Abstract
The potential of compressed CO2 for the extraction of valuable compounds (fatty acids, phenolic compounds and fucoxanthin) from the brown alga, Sargassum muticum, has been explored. Pure supercritical CO2 (scCO2) allowed the extraction of up to 15 % of the ethanol extractables, with the highest yield attained at 10 MPa and 50 °C. The addition of ethanol as cosolvent increased by three both the extraction yields and the radical scavenging activity, and by 90 times the fucoxanthin yield. The extracts obtained with pure carbon dioxide contained higher proportion of ω3 fatty acids than those using with conventional solvents, showing an ω6/ω3 ratio of 2.12 and 2.84 for pure and ethanol modified scCO2, respectively. Selected extracts showed antibrowning activity on B16F10 murine cells and inhibition of lipogenesis in SW872 liposarcoma cells, although cytotoxic effects were observed at 50 μg mL−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sargassum muticum (Yendo) Fensholt is one of the most aggressive invasive macroalgae in European waters and on the West Coast of America. This alga has been associated with changes in biodiversity by competing for light, space, and resources with autochthonous algae. Due to the unsuccessful eradication of S. muticum by hand removal, physical or chemical methods, seasonal harvesting has been suggested as a control strategy. Further integral valorisation of the algal components is desired.
Brown seaweeds contain different bioactives, such as polysaccharides, polyphenols, polyunsaturated fatty acids (PUFA), fucosterol, and fucoxanthin (D’Orazio et al. 2012). Despite the low lipid content in brown algae, this fraction might contain higher levels of essential polyunsaturated fatty acids than traditional earth vegetables. The beneficial health effects of ω3 PUFAs are associated to the improvement of immune and endothelial functions, antiarrhythmic effects, reduction in platelet aggregation and serum triglyceride concentrations, and to their antihypertensive, antiinflammatory, and anticancer actions.
Fucoxanthin is a yellowish brown xanthophyll found in the chloroplasts of brown algae and has recently received attention as a free radical scavenger (Fung et al. 2013) with antiphotoaging, anticarcinogenic, antiinflammatory, antimalaria, and antidiabetic properties (D’Orazio et al. 2012). Fucoxanthin shows specific physiological effects based on unique antiobesity mechanisms that makes it a different and efficient therapy agent (Maeda et al. 2005).
Suitable operation conditions during extraction and purification stages are required, since polyunsaturated fatty acids are thermolabile and fucoxanthin is highly susceptible to heat, low pH, and light and could be affected by the presence of coextractives (Piovan et al. 2013). Extraction and purification of fucoxanthin from Saccharina (Laminaria) japonica using conventional solvents (methanol, acetone, ethanol) (Kanazawa et al. 2008) and from Eisenia bicyclis using pressurized liquid extraction have been reported (Shang et al. 2011). Supercritical CO2 (scCO2) was used for the extraction of fatty acids from Sargassum hemiphyllum (Cheung et al. 1998), oil, fucoxanthin, and polyphenols from Undaria pinnatifida (Roh et al. 2008), and to aid in the formation of particles with functional pigments (Kwon et al. 2011). Extraction of carotenoids, lipids, and antioxidants with scCO2 has been extensively studied and extraction experiments at laboratory scale have been performed at 7.8–70 MPa, 40–76 °C, and 6–500 g CO2 g−1 algae (Crampon et al. 2011). Supercritical CO2 extraction offers advantages derived from the milder operational conditions, due to the low critical temperature of carbon dioxide (31 °C), the adjustable characteristics and the enhanced internal mass transfer properties. This green solvent is frequently used in the food and pharmaceutical industries, being nontoxic, nonflammable, inexpensive, and easily separable from the extracts.
The aim of this study was to investigate the effect of the major operational conditions on the scCO2 extraction of fatty acids, fucoxanthin and phenolics from S. muticum and to evaluate the influence of ethanol as modifier on the yields, composition, and radical scavenging of the extracts. In addition, some properties of the extracts in relation to cosmeceutical and pharmaceutical applications were explored.
Materials and methods
S. muticum, collected in Cape Estai (Pontevedra, Spain) in December 2011, was cleaned from epiphytes and sand and washed with tap water. Pretreatments were selected to facilitate the extraction; algae were freeze-dried in order to avoid the degradation of thermolabile compounds, and comminuted (<0.5 mm) to destroy cell walls and to overcome mass-transfer limitations (Crampon et al. 2011). Ground algae were stored in plastic bags in a dry and dark place until use. Carbon dioxide, 99.99 % purity, was purchased from Praxair (Spain).
Conventional solvent extraction
The freeze-dried alga was contacted with absolute ethanol at a liquid of solid ratio of 10 (v/w) at 40 °C during 12 h on shaking extractors protected from light to limit the possibility of oxidation. Solids were separated by filtration through Whatman No.1 filter paper.
Compressed and supercritical fluid extraction
The ground alga (10 g) was packed with glass beads into an extractor (Thar Designs SFE-1000 F-2-C10, Pittsburgh, USA) with a 1-L cylinder extractor and two 500-mL separators. The CO2 was precooled with a circulating bath (PolyScience, USA, model 9506) before being pumped by a P-200A piston pump (Thar Design Inc) at a flow rate of 25 g CO2 min−1. The cosolvent was pumped by a HPLC pump (Scientific Systems, Inc., USA, model Series III), the flow rate was adjusted to achieve modifier concentrations in the range 0.5–10 % (wt). The extraction vessel was covered with polypropylene wool to avoid solid particles from entering the extraction line. The extract was collected during 1 h in the first separator, operating at ambient temperature. Pressure was studied in the range 10–30 MPa and temperature from subcritical conditions at 30 to 50 °C, based on literature information (Roh et al. 2008; Quitain et al. 2013). The separator was further washed with 96 % ethanol to recover the extract. Ethanol was removed in rotavapor and the extracts were kept under N2, at −20 °C in the dark until analysis.
Analytical methods
The total amount of extractable material was gravimetrically determined and the extraction yield was calculated as mass of extract/mass of dry raw material, expressed as percentage.
HPLC analysis
The fucoxanthin content in the extracts was determined by reversed phase HPLC, in an Agilent HPLC 1100 instrument equipped with a Waters Spherisorb ODS-2 column (5 μm, 250 mm × 4.6 mm) and diode-array detector (DAD detector), using acetonitrile:methanol:water (6:2.5:1.5, vol) as the mobile phase at a flow rate of 0.8 mL min−1. A standard of commercial fucoxanthin (Sigma) was used for quantification.
Total lipids were determined gravimetrically after extraction with CHCl2:MeOH. Fatty acid methyl esters (FAME) were prepared by transmethylation of 8–10 mg lipid samples using 1 mL of 1 % NaOH in MeOH and heating at 55 °C during 15 min, addition of 2 mL of 5 % methanolic HCl, heating at 55 °C during 15 min and then adding 1 mL of H2O (Carreau and Dubacq 1978). FAMEs were extracted by hexane, and after evaporating the organic phase under reduced pressure, FAMEs were analyzed by a GC-MS QP 2010 (Shimadzu, Japan). The temperature gradient increased from 50 °C (2 min) at 10 °C min−1 to 240 °C, which was maintained for 27 min. FAMEs in the sample were identified by comparing their mass spectra with those of authentic standards and NIST MS Search 2.0 library. The results of two independent determinations are expressed as a percentage of total FAME.
Melanin biosynthesis inhibition
To identify extracts or compounds that inhibit production of melanin, B16F10 murine melanoma cells (ATCC-CRL-6475) were cultured in phenol red free Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich, Spain), supplemented with 10 % fetal bovine serum (FBS, Sigma-Aldrich), 1 % penicillin/streptomycin (Hyclone, Thermo Scientific, SV30010) and 1 % l-glutamine (Sigma-Aldrich). The medium was stored at 4–8 °C. Approximately 5 mg of extract was dissolved in 9:1 methanol:DMSO (Panreac, Spain) and dispensed in 96-well plates to prepare concentrations between 50 and 1,000 μg mL−1. Arbutin was used as control. Confluent cultures of B16F10 melanoma cells were rinsed in 4–6 mL phosphate-buffered saline (PBS), which was removed after washing. After the addition of 2 mL trypsin, cells were incubated during 2 min and disaggregated. Cells were centrifuged and resuspended in 10 mL culture medium and then counted in a Neubauer chamber after staining with trypan blue to differentiate viable cells. The negative control was DMEM without cells and the positive was the cell suspension (2 × 104 cells mL−1). Plates were incubated during 96 h at 37 °C and 5 % CO2. The melanin production was spectrophotometrically measured at 405 nm.
Lipogenesis inhibition activity
Human liposarcoma SW872 cells (HTB92) were cultivated in DMEM/Ham’s F12 (3/1 v/v) medium (Sigma) containing 10 % fetal bovine serum (FBS, 1 %) (Sigma-Aldrich), 1 % nonessential amino acids and 1 % P/S (50 U mL−1 penicillin; 50 mg mL−1 streptomycin) (Hyclone, Thermo Scientific). Culture media were stored at 4–8 °C and warmed at 37 °C before use. Cells were grown under 37 °C and 5 % CO2. Approximately 5 mg extract were dissolved in 9:1 methanol:DMSO (Panreac) and dispensed in 96-well plates to reach the desired concentrations, at a final volume of 200 μL per well. Liposarcoma SW872 cells were grown to confluence in T-75 flasks, then were washed with 4–6 mL PBS and incubated with 2 mL trypsin during 2 min. Cells were seeded at 9 × 104 cells per well and incubated during 96 h at 37 °C and 5 % CO2. Two controls were prepared with confluent SW872 cells at 9 × 104 cells per well, a positive one at 48 h, when the lipids production starts, and a negative control at 4 h, when no lipids were formed. Two additional negative controls were prepared, nonconfluent SW872 cells (seeded at 4.5 × 104 cells per well) and human colon carcinoma HCT-116 cells, to provide readings of basal fluorescence, corresponding to nonlipid producing cells. The AdipoRed Assay (Lonza, PT-7009) was used, measuring the intracellular lipids by fluorescence, with excitation at 485 nm and emission at 572 nm (or 532 nm).
Cell viability
The cell viability was determined by a colorimetric assay based on the reduction of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT reagent) (Sigma-Aldrich) to formazan by mitochondrial dehydrogenases in viable cells. To previously grown cells, 50 μL of MTT (1 mg mL−1) solution were dispensed and incubated in a humidified atmosphere of 5 % of CO2 during 2 h at 37 °C. Then, the medium was removed and 100 μL of pure DMSO was added to solubilize the colored compounds generated. A negative control (DMEM without cells) and a positive control (untreated cells) provided minimum and maximum supervivence values, respectively. Absorbance at 490 nm of the dissolved solution was measured to estimate the relative cell viability.
Results
Conventional solvent extraction
The 96 % ethanol soluble fraction of S. muticum collected in winter was 3.15 % (dry weight) and the fucoxanthin extraction yield was 0.55 mg g−1 dry alga; the fucoxanthin content in the dry extract was 1.75 % wt. In the ethanol extracts, the phenolic compounds accounted for 1–2 % of the dry weight of the extract, being equivalent to 18.9 mg Trolox g−1 extract. Aqueous solutions of other solvents, such as methanol:water, acetone:water, and isopropanol:water mixtures could provide higher yields (13–18 %), but these latter have been avoided in this work due to toxicity aspects. Total lipids obtained after extraction with CHCl2:MeOH accounted for 2.15 ± 0.19 %.
Extraction with pure CO2
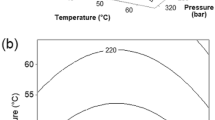
Data in Fig. 1 shows that using pure scCO2 the global extraction yield ranged from 0.2 to 0.37 g 100 g−1 dry algae in experiments at 30–50 °C and 10–30 MPa during 1 h. Extracts presented a brown greenish color and oily aspect. Except at subcritical temperature, the increased pressure favored the fucoxanthin extraction yield, with a maximum of 1.5 mg (100 g)−1 S. muticum at 50 °C, although the purity of fucoxanthin in the dried extract was very low. The phenolic content in the supercritical extracts, except those obtained at 50 °C, was close to 4 % of the dry weight. The extracts obtained at 15–30 MPa showed ABTS radical scavenging activities in the range 0.06–0.07 g Trolox g−1 extract, values significantly lower than those of BHA and BHT (1.8 and 2.1, respectively).
Effect of pressure and temperature during scCO2 extraction of a total extraction yield, b fucoxanthin extraction yield, c phenolic content, and d ABTS radical scavenging capacity of the extracts from Sargassum muticum obtained without modifier (dotted lines) and with 10 % ethanol (continuous lines). Black diamond 30 °C, black square 40 °C, black triangle 50 °C
Extraction with ethanol modified CO2
The low extraction yields of lipids and fucoxanthin in processes using pure CO2 suggested that the addition of cosolvent could enhance the yields. The effect of ethanol was evaluated at operational conditions (10 MPa and 40 °C) leading to highest soluble extraction yields, intermediate phenolic content, and antioxidant activity, although the fucoxanthin content in those extracts was the lowest. The influence of ethanol concentration in the range 0.5–10 % (wt) of the solvent is shown in Fig. 2. A significant improvement was observed in total yields (up to 3 times higher), radical scavenging capacity (up to 2.5 times), and the fucoxanthin extraction yield (up to 90 times). However, due to the lower process selectivity, the phenolic content with modifier was only 1.5 times higher than with pure CO2. Based on this information, extraction experiments using 10 % ethanol as modifier during scCO2 processing of S. muticum were performed. The influence of pressure and temperature is presented in the upper series of Fig. 1, showing the improved performance over pure scCO2. Using modifier, a moderate influence of the major process variables (pressure and temperature) was observed. Optimal conditions for fucoxanthin extraction yield, phenolic content, and radical scavenging properties of the extract were fixed at 50 °C and 20 MPa. Under these operation conditions the influence of the extraction time was assessed using pure and ethanol modified scCO2 and is shown in Fig. 3. Maximum soluble extraction yields with pure scCO2 were attained at 60 min and with cosolvent at 40 min.
Composition of selected extracts
Major components in the scCO2 extracts of S. muticum are lipids and this fraction presents high levels of essential polyunsaturated fatty acids and a nutritionally beneficial ω6:ω3 ratio of 1.2–2.95 (unpublished data). Data in Fig. 4 show that the major PUFAs were linoleic acid (18:2, ω6) and α-linolenic acid (18:3, ω3). Stearidonic acid (18:4, ω3) was not found although it was identified in the original material. Arachidonic acid (20:4, ω6) and linoleic (C18:2, ω6) content were sensitively increased in pure scCO2. Eicosapentanoic acid (20:5, ω3) was not found in CSE and accounted for 1.8 % of scCO2 extracts. Supercritical extracts obtained with pure scCO2 contained more PUFAs, ω6 and ω3 than the conventional ethanolic extracts (e-CSE) and the modified supercritical CO2. The ω3 content was higher in scCO2 extracts and similar to those in the initial alga, whereas in the extracts from e-CSE and from e-scCO2 was similar.
Other properties
Inhibition of melanin production in B16F10 cells, lipogenesis in liposarcoma SW872 cells, and cytotoxicity in both cell lines are shown in Table 1. The scCO2 S. muticum extracts showed inhibitory action on melanin production at all tested concentrations. But the lower activity observed at 500–1,000 μg mL−1 could be due to the interference in the color of the extract and also to their cytotoxicity.
The e-scCO2 extract was an active inhibitor of lipogenesis at 250 μg mL−1, but was highly cytotoxic towards SW872 cells, with IC50 < 50 μg mL−1. The high values observed for the supercritical extract at 100 and 250 μg mL−1 could be due to the color of the extract, which interfered with the readings and could give false values for lipid production. Extract from e-CSE was not active.
Discussion
Extraction of the phenolic and polysaccharide fractions was previously addressed for the valorization of S. muticum collected in summer from this coast (González-López et al. 2012; Balboa et al. 2013a). Samples collected in winter were selected to evaluate the extraction of fucoxanthin, since the xanthophyll content is maximum in winter–spring, and fucoxanthin is the major carotenoid, accounting for 75 % of the measured xanthophylls (Lewey and Gorham 1984). These authors suggested that the decrease in the pigments content per unit dry weight observed in spring could be due to the lower production rate of new pigments in relation to the growth, or the photobleaching or photodegradation of the pigments during summer. In the collection area, the lipid content was relatively constant along the year, slightly higher in summer than in winter, with values comparable to those reported for other Sargassum sp. (Noviendri et al. 2011).
The phenolic purity in the 96 % ethanol extracts was in the range of those found in extracts for other brown algae (Boonchum et al. 2011). Similar fucoxanthin content was reported for brown algae with 0.68 mg g−1 in U. pinnatifida (Quitain et al. 2013), 0.73–1.01 mg g−1 in Sargassum sp. (Noviendri et al. 2011), and 4.50 mg g−1 in Sargassum horneri (Nomura et al. 2013). Both the extraction yields and the fucoxanthin content reported in U. pinnatifida extracts were four and nine times higher, respectively (Quitain et al. 2013). The best conditions of extraction of fucoxanthin from brown seaweeds with pure scCO2 were selected at 20 MPa and 45 °C (Kwon et al. 2011) and at 40 MPa and 40 °C (Quitain et al. 2013). Despite using cosolvent, operation under analogous severity, 20 MPa and 50 °C, was reported (Roh et al. 2008). Optimal conditions for oil extraction were 38 MPa and 50 °C for S. hemiphyllum (Cheung et al. 1998), and 30 MPa and 50 °C with 3.0 % (v/v) ethanol as cosolvent for U. pinnatifida (Roh et al. 2008). However, for this latter alga, optimal conditions for phenolics extraction were 25 MPa and 50 °C (Roh et al. 2008).
A slight effect of the operational conditions on the total yield was also found for U. pinnatifida (Quitain et al. 2013). However, pressure is usually a highly influencing variable (Crampon et al. 2011). A clear beneficial effect of the increased pressure, due to increased solvent density of scCO2, was observed on the lipid extraction from S. hemiphyllum (Cheung et al. 1998) and from U. pinnatifida (Roh et al. 2008). Whereas at higher pressures, an increase of temperature led to higher extraction yield of carotenoids and lipids, at lower pressures led to the opposite effect. This retrograde behaviour shows decreased solubility with temperature at pressures lower than the crossover value and increased solubility with temperature at higher pressures (Cheung et al. 1998; Crampon et al. 2011).
The total yield was only 15 % of the ethanol extractables, whereas supercritical extraction yields comparable to those obtained with Soxhlet were reported for S. hemiphyllum (Cheung et al. 1998) and for U. pinnatifida (Crespo and Yusty 2006), operating at pressures and temperatures equal or higher than 30 MPa and 40 °C.
The extracts showed lower ABTS radical scavenging capacity than synthetic antioxidants, a fact already observed (Balboa et al. 2013b); also, aqueous soluble compounds are expected to be more active than ethanol extractables (Boonchum et al. 2011). The positive correlations between phenolic contents and antioxidant activities in brown algae have often been reported (Balboa et al. 2013b), but macroalgae contain a variety of antioxidant compounds such as tocopherols, ascorbic acid, carotenoids, phospholipids, chlorophyll-related compounds, and polysaccharides. The available commercial fucoxanthin products are mainly constituted of complex extracts.
Ethanol is the most frequently used solvent to increase the polarity of scCO2 and phenolic compounds are among the bioactives in macroalgae that could be expected to be benefited by increased polarity. This strategy was proposed to enhance both phenolic extraction yields and antioxidant activity, since polar compounds are more active in algae (Boonchum et al. 2011) and ethanol modified scCO2 has also been successfully used for the extraction of fucoxanthin (Roh et al. 2008). With 3 % ethanol increased yields from U. pinnatifida were obtained with pressure in the range 8–25 MPa and with temperatures up to 50 °C; up to 0.75 μg of fucoxanthin 100 g−1 were extracted at 20 MPa and 50 °C, whereas the maximum amount of polyphenols was found at 25 MPa and 60 °C (Roh et al. 2008). Temperature and ethanol concentration significantly influenced the pressurized liquid extraction efficiency of fucoxanthin from E. bicyclis, with a maximum of 0.42 mg fucoxanthin g-1 at 110 °C and 90 % ethanol (Shang et al. 2011). Pressure and temperature during scCO2 extraction showed a minor effect on the lipid yield (45–64 % of the total), but these variables had significant effects on astaxanthin extraction yield. The use of 15 % ethanol as modifier increased the oil and astaxanthin recoveries (Sánchez-Camargo et al. 2012).
Algal oil is generally composed of fatty acids, from C14:0 to C20:0, and the selectivity of scCO2 can slightly modify the composition of the oil. Neutral lipids may be efficiently extracted at pressures in the range of 30–50 MPa and temperatures in the range of 40–70 °C (Crampon et al. 2011). The use of cosolvent increases the extraction yields of polar lipids (phospholipids and glycolipids) and pigments.
Literature reports on the effect of entrainer (5–15 % ethanol) on the increased concentration of ω3 fatty acids (mainly eicosapentaenoic and docosahexaenoic acid) and the decreased concentration of the saturated fatty acids from shrimp waste under conditions (300 bar, 50 °C and 15 % wt ethanol) leading to the highest lipids recoveries (Sánchez-Camargo et al. 2012). Supercritical fluid extraction of S. hemiphyllum allowed similar extraction yields as Soxhlet. The concentrations of the total PUFAs increased and concentration of total saturated fatty acids decreased with increasing pressure and solvent density. The total ω3 fatty acids were higher than in Soxhlet-extracted samples and docosapentaenoic acid and docosahexaenoic acid contents were maximal at high pressure and 40–50 °C (Cheung et al. 1998).
Tyrosinase, with a key role in the initial steps of melanin biosynthesis, is responsible for pigmentation in mammals. Inhibitors of tyrosinase are used for controlling food browning reactions caused by tyrosinase and for depigmenting functions in cosmetic and pharmaceutical products. Toxicity or undesirable side effects of some synthetic tyrosinase inhibitors has increased the interest in the identification of natural compounds for skin lighting purposes. A number of natural phenolic compounds have been studied as depigmenting agents, including some phlorotannin derived components (Yoon et al. 2009; Kang et al. 2012).
S. muticum scCO2 extracts showed antimelanoma activity similar to those reported for some solvent extracts of other species. Sargassum polycystum ethanolic extract, hexane, and ethyl acetate fractions reduced cellular tyrosinase activity in a dose-dependent manner. In the cell viability (MTT) assay, hexane fraction did not have any cytotoxicity at 100 μg mL−1, but reduced viable cells at higher doses. The cytotoxic effect of the ethanolic extract was low, whereas that of the ethyl acetate fraction was important (Chan et al. 2011). Also, the ethyl acetate extracts of Sargassum thunbergii showed in vitro cytotoxicity against B16F10 cells in the MTT assay (Kim et al. 2009).
The control compound arbutin was more effective (IC50 = 0.04 μg mL−1) than the S. muticum extracts evaluated in the present work. However, arbutin was less efficient than dieckol in assays with mushroom tyrosinase (Kang et al. 2012) or than 7-phloroeckol (50 μg mL−1) with mouse B16F10 melanoma cells (Yoon et al. 2009). Some brown algal components present in the extracts have confirmed antibrowning activity, i.e., the volatile compounds in the essential oils from green, brown, and red algae (Kajiwara et al. 2006). However, linoleic acid did not reduce tyrosinase activity at noncytotoxic ranges (Maeda and Fukuda 1991). Fucoxanthin dose-dependently reduced the proliferation of B16F10 cells accompanied by the induction of cell cycle arrest and apoptosis, and the antitumor effect of fucoxanthin was confirmed in vivo in mice (Kim et al. 2013). The ethanolic and ethyl acetate solubles from Ecklonia cava and purified phlorotannins (phloroglucinol, dioxinodehydroeckol, and 7-phloroeckol) inhibited melanin formation in B16F10 melanoma cells (Yoon et al. 2009).
Cell viability with the MTT assay was significantly influenced by phenolics from terrestrial sources. The percentages of normal cells decreased and apoptotic cells increased with increasing concentrations of quercetin, which was a potent inhibitor of SW 872 human liposarcoma cells, with IC50 68.2 μM (Huang et al. 2006). The flavedo extracts from citrus fruit showed cytotoxic effects on SW872 cells at 0.75–1 % (Ramful et al. 2010), but albedo and pulp extracts had no adverse effects on cell viability.
In conclusion, supercritical technology proved successful for the extraction of a mixture of fatty acids, phenolic compounds, and fucoxanthin from S. muticum. These high-added value compounds can be proposed for cosmetic, pharmaceutical, and nutraceutical products. The use of ethanol as modifier allowed increased extraction yields, particularly of fucoxanthin. The addition of pure carbon dioxide provided extracts with ω6/ω3 ratio lower than in when ethanol was used either in conventional or in supercritical extraction, and the oil composition was more similar to the original algal oil.
References
Balboa EM, Rivas S, Moure A, Domínguez H, Parajó JC (2013a) Simultaneous extraction and depolymerization of fucoidan from Sargassum muticum in aqueous media. Mar Drugs 11:4612–4627
Balboa EM, Conde E, Moure A, Falqué E, Domínguez H (2013b) In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem 138:1764–1785
Boonchum W, Peerapornpisal Y, Kanjanapothi D, Pekkoh J, Pumas C, Jamjai U, Amornlerdpison D, Noiraksar T, Vacharapiyasophon P (2011) Antioxidant activity of some seaweed from the Gulf of Thailand. Int J Agric Biol 13:95–99
Carreau JP, Dubacq JP (1978) Adaptation of a macro-scale method to the micro-scale for fatty acid methyl transesterification of biological lipid extracts. J Chromatogr 151:384–390
Chan YY, Kim KH, Cheah SH (2011) Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J Ethnopharmacol 137:1183–1188
Cheung PCK, Leung AYH, Ang PO (1998) Comparison of supercritical carbon dioxide and Soxhlet extraction of lipids from a brown seaweed, Sargassum hemiphyllum (Turn.) C. Ag. J Agric Food Chem 46:4228–4232
Crampon C, Boutin O, Badens E (2011) Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind Eng Chem Res 50:8941–8953
Crespo MOP, Yusty MAL (2006) Comparison of supercritical fluid extraction and Soxhlet extraction for the determination of aliphatic hydrocarbons in seaweed samples. Ecotoxicol Environ Saf 64:400–405
D'Orazio N, Gemello E, Gammone MA, De Girolamo M, Ficoneri C, Riccioni G (2012) Fucoxantin: a treasure from the sea. Mar Drugs 10:604–616
Fung A, Hamid N, Lu J (2013) Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem 136:1055–1062
González-López N, Moure A, Domínguez H (2012) Hydrothermal fractionation of Sargassum muticum biomass. J Appl Phycol 24:1569–1578
Huang SL, Hsu CL, Yen GC (2006) Growth inhibitory effect of quercetin on SW 872 human liposarcoma cells. Life Sci 79:203–209
Kajiwara T, Matsui K, Akakabe Y, Murakawa T, Arai C (2006) Antimicrobial browning-inhibitory effect of flavor compounds in seaweeds. J Appl Phycol 18:413–422
Kanazawa K, Ozaki Y, Hashimoto T, Das SK, Matsushita S, Hirano M, Okada T, Komoto A, Mori N, Nakatsuka M (2008) Commercial-scale preparation of biofunctional fucoxanthin from waste parts of brown sea algae Laminaria japonica. Food Sci Technol Res 14:573–582
Kang SM, Heo SJ, Kim KN, Lee SH, Yang HM, Kim AD (2012) Molecular docking studies of a phlorotannin, dieckol isolated from Ecklonia cava with tyrosinase inhibitory activity. Bioorg Med Chem 20:311–316
Kim KN, Ham YM, Moon JY, Kim MJ, Kim DS, Lee WJ, Lee NH, Hyun CG (2009) In vitro cytotoxic activity of Sargassum thunbergii and Dictyopteris divaricata (Jeju Seaweeds) on the HL-60 tumour cell line. Int J Pharmacol 5:298--306
Kim KN, Ahn G, Heo SJ, Heo SJ, Kang SM, Kang MC, Yang HM, Kim D, Roh SW, Kim SK, Jeon BT, Park PJ, Jung WK, Jeon YJ (2013) Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Env Toxicol Pharmacol 35:39–46
Kwon KT, Uddin MS, Jung GW, Chun BS (2011) Preparation of micro particles of functional pigments by gas-saturated solution process using supercritical carbon dioxide and polyethylene glycol. Korean J Chem Eng 28:2044–2049
Lewey SA, Gorham J (1984) Pigment composition and photosynthesis in Sargassum muticum. Mar Biol 80:109–115
Maeda K, Fukuda M (1991) In vitro effectiveness of several whitening cosmetic components in human melanocytes. J Soc Cosmet Chem 42:361–368
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K (2005) Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun 332:392–397
Nomura M, Kamogawa H, Susanto E, Kawagoe C, Yasui H, Saga N, Hosokawa M, Miyashita K (2013) Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J Appl Phycol 25:1159–1169
Noviendri D, Jaswir I, Salleh HM, Taher M, Miyashita K, Ramli N (2011) Fucoxanthin extraction and fatty acid analysis of Sargassum binderi and S. duplicatum. J Med Plants Res 5:2405–2412
Piovan A, Seraglia R, Bresin B, Caniato R, Filippini R (2013) Fucoxanthin from Undaria pinnatifida: photostability and coextractive effects. Molecules 18:6298–6310
Quitain AT, Kai T, Sasaki M, Goto M (2013) Supercritical carbon dioxide extraction of fucoxanthin from Undaria pinnatifida. J Agric Food Chem 61:5792–5797
Ramful D, Tarnus E, Rondeau P, Da Silva CR, Bahorun T, Bourdon E (2010) Citrus fruit extracts reduce advanced glycation end products (AGEs)- and H2O2-induced oxidative stress in human adipocytes. J Agric Food Chem 58:11119–11129
Roh MK, Uddin MS, Chun BS (2008) Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using supercritical carbon dioxide with co-solvent. Biotechnol Bioproc Eng 13:724–729
Sánchez-Camargo AP, Meireles MAA, Ferreira ALK, Saito E, Cabral FA (2012) Extraction of ω-3 fatty acids and astaxanthin from Brazilian redspotted shrimp waste using supercritical CO2 + ethanol mixtures. J Supercrit Fluids 61:71–77
Shang YF, Kim SM, Lee WJ, Um BH (2011) Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J Biosci Bioeng 111:237–241
Yoon NY, Eom TK, Kim MM, Kim SK (2009) Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J Agric Food Chem 57:4124–4129
Acknowledgments
The authors are grateful to the Ministry of Science and Innovation of Spain (Res. Proj. Ref. CTM2012-38095) and to Xunta de Galicia (INBIOMED project) for the financial support. Both projects were partially funded by the FEDER Program of the European Union (“Unha maneira de facer Europa”). The authors thank Miguel Estévez for his skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Conde, E., Moure, A. & Domínguez, H. Supercritical CO2 extraction of fatty acids, phenolics and fucoxanthin from freeze-dried Sargassum muticum . J Appl Phycol 27, 957–964 (2015). https://doi.org/10.1007/s10811-014-0389-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0389-0