Abstract
Plants and algae are the main sources of natural bioactive compounds used in the food and pharmaceutical industries. It is very important to achieve an efficient and safe technique to recover bioactive compounds while maintaining their quality and properties. Subcritical water extraction is the most promising engineering approach that offers an environmentally friendly technique for extracting various compounds from plants and algae. Application of pressurized water and high temperature in subcritical phase is able to modify the dielectric constant and polarity of the solvent which then contributes to a better extraction process. The technique improves the mass transfer rate and preserves the biological potency of the extracts. This article reviews current studies on the extraction of bioactive compounds from various species of plants and algae using the subcritical water technique and discusses its effects and benefits for the food and pharmaceutical industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants and algae provide an almost inexhaustible source of natural bioactive compounds. The development and production of natural products has become of great interest to the food and pharmaceutical industries. Therefore, recovering bioactive compounds from plants and algae has also become a vital topic in many industries including research laboratories.
Extraction technologies have been developed to separate desirable compounds from plants and algae to obtain highly purified products. Bioactive compounds can be identified in algae and various parts of plants such as the stem, flowers, fruits or leaves. Selecting an effective and appropriate extraction method is very important for quantitative and qualitative studies of the compound extraction process. There are many factors influencing the extraction process involving a solid matrix and a solvent such as the matrix properties of the plant components, solvent, temperature, pressure, ratio of solvent to matrix, and time applied [1].
Many extraction techniques have been used for the discovery of various bioactive compounds such as Soxhlet extraction, maceration, and hydro-distillation. However, these techniques can be time consuming and costly, consume large quantities of highly pure solvent, poor selectivity, and have a risk of decomposition of thermolabile compounds [2]. These difficulties have led to the development of the subcritical water extraction (SWE) technique which is also known as pressurized hot water extraction (PHWE) and superheated water extraction (SHWE). SWE is an environmentally friendly approach that uses water as the solvent and high temperatures and pressures to achieve the safe, rapid, and efficient extraction of the required compounds from the plant and algae matrices.
This article reviews recent progress in the application of SWE to various plants and algae species for use in the food and pharmaceutical industries. The first section highlights the properties of SWE, its mechanism, and its benefits. The second section discusses how the subcritical water technique is applied to plant extraction, the species of plant used, and the compounds recovered. This is followed by an overview of the SWE technique for recovering bioactive compounds from algae species. The effects of the SWE process on some biological activities of the extracted compounds are also discussed. The review concludes by examining the outlook for improving this particular technique in terms of its applications, products, and process design.
Subcritical Water Extraction (SWE): Properties and Benefits
Extraction processes for recovering bioactive compounds have been developed to get highly purified products and make them available for an extensive range of applications. These technologies offer an innovative approach to increase the production of these compounds [3]. Carefully managing parameters such as pressure, time, and temperature, together with selecting an appropriate solvent, are both extremely important during the extraction process [4]. These parameters and the initial materials can affect the composition and properties of the extracted compounds, especially relevant for processes in the pharmaceutical and food industries.
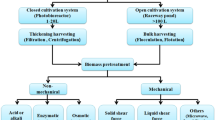
The SWE setup consists of a solvent reservoir with a high-pressure pump for the input of the water into the system, a cell or column within an oven where the extraction occurs and a valve to maintain the pressure within the system. The extraction column was heated in a gas chromatograph oven, and a pump was used to pump the water into the column. Pressurized sample column possibly kept the water in liquid phase at the high extraction temperatures. Static extraction occurs with the system’s valves fully closed [2, 5, 6]. The basic diagram for a SWE process used for plant and algae is presented in Fig. 1. The extraction process involved three sequential steps that started with diffusion of solutes from the transition components to the surface. Then, the solutes compound will be transferred into the solvent and followed by the elution of the solutes compound out of the extraction column [7, 8].
The SWE process involves the application of water at temperatures higher than its boiling point under high pressure to maintain its liquid state. The temperature applied during extraction process has an important impact on the extraction efficiency and selectivity. The temperature above the normal boiling point of water was normally used in SWE. As a result, physical advantages including high diffusion, low viscosity, and low surface tension are achieved with this elevated temperature condition. In addition, increases in vapor pressures and rapid thermal desorption of target compounds from matrices could also enhance the extraction efficiency. In this condition, the physical and chemical properties of water as a solvent, such as its dielectric constant and solubility parameter, will change dramatically. At room temperature (25 °C), the dielectric constant of water is around 80 and decreases to about 33 at 200 °C. This value is similar to some organic solvents such as ethanol or methanol [2, 3, 9]. Consequently, this shows that SWE can be applied for extracting nonpolar compounds and water can be used to replace organic solvents. Nevertheless, the variation in dielectric constant for each type of compounds must be considered.
The solubility parameter of water as a solvent can be modified by manipulating the temperature. Increasing the temperature favors the solubility of the compound and modification on its selectivity [5]. Srinivas et al. [10] reported on Hansen three-dimensional solubility parameter concept to optimize the extraction of molecularly complex solutes using subcritical fluid solvents. Hansen solubility spheres have been used to characterize and quantify the solute–subcritical water interactions as a function of temperature. The use of group contribution methods in collaboration with computerized algorithms to plot the Hansen spheres provides a quantitative prediction tool for optimizing the design of extraction conditions. They use three examples on SWE process, which are betulin from birch bark, niacin from spent brewer’s yeast, and flavonoids from grape pomace to test the applicability of the predictive method. From the result, it can be seen that the method can be used to estimate conditions for solute–solvent miscibility, an optimum temperature range for conducting extractions under pressurized conditions, and approximate extraction conditions of solutes from natural matrices, which makes it possible to design the extraction conditions required for processing of natural products using SWE [10, 11]. In addition, by increasing the temperature, the viscosity and surface tension of the water decrease, while the diffusivity increases, allowing a deep penetration of the solvent into the matrix, thus enhancing the efficiency and speed of the extraction process [6]. This means that the mass transfer rate from the solid phase to the subcritical water is improved.
On the other hand, elevated pressures were applied to keep the water from boiling and maintaining its liquid phase. However, varying the pressure did not result in any changes and often has little effect on the extraction efficiency of SWE. The time for the extraction is depended on the temperature and the nature of the matrix and target compounds. For SWE, the extraction times were very short compared with any other conventional extraction techniques. But, a long heating time during the extraction may cause the compound degradation. Therefore, optimization of time is very important to construct the SWE process [6–8].
SWE offers a series of important advantages over other techniques: the higher quality of the extracts, a faster process, a reduction in the amount of solvents used, and its cost. Additionally, by using water instead of organic solvents, SWE is a more environmentally friendly extraction process for extracting functional food or pharmaceutical ingredients that are beneficial to health [12]. Moreover, due to some reports on interactions among the compounds during SWE and the structure of newly formed antioxidants, further research must be developed to investigate individual roles of the compounds to the whole antioxidant capacity or any other biological activities of the extracts [3, 13].
Extraction of Bioactive Compounds from Plants
Plants contain many biocompounds with therapeutic potential. These are the result of stresses, such as fluctuations in light and temperature, or changing exposure to seawater by the tide. As a consequence, they have adapted by developing many biochemicals that help them thrive [14, 15]. The biochemicals perform as a mechanical support as well as immunological signals that can be harnessed for use in the food and pharmaceutical fields. Biochemical processing companies have identified the potential of biochemicals in plants and have developed various extraction methods to obtain these biochemicals for use in industry [16].
In the industrial synthesis of organic chemicals, plants play an important role as the source of basic chemicals which will then be used in various applications including food, pharmaceuticals, and in research laboratories. Some studies on extracting beneficial compounds from plants using the SWE technique are summarized in Table 1.
Historically, essential oils from various plants have been applied over a range of uses from food preservation [55] to skin treatments and cancer remedies [56, 57]. Recently, SWE has been used to extract essential oils from plants, including fennel. Gamiz-Gracia and Luque de Castro [17] compared the SWE method with both hydro-distillation and dichloromethane extraction, and a better outcomes have been acquired regarding to the speed, productivity, purity, and feasibility of controlling the composition of the extract. SWE was faster than the other conventional methods, taking 50 min, while hydro-distillation and manual dichloromethane extraction required 4 and 24 h, respectively [17].
Essential oils from Thymbra spicata leaves were also been extracted using subcritical water by Ozel et al. [18]. Due to their report, SWE presented the highest extraction efficiency and minimum time by a complete extraction of 20 min [18]. Other results from investigations using SWE for Shirazi thyme (Zataria multiflora Boiss.) and Coriander (Coriandrum sativum) confirmed that SWE took less time and provided a higher quality of extract compared with the other techniques tested [19, 20].
SWE has also been used to obtain extracts with a high phenolic content from plants. Phenolic compounds form a major group of plant secondary metabolites and occur widely with a diverse range of structures [58]. They can occur as glycones or glycosides, as monomers or highly polymerized structures, and as free or matrix-bound compounds [58, 59]. Nevertheless, phenolic compounds are not uniformly distributed in the plant matrix and vary considerably in terms of their stability, which complicates the processes of their extraction and isolation. Therefore, these procedures must be optimized, depending on the nature of the sample chosen and the target analytes [60, 61].
There have been few studies on the extraction of phenolic compounds from plants using SWE. These include extraction from mango leaves [21], pomegranate [22], rice bran [23], potato peel [24], cinnamon [26], citrus pomaces [27], golden oyster mushroom [28], oregano [30], and marigold flowers [31]. These studies have indicated that SWE is a promising technique for the successful preparation and isolation of phenolic compounds from the matrices of various plants.
Flavonoids are extensively distributed in plants and possess many roles and functions. Variability, low toxicity, and wide distribution of flavonoids compared with other active compounds make the animals, as well as humans, ingest significant amounts of it in their diet [62]. A study on extracting flavonoids from various plants (onion skins, Saururus chinensis, seabuckthorn leaves, parsley, carrots, and lemon, orange, and grapefruit peels) determined the optimum conditions of time and temperature for SWE and their dependence on the chemical structure of the flavonoid. This showed that the extraction of a flavonoid was greatly influenced by both the extraction conditions of the subcritical water solvent and the structure of the flavonoid. SWE at higher temperatures increased the thermal agitation and hence decreased the strength of the hydrogen bonds, which led to the more efficient extraction of nonpolar hydrophobic compounds compared with polar hydrophilic compounds [34]. Quercetin is one of the flavonoids that naturally occur in plants and commonly extracted using ethanol or aqueous-based ethanol and methanol solutions. However, subcritical water could be an excellent alternative medium for extracting quercetin due to its temperature-dependent selectivity, environmental acceptability, efficiency, and lower cost. Few studies have been done on extracting this compound through SWE including onion waste, Polygonum hydropiper and onion skin [35–37]. Quercetin has many biological activities and known to have had a powerful antioxidant activity both in vitro and in vivo. Turner et al. [35] implemented SWE with biocatalytic conversion to produce quercetin. The conversion process by using Thermotoga neapolitana β-glucosidase enzyme converted quercetin glycosides to active quercetin in <10-min reaction. The study demonstrated rapid method to determine the content of quercetin and isorhamnetin in onion samples and is environmentally sustainable as it only uses water as solvent and enzymes as catalysts.
Polysaccharides are also a major constituent of plants. Chao et al. [39] extracted valuable natural polysaccharides from Lucium barbarum using SWE with the assistance of ultrasonication as a pre-treatment. Their study showed that the maximum recovery of polysaccharides was obtained at an extraction temperature of 100 °C for 53 min. The process was very efficient because of its easy pre-treatment and the high yields of extracted polysaccharides [39]. Polysaccharides have also been recovered from Ganoderma lucidum (Lingzhi mushroom) using SWE [40]. During the extraction process, the thermal softening of Ganoderma lucidum allowed the removal of the polysaccharides and protected other components through hydrolysis. There have also been studies on extracting cellulose and hemicellulose using SWE from citrus junos peels and oil palm fronds [41, 42]. The highest cellulose content obtained from citrus junos peel was up to 80 % using a 200 °C treatment temperature, and from oil palm, a maximum hemicellulose yield of 69.60 % at a temperature of 190 °C was obtained. Both these studies indicate that SWE can be applied efficiently and that its parameters manipulated to maximize the yield of compounds.
Monosaccharides and oligosaccharides can also be extracted using the SWE technique from palm oil fronds [38] and coconut plants [43]. Monosaccharide and oligosaccharides are produced by the decomposition of carbohydrates and can be used as a biofuel, food additive, or pharmaceutical products. The solubilized monosaccharide can also be converted and produce other polymers [63]. For palm oil, the highest monosaccharide content was successfully extracted using SWE at a temperature of 190 °C with a treatment time of 10 min, while for coconut, the highest yield of mono- and oligosaccharides extracts was obtained at a temperature of 250 °C for 10 min. The yield and ratio of saccharide components can also be manipulated by varying the proportion of raw materials to water used in the SWE process [43].
Apart from the examples above, SWE has also been used to extract many other compounds from various plants sources such as pectin [44], oil [47], reducing sugars [48], resorcinol and chavibetol [50], mannitol [51], asiatic acid [52], mangiferin [53], and carotenoids [54]. All these compounds have beneficial properties that can be applied in the food and pharmaceutical industries. These studies also suggest the SWE technique as the preferred approach for extracting these compounds from the original plant matrices as it has been shown that subcritical extraction with water enhanced the efficiency of extracting the target compounds. Compared with other organic solvents, using water is also cost-effective, environmentally friendly, and safe regarding its toxicity, flammability, and availability [51].
Extraction of Bioactive Compounds from Algae
Algae exist as a wide range of autotrophic organisms that grow using photosynthesis in a process similar to land-based plants. The unicellular structure of algae permits the easy conversion of solar energy into chemical energy [2, 64, 65]. Algae also possess greater sustainable and commercial advantages over other feed stocks. They are very attractive as a natural source of bioactive compounds as they have the potential to generate the compounds in cultures which allow the production of complex molecular structures [66–71].
The ability of algae to synthesize a variety of bioactive chemicals is a result of their numerous physiological, biochemical, and molecular strategies to cope with stress. As photosynthetic organisms, algae contain chlorophyll that can be applied in the food and cosmetic fields [72–74]. In the pharmaceutical industry, several types of algae have been used to create bioactive compounds such as antioxidants and antibiotics [75, 76]. Algae are consumed as nutritional supplements by humans as they are high in protein, vitamins, and polysaccharides [77, 78].
Compared with studies on plants, those on SWE of algae have focused mostly on the biological activities of the compounds extracted. Rodríguez-Meizoso et al. [5] have described the antioxidant and antimicrobial activity of compounds extracted from Haematococcus pluvialis microalgae using SWE. Their results showed that the extraction temperature greatly influenced the extraction yield and antioxidant activity. At 200 °C, the extraction yield was more than 30 % (dry weight basis) and the extracts exhibited the highest antioxidant activity. However, the extraction temperature did not significantly affect the antimicrobial activity of the extracts. In addition, a major issue for extraction from microalgae is breaking down the protective barrier (cell wall) which slows down and inhibits the extraction and bioavailability of bioactive compounds [79]. In this case, the microalgae samples were freshly pre-treated by freezing with liquid nitrogen and mashing them in a ceramic mortar to improve the extraction yield [5].
In another study by Plaza et al. [13] using SWE, raising the extraction temperature improved the antioxidant capacity of natural extracts. They also verified the neoformation of antioxidants during SWE from different natural products, such as Chlorella vulgaris, C. abies-marina, Porphyra spp., S. vulgare, S. muticum, U. pinnatifida, and H. incurvus algae. The antioxidant capacity of the extracts obtained at 200 °C was significantly higher than those extracted at 100 °C. The increases in antioxidant capacity were caused by the production of new compounds derived from Maillard reactions and caramelization during the SWE process [13].
Effects of SWE on In Vitro Biological Assessment of Plants/Algae Extracts
Characterizing bioactive compounds extracted using SWE has also been determined by assessing their in vitro biological activities including their antioxidant and antimicrobial activities. Table 2 summarizes the studies on the biological activities of compounds extracted from plants or algae using SWE.
The term antioxidant refers to any compound that hinders the reaction of a substance with dioxygen or any compounds that inhibit free radical reaction [87, 88]. Most antioxidant compounds that exist naturally in plants have been classified as free radical scavengers [89]. Because of their positive effects, antioxidants have gained importance as health promoters to ameliorate cardiovascular problems, atherosclerosis, cancers, and the aging process [90].
Interest in identifying antioxidant compounds from plants and algae which pharmacologically have only slight or no side effects has increased worldwide, especially for use in protective medicines and the food industry [91]. Incorporating antioxidant constituents into the normal daily diet can support the human body in resisting some diseases [92, 93]. The protective mechanisms of antioxidants can inhibit the accumulation of reactive oxygen species and free radicals and so counteract their cell-damaging activities [94]. The antioxidant capacity of plants and algae products is of growing interest (Table 2), especially in the beverage market segment of functional foods and in pharmaceutical applications for treating oxidation-associated diseases such as inflammation [95, 96].
The antioxidant capacity of rosemary plant extracts obtained by the SWE technique has been evaluated and measured using a free radical method or 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. Ibanez et al. [80] showed that the antioxidant capacity of rosemary extracts obtained by SWE was very high and comparable to that using supercritical fluid extraction [80, 97]. In their study, the effect of temperature on extraction efficiency was investigated with values of antioxidant activity from SWE extracts at around 11.3 µg/mL for all the temperatures tested [80]. The compounds contributing the highest antioxidant activity in the extracts were carnosic acid, carnosol, rosmanol, and other polar compounds. The inhibition of lipid peroxidation by the compounds was effective for protecting biological systems against oxidative stresses [98].
Seabuckthorn (Hippophae rhamnoides) leaves are also an antioxidant agent that has been evaluated using both DPPH and FRAP (ferric reducing ability of plasma) assays. Compared with extracts obtained from other extraction methods (Soxhlet and maceration extraction), SWE gave higher antioxidant activity and illustrated its advantages for obtaining formulations of high antioxidant compounds [81].
The antioxidant capacities of extracts from Mangifera indica leaves obtained from SWE compared with those from supercritical fluid extraction were higher [21]. Antioxidant activity of extracts and standard compounds, which are (+)-α-tocopherol, mangiferin, and quercetin, are determined by DPPH assay. Using SWE, the antioxidant capacity of the extracts were better than (+)-α-tocopherol and similar to that of the standard compounds, mangiferin (7.80 DPPH g/g dry extract) and quercetin (7.06 DPPH g/g dry extract). They concluded that SWE was more efficient for extracting phenolic compounds with potent antioxidant activity than supercritical fluid (carbon dioxide) extraction.
Other studies have reported the antioxidant capacity of extracts from various plants such as onion peel [82], Carya cathayensis Sarg. (Chinese hickory) [49], green coffee beans [84], and Haematococcus pluvialis algae [5]. They have shown that the antioxidant activities of the compounds extracted using SWE were always higher compared with using other extraction methods. The studies also proved that the antioxidant capacity was correlated with the content of phenolic compounds present. It was also be derivable to the synergistic effects of the various polyphenols including flavonoids in the plant itself such as onion peels [82]. However, further studies are required to determine all the elements in extracts that contribute to the antioxidant capacity. Finally, it also shows that plants contain strong antioxidant activity and that SWE is an efficient method to extract the antioxidant components [84].
SWE of algae (C. vulgaris, C. abies-marina, Porphyra spp., S. vulgare, S. muticum, U. pinnatifida, and H. incurvus) and plants (rosemary, thyme, and verbena) was studied with the aim of determining whether antioxidants occurred naturally in the raw samples or were formed during extraction by the Maillard reaction and other chemical events [13]. The results showed that antioxidants with various physicochemical properties could be formed during the SWE extraction process. In addition, they also studied the formation of neoantioxidants derived from Maillard reaction in glycation model systems [99]. Other reports have also suggested that antioxidants are formed during Maillard reactions and caramelization [100, 101].
Some studies have assessed in vitro the antimicrobial activities of compounds extracted by SWE, as well as antibacterial, antiviral, and anticancer activities [102]. Lee et al. [82] evaluated the antimicrobial effects of onion peel extract prepared using SWE. The microorganisms used are Staphylococcus aureus KCCM 40501, KCCM 32395, and KCCM 11335. Staphylococcus aureus is a Gram-positive bacterium and one of the pathogens that is most commonly associated with food-borne illnesses [103]. Some strains are resistant to some antibiotics used in hospitals and so are referred to as ‘super-bacteria’ or ‘methicillin-resistant Staphylococcus aureus (MRSA).’ The effect of SWE extract on various strains of Staphylococcus aureus has been investigated using quercetin as a standard [104]. This showed that the antimicrobial effect of the onion peel extract using SWE was greater than using quercetin at a similar concentration because of the carbohydrates in the extracts that can be used as nutrients for the growth of Staphylococcus aureus strains [82].
Another report evaluated the antimicrobial activity of Haematococcus pluvialis algae extracts against microorganisms that importance in the food industry as they can cause spoilage and some are pathogens, including Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 11775, Candida albicans ATCC 60193, and Aspergillus niger ATCC 16404 [5]. The algae extracts exhibited good antimicrobial activity against bacteria and yeast, at all the temperatures tested during the study. Analyzing the effects of extraction temperature on the antimicrobial activity of the extracts from the algae showed that changing the temperature produced no significant effects. However, for bacteria, using lower temperatures (50 or 100 °C) could enhance the extraction of compounds with antimicrobial activity [5].
Using SWE on Brassica juncea (mustard greens) has been studied to evaluate the antiviral effects of the extracts against the influenza virus, A/H1N1, being applied as a supplement in nonfat milk for use as an ‘antiviral food’ [85]. At the maximum nontoxic concentrations, the extracts had a higher antiviral activity against the virus than n-hexane, ethanol, or hot water (80 °C). Adding 0.5 mg/mL of Brassica juncea extract to the culture medium led to a cell viability (% antiviral activity) of 50.35 % for Madin-Darby canine kidney cells infected with the A/H1N1 influenza virus. A nonfat milk, supplemented with 0.28 mg/mL of Brassica juncea extract from SWE, showed 39.62 % antiviral activity against the A/H1N1 influenza virus. Therefore, the use of a Brassica juncea extract prepared by SWE as a food supplement might help protect against influenza viral infection. The antiviral activity of Brassica juncea extracts was maintained in nonfat milk, so it may be used as a food supplement in dairy products to prevent infection caused by the influenza virus.
Another study on the antibacterial activity of ginseng extracts produced from its stems and leaves compared the use of hot water and ethanol extraction with SWE [86]. All the ginseng extracts hindered the growth of Bacillus cereus, Salmonella enteritidis, Escherichia coli, and Listeria monocytogenes. They found that adding ginseng extracts prepared by SWE contributed to the disruption of the bacterial cell membranes. Ginseng extracts with antibacterial activity have a huge potential to be used as a food additive against food-borne pathogens, and SWE was very suitable and efficient at discovering ginseng extracts that inhibit bacterial growth [86].
The possible relevance of compounds extracted using the SWE process for antiproliferative activity has also been studied. Compounds derived from some reactions during the SWE process for olive leaves, such as 5-hydroxymethylfurfural (HMF), have been investigated by Herrero et al. [9]. Various extraction temperatures were used to evaluate the anticancer activity of the extracts to determine to what extent the HMF generated during the SWE process was responsible for the bioactivity of the extracts. In another aspect of this study, two different human colon adenocarcinoma cell culture models, HT-29 and SW-480 cells, were used to assess the anticancer activity of the olive leaves extracts. Observation using the MTT (colorimetric) assay showed that the proliferation of the two cell lines was not affected, regardless of the concentration of HMF in the range studied. This suggested that the presence of this compound had no influence on the extract’s antiproliferative activity against the colorectal adenocarcinoma HT-29 and SW-480 cells and demonstrated the safety of the SWE process [9].
Conclusion and Future Outlooks
In conclusion, natural bioactive compounds extracted from plants and algae are the preferred alternatives for use in the food and pharmaceutical industries. The extraction of the compounds is being studied and developed extensively to maintain their quality and properties while achieving an efficient and safe extraction process.
The extraction of various compounds from plants and algae using SWE provides a superior process, especially in environmental terms. In addition, SWE offers improvements in the mass transfer rate and enhances the permeability of solvent into the cells giving the advantages of higher extraction yields, shorter extraction times, and a minimal or no effect on the activity and structure of the bioactive compounds extracted.
Recently, food and pharmaceutical products obtained from natural sources have become popular because of changes in eating habits and concerns about health. Therefore, research and development on extracting these compounds, especially using SWE processes, must continue to make progress to improve the quality and properties of these bioactive compounds and to design safer and more environmentally friendly processes. SWE technology is developing toward scaled-up operation and design of industrial-scale equipment to extract high volume of desirable compounds from various natural sources, especially for pharmaceutical and food applications. It was started by laboratory and pilot-scale system development to optimize the process key parameters such as temperature, pressure, flow rate, or pH to achieve desirable extraction efficiency or rate.
References
Hernández Y, Lobo MG, González M (2009) Factors affecting sample extraction in the liquid chromatographic determination of organic acids in papaya and pineapple. Food Chem 114:734–741
Ibañez E, Herrero M, Mendiola J, Castro-Puyana M (2012) Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates. In: Hayes M (ed) Marine bioactive compounds. Springer, New York, pp 55–98
Joana Gil-Chávez G, Villa JA, Fernando Ayala-Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM, González-Aguilar GA (2013) Technologies for Extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci Food Saf 12:5–23
Azmir J, Zaidul I, Rahman M, Sharif K, Mohamed A, Sahena F, Jahurul M, Ghafoor K, Norulaini N, Omar A (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117:426–436
Rodríguez-Meizoso I, Jaime L, Santoyo S, Señoráns F, Cifuentes A, Ibáñez E (2010) Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. J Pharm Biomed Anal 51:456–463
Ong ES, Cheong JSH, Goh D (2006) Pressurized hot water extraction of bioactive or marker compounds in botanicals and medicinal plant materials. J Chromatogr A 1112:92–102
Teo CC, Tan SN, Yong JWH, Hew CS, Ong ES (2010) Pressurized hot water extraction (PHWE). J Chromatogr A 1217:2484–2494
Turner C, Ibañez E (2012) Pressurized hot water extraction. In: Lebovka N, Vorobiev E, Chemat F (eds) Enhancing extraction processes in the food industry. CRC Press, Boca Raton, pp 223–254
Herrero M, Cifuentes A, Ibanez A (2006) Sub-and supercritical fluid extraction of functional ingredients from different natural sources: plants, food-by-products, algae and microalgae: a review. Food Chem 98:136–148
Srinivas K, King J, Monrad J, Howard L, Hansen C (2009) Optimization of subcritical fluid extraction of bioactive compounds using Hansen solubility parameters. J Food Sci 74:342–354
King JW, Srinivas K (2009) Multiple unit processing using sub- and supercritical fluids. J Supercrit Fluids 47:598–610
Huie CA (2002) Review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal Bioanal Chem 373:23–30
Plaza M, Amigo-Benavent M, Del Castillo MD, Ibáñez E, Herrero M (2010) Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res Int 43:2341–2348
Harborne JB, Baxter H (1993) Phytochemical dictionary. A handbook of bioactive compounds from plants. Taylor & Francis Limited, New York
Schieber A, Stintzing FC, Carle R (2001) By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci Technol 12:401–413
Hammed AM, Jaswir I, Amid A, Alam Z, Asiyanbi-H TT, Ramli N (2013) Enzymatic hydrolysis of plants and algae for extraction of bioactive compounds. Food Rev Int 29:352–370
Gámiz-Gracia L, Luque de Castro MD (2000) Continuous subcritical water extraction of medicinal plant essential oil: comparison with conventional techniques. Talanta 51:1179–1185
Ozel MZ, Gogus F, Lewis AC (2003) Subcritical water extraction of essential oils from Thymbra spicata. Food Chem 82:381–386
Khajenoori M, Asl AH, Hormozi F, Eikani MH, Bidgoli HN (2009) Subcritical water extraction of essential oils from Zataria multiflora boiss. J Food Process Eng 32:804–816
Eikani MH, Golmohammad F, Rowshanzamir S (2007) Subcritical water extraction of essential oils from coriander seeds (Coriandrum sativum L.). J Food Eng 80:735–740
Fernández-Ponce MT, Casas L, Mantell C, Rodríguez M, Martínez de la Ossa E (2012) Extraction of antioxidant compounds from different varieties of Mangifera indica leaves using green technologies. J Supercrit Fluids 72:168–175
He L, Zhang X, Xu H, Xu C, Yuan F, Knez Z, Novak Z, Gao Y (2012) Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC–ABTS+ assay. Food Bioprod Process 90:215–223
Wataniyakul P, Pavasant P, Goto M, Shotipruk A (2012) Microwave pretreatment of defatted rice bran for enhanced recovery of total phenolic compounds extracted by subcritical water. Bioresour Technol 124:18–22
Singh PP, Saldaña MDA (2011) Subcritical water extraction of phenolic compounds from potato peel. Food Res Int 44:2452–2458
Alvarez VH, Cahyadi J, Xu D, Saldaña MDA (2014) Optimization of phytochemicals production from potato peel using subcritical water: experimental and dynamic modeling. J Supercrit Fluids 90:8–17
Khuwijitjaru P, Sayputikasikorn N, Samuhasaneetoo S, Penroj P, Siriwongwilaichat P, Adachi S (2012) Subcritical water extraction of flavoring and phenolic compounds from cinnamon bark (Cinnamomum zeylanicum). J Oleo Sci 61:349–355
Kim JW, Nagaoka T, Ishida Y, Hasegawa T, Kitagawa K, Lee SC (2009) Subcritical water extraction of nutraceutical compounds from citrus pomaces. Sep Sci Technol 44:2598–2608
Jo EK, Heo DJ, Kim JH, Lee YH, Ju YC, Lee SC (2013) The effects of subcritical water treatment on antioxidant activity of golden oyster mushroom. Food Bioprocess Technol 6:2555–2561
Gong Y, Zhang X, He L, Yan Q, Yuan F, Gao Y (2013) Optimization of subcritical water extraction parameters of antioxidant polyphenols from sea buckthorn (Hippophaë rhamnoides L.) seed residue. J Food Sci Technol 1:1–9
Rodríguez-Meizoso I, Marin FR, Herrero M, Señorans FJ, Reglero G, Cifuentes A, Ibáñez E (2006) Subcritical water extraction of nutraceuticals with antioxidant activity from oregano. Chemical and functional characterization. J Pharm Biomed Anal 41:1560–1565
Xu H, Wang W, Jiang J, Yuan F, Gao Y (2014) Subcritical water extraction and antioxidant activity evaluation with on-line HPLC-ABTS·+ assay of phenolic compounds from marigold (Tagetes erecta L.) flower residues. J Food Sci Technol 1:1–9
Cheigh CI, Yoo SY, Ko MJ, Chang PS, Chung MS (2015) Extraction characteristics of subcritical water depending on the number of hydroxyl group in flavonols. Food Chem 168:21–26
Cheigh CI, Chung EY, Chung MS (2012) Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J Food Eng 110:472–477
Ko MJ, Cheigh CI, Chung MS (2014) Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem 143:147–155
Turner C, Turner P, Jacobson G, Almgren K, Waldebäck M, Sjöberg P, Karlsson EN, Markides KE (2006) Subcritical water extraction and β-glucosidase-catalyzed hydrolysis of quercetin glycosides in onion waste. Green Chem 8:949–959
Ko MJ, Cheigh CI, Cho SW, Chung MS (2011) Subcritical water extraction of flavonol quercetin from onion skin. J Food Eng 102:327–333
Lekar A, Borisenko S, Filonova O, Vetrova E, Sushkova S, Borisenko N (2013) Subcritical water extraction of quercetin from Polygonum hydropiper L. Middle East J Sci Res 17:252–255
Norsyabilah R, Hanim S, Norsuhaila M, Noraishah A, Kartina S (2013) Subcritical water extraction of monosaccharides from oil palm fronds hemicelluloses. Malays J Anal Sci 17:272–275
Chao Z, Ri-fu Y, Tai-qiu Q (2013) Ultrasound-enhanced subcritical water extraction of polysaccharides from Lycium barbarum L. Sep Purif Technol 120:141–147
Matsunaga Y, Machmudah S, Kanda WH, Sasaki M, Goto M (2014) Subcritical water extraction and direct formation of microparticulate polysaccharides powders from Ganoderma lucidum. Int J Technol 5:40–50
Tanaka M, Takamizu A, Hoshino M, Sasaki M, Goto M (2012) Extraction of dietary fiber from Citrus junos peel with subcritical water. Food Bioprod Process 90:180–186
Hanim SS, Norsyabilah R, Suhaila MHN, Noraishah A, Kartina S (2012) Effects of temperature, time and pressure on the hemicelluloses yield extracted using subcritical water extraction. Procedia Eng 42:562–565
Khuwijitjaru P, Pokpong A, Klinchongkon K, Adachi S (2014) Production of oligosaccharides from coconut meal by subcritical water treatment. Int J Food Sci Technol 49:1946–1952
Wang X, Chen Q, Lü X (2014) Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll 38:129–137
Chen HM, Fu X, Luo ZG (2015) Properties and extraction of pectin-enriched materials from sugar beet pulp by ultrasonic-assisted treatment combined with subcritical water. Food Chem 168:302–310
Ndlela SC, de Moura JMLN, Olson NK, Johnson LA (2012) Aqueous extraction of oil and protein from soybeans with subcritical water. J Am Oil Chem Soc 89:1145–1153
Ravber M, Knez Z, Škerget M (2015) Simultaneous extraction of oil- and water-soluble phase from sunflower seeds with subcritical water. Food Chem 166:316–323
Prado JM, Forster-Carneiro T, Rostagno MA, Follegatti-Romero LA, Maugeri Filho F, Meireles MAA (2014) Obtaining sugars from coconut husk, defatted grape seed, and pressed palm fiber by hydrolysis with subcritical water. J Supercrit Fluids 89:89–98
Shimanouchi T, Ueno S, Yang W, Kimura Y (2014) Extraction of reducing sugar with anti-oxidative scavengers from peels of Carya cathayensis Sarg.: use of subcritical water. Environ Eng Res 19:41–45
Musa T, Sanagi M, Wan Ibrahim W, Ahmad F, Aboul-Enein H (2014) Determination of 4-allyl resorcinol and chavibetol from piper betle leaves by subcritical water extraction combined with high-performance liquid chromatography. Food Anal Methods 7:893–901
Ghoreishi SM, Shahrestani RG (2009) Subcritical water extraction of mannitol from olive leaves. J Food Eng 93:474–481
Kim WJ, Kim J, Veriansyah B, Kim JD, Lee YW, Oh SG, Tjandrawinata RR (2009) Extraction of bioactive components from Centella asiatica using subcritical water. J Supercrit Fluids 48:211–216
Kim WJ, Veriansyah B, Lee YW, Kim J, Kim JD (2010) Extraction of mangiferin from Mahkota Dewa (Phaleria macrocarpa) using subcritical water. J Ind Eng Chem 16:425–430
Cardenas-Toro FP, Forster-Carneiro T, Rostagno MA, Petenate AJ, Maugeri Filho F, Meireles MAA (2014) Integrated supercritical fluid extraction and subcritical water hydrolysis for the recovery of bioactive compounds from pressed palm fiber. J Supercrit Fluids 93:42–48
Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M (2011) Use of essential oils in bioactive edible coatings: a review. Food Eng Rev 3:1–16
Prabuseenivasan S, Jayakumar M, Ignacimuthu S (2006) In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med 6:39
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253
Balasundram N, Sundram K, Samman S (2006) Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem 99:191–203
Umar Lule S, Xia W (2005) Food phenolics, pros and cons: a review. Food Rev Int 21:367–388
Santos-Buelga C, Gonzalez-Manzano S, Dueñas M, Gonzalez-Paramas A (2012) Extraction and isolation of phenolic compounds. In: Sarker SD, Nahar L (eds) Natural products isolation. Humana Press, New York City, pp 427–464
Shi J, Nawaz H, Pohorly J, Mittal G, Kakuda Y, Jiang Y (2005) Extraction of polyphenolics from plant material for functional foods—engineering and technology. Food Rev Int 21:139–166
Xiao B, Sun X, Sun R (2001) Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym Degrad Stab 74:307–319
Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12:29–36
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Berry J (2011) Marine and freshwater microalgae as a potential source of novel herbicides. In: Kortekamp A (ed) Herbicides and environment. InTech, Rijeka, pp 705–734
Cifuentes A, Herrero M, Ibáñez E, Jaime L, Martín Álvarez PJ (2006) Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. J Agric Food Chem 54:5597–5603
Jaime L, Mendiola JA, Herrero M, Soler-Rivas C, Santoyo S, Senorans FJ, Cifuentes A, Ibanez E (2005) Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J Sep Sci 28:2111–2120
Mendiola JA, Marín FR, Hernández SF, Arredondo BO, Señoráns FJ, Ibañez E, Reglero G (2005) Characterization via liquid chromatography coupled to diode array detector and tandem mass spectrometry of supercritical fluid antioxidant extracts of Spirulina platensis microalga. J Sep Sci 28:1031–1038
Rodriguez-Meizoso I, Jaime L, Santoyo S, Cifuentes A, Garcia-Blairsy Reina G, Senorans FJ, Ibanez E (2008) Pressurized fluid extraction of bioactive compounds from Phormidium species. J Agric Food Chem 56:3517–3523
Ozkan A, Berberoglu H (2013) Physico-chemical surface properties of microalgae. Colloids Surf B Biointerfaces 112:287–293
Xin L, Hong-ying H, Yu-ping Z (2011) Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour Technol 102:3098–3102
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Hong Y, Hu HY, Xie X, Sakoda A, Sagehashi M, Li FM (2009) Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat Toxicol 91:262–269
Rasmussen RS, Morrissey MT (2007) Marine biotechnology for production of food ingredients. Adv Food Nutr Res 52:237–292
Harun R, Singh M, Forde GM, Danquah MK (2010) Bioprocess engineering of microalgae to produce a variety of consumer products. Renew Sustain Energy Rev 14:1037–1047
Garcia-Casal MN, Ramírez J, Leets I, Pereira AC, Quiroga MF (2009) Antioxidant capacity, polyphenol content and iron bioavailability from algae (Ulva sp., Sargassum sp. and Porphyra sp.) in human subjects. Br J Nutr 101:79–85
Carballo-Cárdenas EC, Tuan PM, Janssen M, Wijffels RH (2003) Vitamin E (α-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol Eng 20:139–147
Jones AC, Gu L, Sorrels CM, Sherman DH, Gerwick WH (2009) New tricks from ancient algae: natural products biosynthesis in marine cyanobacteria. Curr Opin Chem Biol 13:216–223
Barba F, Grimi N, Vorobiev E (2015) New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng Rev 7:45–62
Ibanez E, Kubátová A, Señoráns FJ, Cavero S, Reglero G, Hawthorne SB (2003) Subcritical water extraction of antioxidant compounds from rosemary plants. J Agric Food Chem 51:375–382
Kumar MSY, Dutta R, Prasad D, Misra K (2011) Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem 127:1309–1316
Lee K, Kim KT, Nah SY, Chung MS, Cho S, Paik HD (2011) Antimicrobial and antioxidative effects of onion peel extracted by the subcritical water. Food Sci Biotechnol 20:543–548
Lee K, Kim KT, Kim H, Chung MS, Chang PS, Park H, Pai HD (2014) Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water, and subcritical water extraction. Food Sci Biotechnol 23:615–621
Narita Y, Inouye K (2012) High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem 135:943–949
Lee NK, Lee JH, Lim SM, Lee KA, Kim YB, Chang PS, Paik HD (2014) Short communication: antiviral activity of subcritical water extract of Brassica juncea against influenza virus A/H1N1 in nonfat milk. J Dairy Sci 97:5383–5386
Lee KA, Kim WJ, Kim HJ, Kim KT, Paik HD (2013) Antibacterial activity of Ginseng (Panax ginseng C. A. Meyer) stems–leaves extract produced by subcritical water extraction. Int J Food Sci Technol 48:947–953
Jarrar N, Abu-Hijleh A, Adwan K (2010) Antibacterial activity of Rosmarinus officinalis L. alone and in combination with cefuroxime against methicillin–resistant Staphylococcus aureus. Asian Pac J Trop Med 3:121–123
Ezeigbo I, Ezeja M, Madubuike K, Ifenkwe D, Ukweni I, Udeh N, Akomas S (2012) Antidiarrhoeal activity of leaf methanolic extract of Rauwolfia serpentina. Asian Pac J Trop Biomed 2:430–432
Avato P, Bucci R, Tava A, Vitali C, Rosato A, Bialy Z, Jurzysta M (2006) Antimicrobial activity of saponins from Medicago sp.: structure-activity relationship. Phytother Res 20:454–457
Miliauskas G, Venskutonis PR, van Beek TA (2004) Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 85:231–237
Moon JK, Shibamoto T (2009) Antioxidant assays for plant and food components. J Agric Food Chem 57:1655–1666
Chairman K, Singh AJAR, Alagumuthu G (2012) Cytotoxic and antioxidant activity of selected marine sponges. Asian Pac J Trop Dis 2:234–238
Kelman D, Posner EK, McDermid KJ, Tabandera NK, Wright PR, Wright AD (2012) Antioxidant activity of Hawaiian marine algae. Mar Drugs 10:403–416
Aruoma OI (2003) Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res Fundam Mol Mech Mutagen 523–524:9–20
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Qi H, Zhang Q, Zhao T, Chen R, Zhang H, Niu X, Li Z (2005) Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol 37:195–199
Senorans F, Ibanez E, Cavero S, Tabera J, Reglero G (2000) Liquid chromatographic–mass spectrometric analysis of supercritical-fluid extracts of rosemary plants. J Chromatogr A 870:491–499
Haraguchi H, Saito T, Okamura N, Yagi A (1995) Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Med 61:333–336
Plaza M, Amigo-Benavent M, del Castillo MD, Ibáñez E, Herrero M (2010) Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res Int 43:1123–1129
Atrooz OM (2008) The effects of Maillard reaction products on apple and potato polyphenoloxidase and their antioxidant activity. Int J Food Sci Technol 43:490–494
Şahin H, Topuz A, Pischetsrieder M, Özdemir F (2009) Effect of roasting process on phenolic, antioxidant and browning properties of carob powder. Eur Food Res Technol 230:155–161
Mayer AM, Gustafson KR (2003) Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int J Cancer 105:291–299
Bang W, Hanson D, Drake M (2008) Effect of salt and sodium nitrite on growth and enterotoxin production of Staphylococcus aureus during the production of air-dried fresh pork sausage. J Food Prot 71:191–195
Bonaccorsi P, Carist C, Gargiulli C, Leuzzi U (2008) Flavonol glucosides in Allium species: a comparative study by means of HPLC–DAD–ESI–MS–MS. Food Chem 107:1668–1673
Acknowledgments
The authors gratefully acknowledge Universiti Putra Malaysia and financial support from the Fundamental Research Grant Scheme (FRGS) provided by the Ministry of Education Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zakaria, S.M., Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng Rev 8, 23–34 (2016). https://doi.org/10.1007/s12393-015-9119-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-015-9119-x