Abstract
Forkhead box R2 (FOXR2), a member of forkhead box (FOX) family, has been identified as an oncogene in medulloblastoma and breast cancer recently. However, the expression and function of FOXR2 in hepatocellular carcinoma cell (HCC) are still unclear. Here, we report that FOXR2 is frequently upregulated in 25/42 (59.5 %) of HCC specimens compared with neighboring non-cancerous tissues in messenger RNA (mRNA) level and further confirmed by immunohistochemistry analysis in protein level. Cellular function analyses revealed that FOXR2 promoted cell growth and colony formation, whereas knockdown of FOXR2 by RNA inference inhibited cell growth and decreased the growth ability of HCC cells in soft agar. Moreover, we also found FOXR2 overexpression facilitated the development of tumor xenografts in nude mice model. In addition, we validated β-catenin, Skp2, c-Myc, and Gli-1 as the potential downstream effectors of FOXR2 in the regulation of cell proliferation and malignancy by quantitative real-time PCR analysis. Collectively, our data suggest that FOXR2 promotes cell proliferation and malignancy in HCC and could be a novel promising therapeutic target for this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) serves as one of the most lethal cancers and the third leading cause of cancer-related death worldwide [1]. Although surgical resection is the main approach for HCC treatment, the overall survival of HCC remains poor due to the high rate of recurrence, metastasis, and lack of effective systemic chemotherapy. Currently, gene target therapy has been providing a new approach for treating cancers, which calls for a novel molecular marker as a treatment target [2]. Therefore, it is significant to find out an efficient and specific biomarker and figure out its molecular mechanism in the process of HCC development.
Forkhead box (FOX) family members are implicated in a broad range of biological processes, such as embryogenesis, proliferation, differentiation, apoptosis, transformation, longevity, metabolic homeostasis, and tumorigenesis [3–5]. At least 43 members constitute the human FOX family, from FOXA1 to FOXQ1 [6]. All the protein members can function as transcriptional factors regulating gene expression as the initial definition by containing a conserved DNA-binding motif (WD) of 100 amino acids. A great number of literatures have shown that the human FOX gene family is associated with carcinogenesis, whether promoting or suppressing cancer progression. For example, FOXM1 is a widely accepted oncogene and overexpressed in a series of neoplasm [7, 8]. FOXD3 is another FOX protein, which is involved in the suppression of cancer development. Reports have demonstrated that FOXD3 was downregulated in lung cancer, melanoma, and neuroblastoma for induction of tumorigenesis [9–11].
Forkhead box R2 (FOXR2, also named FOXN6), an atypical member of FOX gene family, has been little studied in cancer. One previous study has shown that human FOXR2 messenger RNA (mRNA) was expressed in breast cancer cell line and primary breast cancer [12]. Recently, FOXR2 is found to be highly expressed in breast cancer samples and correlates with poor prognosis [13]. In medulloblastoma, FOXR2 was also identified as an oncogene [14]. Notably, FOXR2 was strongly expressed in a small subset of medulloblastomas belonging to the SHH subgroup of medulloblastoma, indicating that FOXR2 may participate in SHH signaling pathway to mediate cancer development [15].
In the present study, we provide evidences that FOXR2 expression is frequently elevated in HCC samples compared with the adjacent normal liver tissues. We also conducted several in vitro and in vivo functional studies to confirm the oncogenic role of FOXR2 in regulating cell growth and malignancy, as well as the possible underlying mechanisms.
Materials and methods
Tissue specimens and cell culture
A total of 42 pairs of HCC and adjacent non-tumor tissues were obtained from HCC patients by way of surgery and with informed consent in Shanghai East Hospital. After resection, all the samples were strictly paired and frozen in liquid nitrogen and stored at −80 °C prepared for RNA extraction. The diagnoses of these liver cancer samples were determined by pathologists according to the criteria of the WHO. The use of human samples was approved by the Ethics Committee of the Shanghai East Hospital. The HCC cell lines Huh7, YY-8103, L02, Hep3B, HCC-LM3, and WRL68 were taken from our laboratory stocks. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) (Gibco, USA) and antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin). All cell lines were cultured in humidified incubator at 37 °C with 5 % CO2.
Immunohistochemistry
The paraffin-embedded tissue samples were collected from 30 HCC patients who had undergone initial surgery at the Shanghai East Hospital between 2014 and 2015. The slides were deparaffinized, rehydrated, and placed into citric acid buffer (pH 6.0, 0.1 M) for heating for 10 min. The endogenous peroxidase activity was then blocked by incubation with 3 % H2O2 for 10 min. Afterwards, sections were incubated with blocking buffer (Beyotime, China) for 1 h and then incubated overnight at 4 °C with FOXR2 antibody (1;100, Proteintech, Wuhan, China). The slides were subsequently incubated with the secondary antibody for 30 min at room temperature and then developed with the 3,3′-diaminobenzidine (DAB, Maixin-Bio, China) solution for 5 min followed by counterstaining with hematoxylin. Negative controls were incubated in blocking buffer alone. These results were only considered if these control samples demonstrated a negative staining.
RNA isolation and quantitative real-time PCR
RNA isolation was performed using Trizol reagent (Invitrogen, CA, USA) from HCC samples or cell lines. The PrimeScript®RT reagent Kit with gDNA Eraser (Takara, Otsu, Japan) was used to transcribe RNA into complementary DNA (cDNA) reversely according to the manufacturer’s recommendations. Quantitative real-time PCR (qRT-PCR) was run on ABI 7500 Real Time System with SYBER Green reagent (Takara, Japan). The following primer pairs were used to specially amplify the FOXR2, β-actin, Skp2, Gli-1, c-Myc, and β-catenin. FOXR2-F: 5′-TCAGTGTGCAGGAGATCTAC-3′ and FOXR2-R: 5′-TACCAAGATCAAAGAGAGAGGTCAAC-3′; β-actin-F: 5′-CCTGGCACCCAGCACAATG-3′ and β-actin-R: 5′- GGGCCGGACTCGTCATACT-3′; Skp2-F: 5′-CCAGCAAGACTTCTGAACTGC-3′ and Skp2-R: 5′-GAGGCACAGACAGGAAAAGA-3′; Gli-1-F: 5′-TCTGGACATACCCCACCTCCCTCTG-3′ and Gli-1-R: 5′-ACTGCAGCTCCCCCAATTTTTCTGG-3′; c-Myc-F: 5′-AGCCCACTGGTCCTCAAGA-3′ and c-Myc-R: 5′-CCTCTTACAGTTCTCCGCTTG-3′; β-catenin-F: 5′-TTGAAAATCCAGCGTGGACA-3′ and β-catenin-R: 5′-TCGAGTCATTGCATACTGTC-3′. The mRNA level of each sample was normalized to β-actin prior to comparative analysis using 2−△Ct method.
Construction of recombinant plasmid and RNA interference
The full-length cDNA of FOXR2 (NM_198451.3) was amplified from human liver cDNA library and cloned into the pcDNA3.1-myc expression vector to generate pcDNA3.1-FOXR2 expression plasmid. Accurate reading frame insertion was verified by DNA sequencing. Target-specific small inference RNAs (siRNAs) against FOXR2 (si-1, si-2) as well as a control (siNC) were chemically synthesized by GenePharma, Shanghai, China. The sense sequences for si-1: 5′-GCUCCCUAGAUGAGAUACAdTdT-3′; for si-2: 5′-GGUGUUAAGUAAAGUUAGAdTdT-3′; for siNC: 5′-UUCUCCGAACGUGUCACGUdTdT-3′. To stably overexpress or knock down FOXR2 expression, lentiviruses carrying FOXR2 ORF or shRNA (corresponding to si-1) were packaged and purchased from GenePharma, Shanghai. Stably infected cell lines were isolated by puromycin selection.
Cell transfection
Cell transfection with plasmids or siRNAs was performed with Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Western blot analysis
The Western blotting protocol was described previously [16]. Briefly, cell lysates were prepared using cold RIPA buffer with protease inhibitor cocktail (Roche, USA). The proteins were subjected to electrophoresis in SDS-PAGE (10 % w/v) and transferred onto a polyvinylidene difluoride membrane. The membrane was blocked in 5 % blocking buffer for 2 h at room temperature and then incubated with the primary antibody in PBST overnight at 4 °C. Incubation with the secondary antibody was performed for 1 h at room temperature. Anti-FOXR2 (Proteintech, Wuhan, China) and β-actin (Santa Cruz, USA) antibodies were used in this study. Detection of proteins was achieved by using the Bio-Rad Clarity Western chemiluminescence system (Bio-Rad, USA) according to the manufacturer’s instructions.
Cell proliferation and colony formation assay
HCC cells were seeded at 3000–4000 cells per well of 96-well plates in triplicate. Cell viability was measured every day using Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Japan) according to the manufacturer’s instructions. For the colony formation assay of L02 cells, 6000 cells were plated into each well of a six-well plate and were maintained in DMEM containing 10 % FBS and 0.6 mg/mL G418 (Life Technologies, USA) to allow colony formation. After 2 weeks, colonies were fixed with methanol and stained with 0.1 % crystal violet (Sigma, USA) in PBS for 15 min. The visible colonies were manually counted. Triplicate wells were measured for each treatment group.
Soft agar colony formation assay
HCC cells transfected with siRNAs against FOXR2 were grown in medium containing 1 % base agar and 0.5 % top agar. Additional culture media were overlaid every 4–5 days. After 10–14 days of culture, colonies were enumerated and pictures of the colonies were taken. The experiments were independently repeated at least three times.
Tumorigenicity assay in nude mice
Four- to five-week-old male nude mice (strain BALB/c) were obtained from SLAC Laboratories Animal, Shanghai, China, to study the role of FOXR2 in growth of xenograft tumor. For tumor growth assessment experiments, one million from stably infected cells (YY-8103-LV-FOXR2, WRL68-LV-shFOXR2) and appropriate control cells were subcutaneously implanted into the dorsal flank of the same mouse respectively. Tumor size was measured twice a week using calipers from 4 to 30 days after implantation, and the tumor volumes were estimated using the formula volume (mm3) = [width2 (mm2) × length (mm)]/2. Mice with tumors were sacrificed by cervical dislocation, and the tumor pairs were harvested and weighed. All animal handling and experimental procedures were approved by the Ethics Committee of Shanghai East Hospital.
Statistical analysis
Statistical analyses were performed using PRISM version 5.0 software (GraphPad Software, Inc, CA, USA). Only p < 0.05 was considered significant. “*” indicates p < 0.05; “**” indicates p < 0.01. All data were expressed as the means ± standard deviation (SD) and were analyzed using Student’s t test to compare two groups of in vitro and in vivo data.
Results
FOXR2 is frequently upregulated in HCC
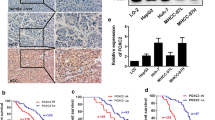
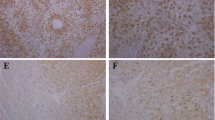
A quantitative real-time PCR (qRT-PCR) was performed on 42 pairs of clinical samples from HCC patients (tumor tissues and matched adjacent non-tumor liver tissues) and determined their FOXR2 mRNA levels. As a result, FOXR2 exhibited higher expression pattern in most of tumor tissues than in the matched adjacent non-tumor liver tissues (p < 0.01, Fig. 1a), where FOXR2 was upregulated in 25/42 (59.2 %) of the HCC specimens at more than 1.5-fold higher levels (Fig. 1b). To confirm this result in protein level, we explored FOXR2 expression in additional 30 pairs of human HCC specimens and non-tumor livers by immunohistochemical staining with a specific antibody against FOXR2. The resulting data showed that FOXR2 was significantly overexpressed in 46.7 % (14/30) of HCC tissues compared with their non-tumor tissues consistent with the qRT-PCR results. Two representative cases of the clinic samples handled with immunohistochemical staining were shown in Fig. 1c. However, the upregulation of FOXR2 in these patients was not statistically correlated with any clinicopathological characteristic. Collectively, this aberrant upregulation of FOXR2 in cancer tissues implicates it could play an important role in HCC.
Expression pattern of FOXR2 in HCC specimens. a qRT-PCR was conducted to detect the expression of FOXR2 in 42 pairs of HCCs (C) and non-HCC tissues (N). For each sample, the relative FOXR2 mRNA level was normalized to that of β-actin. p value as indicated was calculated by paired t test. **p < 0.01. b The change fold (C/N) of FOXR2 expression in each sample pair was respectively indicated as a column. c Two representative clinical cases of immunohistochemical staining with anti-FOXR2 antibody. Magnification ×200
FOXR2 overexpression significantly promotes HCC cell proliferation
To investigate the role of FOXR2 in HCC cell growth, pcDNA3.1-FOXR2 plasmid was transiently transfected into four HCC cell lines including Hep3B, Huh7, YY-8103, and L02. The Western blot results indicated that FOXR2 were successfully overexpressed in the four cell lines at protein level (Fig. 2a). Further, we observed that FOXR2 overexpression significantly increased the cell viability of each HCC cell line compared with the empty vector control group (Fig. 2b). Moreover, colony formation assays were carried out to further confirm the promotion of FOXR2 on cell growth. As shown in Fig. 2c, FOXR2 overexpression facilitated more colonies formed in L02 cells, supporting the notion from the cell viability assays that FOXR2 overexpression induces HCC cell growth.
FOXR2 overexpression promotes HCC cell proliferation and colony formation. a Western blot assay was used to detect ectopic expression of FOXR2 in Hep-3B, Huh7, YY-8103, and L02 cells, where β-actin was used as a loading control. b Ectopic expression of FOXR2 promoted cell proliferation in the above cells; cells carrying empty vector were used as a control. The experiments were repeated at least three times, and the points represent the mean values of triplicate wells (mean ± SD). c Exogenous FOXR2 expression promoted colony formation of L02 cells. *p < 0.05, **p < 0.01
Silencing of FOXR2 suppresses cell growth and malignancy
To evaluate whether downregulating FOXR2 expression suppresses HCC cell growth, two small interference RNAs (siRNAs), si-1 and si-2, against FOXR2 were chemically synthesized. The interference efficiency of these two siRNAs was examined by qRT-PCR and Western blot. Figure 3a demonstrated the efficient knockdown of FOXR2 at both protein level and RNA level in WRL68 and HCC-LM3 cells. As expected, FOXR2 knockdown reduced the cell viability of the two HCC cell lines compared with the control siNC (Fig. 3b). Next, we tested the effect of FOXR2 silencing on cell growth in soft agar. As known, this ability of cancer cells in soft agar growth reflects their malignancy. Compared with those cells transfected with siNC, the cells transfected with si-1 and si-2 formed smaller and fewer colonies in soft agar (Fig. 3c), indicating that FOXR2 knockdown decreased cancer cell malignancy. These data above strongly suggest that FOXR2 is involved in the regulation of cell growth and malignancy.
Silencing of FOXR2 by siRNAs inhibits cell growth. a Efficiency of FOXR2 knockdown with si-1 and si-2 in WRL68 and HCC-LM3 by Western blot and qRT-PCR analysis. b Silencing FOXR2 weakened cell growth of WRL68 and HCC-LM3 cells by CCK-8 method. c Silencing FOXR2 suppressed colony formation of the two cells as indicated in soft agar. *p < 0.05, **p < 0.01
FOXR2 promotes the tumorigenicity of HCC cells in vivo
To further elucidate the effect of FOXR2 on tumorigenicity in vivo, tumor formation assay in athymic nude mice was employed. As shown in Fig. 4a, the tumors derived from YY-8103 cells stably expressing FOXR2 were much bigger in size and significantly higher in weight than those of tumors formed from control cells (Fig. 4a). On the other hand, when we performed the same experiment with WRL68 cells stably silencing FOXR2, the tumors came from these cells showed a significant decrease in size and weight than control (Fig. 4b). These results suggest that FOXR2 plays an important role in tumorigenicity of HCC cells in vivo.
FOXR2 promotes tumor growth of subcutaneous xenograft of HCC cells in nude mice. A total of 1 × 106 YY-8103 cells stably expressing FOXR2 (a) or WRL68 cells stably silencing FOXR2 (b) were injected into nude mice (n = 5), respectively, while those with empty vector were used as controls. Tumor dimensions were examined using a digital caliper twice a week. The tumor growth process was observed for at least 4 weeks. Nude mice were executed in the end, and tumors were excised and weighed. *p < 0.05, **p < 0.01
FOXR2 expression levels are associated with β-catenin, Skp2, c-Myc, and Gli-1
To address the molecular mechanisms by which FOXR2 contributes to HCC cell proliferation and malignancy, we examined the expression of several tumor growth-related genes by qRT-PCR, including β-catenin, CyclinD1, Skp2, c-Myc, Gli-1, P21, FAK, HMGA2, P53, PTEN, STAT3, and FOXM1. The data indicated that the expression levels of β-catenin, Skp2, c-Myc, and Gli-1 were obviously perturbed with FOXR2 overexpression or knockdown in those cell lines tested. As shown in Fig. 5, when FOXR2 was overexpressed in YY-8103 and L02 cells, the four genes were also upregulated (except β-catenin and Gli-1 expression in YY-8103 cells), while FOXR2 was knocked down in WRL68 and HCC-LM3 cells, the four genes were along with downregulation, demonstrating that FOXR2 may transcriptionally regulate the expression of these genes. The consistent effects of FOXR2 on other genes were not observed in all four cell lines (data not shown). These findings demonstrate that β-catenin, Skp2, c-Myc, and Gli-1 may be involved in FOXR2-mediated cell proliferation and malignancy.
β-catenin, Skp2, c-Myc, and Gli-1 are regulated by FOXR2 in liver cancer cells. β-catenin, Skp2, c-Myc, Gli-1, as well as FOXR2 expression were detected by qRT-PCR in the stably infected cell lines as indicated respectively. With FOXR2 overexpression or knockdown, all these four genes expression were affected at transcription level accordingly
Discussion
As known, FOX gene family have involved to acquire a specialized function in many key biological processes. It is an ambitious project to research in detail the contribution of all of the FOX genes to tumor. In this study, we provided sufficient evidence to characterize the expression and role of one FOX gene, FOXR2, in the regulation of HCC development for the first time. Our data indicated that, in HCC clinical samples, FOXR2 was frequently upregulated in mRNA and protein level, consistent with other cancers such as breast cancer and medulloblastoma reported [13, 14]. These results strongly suggest that FOXR2 may act as a biomarker for cancer diagnosis and prediction. However, unlike in breast cancer, FOXR2 expression was not related to age, gender, tumor stage, tumor size, tumor number, tumor differentiation, and other clinicopathological parameters in our case. This may be owing to no sufficient clinical samples for statistics. Further study should be conducted to collect a large population of clinical samples of liver cancer in the future.
Regarding the roles of FOXR2 in HCC development, we used colony formation and cell viability assays to demonstrate increased or decreased expression of FOXR2 in HCC cell lines that significantly influenced cell proliferation. Also, we found that FOXR2 knockdown could decrease the ability of cell growth in soft agar which reflects tumor cell malignancy. In accordance with the data in vitro, it was observed that FOXR2 expression accelerated tumorigenecity of HCC cells in nude mice. On the whole, our findings provide new insights into the roles of FOXR2 on tumor cell proliferation and malignancy in HCC.
Due to the significant effect of FOXR2 gene on HCC cell growth and malignancy, it is necessary to address the underlying mechanisms by which FOXR2 induces tumorigenesis. We speculated that FOXR2 may regulate some crucial genes expression involved in cancer cell proliferation, malignancy, or metastasis. Therefore, we selected 12 genes, CTNNB1 (β-catenin), CyclinD1, Skp2, c-Myc, Gli-1, CDKN1A (P21), FAK, HMGA2, TP53, PTEN, STAT3, and FOXM1, as the candidates. qRT-PCR data demonstrated β-catenin, Skp2, c-Myc, and Gli-1 were modulated by FOXR2. The four factors are all well known responsible for cancer progression. β-catenin acts as a key protein in Wnt signal pathway, and the nuclear translocation of β-catenin activates some oncogenes in various carcinoma including gastric cancer, gynecologic cancer, and hepatoma [17, 18]. Skp2 is overexpressed in solid tumors and hematological malignancies and this oncoprotein could attach ubiquitin to its target proteins and destroy them through the 26S proteasome [19]. Besides, c-Myc plays an important role in the occurrence and progression of various tumors through transcriptionally regulating target gene, especially the cell cycle suppressor genes [20]. Gli-1 is central to the hedgehog pathway. One study showed that silencing Gli-1 induced apoptosis in Huh7 cells by downregulating Bcl-2 [21]. Our findings implied that FOXR2 may contribute to HCC through these signaling pathways mediated by the four factors in HCC.
In summary, we present data that FOXR2 is upregulated in HCC and plays oncogenic roles in cell proliferation and malignancy. Our findings shed light on the pathogenesis of HCC possibly induced by FOXR2 and provide a potential therapeutic target for HCC treatment.
References
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004.
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59.
Jackson BC, Carpenter C, Nebert DW, Vasiliou V. Update of human and mouse forkhead box (FOX) gene families. Hum Genomics. 2010;4:345–52.
Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–40.
Katoh M, Igarashi M, Fukuda H, Nakagama H, Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328:198–206.
Kuda M, Kohashi K, Yamada Y, Maekawa A, Kinoshita Y, Nakatsura T, Iwamoto Y, Taguchi T, Oda Y. FOXM1 expression in rhabdomyosarcoma: a novel prognostic factor and therapeutic target. Tumour Biol: J Int Soc Oncodevelopmental Biol Med. 2015
Halasi M, Gartel AL. FOX(M1) news—it is cancer. Mol Cancer Ther. 2013;12:245–54.
Yan JH, Zhao CL, Ding LB, Zhou X. FOXD3 suppresses tumor growth and angiogenesis in non-small cell lung cancer. Biochem Biophys Res Commun. 2015;466:111–6.
Weiss MB, Abel EV, Dadpey N, Aplin AE. FOXD3 modulates migration through direct transcriptional repression of TWIST1 in melanoma. Mol Cancer Res: MCR. 2014;12:1314–23.
Li D, Mei H, Qi M, Yang D, Zhao X, Xiang X, et al. FOXD3 is a novel tumor suppressor that affects growth, invasion, metastasis and angiogenesis of neuroblastoma. Oncotarget. 2013;4:2021–44.
Tian HP, Lun SM, Huang HJ, He R, Kong PZ, Wang QS, et al. DNA methylation affects the SP1-regulated transcription of FOXF2 in breast cancer cells. J Biol Chem. 2015;290:19173–83.
Song H, He W, Huang X, Zhang H, Huang T. High expression of FOXR2 in breast cancer correlates with poor prognosis. Tumour Biol: J Int Soc Oncodevelopmental Biol Med. 2015.
Koso H, Tsuhako A, Lyons E, Ward JM, Rust AG, Adams DJ, et al. Identification of FoxR2 as an oncogene in medulloblastoma. Cancer Res. 2014;74:2351–61.
Rahrmann EP, Watson AL, Keng VW, Choi K, Moriarity BS, Beckmann DA, et al. Forward genetic screen for malignant peripheral nerve sheath tumor formation identifies new genes and pathways driving tumorigenesis. Nat Genet. 2013;45:756–66.
Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D, et al. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009;69:3501–9.
Zhang X, Hao J. Development of anticancer agents targeting the Wnt/beta-catenin signaling. Am J Cancer Res. 2015;5:2344–60.
Song X, Xin N, Wang W, Zhao C. Wnt/beta-catenin, an oncogenic pathway targeted by H. pylori in gastric carcinogenesis. Oncotarget. 2015;6:35579–88.
Hao Z, Huang S. E3 ubiquitin ligase Skp2 as an attractive target in cancer therapy. Front Biosci (Landmark ed). 2015;20:474–90.
Ott G. Impact of MYC on malignant behavior. Hematol / Educ Program Am Soc Hematol Am Soc Hematol Educ Program. 2014;2014:100–6.
Chen XL, Cao LQ, She MR, Wang Q, Huang XH, Fu XH. Gli-1 siRNA induced apoptosis in Huh7 cells. World J Gastroenterol. 2008;14:582–9.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81302064), Shanghai Natural Science Foundation of China (13ZR1434000), Specialized Research Fund for the Doctoral Program of Higher Education (20130072120060), and Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (PWZxq2014-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Sample tissues were obtained from HCC patients by way of surgery and with informed consent in Shanghai East Hospital. The use of human samples and all animal handling and experimental procedures were approved by the Ethics Committee of the Shanghai East Hospital.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Wang, X., He, B., Gao, Y. et al. FOXR2 contributes to cell proliferation and malignancy in human hepatocellular carcinoma. Tumor Biol. 37, 10459–10467 (2016). https://doi.org/10.1007/s13277-016-4923-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4923-3