Abstract
Hepatocellular carcinoma (HCC) is a major health concern with a high morbidity and mortality rate worldwide. However, the mechanism underlying hepatocarcinogenesis remains unclear. Forkhead box P2 (FOXP2) has been implicated in various human cancer types. However, the role of FOXP2 in HCC remains unknown. Western blot and immunohistochemistry were used to measure the expression of FOXP2 protein in HCC and adjacent normal tissues in 50 patients. Wound healing and transwell assays were used to determine the cell invasion ability. We showed that the level of FOXP2 was significantly reduced in HCC compared with the adjacent non-tumorous tissue. There was statistical significance between the expression of FOXP2 and vein invasion (P = 0.017), number of tumor nodes (P = 0.028), and AFP (P = 0.033). Low expression of FOXP2 correlated with poor survival. Moreover, wound healing and transwell assays showed that FOXP2 could decrease cell invasion and affect the expression of vimentin and E-cadherin. Our results suggested that FOXP2 expression was downregulated in HCC tumor tissues, and reduced FOXP2 expression was associated with poor overall survival. In addition, downregulation of FOXP2 significantly enhanced cell invasiveness. These findings uncover that FOXP2 might be a new prognostic factor and be closely correlated with HCC cell invasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a major health concern with a high morbidity and mortality rate worldwide [1], with nearly 600,000 deaths occurring worldwide each year [2]. Liver transplantation and surgical resection are effective treatment of early-stage HCC. However, the overall survival for patients with HCCs remains poor [3]. In order to improve the overall survival of HCC patients, there is an urgent need to find novel valuable prognostic biomarkers and therapeutic targets for early diagnosis and effective treatment of HCC [4].
Transcription factors of the forkhead box P (FOXP1–4) family have been implicated in several types of human tumors [5]. Forkhead box P1 (FOXP1) has a range of functions and has been documented to play a role in the development of various solid tumor types [6–11]. FOXP1 can act as either a tumor suppressor or an oncogene [12]. FOXP1 protein was lost in breast cancer [13, 14], renal cell carcinoma [15], prostate cancer [16, 17], and early endometrial cancer [18]. In contrast, FOXP1 protein was overexpressed and as an oncogene in hepatocellular carcinoma [19]. FOXP3 was reportedly expressed in human melanoma cells and pancreatic cancer cells, whereas it was not observed in normal melanocytes and pancreatic duct cells [20, 21]. FOXP4 expression was reported to be inactivated by translocation in several breast cancer cell lines [22] and downregulated in kidney cancer [23].
Forkhead box P2 (FOXP2) is a member of forkhead box P family, which shares a common DNA-binding consensus sequence with other FOXP members [24–26]. Some studies reported that FOXP2 was highly expressed in the colon, ovary, liver intestine, and brain [27], as well as developing heart, lung, and gut tissues [28]. FOXP2 has mainly been reported to regulate speech and language development and neuronal development [29]. Apart from this, a number of evidence have indicated that FOXP2 has also been implicated in cancer development. Studies showed that FOXP2 overexpression served as a strong discriminator between normal lymphocytes and multiple myeloma [27]. In a recent study, FOXP2 has been suggested to play a role in prostate cancer development. Notably, FOXP2 expression is significantly correlated with tumor aggressiveness, especially in nonfusion type prostate cancer [30]. FOXP2 was reported to play an important role in breast cancer carcinogenesis, depletion of FOXP2 was adequate in promoting tumor initiation, and metastasis in BCCs and repressed FOXP2 expression are closely correlated with poor survival in breast cancer [31]. However, the role of FOXP2 expression in HCC remains to be evaluated.
In the present study, we investigated the biological and clinical significance of FOXP2 expression in human HCC. We found that FOXP2 expression was significantly reduced in human HCC specimens, and the downregulation of FOXP2 significantly enhanced cell invasiveness and regulated the expression of vimentin and E-cadherin. Our results imply that the repression of FOXP2 was associated with augmented cancer cell invasiveness and poor patient survival in human HCC.
Materials and methods
Patients and tissue samples
Fifty HCC sections and 8 HCC tissue samples from patients who underwent surgery without postoperative systemic chemotherapy were obtained from the Surgery Department at the Affiliated Hospital of Nantong University, between 2004 and 2005. Formalin-fixed and paraffin-embedded tissue specimens and clinicopathological data from these patients were collected. The main clinical and pathologic variables are shown in Table 1. Forty patients were men while 10 were women, and their average age was 43 years (range, 21–65). Tumors were classified as well (grade I; n = 11), moderately (grade II; n = 35), or poorly (grade III; n = 4) differentiated. The follow-up time was 5 years, with a range of 1–80 months.
Immunohistochemistry
In brief, sections were first deparaffinized in xylene and rehydrated through graded ethanol. For antigen retrieval, the sections were boiled in citrate buffer (0.01 M, pH 6.0) for 10 min at 105 °C. After endogenous peroxidase activity was blocked with 3 % H2O2 in PBS, 10 % goat serum was used to block any non-specific reactions for 1 h at room temperature and followed by incubation with a rabbit polyclonal anti-FOXP2 (1:50; Santa Cruz Biotechnology) for 2 h at room temperature. Then, the sections were washed with PBS twice and incubated with secondary antibodies (biotin-labeled; Santa Cruz) and third antibodies (peroxidase-labeled; Santa Cruz) and stained with DAB. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted in resin mount. Negative control sections were incubated with using a non-specific immunoglobulin IgG (diluted 1:50; Santa Cruz Biotechnology) instead of the primary antibody.
Immunohistochemical evaluation
For the assessment of FOXP2 expression, five high-power fields were randomly chosen and cell staining was examined under high-power magnification. More than 500 cells were counted to determine the labeling index (LI) [32]. The FOXP2 immunostaining score was calculated as both the staining intensity and the percentage of positively stained tumor cells. The intensity of FOXP2 staining was scored as no staining(0), weakly (1), moderately (2), or strongly (3). The percent positivity of FOXP2 was scored as follows:<10 %(0), 10–30 % (1), 31–50 % (2), 51–70 % (3), ≥71 % (4). The immunostaining score was calculated as the staining intensity score × the percentage positive score. Scores 0–4 were counted as low expression, while 5–12 were counted as overexpression [33].
Western blot analysis
Before immunoblotting, cells were washed three times with ice-cold PBS, resuspended in lysis buffer (0.5 % Nonidet P-40, 100 mM NaF, 200 μM Na3VO4, protease inhibitor mixture, 120 mM NaCl, 50 mM Tris–HCl), and then incubated at 4 °C for 20 min while rocking. Lysates were cleared by centrifugation (10 min, 12,000 rpm, 4 °C) collected the supernatant and examined protein concentrations with a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). The supernatant was diluted in 2× SDS loading buffer and boiled, and 50 μg of total protein was resolved by SDS–PAGE and transferred onto a polyvinylidene fluoride membrane (Immobilon; Millipore). The membranes were firstly blocked with 5 % dried skim milk in TBST (150 mM NaCl, 20 mM Tris, 0.05 % Tween-20) and then incubated with polyclonal antibody against using the primary antibodies described at room temperature for 2 h. Antibodies used were as follows: anti-FOXP2 (1:1000; Santa Cruz Biotechnology); anti-E-cadherin (1:1000; Santa Cruz Biotechnology); anti-vimentin (1:1000; Santa Cruz Biotechnology); anti-GAPDH (1:1,000; Sigma). After three times of washes, filters were incubated with horseradish peroxidase-linked IgG secondary human anti-mouse or anti-rabbit antibodies (1:1,000; Pierce). Detection of immunocomplexes was performed with an enhanced chemiluminescence system (NEN Life Science Products, Boston, MA, USA) [34].
Cell culture
The human HCC cell lines, SK-Hep1, Huh7, and SMCC-7721, and the L02 normal liver cell line were obtained from our laboratory and maintained in DMEM supplemented with 10 % fetal bovine serum (FBS), streptomycin 100 μg/mL, and penicillin 100 U/mL at 37 °C in 5 % CO2.
Plasmid constructs ShRNA and transfection
The RNAi species for the FOXP2 knockdown were synthesized by Enechem (Shanghai, China). The RNA interference (RNAi) FOXP2 sequence targeted were as follows: ShRNA1: 5′-TAAGTAATCCTGGACTGAT-3′; ShRNA2: 5′-AACTTGGAAGAATGCAGTA-3′; ShRNA3: 5′-TTAACAATGAACACGCATT-3′; ShRNA4: 5′-AGCAAACAAGTGGATTGAA-3′; and control: 5′-UUCUCCGAACGUGUCACGU-3′. Cell transfection was performed using Lipofectamine 2000 according to the manufacturer’s instructions.
Wound healing assays
The cells were seeded in 6-well plates and grown to confluence overnight. Forty-eight hours after transfection, cells were serum starved for 12 h and then scratched within the confluent cell layer using the fine end of a 10-μl pipette tip. They were also photographed under 20× objective lens every 24 h by inverted Leica phase-contrast microscope (Leica DFC 300 FX).
Transwell invasion assays
Cell invasion assay was performed in transwell chamber (Corning, 8 μm pore size). BD Matrigel Basement Membrane Matrix was assessed according to the manufacturer’s recommended protocol. Then, 0.5 ml of serum-free medium was placed in the upper chamber, and DMEM with 10 % FBS was added to the bottom chambers. Equal numbers (1 × 105) of cells were plated in the upper chamber of quadruplicate wells and incubated at 37 °C for 72 (SMCC-7721 cell) and 96 h (Huh7 cell). Cells were then fixed with paraformaldehyde, stained with crystal violet, to visualize the nuclei. Experiments were repeated four times.
Statistical analysis
All experiments were repeated at least three times. Data were presented as (mean ± SD). Statistical analysis was performed by using the SPSS 16.0 (standard version 16.0; SPSS, Chicago, IL, USA). P < 0.05 was considered significant. Expression of FOXP2 in HCC samples was analyzed by using x 2 test. Survival analysis was undertaken using the Kaplan–Meier method.
Results
FOXP2 expression in HCC
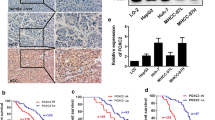
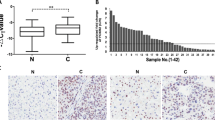
We first determined the expression of FOXP2 in 8 paired adjacent normal tissues and HCC biopsy samples. The expression of FOXP2 was dramatically reduced in tumor tissues compared with the adjacent normal tissues. GAPDH was used as a control for protein loading and integrity (Fig. 1a). We next examined the expression of FOXP2 in a panel of 3 HCC cell lines and a L02 normal liver cell line. It was found that FOXP2 was significantly highly expressed in SMCC-7721 and Huh7 HCC cell lines (Fig. 1b). These findings suggested that FOXP2 was downregulated in HCC specimens.
FOXP2 is lowly expressed in HCC tissues. a Western blot analysis showed that the expression of FOXP2 was decreased in HCC tissues. The bar chart demonstrates the ratio of FOXP2 protein to GAPDH by densitometry. (*P < 0.05). b FOXP2 protein had a low expression in the human hepatocellular carcinoma cell lines Huh7 and SMCC-7721 cells, compared with the normal liver cell line L02
Immunohistochemical analysis of FOXP2 expression in HCC clinical samples and its relationship to clinicopathological parameters
To determine whether the level of FOXP2 protein was associated with the clinicopathological parameters of HCC, immunohistochemical analysis of FOXP2 expression was carried out on tissue microarrays that contained 50 samples from HCC patients. FOXP2 expression in HCC was scored <5 as low expression, while FOXP2 expression in HCC was scored ≥5 as high expression. As shown in Fig. 2, representative examples of FOXP2 reactivity were observed in HCC specimens with the vein invasion. The relationship between FOXP2 expression and clinicopathologic parameters was further analyzed. There was statistical significance between the expression of FOXP2 and vein invasion (P = 0.017) and number of tumor nodes (P = 0.028) and AFP (P = 0.033) (Table 1). There were no statistical correlations between FOXP2 expression and the remaining clinicopathologic parameters, such as age, histological grade, cirrhosis, tumor size, capsular formation, HBsAg, cirrhosis, metastasis, and gender (P > 0.05).
FOXP2 expression and patient survival
We carried out Kaplan–Meier survival analysis to study the correlation between FOXP2 expression and patients’ survival. According to the Kaplan–Meier survival analysis, patients with low FOXP2 expression were likely to have a significantly shorter overall survival (Fig. 3, P < 0.05).
Univariate and multivariate analyses of prognostic variables in HCC patients
We carried out Cox proportional hazards model to examine the impact of FOXP2 expression and other clinicopathological parameters on the survival of HCC patients. FOXP2 expression was a significant prognostic factor in the univariate analysis (Table 2). The prognostic merit of FOXP2 expression was further examined using multivariate analysis. Results showed that FOXP2 could serve as independent predictor of patients’ survival. Thus, FOXP2 expression would be closely correlated with the overall survival of patients with HCC (P < 0.05, Table 2).
FOXP2 regulated the invasion in HCC cells
Based on the finding that FOXP2 was associated with vein invasion in HCC, we further studied the potential influence of FOXP2 on HCC cell invasion ability. We used shRNA to knockdown FOXP2 expression in the SMCC-7721 cells. SMCC-7721 cells were transiently transfected with FOXP2-ShRNA1–4 or control ShRNA. Forty-eight hours after transfection, FOXP2-ShRNA1 showed effective FOXP2 interference in the SMCC-7721 cells (>50 %) compared with control ShRNA (Fig. 4a). Wound healing assay showed that depletion of FOXP2 could promote cell invasion ability compared to control group in SMCC-7721 and Huh7 cells (Fig. 4b, c). Consistently, invasion assay demonstrated that silencing FOXP2 expression in SMCC-7721 cells and Huh7 cells could increase the number of invaded cells compared with control groups (Fig. 5a). We detected protein levels of EMT-associated genes in SMCC-7721 and Huh7 cells. Knocking down FOXP2 expression reduced the expression of E-cadherin but enhanced vimentin protein expression in SMCC-7721 and Huh7 cells (Fig. 5b). Thus, our data revealed that FOXP2 might regulate the invasion of HCC cells.
FOXP2 modulated the invasive ability of HCC cells. a Forty-eight hours after transiently transfected with FOXP2-ShRNA1–4 or control ShRNA, FOXP2 protein expression was detected using Western blot analysis in SMCC-7721 cells. Western blotting showed that FOXP2-ShRNA1 exhibited the best knockout efficiency. The bar chart below indicated the ratio of FOXP2 protein to GAPDH by densitometry. b, c Wound healing assay using SMCC-7721 and Huh7 cells. Migration of the cells to the wound was visualized at 0, 24, and 48 h with an inverted Leica phase-contrast microscope (200× magnification). The bar chart shows that the distance of cell migration (*P < 0.05)
FOXP2 expression inhibited cell invasion using transwell assays. a Interference of FOXP2 promoted the invasion of SMCC-7721 cells and Huh7 cells. The bar chart showed that the number of invasive cells was significantly higher in SMCC-7721 and Huh7 cells transfected with ShRNA than that of cells transfected with control cells. (*P < 0.05). b The effects of FOXP2 depletion on the expression of E-cadherin and vimentin. Knocking down FOXP2 expression reduced the expression of E-cadherin whereas enhanced vimentin protein expression in SMCC-7721 and Huh7 cells
Discussion
Forkhead box (Fox) family has becoming a promising therapeutic option for cancer therapy. FOXC1 inhibited the in vitro invasion and the in vivo pulmonary metastasis of MDA-MB-231HM cells [35]. FOXC2 played a central role in promoting invasion [36]. Downregulation of FOXM1 could lead to the inhibition of invasion of pancreatic cancer cells and U2OS osteosarcoma cells [37, 38]. Overexpression of FOXP3 inhibited cell invasion in epithelial ovarian cancer [39, 40]. FOXO3a promoted tumor cell invasion through the induction of MMPs [41]. However, the role of FOXP2 in the regulation of tumor metastasis and invasion remains virtually unknown.
In this study, we first detected FOXP2 protein expression in HCC tissue specimens and HCC cell lines using Western blot analysis. We found that FOXP2 was decreased in most primary HCC tumor tissues. We also found that patients with a low FOXP2 expression were likely to have a significantly shortened overall survival. In addition, multivariate analysis suggested that FOXP2 protein was an independent prognostic factor for overall survival. These findings uncovered that FOXP2 could be used as a new predictor of the prognosis of patients with HCC.
Next, we determined the potential role of FOXP2 in tumor cell invasion ability. We found that the downregulation of FOXP2 significantly enhanced cell invasiveness by wound healing assay and transwell assay. Increasing evidence indicates that epithelial to mesenchymal transition (EMT) plays an important role in tumor progression [42] and is postulated to be involved in tumor cell invasion and metastasis including HCC [43–47]. We examined the levels of EMT-associated proteins in SMCC-7721 and Huh7 cells. Depletion of FOXP2 expression reduced the expression of E-cadherin whereas enhanced the expression of vimentin in SMCC-7721 and Huh7 cells.
FOXP2 has been suggested to play a role in breast cancer. Studies have shown that MSC-deregulated microRNAs constitute a network that depresses the expression of FOXP2. Depletion of FOXP2 can cause the cancer cells to metastasize much more vigorously in BCCs [31]. Our data show that the downregulation of FOXP2 significantly enhanced cell invasiveness. Our study showed that FOXP2 expression had similar function in HCC.
In conclusion, our study suggests that FOXP2 plays an important role in HCC carcinogenesis especially in tumor invasion. Therefore, FOXP2 may serve as a novel molecular target for the detection and treatment of HCC.
References
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17.
Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–58.
Poon RT, Ng IO, Fan ST, Lai EC, Lo CM, Liu CL, et al. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001;19:3037–44.
Frau M, Biasi F, Feo F, et al. Prognostic markers and putative therapeutic targets for hepatocellular carcinoma. Mol Aspects Med. 2010;31:179–93.
Katoh M. Human FOX, gene family (Review). Int J Oncol. 2004;25:1495–500.
Koon HB, Ippolito GC, Banham AH, et al. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2007;11:955–65.
Green MR, Gandhi MK, Courtney MJ, et al. Relative abundance of full-length and truncated FOXP1 isoforms is associated with differential NF kappaB activity in follicular lymphoma. Leuk Res. 2009;33:1699–702.
Banham AH, Beasley N, Campo E, et al. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61:8820–9.
Wang B, Weidenfeld J, Lu MM, et al. FOXP1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–87.
Hu H, Wang B, Borde M, et al. FOXP1 is an essential transcriptional regulator of B cell development. Nat Immunol. 2006;7:819–26.
Shu W, Lu MM, Zhang Y, et al. FOXP2 and FOXP1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000.
Li C, Tucker PW. DNA-binding properties and secondary structural model of the hepatocyte nuclear factor 3/fork head domain. Proc Natl Acad Sci U S A. 1993;90:11583–7.
Fox SB, Brown P, Han C, et al. Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor alpha and improved survival in primary human breast carcinomas. Clin Cancer Res. 2004;10:3521–7.
Bates GJ, Fox SB, Han C, et al. Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptor-beta in primary invasive breast carcinomas. Breast Cancer Res Treat. 2008;111:453–9.
Toma MI, Weber T, Meinhardt M, et al. Expression of the forkhead transcription factor FOXP1 is associated with tumor grade and Ki67 expression in clear cell renal cell carcinoma. Cancer Investig. 2011;29:123–9.
Banham AH, Boddy J, Launchbury R, et al. Expression of the forkhead transcription factor FOXP1 is associated both with hypoxia inducible factors (HIFs) and the androgen receptor in prostate cancer but is not directly regulated by androgens or hypoxia. Prostate. 2007;67:1091–8.
Takayama K, Horie-Inoue K, Ikeda K, et al. FOXP1 is an androgen-responsive transcription factor that negatively regulates androgen receptor signaling in prostate cancer cells. Biochem Biophys Res Commun. 2008;374:388–93.
Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Loss of expression and nuclear/cytoplasmic localization of the FOXP1 forkhead transcription factor are common events in early endometrial cancer: relationship with estrogen receptors and HIF-1α expression. Mod Pathol. 2006;19:9–16.
Zhang Y, Zhang S, et al. Prognostic significance of FOXP1 as an oncogene in hepatocellular carcinoma. J Clin Pathol. 2012;65:528–33.
Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, et al. The regulatory T cell-associated transcription factor FOXP3 is expressed by tumor cells. Cancer Res. 2008;68:3001–9.
Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, et al. FOXP3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–50.
Howarth KD, Blood KA, Ng BL, et al. Array painting reveals a high frequency of balanced translocations in breast cancer cell lines that break in cancer-relevant genes. Oncogene. 2008;27:3345–59.
Teufel A, Wong EA, Mukhopadhyay M, et al. FOXP4, a novel forkhead transcription factor. Biochim Biophys Acta. 2003;1627:147–52.
Shu W, Yang H, Zhang L, et al. Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem. 2001;276:27488–97.
Wang B, Lin D, et al. Multiple domains define the expression and regulatory properties of FOXP1 forkhead transcriptional repressors. J Biol Chem. 2003;278:24259–68.
Li S, Weidenfeld J, et al. Transcriptional and DNA binding activity of the FOXP1/2/4 family is modulated by heterotypic and homotypic protein interactions. Mol Cell Biol. 2004;24:809–22.
Campbell AJ, Lyne L, Brown PJ, et al. Aberrant expression of the neuronal transcription factor FOXP2 in neoplastic plasma cells. Br J Haematol. 2010;149:221–30.
Schroeder DI, Myers RM. Multiple transcription start sites for FOXP2 with varying cellular specificities. Gene. 2008;413:42–8.
Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–23.
Stumm L, Burkhardt L, Steurer S, et al. Strong expression of the neuronal transcription factor FOXP2 is linked to an increased risk of early PSA recurrence in ERG fusion-negative cancers. J Clin Pathol. 2013;66:563–8.
Benjamin GC, Antoine C, George WB, et al. MSC-regulated MicroRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell. 2014;15:762–74.
Xu X, Yamamoto H, Sakon M, Yasui M, Ngan CY, Fukunaga H, et al. Over-expression of CDC25A phosphatase is associated with hyper-growth activity and poor prognosis of human hepatocellular carcinomas. Clin Cancer Res. 2003;9(5):1764–72.
Yu C, Chen K, Zheng H, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30:894–901.
Wang Y, Yang S, Ni Q, He S, Zhao Y, Yuan Q, et al. Over-expression of forkhead box J2 can decrease the migration of breast cancer cells. J Cell Biochem. 2012;113(8):2729–37.
Du J, Li L, Ou Z, Kong C, Zhang Y, Dong Z, et al. FOXC1, a target of polycomb, inhibits metastasis of breast cancer cells. Breast Cancer Res Treat. 2011;131:65–73.
Malin D, Kim IM, Boetticher E, Kalin TV, Ramakrishna S, Meliton L, et al. Forkhead box F1 is essential for migration of mesenchymal cells and directly induces integrin-beta3 expression. Mol Cell Biol. 2007;27:2486–98.
Wang IC, Chen YJ, Hughes DE, Ackerson T, Major ML, Kalinichenko VV, et al. FOXM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness. J Biol Chem. 2008;283:20770–8.
Bao B, Wang Z, Ali S, Kong D, Banerjee S, Ahmad A, et al. Over-expression of FOXM1 leads to epithelial–mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296–306.
Tallon B, Bhawan J. FOXP3 expression is increased in cutaneous squamous cell carcinoma with perineural invasion. J Cutan Pathol. 2010;37:1184–5.
Zhang HY, Sun H. Up-regulation of FOXP3 inhibits cell proliferation, migration and invasion in epithelial ovarian cancer. Cancer Lett. 2010;287:91–7.
Storz P, Doppler H, Copland JA, Simpson KJ, Toker A. FOXO3a promotes tumor cell invasion through the induction of matrix metalloproteinases. Mol Cell Biol. 2009;29:4906–17.
Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–205.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–74.
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–76.
Chen L, Chan TH, Yuan YF, Hu L, Huang J, Ma S, et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J Clin Invest. 2010;120:1178–91.
Compliance with ethical standards
We investigated 50 cases of liver cancer provided by the Surgery Department at the Affiliated Hospital of Nantong University. Ethics committee approval was obtained from the Institutional Ethics Committee of Affiliated Hospital of Nantong University to the commencement of the study, and written informed consent was obtained from every patient.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, X., Zhou, H., Zhang, T. et al. Downregulation of FOXP2 promoter human hepatocellular carcinoma cell invasion. Tumor Biol. 36, 9611–9619 (2015). https://doi.org/10.1007/s13277-015-3701-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3701-y