
Galamba N et al (2019) Solubility of polar and nonpolar aromatic molecules in subcritical water: the role of the dielectric constant. J Chem Theory Comput 15(11):6277–6293
Wiboonsirikul J, Adachi S (2008) Extraction of functional substances from agricultural products or by-products by subcritical water treatment. Food Sci Technol Res 14(4):319–319
Plugatyr A, Svishchev IM (2011) Molecular diffusivity of phenol in sub- and supercritical water: application of the split-flow Taylor dispersion technique. J Phys Chem B 115(11):2555–2562
Sasaki M et al (2000) Dissolution and hydrolysis of cellulose in subcritical and supercritical water. Ind Eng Chem Res 39(8):2883–2890
Getachew AT, Chun BS (2017) Molecular modification of native coffee polysaccharide using subcritical water treatment: structural characterization, antioxidant, and DNA protecting activities. Int J Biol Macromol 99:555–562
Zakaria SM, Kamal SMM (2016) Subcritical water extraction of bioactive compounds from plants and algae: applications in pharmaceutical and food ingredients. Food Engineering Reviews 8(1):23–34
Rodríguez-Meizoso I et al (2010) Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. J Pharm Biomed Anal 51(2):456–463
Santoyo S et al (2010) Pressurized liquid extraction as an alternative process to obtain antiviral agents from the edible microalga Chlorella vulgaris. J Agric Food Chem 58(15):8522–8527
Santoyo S et al (2011) Pressurized liquids as an alternative green process to extract antiviral agents from the edible seaweed Himanthalia elongata. J Appl Phycol 23(5):909–917
Santoyo S et al (2012) Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J Appl Phycol 24(4):731–741
Plaza M et al (2012) Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. LWT-Food Science and Technology 46(1):245–253
Bueno M et al (2020) Compressed CO2 technologies for the recovery of carotenoid-enriched extracts from Dunaliella salina with potential neuroprotective activity. ACS Sustain Chem Eng 8(30):11413–11423
Crampon C, Boutin O, Badens E (2011) Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind Eng Chem Res 50(15):8941–8953
Crampon C et al (2013) Influence of pretreatment on supercritical CO2 extraction from Nannochloropsis oculata. J Supercrit Fluid 79:337–344
Turner C, King JW, Mathiasson L (2001) Supercritical fluid extraction and chromatography for fat-soluble vitamin analysis. J Chromatogr A 936(1–2):215–237
Castro-Puyana M et al (2013) Subcritical water extraction of bioactive components from algae. Functional ingredients from algae for foods and nutraceuticals. Elsevier, pp 534–560
Plaza M et al (2010) Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res Int 43(4):1123–1129
Herrero M et al (2005) Capillary electrophoresis-mass spectrometry of Spirulina platensis proteins obtained by pressurized liquid extraction. Electrophoresis 26(21):4215–4224
Hawthorne SB, King JW (2018) Principles and practice of analytical SFE. Practical Supercritical Fluid Chromatography and Extraction. Routledge, pp 219–282
Michalak, I., et al., Supercritical algal extracts: a source of biologically active compounds from nature. Journal of Chemistry, 2015. 2015.
Mercer P, Armenta RE (2011) Developments in oil extraction from microalgae. Eur J Lipid Sci Technol 113(5):539–547
Aravena, R.I. and J.M. del Valle (2012) Effect of microalgae preconditioning on supercritical CO2 extraction of astaxanthin from Haematococcus pluvialis. in Proceedings of the 10th International Symposium of Supercritical Fluids, San Francisco, CA, USA
Safi C et al (2014) Extraction of lipids and pigments of Chlorella vulgaris by supercritical carbon dioxide: influence of bead milling on extraction performance. J Appl Phycol 26(4):1711–1718
Fujii K (2012) Process integration of supercritical carbon dioxide extraction and acid treatment for astaxanthin extraction from a vegetative microalga. Food Bioprod Process 90(4):762–766
Men’shova, R., et al., Effect of pretreatment conditions of brown algae by supercritical fluids on yield and structural characteristics of fucoidans. Chemistry of Natural Compounds, 2013. 48(6): p. 923–926.
Patil PD et al (2018) Extraction of bio-oils from algae with supercritical carbon dioxide and co-solvents. J Supercrit Fluid 135:60–68
Cheng C-H et al (2011) Comparative study of lipid extraction from microalgae by organic solvent and supercritical CO2. Biores Technol 102(21):10151–10153
Dejoye C et al (2011) Combined extraction processes of lipid from Chlorella vulgaris microalgae: microwave prior to supercritical carbon dioxide extraction. Int J Mol Sci 12:9332–9341
Balboa EM, Moure A, Domínguez H (2015) Valorization of Sargassum muticum biomass according to the biorefinery concept. Mar Drugs 13(6):3745–3760
Pan JL et al (2012) Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng Life Sci 12(6):638–647
Ibañez E et al (2012) Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates. Marine bioactive compounds. Springer, pp 55–98
Khaw K-Y et al (2017) Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: a review. Molecules 22(7):1186
Plaza M, Cifuentes A, Ibáñez E (2008) In the search of new functional food ingredients from algae. Trends Food Sci Technol 19(1):31–39
Yen H-W et al (2015) Supercritical fluid extraction of valuable compounds from microalgal biomass. Biores Technol 184:291–296
Vandamme EJ and Revuelta JL (2016) Vitamins, biopigments, antioxidants and related compounds: a historical, physiological and (bio) technological perspective. Industrial biotechnology of vitamins, biopigments, and antioxidants
Zhang J et al (2014) Microalgal carotenoids: beneficial effects and potential in human health. Food Funct 5(3):413–425
Liau B-C et al (2010) Supercritical fluids extraction and anti-solvent purification of carotenoids from microalgae and associated bioactivity. The Journal of Supercritical Fluids 55(1):169–175
Yen H-W, Chiang W-C, Sun C-H (2012) Supercritical fluid extraction of lutein from Scenedesmus cultured in an autotrophical photobioreactor. J Taiwan Inst Chem Eng 43(1):53–57
Cardoso L et al (2012) Extraction of carotenoids and fatty acids from microalgae using supercritical technology. Am J Anal Chem 3(12A):877–883
Abrahamsson V, Rodriguez-Meizoso I, Turner C (2012) Determination of carotenoids in microalgae using supercritical fluid extraction and chromatography. J Chromatogr A 1250:63–68
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30(3):709–732
Atabani AE et al (2012) A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew Sustain Energy Rev 16(4):2070–2093
Solana M, Rizza C, Bertucco A (2014) Exploiting microalgae as a source of essential fatty acids by supercritical fluid extraction of lipids: comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. The Journal of Supercritical Fluids 92:311–318
Li Y et al (2014) A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb Cell Fact 13(1):1–9
Sarkar S et al (2020) Priority-based multiple products from microalgae: Review on techniques and strategies. Crit Rev Biotechnol 40(5):590–607
Soh L, Zimmerman J (2011) Biodiesel production: the potential of algal lipids extracted with supercritical carbon dioxide. Green Chem 13(6):1422–1429
Zhang, H., et al., Fucoxanthin: a promising medicinal and nutritional ingredient. Evidence-based complementary and alternative medicine, 2015. 2015.
Kanda H et al (2014) Extraction of fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar Drugs 12(5):2383–2396
Kim SM et al (2012) A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl Biochem Biotechnol 166(7):1843–1855
Quitain AT et al (2013) Microwave–hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide deoiled Undaria pinnatifida. Ind Eng Chem Res 52(23):7940–7946
Conde E, Moure A, Domínguez H (2015) Supercritical CO2 extraction of fatty acids, phenolics and fucoxanthin from freeze-dried Sargassum muticum. J Appl Phycol 27(2):957–964
Klejdus B et al (2010) Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J Chromatogr A 1217(51):7956–7965
Wahyudiono W, Machmudah S, Goto M (2013) Utilization of sub and supercritical water reactions in resource recovery of biomass wastes. Engineering Journal 17(1):1–12
Plaza M, Turner C (2015) Pressurized hot water extraction of bioactives. TrAC, Trends Anal Chem 71:39–54
Cheng Y et al (2021) Subcritical water extraction of natural products. Molecules 26(13):4004
Zhang C et al (2016) Enhancing the performance of co-hydrothermal liquefaction for mixed algae strains by the Maillard reaction. Green Chem 18(8):2542–2553
Lee W-K, Namasivayam P, Ho C-L (2014) Effects of sulfate starvation on agar polysaccharides of Gracilaria species (Gracilariaceae, Rhodophyta) from Morib Malaysia. J Appl Phycol 26(4):1791–1799
Machmudah S et al (2017) Sub-and supercritical fluids extraction of phytochemical compounds from Eucheuma cottonii and Gracilaria sp. Chem Eng Trans 56:1291–1296
Vázquez-Delfín E, Robledo D, Freile-Pelegrín Y (2014) Microwave-assisted extraction of the Carrageenan from Hypnea musciformis (Cystocloniaceae, Rhodophyta). J Appl Phycol 26(2):901–907
Alvarez-Rivera G et al (2020) Chapter 13 - Pressurized liquid extraction. Liquid-phase extraction. Elsevier, pp 375–398
Picó Y (2012) 3.28 - Recent advances in sample preparation for pesticide analysis, in Comprehensive sampling and sample preparation, J. Pawliszyn, Editor, Academic Press: Oxford. p. 569–590
de Jesus Raposo, M.F., A.M.M.B. de Morais, and R.M.S.C. de Morais, Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life sciences, 2014. 101(1–2): p. 56–63.
Awaluddin, S., et al., Subcritical water technology for enhanced extraction of biochemical compounds from Chlorella vulgaris. BioMed research international, 2016. 2016.
Sarkar S, et al. (2019) Integrated approach for the sustainable extraction of carbohydrates and proteins from microalgae, in Sustainable downstream processing of microalgae for industrial application, CRC Press. p. 201-227
Jönsson M et al (2020) Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 25(4):930
Tsubaki S, et al. (2016) Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food chemistry. 210: p. 311–316
Alboofetileh M et al (2019) Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int J Biol Macromol 128:244–253
Ahmed ABA et al (2014) Pharmaceutical, cosmeceutical, and traditional applications of marine carbohydrates. Adv Food Nutr Res 73:197–220
Meillisa A, Woo H-C, Chun B-S (2015) Production of monosaccharides and bio-active compounds derived from marine polysaccharides using subcritical water hydrolysis. Food Chem 171:70–77
Yun EJ et al (2016) Pretreatment and saccharification of red macroalgae to produce fermentable sugars. Biores Technol 199:311–318
Din SS et al (2019) Extraction of agar from Eucheuma cottonii and Gelidium amansii seaweeds with sonication pretreatment using autoclaving method. J Oceanol Limnol 37(3):871–880
Li Y-X et al (2011) Phlorotannins as bioactive agents from brown algae. Process Biochem 46(12):2219–2224
Wang T et al (2010) Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT-Food Sci Technol 43(9):1387–1393
López A et al (2011) The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem 125(3):1104–1109
Plaza M et al (2010) Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res Int 43(10):2341–2348
Reddy HK et al (2014) Subcritical water extraction of lipids from wet algae for biodiesel production. Fuel 133:73–81
Thiruvenkadam S, et al. (2018) Subcritical water extraction of Chlorella pyrenoidosa: Optimization through response surface methodology. BioMed research international, 2018
Zainan NH, et al. (2019) Biochemical analysis and potential applications of aqueous and solid products generated from subcritical water extraction of microalgae Chlorella pyrenoidosa biomass. J Appl Phycol: p. 1–16
Mendiola JA, et al. (2007) Use of supercritical CO2 to obtain extracts with antimicrobial activity from Chaetoceros muelleri microalga. A correlation with their lipidic content. Eur Food Res Technol 224(4):505–510
Cheung PC (1999) Temperature and pressure effects on supercritical carbon dioxide extraction of n-3 fatty acids from red seaweed. Food Chem 65(3):399–403
Macías-Sánchez M et al (2010) Supercritical fluid extraction of carotenoids from Scenedesmus almeriensis. Food Chem 123(3):928–935
Herrero M et al (2013) Compressed fluids for the extraction of bioactive compounds. TrAC, Trends Anal Chem 43:67–83
Gilbert-López B et al (2017) Green compressed fluid technologies for downstream processing of Scenedesmus obliquus in a biorefinery approach. Algal Res 24:111–121
Sánchez‐Camargo AdP, et al. (2018) Development of green extraction processes for Nannochloropsis gaditana biomass valorization. Electrophoresis 39(15):1875–1883
Reyes FA et al (2014) Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J Supercrit Fluid 92:75–83
Herrero M et al (2015) Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC, Trends Anal Chem 71:26–38
Koo SY et al (2012) Optimization of pressurized liquid extraction of zeaxanthin from Chlorella ellipsoidea. J Appl Phycol 24(4):725–730
Gilbert-López B et al (2015) Downstream processing of Isochrysis galbana: a step towards microalgal biorefinery. Green Chem 17(9):4599–4609
Rodríguez-Meizoso I et al (2012) Life cycle assessment of green pilot-scale extraction processes to obtain potent antioxidants from rosemary leaves. J Supercrit Fluid 72:205–212
Righi S et al (2011) Comparative cradle-to-gate life cycle assessments of cellulose dissolution with 1-butyl-3-methylimidazolium chloride and N-methyl-morpholine-N-oxide. Green Chem 13(2):367–375
Cardenas-Toro FP et al (2015) Pressurized liquid extraction and low-pressure solvent extraction of carotenoids from pressed palm fiber: experimental and economical evaluation. Food Bioprod Process 94:90–100
Wang, H.-C., et al., Continuous extraction of lipids from Schizochytrium sp. by CO2-expanded ethanol. Bioresource technology, 2015. 189: p. 162–168.
Sánchez-Camargo A et al (2016) Supercritical antisolvent fractionation of rosemary extracts obtained by pressurized liquid extraction to enhance their antiproliferative activity. J Supercrit Fluid 107:581–589
Vardakas A (2020) A new process for enzyme-assisted subcritical water extraction of rice husk polyphenols. University of Food Technologies: Plovdiv, Bulgaria. p. 76.
Palavra A et al (2011) Supercritical carbon dioxide extraction of bioactive compounds from microalgae and volatile oils from aromatic plants. J Supercrit Fluid 60:21–27
Tang S et al (2011) Study on supercritical extraction of lipids and enrichment of DHA from oil-rich microalgae. J Supercrit Fluid 57(1):44–49
Nobre BP, et al. (2013) A biorefinery from Nannochloropsis sp. microalga–extraction of oils and pigments. Production of biohydrogen from the leftover biomass. Bioresource Technol 135:128–136
Golmakani M-T et al (2012) Expanded ethanol with CO2 and pressurized ethyl lactate to obtain fractions enriched in γ-linolenic acid from Arthrospira platensis (Spirulina). J Supercrit Fluid 62:109–115
Billakanti JM, et al. (2012) Extraction of fucoxanthin from Undaria pinnatifida using enzymatic pre-treatment followed by DME and EtOH co-solvent extraction. in 10th International Symposium on Supercritical Fluids. CASSS: Emeryville, CA, USA
Pérez-López P et al (2014) Comparative environmental assessment of valorization strategies of the invasive macroalgae Sargassum muticum. Biores Technol 161:137–148
Zheng J et al (2012) Chemical composition and antioxidant/antimicrobial activities in supercritical carbon dioxide fluid extract of Gloiopeltis tenax. Mar Drugs 10(12):2634–2647
Rodriguez-Jasso RM et al (2014) Chemical composition and antioxidant activity of sulphated polysaccharides extracted from Fucus vesiculosus using different hydrothermal processes. Chem Pap 68(2):203–209
González-López N, Moure A, Domínguez H (2012) Hydrothermal fractionation of Sargassum muticum biomass. J Appl Phycol 24(6):1569–1578
Saravana PS et al (2016) Evaluation of the chemical composition of brown seaweed (Saccharina japonica) hydrolysate by pressurized hot water extraction. Algal Res 13:246–254
Funding
This study is financially supported by the Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India (Project Number: CRG/2018/002479).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkar, S., Gayen, K. & Bhowmick, T.K. Green extraction of biomolecules from algae using subcritical and supercritical fluids. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-02309-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-02309-3